Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.601
Peer-review started: October 3, 2023
First decision: December 6, 2023
Revised: December 28, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 27, 2024
Processing time: 144 Days and 22 Hours
Gastric stromal tumors, originating from mesenchymal tissues, are one of the most common tumors of the digestive tract. For stromal tumors originating from the muscularis propria, compared with conventional endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFTR) can remove deep lesions and digestive tract wall tumors completely. However, this technique has major limitations such as perforation, postoperative bleeding, and post-polypec
A 47-year-old male patient had a hemispherical protrusion found during gastric endoscopic ultrasonography, located at the upper gastric curvature adjacent to the stomach fundus, with a smooth surface mucosa and poor mobility. The lesion was 19.3 mm × 16.1 mm in size and originated from the fourth ultrasound layer. Computed tomography (CT) revealed no significant evidence of lymph node enlargement or distant metastasis. Using conventional ESD technology for mucosal pre-resection, exposed EFTR was performed to resect the intact tumor in order to achieve a definitive histopathological diagnosis. Based on its morphology and immunohistochemical expression of CD117 and DOG-1, the lesion was proven to be consistent with a gastric stromal tumor. Six days after exposed EFTR, CT showed a large amount of encapsulated fluid and gas accumulation around the stomach. In addition, gastroscopy suggested intracavitary bleeding and abdominal puncture drainage indicated serosal bleeding. Based on these findings, the patient was diagnosed with serosal bleeding resulting in encapsulated abdominal hemorrhage after exposed EFTR for a gastric stromal tumor. The patient received combined treatments, such as hemostasis under gastroscopy, gastrointestinal decompression, and abdominal drainage. All examinations were normal within six months of follow-up.
This patient developed serous surface bleeding in the gastric cavity following exposed EFTR. Serosal bleeding resulting in an encapsulated hemoperitoneum is rare in clinical practice. The combined treatment may replace certain surgical techniques.
Core Tip: Postoperative bleeding is a common complication after exposed endoscopic full-thickness resection (EFTR), and is most commonly seen in the gastrointestinal tract. In the present case, following exposed EFTR for a gastric stromal tumor, bleeding was noted in both the gastric cavity and the serosal surface, which resulted in an encapsulated hemoperitoneum. The patient subsequently recovered after comprehensive treatment with gastrointestinal decompression, endoscopic hemostasis, B-ultrasound-guided abdominal puncture drainage, and anti-infection therapy.
- Citation: Lu HF, Li JJ, Zhu DB, Mao LQ, Xu LF, Yu J, Yao LH. Postoperative encapsulated hemoperitoneum in a patient with gastric stromal tumor treated by exposed endoscopic full-thickness resection: A case report. World J Gastrointest Surg 2024; 16(2): 601-608
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/601.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.601
Gastric stromal tumors, which are derived from mesenchymal tissues, are one of the most commonly diagnosed gastrointestinal tumors[1-4]. They typically occur in the gastric fundus, anterior wall of the gastric corpus, and anterior wall of the gastric antrum[5]. On account of their origin in the stomach wall, gastric stromal tumors can be classified into stromal tumors originating from the muscularis mucosa and those originating from the muscularis propria[1,6]. Endoscopic submucosal dissection (ESD) has been extensively performed to treat those tumors derived from the superficial muscularis mucosa[7,8]. By contrast, for gastric stromal tumors derived from the deep layers of the muscularis propria, especially those that grow outside the cavity, ESD may result in perforation and incomplete tumor excision[9].
Thus, muscularis propria-derived gastric stromal tumors are regarded as contraindications to endoscopic resection[10,11]. Moreover, these tumors are clinically removed by surgical or laparoscopic procedures. Recently, exposed endoscopic full-thickness resection (EFTR) for the treatment of gastric stromal tumors originating from the muscularis propria has obtained satisfactory therapeutic effects[12-14]. This report describes a case of postoperative encapsulated hemoperitoneum in a patient with a gastric stromal tumor treated by exposed EFTR.
