Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.451
Peer-review started: November 21, 2023
First decision: December 5, 2023
Revised: December 16, 2023
Accepted: January 23, 2024
Article in press: January 23, 2024
Published online: February 27, 2024
Processing time: 96 Days and 6.6 Hours
Colorectal cancer (CRC) has one of the highest morbidity and mortality rates among digestive tract tumors. Intra-abdominal infection (IAI) is a common postoperative complication that affects the clinical outcomes of patients with CRC and hinders their rehabilitation process. However, the factors influencing abdominal infection after CRC surgery remain unclear; further, prediction models are rarely used to analyze preoperative laboratory indicators and postoperative complications.
To explore the predictive value of preoperative blood markers for IAI after radical resection of CRC.
The data of 80 patients who underwent radical resection of CRC in the Anorectal Surgery Department of Suzhou Hospital affiliated with Anhui Medical University were analyzed. These patients were categorized into IAI (n = 15) and non-IAI groups (n = 65) based on whether IAI occurred. Influencing factors were compared; general data and laboratory indices of both groups were identified. The relationship between the indicators was assessed. Further, a nomogram prediction model was developed and evaluated; its utility and clinical applicability were assessed.
The risk factors for IAI after radical resection of CRC were neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and carcinoembryonic antigen (CEA) levels. NLR was correlated with PLR and SII (r = 0.604, 0.925, and 0.305, respectively), while PLR was correlated with SII (r = 0.787). The nomogram prediction model demonstrated an area under the curve of 0.968 [95% confidence interval (CI): 0.948-0.988] in the training set (n = 60) and 0.926 (95%CI: 0.906-0.980) in the validation set (n = 20). The average absolute errors of the calibration curves for the training and validation sets were 0.032 and 0.048, respectively, indicating a good model fit. The decision curve analysis curves demonstrated high net income above the 5% threshold, indicating the clinical practicality of the model.
The nomogram model constructed using NLR, PLR, SII, and CEA levels had good accuracy and reliability in predicting IAI after radical resection of CRC, potentially aiding clinical treatment decision-making.
Core tip: Intra-abdominal infection (IAI) is a common and serious complication following the radical resection of colorectal cancer (CRC) that affects the efficacy of surgery, prolongs hospital stay, and hinders the postoperative rehabilitation process of patients. In this study, the clinical data of 80 patients who underwent radical resection for CRC were retrospectively analyzed. Based on whether IAI occurred, patients were divided into IAI and non-IAI groups. The relationship between IAI and preoperative neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, systemic immune-inflammation index, and carcinoembryonic antigen levels in patients after radical resection of CRC was studied, and a prediction model with good prediction accuracy was developed.
- Citation: Liu CQ, Yu ZB, Gan JX, Mei TM. Preoperative blood markers and intra-abdominal infection after colorectal cancer resection. World J Gastrointest Surg 2024; 16(2): 451-462
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/451.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.451
Colorectal cancer (CRC) is a common pernicious tumor of the alimentary system. In Western countries, such as Europe and the United States, CRC is the second leading cause of death from malignant tumors[1], and its morbidity and mortality rates are the highest among those of digestive tract tumors in China[2]. CRC treatment options include surgery, chemotherapy, teletherapy, and immunotherapy. Radical resection is the only curative treatment available for CRC. With advancements in CRC diagnosis and therapy, early detection rates and the number of radical surgeries are gradually increasing. Consequently, patients’ survival time and health-related quality of life have also improved[3]. However, patients may experience complications after radical surgery, including postoperative bleeding, anastomotic leakage, incision infections, and abdominal infections. Among these complications, abdominal infection is the most common and severe postoperative complication of radical CRC resection. Studies have shown that abdominal infections can cause prolonged hospital stays and trigger systemic inflammatory response syndrome in patients with CRC, which can lead to sepsis and death in severe cases[4,5].