A 47-year-old male patient was first admitted to our department for further evaluation and treatment of a gastric mass incidentally discovered by gastroscopy during a routine health check-up. One week after undergoing exposed EFTR, the patient was re-admitted to our department due to fatigue and anorexia for 2 d.
The patient did not have symptoms such as abdominal pain, diarrhea, nausea, or vomiting.
The patient had a history of hypertension and took medication regularly.
The patient was a non-smoker and did not drink alcohol. He had no significant family history.
Both during the first and second hospitalization, physical examination revealed no obvious abnormalities, and his abdomen was soft without tenderness and no palpable mass.
Before exposed EFTR in the first hospitalization, laboratory tests demonstrated normal routine blood tests, coagulative function, liver function, and renal function. Moreover, common serum tumor markers, including alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9, were normal.
Following exposed EFTR in the first hospitalization, on the day after surgery, the patient’s temperature was 37.5 °C, and routine blood tests showed the following: White blood cell (WBC) count: 14.5 × 109/L; neutrophil/granulocyte (NE) ratio: 85.6%; hemoglobin (HB): 127 g/L; and hypersensitive C reactive protein (hsCRP): 25.89 mg/L, which suggested post-polypectomy syndrome with fever and signs of inflammation. On the third day after surgery, all laboratory examinations showed normal results.
During the second hospitalization, laboratory examinations showed WBC count: 19.7 × 109/L; NE ratio: 82.9%; HB: 134 g/L; and hsCRP: 206.06 mg/L. Blood chemistry tests demonstrated signs of inflammation/infection. On the second day, laboratory examinations revealed WBC count: 12.3 × 109/L; NE ratio: 87.6%; HB: 114 g/L; and hsCRP: 161.16 mg/L. Blood tests indicated inflammatory and infectious complications; therefore, abdominal puncture fluid culture and blood culture were performed. Both aerobic and anaerobic cultures of the puncture fluid indicated infection with Klebsiella pneumoniae and Streptococcus parahaemolyticus, sensitivity to carbapenems, and negative blood culture.
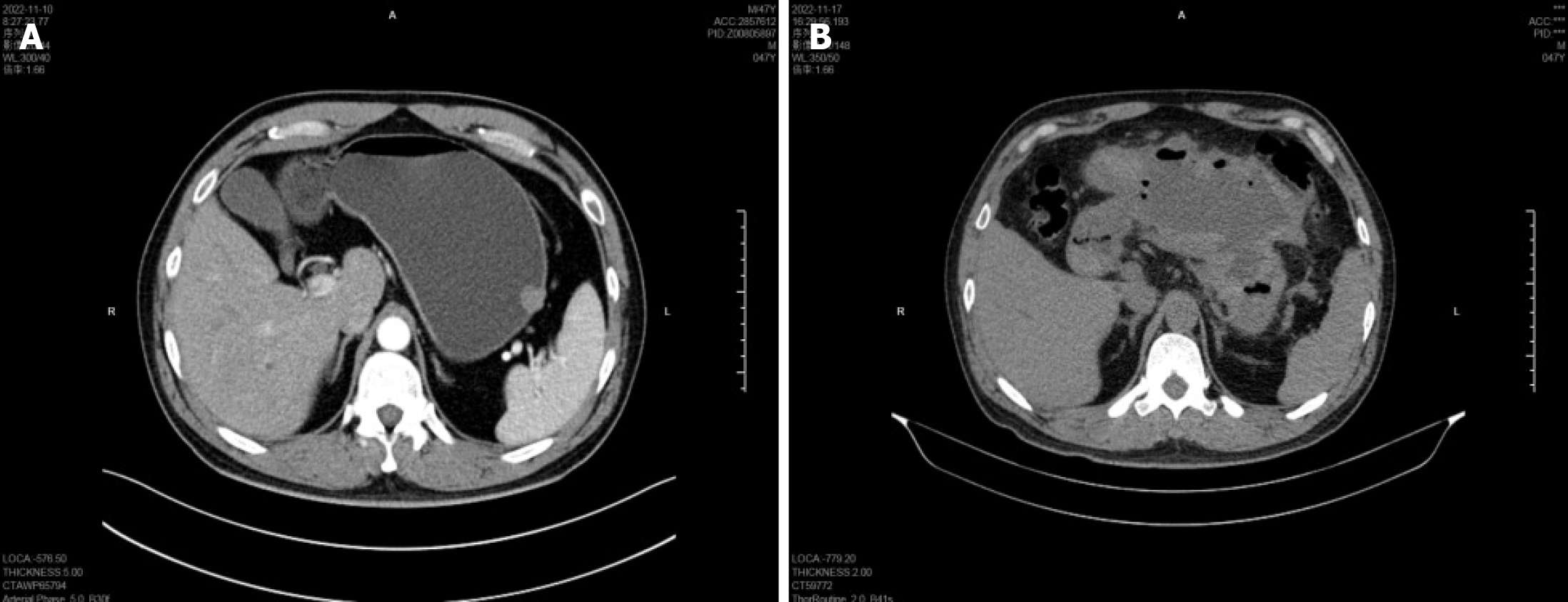
Prior to exposed EFTR, endoscopic examination revealed a hemispherical protrusion, approximately 16 mm in diameter, located at the upper gastric curvature adjacent to the stomach fundus, with a smooth surface mucosa and poor mobility (Figure 1A). Gastric endoscopic ultrasonography (EUS) examination revealed a mass 19.3 mm × 16.1 mm in size, derived from the muscularis propria, which grew outside the cavity. The echo pattern of the mass was uniform and at a low level (Figure 1B); however, the results of EUS were insufficient for diagnosis. Gastric computed tomography (CT) illustrated a well-demarcated mass 16 mm × 15 mm in size, demonstrating mild enhancement, with an intact mucosal line. Additionally, the CT scan showed no significant evidence of lymph node enlargement or distant metastasis (Figure 2A).
Following exposed EFTR, abdominal CT illustrated that titanium endoclips remained in the stomach, with a large amount of encapsulated fluid and gas accumulation around the stomach (Figure 2B). Emergency gastroscopy also revealed a small amount of dark red bloody liquid in the lower part of the stomach and duodenum. After surgery, multiple metal endoclips were observed on the surface of the wound, with obvious swelling of the anal mucosa and a small amount of bleeding on the surface. The wound was cauterized with hemostatic forceps and re-clamped with metal endoclips. Ultrasound examination