The interaction between systemic inflammation and the local immune response is considered the seventh marker of cancer and has been confirmed to be related to the emergence and advancement of several malignancies[6]. Several studies have shown that relevant inflammatory indicators, including the neutrophil-lymphocyte ratio (NLR)[7], platelet (PLT)-lymphocyte ratio (PLR)[8], and systemic immune-inflammation index (SII)[9], can be used as prognostic factors for various tumors. Among them, NLR is a clear indicator of the systemic inflammatory response, which can monitor the dynamic balance between pro-tumor and anti-tumor immunity in patients by combining neutrophil and lymphocyte (LY) factors. Previous research has shown that the NLR is significantly associated with the prognosis of various common tumors, such as CRC[10], lung cancer[11], gynecological cancer[12], and esophageal cancer[13]. PLR, an indicator derived from PLT and LY counts, can also help monitor the dynamic balance between pro-tumor and anti-tumor effects in patients with tumors and is related to the pathology and prognosis of various clinical tumors[14,15]. A newer indicator, SII, which incorporates neutrophils, PLTs, and LYs, was proposed to report the immune inflammatory response within the system[16]. Chen et al[17] confirmed the predictive value of SII in patients with CRC. Carcinoembryonic antigen (CEA) is a tumor marker. It is an acidic glycoprotein with human embryonic antigen characteristics and assists in tumor cell aggregation, adhesion, invasion, and metastasis[18,19]. Zhai et al[20] found that elevated serum CEA levels in patients are correlated with postoperative complications. However, the specific factors influencing abdominal infections after CRC surgery remain unclear, with few studies covering the complete spectrum of these factors. Therefore, it is necessary to identify the markers that can accurately predict the occurrence of abdominal infection in patients with CRC after radical resection of CRC and help select optimal treatment strategies to improve patient outcomes.
Currently, prediction models can be visualized using a column graph and have been widely used as a reliable tool for risk prediction[21] and have a good guiding value in disease prediction. In clinical practice, prediction models are mostly used to predict the prognosis and survival of patients with CRC[21-24]; however, they are rarely used to analyze preoperative laboratory indicators and postoperative complications.
Therefore, this study aimed to explore the correlation between preoperative NLR, PLR, SII, and CEA levels and intra-abdominal infections (IAIs) in patients after radical CRC resection and develop a prediction model to identify risk factors for abdominal infection after radical CRC resection. This study provides insights for clinicians regarding the treatment of complications after CRC surgery.
This retrospective study included 80 patients who were admitted to the Anorectal Surgery Department of Suzhou Hospital, affiliated with Anhui Medical University, between January 2021 and March 2023 and underwent radical surgery for CRC. The research process is illustrated in Figure 1. Based on whether they had postoperative IAI, the patients were divided into an IAI group (n = 15) and a non-IAI group (n = 65). The inclusion criteria were as follows: (1) CRC diagnosis confirmed by colonoscopy before operation and pathological examination after operation; (2) complete clinical and pathological data, including NLR, PLR, SII, and CEA test results from peripheral blood taken 1 wk before surgery; (3) ability to communicate properly; (4) no underlying conditions causing malnutrition and severe infectious diseases; and (5) must have received postoperative follow-up. The following categories of patients were excluded: (1) Patients with malignant tumors at other sites; (2) patients who have received chemotherapy or other CRC-related treatments prior to this surgery; (3) patients undergoing emergency surgery; and (4) patients with distant metastasis indicated by preoperative examination.
Patient data collection: Data on the sex, age, height, weight, medical history, disease course, intraoperative blood loss, and postoperative complications of the selected patients were recorded. Pathological data, including tumor site, size, degree of differentiation, depth of invasion, metastasis, and tumor type, were also documented. The body mass index (BMI) was calculated using patients’ height and weight upon hospitalization.
Laboratory examination: Within 1 wk before surgery, routine blood and biochemical tests were performed on peripheral blood samples of patients to determine preoperative hemoglobin, red blood cell, albumin, white blood cell, PLT, neutrophilicgranulocyte (NE), LY, cluster of differentiation 45+ cluster of differentiation 3 (CD45+ CD3-), cluster of differentiation 4/cluster of differentiation 8, CEA, alpha-fetopro-tei, squamous cell carcinoma antigen, carbohydrate Antigen 19-9, carbohydrate antigen 125, carbohydrate antigen 15-3, and heat shock protein 90 α values. The NLR, PLR, and SII values were calculated as follows: NLR = NE/LY, PLR = PLT/LY, SII = (NE × PLT)/LY.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0. The Chi-square test was used for the measurement data, and the t-test was used for the counting data. Counting data following a normal distribution were expressed as mean SD, and categorical variables were presented as percentages of positive cases. Logistic regression analysis was employed to determine whether relevant factors were associated with postoperative IAI in patients with CRC, and receiver operating characteristic (ROC) curves were used to confirm these factors. Spearman’s correlation coefficient was used to assess the relationship between the indicators. All tests in the study used a two-tailed approach, and statistical significance was set at P < 0.05.
Based on the results of the multifactor analysis, R software was used to construct a nomogram prediction model. To verify the prediction accuracy, 1000 rounds of bootstrap sampling were used for internal and external validation. Additionally, ROC curves, calibration curves, and decision curve analysis (DCA) were employed to evaluate the prediction efficiency and clinical effectiveness of the nomogram.
Patients in the IAI group exhibited a statistically significant higher mean age than those in the non-IAI group (P < 0.05). However, no significant differences were observed in terms of sex distribution, smoking history, medical history, tumor pathology, and tumor surgical classification (P > 0.05) (Table 1).
| IAI group (%) | Non-IAI group (%) | t/χ2 | P value | |
| Sample size | 15 | 65 | – | – |
| Gender | ||||
| Male | 11 (73.3) | 35 (53.8) | 1.894 | 0.169 |
| Female | 4 (26.7) | 30 (46.2) | ||
| Age | 65.63 ± 13.03 | 57.75 ± 12.86 | -2.119 | 0.046 |
| BMI | 24.05 ± 3.47 | 22.94 ± 2.99 | -1.257 | 0.212 |
| Smoking history | ||||
| Yes | 6 (40.0) | 20 (30.8) | 0.473 | 0.491 |
| No | 9 (60.0) | 45 (69.2) | ||
| Hypertension | ||||
| Yes | 10 (66.7) | 33 (50.8) | 1.239 | 0.266 |
| No | 5 (33.3) | 32 (49.2) | ||
| Diabetes | ||||
| Yes | 8 (53.3) | 38 (58.5) | 0.131 | 0.717 |
| No | 7 (46.7) | 27 (41.5) | ||
| Preoperative anemia | ||||
| Yes | 12 (80.0) | 47 (72.3) | 0.373 | 0.542 |
| No | 3 (20.0) | 18 (27.7) | ||
| Tumor site | ||||
| Left | 8 (53.3) | 37 (56.9) | 0.064 | 0.801 |
| Right | 7 (46.7) | 28 (43.1) | ||
| Degree of differentiation | ||||
| Medium and above | 9 (60.0) | 30 (46.2) | 0.935 | 0.334 |
| Low | 6 (40.0) | 35 (53.8) | ||
| Infiltration depth | ||||
| T3 + T4 | 8 (53.3) | 38 (58.5) | 0.131 | 0.717 |
| T1 + T2 | 7 (46.7) | 27 (41.5) | ||
| Tumor diameter (cm) | 4.69 ± 1.15 | 4.79 ± 1.21 | 0.293 | 0.770 |
| Lymphatic metastasis | ||||
| Yes | 6 (40.0) | 23 (35.4) | 0.112 | 0.737 |
| No | 9 (60.0) | 42 (64.6) | ||
| Gross classification of tumor | ||||
| Ulcerative | 4 (26.7) | 13 (20.0) | 0.500 | 0.868 |
| Wettability | 7 (46.7) | 31 (47.7) | ||
| Uplift type | 4 (26.7) | 21 (32.3) | ||
| Intraoperative bleeding | 388.58 ± 59.27 | 402.23 ± 57.11 | 0.810 | 0.428 |
A comparison of laboratory indicators between the patient groups revealed that the levels of PLT, NE, CD45+ CD3-, NLP, PLR, SII, and CEA in the IAI group were higher than those in the non-IAI group (P < 0.05). Moreover, LY in the group with IAI was lower than that in the group without IAI (P < 0.05). There was no statistical relevance among the other indicators (Table 2).