showed that a mixed mass of gas and liquid was detected around the upper abdominal cavity and stomach, which was approximately 80 mm × 151 mm in size with an alveolate-like shape inside. These imaging features guided treatment by abdominal puncture drainage and gastrointestinal decompression.
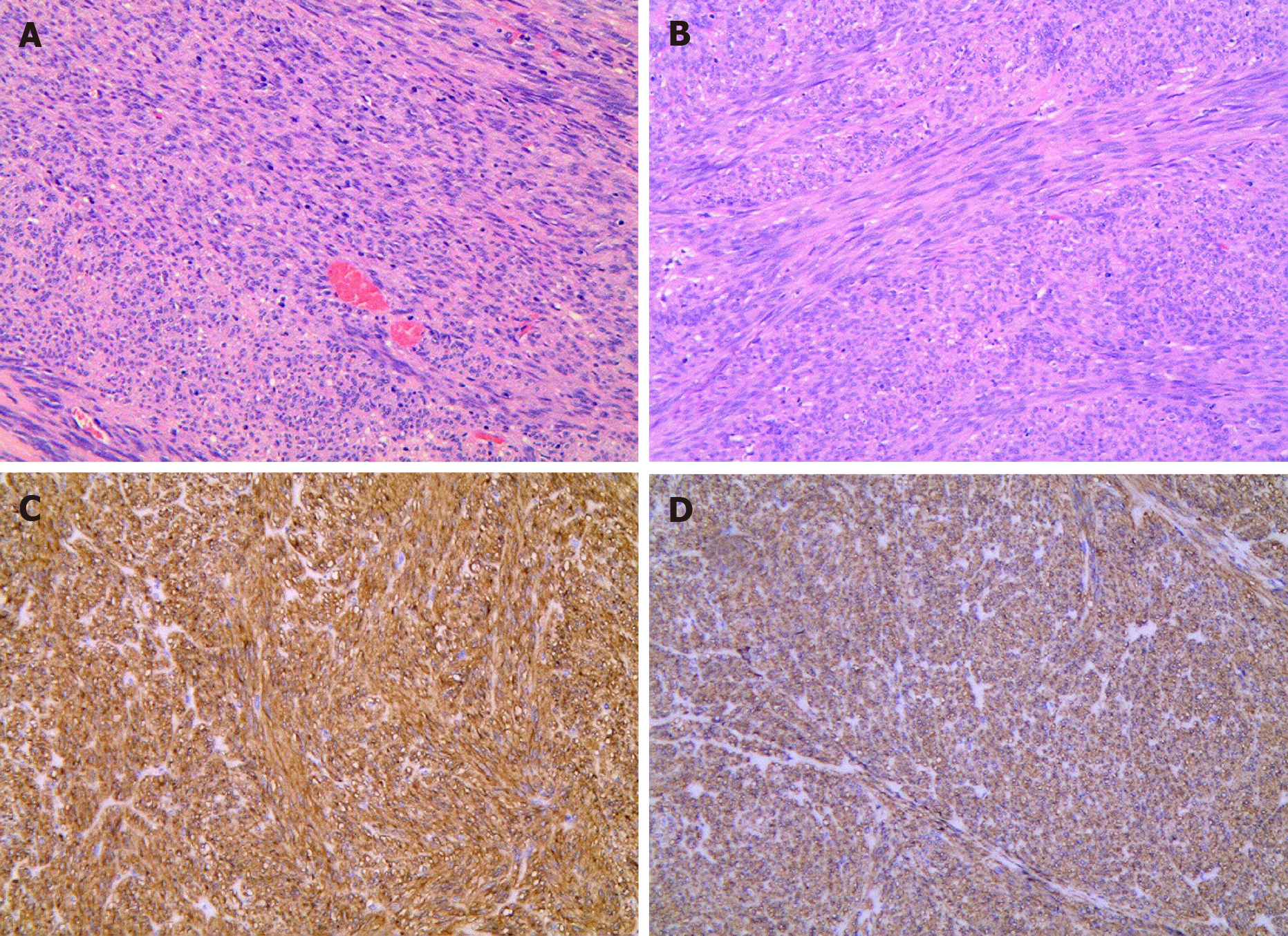
After preliminary examinations, exposed EFTR was performed to complete tumor excision and obtain histopathological results (Figure 3). On gross examination, the tumor was a well-shaped firm mass, white and yellow in color. Histopathological results illustrated that the tumor was located in the muscularis propria and was a spindle cell tumor, arranged in a bundle-like interweaving pattern with mild nuclear atypia (Figure 3A and B). Immunohistochemical staining was performed to determine tumor diagnosis, which displayed positive CD117 and DOG1 (Figure 3C and D). Due to these results, the histopathological diagnosis was in accordance with a gastric stromal tumor.
According to these examinations, during the second hospitalization, the patient was treated for postoperative complications including delayed bleeding, encapsulated hemoperitoneum, and abdominal infection following exposed EFTR for a gastric stromal tumor.
During the first hospitalization, the patient underwent exposed EFTR without laparoscopic assistance under general anesthesia during tracheal intubation on November 11, 2022. Following complete resection of the tumor, a metal clamp was applied to the defect wound in the gastric wall under endoscopy, with gradual clamping of the wound from both sides to the center. During the operation, close observation of changes in the abdomen was of vital significance, with the application of a 20G needle to puncture the right upper abdomen to alleviate pneumoperitoneum.