| Project | IAI group | Non-IAI group | t | P value |
| HGB (g/L) | 126.74 ± 19.58 | 130.29 ± 21.13 | 0.595 | 0.554 |
| RBC (1012/L) | 4.09 ± 0.32 | 4.06 ± 0.28 | -0.267 | 0.790 |
| ALB (g/L) | 38.19 ± 2.13 | 38.11 ± 2.31 | -0.131 | 0.896 |
| WBC (109/L) | 5.63 ± 1.03 | 5.46 ± 0.87 | -0.657 | 0.513 |
| PLT (109/L) | 359.33 ± 30.33 | 291.41 ± 63.94 | -3.997 | 0.000 |
| NE (109/L) | 4.84 ± 0.70 | 3.44 ± 1.11 | -4.684 | 0.000 |
| LY (109/L) | 1.44 ± 0.18 | 1.73 ± 0.34 | 3.174 | 0.002 |
| CD45+ CD3- | 13.41 ± 0.87 | 14.46 ± 1.55 | 2.518 | 0.014 |
| CD4/CD8 | 1.49 ± 0.092 | 1.49 ± 0.11 | 0.137 | 0.891 |
| NLR | 3.43 ± 0.65 | 2.08 ± 0.84 | -5.770 | 0.000 |
| PLR | 253.87 ± 40.95 | 177.65 ± 62.28 | -4.508 | 0.000 |
| SII | 1226.48 ± 245.55 | 611.52 ± 285.96 | -7.691 | 0.000 |
| CEA (μg/L) | 4.32 ± 0.65 | 3.65 ± 0.84 | -2.913 | 0.005 |
| AFP (μg/L) | 3.63 ± 0.94 | 3.57 ± 0.91 | -0.218 | 0.829 |
| SCC (μg/L) | 1.40 ± 0.086 | 1.40 ± 0.097 | 0.038 | 0.970 |
| CA19-9 (Ku/L) | 18.78 ± 0.75 | 18.84 ± 1.24 | 0.169 | 0.866 |
| CA-125 (Ku/L) | 10.64 ± 1.04 | 11.13 ± 1.09 | 1.576 | 0.119 |
| CA15-3 (Ku/L) | 8.26 ± 1.06 | 7.87 ± 0.96 | -1.378 | 0.172 |
| Hsp90α (ng/mL) | 38.21 ± 2.82 | 38.49 ± 2.69 | 0.344 | 0.734 |
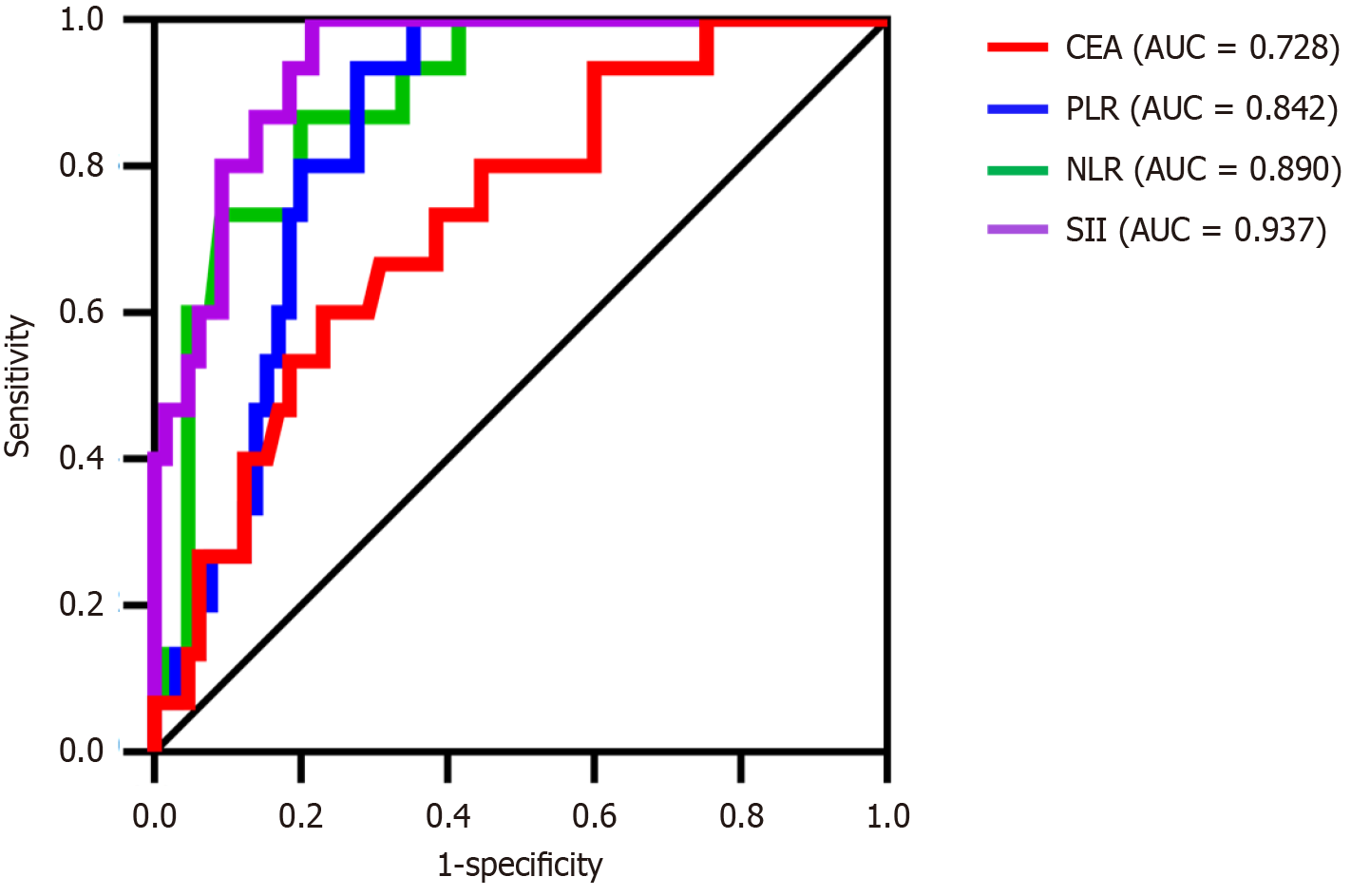