Postoperative treatment included maintaining a semi-reclined position, fasting, gastrointestinal decompression, intravenous infusion of 1.0 g cefotiam for anti-infection, intravenous infusion of omeprazole for acid suppression, and fluid replacement to maintain electrolyte balance. On the first and second day, 150 mL and 400 mL of yellow green liquid was drained by gastrointestinal decompression, respectively. On the third day, the gastrointestinal decompression tube was removed, followed by intake of a liquid diet and then a semi-liquid diet. After drinking and eating, the patient had no abnormal abdominal signs, and all laboratory examinations were normal. He was discharged on the 4th d after surgery.
During the second hospitalization, according to the blood chemistry tests, the patient received treatment with cefoperazone sodium and sulbactam sodium for injection to control infection. An intravenous infusion of omeprazole for acid suppression was also given, and other therapies were adopted to maintain electrolyte balance. Additionally, based on the endoscopic and imaging results, the patient received gastrointestinal decompression, and 210 mL of dark red bloody liquid was intermittently drained over 12 h. The patient was given norepinephrine nasal feeding and hemostasis treatment intermittently. On the second day of hospitalization, the antibiotic was upgraded to meropenem for anti-infection treatment. The subsequent findings of abdominal puncture fluid culture and blood culture demonstrated the correctness of changing the medication. Under ultrasound guidance, a puncture tube was placed to drain abdominal fluid, and dark red bloody liquid was extracted, which further confirmed the diagnosis of postoperative bleeding both in the gastric cavity and on the serosal surface after exposed EFTR, which formed an encapsulated hemoperitoneum in the abdominal cavity. The amount of encapsulated fluid in the abdominal cavity was reduced by gastrointestinal decompression drainage and abdominal puncture drainage, during which 0.9% physiological saline was used for intermittent flushing and drainage.
After comprehensive treatment by endoscopic hemostasis, abdominal puncture and drainage, and anti-infection, this treatment regimen was considered efficacious. The patient gradually recovered following bleeding and abdominal infection caused by exposed EFTR and was ultimately discharged on the 13th d after admission.
Based on the Chinese expert consensus on the endoscopic diagnosis and treatment of gastrointestinal stromal tumor (2020, Beijing), it is recommended that such patients undergo endoscopic examination within 1 year after endoscopic treatment, to evaluate wound healing and tumor recurrence at 3 mo, 6 mo, and 12 mo after surgery. CT scans can be performed every 6-12 mo within 5 years after surgery, but our patient’s compliance was poor. The most recent gastroscopy review at 11 mo after surgery did not show tumor recurrence, and routine blood examinations indicated that hsCRP was completely normal. Additionally, abdominal ultrasound indicated that there was no obvious mass echo or liquid in the abdominal cavity.
For gastric stromal tumors originating from the muscularis propria that grow in an extraluminal manner or are closely connected to the serosal layer, routine ESD is prone to perforation and incomplete tumor resection[9,15-17]. Instead, exposed EFTR without laparoscopic assistance is capable of completely removing the tumor and reducing the recurrence rate[18,19]. Therefore, exposed EFTR, as a new minimally invasive technology, has therapeutic potential for patients with gastrointestinal submucosal tumors[20-23]. In addition, compared with surgical procedures, exposed EFTR has advantages such as less trauma and maintains the original anatomy and function of the stomach to an extent[24,25]. However, the most crucial issue for exposed EFTR is ensuring the complete closure of the gastric resection site in order to decrease the probability of complications, such as delayed hemorrhage, perforation, infections, and abdominal abscesses[26-29]. Furthermore, serious complications may be life-threatening[30]. Thus, the prevention and treatment of complications are of vital significance.
Postoperative hemorrhage is one of the most common complications following exposed EFTR[31,32]. In clinical practice, hemorrhage is divided into intraoperative bleeding and delayed bleeding based on the time of bleeding[33-35]. Moreover, according to the bleeding location, it can be classified into intracavitary bleeding and serosal bleeding[36]. Postoperative bleeding has a close association with the location of the lesion, and patients with tumors located at the junction of the gastric fundus are prone to delayed bleeding after exposed EFTR[37]. In this case, the lesion was located at the junction of the gastric fundus. After surgery, the gastric wall defect was completely clamped from both sides of the wound to the center with metal clips under direct vision via the endoscope, and postoperative hemorrhage subsequently occurred.
Previous reports have shown that postoperative bleeding after exposed EFTR is mainly manifested as hematemesis, melena, hematochezia, and decreased blood pressure[32,37]. However, in this patient, poor appetite and fatigue were the main symptoms, with bleeding in both the gastric cavity and the serous surface. Additionally, serous surface bleeding led to an encapsulated hemoperitoneum in the abdominal cavity, which is relatively rare in clinical practice. The postoperative bleeding in this patient may have been the result of the abundant blood supply to the gastric body and the large diameter of the arteries. The large wound surface in exposed EFTR can easily cause extra-serous blood vessel injury and slow recovery of the full-layer defect of the stomach wall, and the wound surface of the stomach body can easily cause blood vessel exposure or even blood vessel destruction after overeating. All these reasons could have led to postoperative hemorrhage in this patient.
In this case, gastrointestinal decompression drainage of bright red bloody fluid illustrated intracavitary bleeding. Thus, we achieved cessation of bleeding under endoscopy. With regard to a treatment strategy for serous surface bleeding forming an encapsulated hemoperitoneum in the abdominal cavity after exposed EFTR, to date, there is no relevant literature on this issue. Based on clinical experience, we carried out intraperitoneal puncture drainage to drain dark red bloody fluid via abdominal ultrasound. In addition, we adopted intermittent postoperative saline irrigation, clamping, or opening of drainage tubes to monitor bleeding and reduce infection. The encapsulated effusion sac gradually shrank and then disappeared completely.
The combination therapy approach involving abdominal puncture and drainage under ultrasound guidance combined with antibiotics for anti-infection treatment proved to be effective in this patient. There are potential effects when adopting ultrasound-guided puncture and drainage for intra-abdominal hemorrhage. First, it can clarify the nature of intra-abdominal effusion and monitor bleeding. Second, intermittent drainage and flushing can reduce the incidence of infection and facilitate the absorption of intra-abdominal hemorrhage. Third, puncture fluid culture is capable of determining the source of infection and providing guidance for the utilization of sensitive antibiotics. However, reducing postoperative bleeding at the origin is of vital importance. The adoption of closure techniques is capable of achieving a full-thickness post-EFTR defect closure for the purpose of reducing EFTR-related complications[38]. To date, the closure of transmural defects after exposed EFTR is largely achieved through through-the-scope (TTS) clips or clips combined with endoloops. However, owing to the superficial bite of the clips, full thickness defect closure is difficult to achieve by these techniques. Thus, an appropriate closure approach is crucial for exposed EFTR to promote its effectiveness and safety. Compared with both TTS clips and endoloops, the Ovesco over-the-scope clip system and OverStitch endoscopic suturing system have the significant advantages of realizing full-thickness closure and incorporating the muscularis propria layer, but the main limitation of these techniques is their high cost. However, the cost-effectiveness of post-EFTR defect closure using endosuturing requires further investigation in light of its potential capability of reducing complications, hospitalization, and the need for surgery.
Hemorrhage is a common complication after exposed EFTR. In this case, both the gastric cavity and the serosal surface showed bleeding, and the serosal surface bleeding formed an encapsulated hemoperitoneum in the abdominal cavity, which is rare in clinical practice. Clinical diagnosis and treatment require timely gastrointestinal decompression and gastroscopy examination. Early identification and diagnosis of delayed bleeding after exposed EFTR are crucial. While there are no obvious symptoms in the early stage, abdominal CT scan is crucial to identify specific complications. In addition, timely abdominal ultrasound examination and abdominal puncture and drainage under ultrasound guidance in combination with antibiotics for anti-infection are necessary. Our patient experienced delayed bleeding, encapsulated hemoperitoneum, and abdominal infection after exposed EFTR, which resolved after non-surgical treatment.
| 1. | Huang LY, Cui J, Liu YX, Wu CR, Yi DL. Endoscopic therapy for gastric stromal tumors originating from the muscularis propria. World J Gastroenterol. 2012;18:3465-3471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Sharma AK, Kim TS, Bauer S, Sicklick JK. Gastrointestinal Stromal Tumor: New Insights for a Multimodal Approach. Surg Oncol Clin N Am. 2022;31:431-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Serrano C, George S. Gastrointestinal Stromal Tumor: Challenges and Opportunities for a New Decade. Clin Cancer Res. 2020;26:5078-5085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 4. | Arshad J, Costa PA, Barreto-Coelho P, Valdes BN, Trent JC. Immunotherapy Strategies for Gastrointestinal Stromal Tumor. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 298] [Article Influence: 59.6] [Reference Citation Analysis (1)] |
| 6. | Khan S, Cui X, Nasir S, Rafiq SM, Qin B, Bai Q. Advances in endoscopic resection techniques of small gastric tumors originating from the muscularis propria. Front Oncol. 2022;12:1001112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Pasquer A, Poncet G, Rostain F, Rivory J, Hervieu V, Périnel J, Pioche M. Successful non-exposed endoscopic wall-inversion surgery for gastric stromal tumor and gastric ESD for dysplastic lesion during a single procedure. Endoscopy. 2021;53:E452-E454. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Rajravelu RK, Ginsberg GG. Management of gastric GI stromal tumors: getting the GIST of it. Gastrointest Endosc. 2020;91:823-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Du C, Chai N, Linghu E, Li H, Zhai Y, Li L, Tang X, Wang H, Tang P. Clinical outcomes of endoscopic resection for the treatment of gastric gastrointestinal stromal tumors originating from the muscularis propria: a 7-year experience from a large tertiary center in China. Surg Endosc. 2022;36:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Lai EC, Lau SH, Lau WY. Current management of gastrointestinal stromal tumors--a comprehensive review. Int J Surg. 2012;10:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (9)] |
| 12. | Chen T, Zhang YW, Lian JJ, Zhang HB, Xu AP, Li F, Yan XH, Duan BS, Zhao ZY, Chu Y, Shen L, Cao J, Zhang L, Zheng L, Chu SG, Xu MD. No-touch endoscopic full-thickness resection technique for gastric gastrointestinal stromal tumors. Endoscopy. 2023;55:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Yang J, Ni M, Jiang J, Ren X, Zhu T, Cao S, Hassan S, Lv Y, Zhang X, Wei Y, Wang L, Xu G. Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤1.5 cm) gastric GI stromal tumors. Gastrointest Endosc. 2022;95:660-670.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Granata A, Martino A, Ligresti D, Tuzzolino F, Lombardi G, Traina M. Exposed endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors: A systematic review and pooled analysis. Dig Liver Dis. 2022;54:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Longcroft-Wheaton G, Bhandari P. Endoscopic resection of submucosal tumors. Expert Rev Gastroenterol Hepatol. 2015;9:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Hsiao SW, Chen MW, Yang CW, Lin KH, Chen YY, Kor CT, Huang SP, Yen HH. A Nomogram for Predicting Laparoscopic and Endoscopic Cooperative Surgery during the Endoscopic Resection of Subepithelial Tumors of the Upper Gastrointestinal Tract. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Jiao R, Zhao S, Jiang W, Wei X, Huang G. Endoscopic Submucosal Dissection of Gastrointestinal Stromal Tumours: A Retrospective Cohort Study. Cancer Manag Res. 2020;12:4055-4061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Hsu WH, Wu TS, Hsieh MS, Kung YM, Wang YK, Wu JY, Yu FJ, Kuo CH, Su YC, Wang JY, Wu DC, Hu HM. Comparison of Endoscopic Submucosal Dissection Application on Mucosal Tumor and Subepithelial Tumor in stomach. J Cancer. 2021;12:765-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014;12:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan T, Wang X, Lv L, Huo J, Liu D. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. 2017;31:3376-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Duan TY, Tan YY, Wang XH, Lv L, Liu DL. A comparison of submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric fundus submucosal tumors. Rev Esp Enferm Dig. 2018;110:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Andalib I, Yeoun D, Reddy R, Xie S, Iqbal S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc. 2018;32:1787-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Yip HC, Teh JL, Teoh AYB, Chiu P. Pure endoscopic resection versus laparoscopic assisted procedure for upper gastrointestinal stromal tumors: Perspective from a surgical endoscopist. Dig Endosc. 2023;35:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Liu S, Zhou X, Yao Y, Shi K, Yu M, Ji F. Resection of the gastric submucosal tumor (G-SMT) originating from the muscularis propria layer: comparison of efficacy, patients' tolerability, and clinical outcomes between endoscopic full-thickness resection and surgical resection. Surg Endosc. 2020;34:4053-4064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Xiu H, Zhao CY, Liu FG, Sun XG, Sun H, Liu XS. Comparing about three types of endoscopic therapy methods for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Scand J Gastroenterol. 2019;54:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Ren Z, Lin SL, Zhou PH, Cai SL, Qi ZP, Li J, Yao LQ. Endoscopic full-thickness resection (EFTR) without laparoscopic assistance for nonampullary duodenal subepithelial lesions: our clinical experience of 32 cases. Surg Endosc. 2019;33:3605-3611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Høgh A, Deding U, Bjørsum-Meyer T, Buch N, Baatrup G. Endoscopic full-thickness resection (eFTR) in colon and rectum: indications and outcomes in the first 37 cases in a single center. Surg Endosc. 2022;36:8195-8201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Liu L, Xu X, Ye Y, Shi D, Li R, Chen W. Risk factors for conversion from endoscopic resection to laparoscopic resection for gastric gastrointestinal stromal tumors. J Int Med Res. 2023;51:3000605231167796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Liu L, Ye Y, Wang Q, Feng Y, Shi D, Li R, Lu F, He B, Xu X. Risk factors for postoperative complications in endoscopic resection of gastric gastrointestinal stromal tumors: a multi-center analysis. Surg Endosc. 2023;37:6844-6851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Li B, Shi Q, Qi ZP, Yao LQ, Xu MD, Lv ZT, Yalikong A, Cai SL, Sun D, Zhou PH, Zhong YS. The efficacy of dental floss and a hemoclip as a traction method for the endoscopic full-thickness resection of submucosal tumors in the gastric fundus. Surg Endosc. 2019;33:3864-3873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Mori H, Kobara H, Nishiyama N, Masaki T. Current status and future perspectives of endoscopic full-thickness resection. Dig Endosc. 2018;30 Suppl 1:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Sun H, Cao T, Zhang F, Tao K, Xu H. Gastric defect closure after endoscopic full-thickness resection: the closing while dissecting technique. Surg Endosc. 2023;37:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | von Renteln D, Rösch T, Kratt T, Denzer UW, El-Masry M, Schachschal G. Endoscopic full-thickness resection of submucosal gastric tumors. Dig Dis Sci. 2012;57:1298-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Wang X, Xiong G, Qian Y, Wang H, Liu L, Miao L, Fan Z. Complete defect closure of gastric submucosal tumors with purse-string sutures. Surg Endosc. 2014;28:1844-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Guo J, Liu Z, Sun S, Liu X, Wang S, Ge N, Wang G, Qi Y. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015;29:3356-3362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Tada N, Kobara H, Nishiyama N, Fujihara S, Masaki T, Uedo N. Current Status of Endoscopic Full-Thickness Resection for Gastric Subepithelial Tumors: A Literature Review Over Two Decades. Digestion. 2023;104:415-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Granata A, Martino A, Ligresti D, Zito FP, Amata M, Lombardi G, Traina M. Closure techniques in exposed endoscopic full-thickness resection: Overview and future perspectives in the endoscopic suturing era. World J Gastrointest Surg. 2021;13:645-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Duan JX, China; Martino A, Italy; Soldera J, Brazil S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Zhao YQ