The above indicators with notable differences were included in the logistic regression analysis, with the presence of IAI (yes = 1, no = 0) serving as the dependent variable and age, CD45+ CD3-, NLR, PLR, SII, and CEA as the independent variables. The results indicated that NLR, PLR, SII, and CEA were risk factors for IAI in patients with CRC after radical resection of CRC (odds ratio > 1, P < 0.05) (Table 3). The ROC curve was used to evaluate the diagnostic value of each indicator. The highest area under the curve (AUC) of the SII was 0.937 (Table 4 and Figure 2).
| Independent variable | B | S.E. | Wald | P value | OR | 95%CI |
| Age | 0.001 | 0.055 | 0.000 | 0.990 | 1.001 | 0.899-1.114 |
| CD45+ CD3- | 0.355 | 1.185 | 0.090 | 0.765 | 1.426 | 0.140-14.542 |
| NLR | -1.615 | 0.677 | 5.69 | 0.017 | 1.199 | 0.053-1.750 |
| PLR | -0.022 | 0.010 | 5.13 | 0.024 | 1.978 | 0.960-1.997 |
| SII | 0.010 | 0.004 | 7.194 | 0.007 | 1.010 | 1.003-1.018 |
| CEA (μg/L) | 3.131 | 1.414 | 4.903 | 0.027 | 22.905 | 1.433-366.157 |
| Constant | -20.789 | 18.767 | 1.227 | 0.268 | 0.000 | – |
| Independent variable | Cutoff | AUC | Sensitivity | Specificity | Youden index | P value | 95%CI |
| NLR | 2.67 | 0.890 | 0.867 | 0.800 | 0.667 | 0.000 | 0.811-0.969 |
| PLR | 213.18 | 0.842 | 0.933 | 0.723 | 0.656 | 0.000 | 0.757-0.927 |
| SII | 826.24 | 0.937 | 0.215 | 0.785 | 0.785 | 0.000 | 0.886-0.989 |
| CEA (μg/L) | 4.03 | 0.728 | 0.667 | 0.708 | 0.375 | 0.006 | 0.595-0.861 |
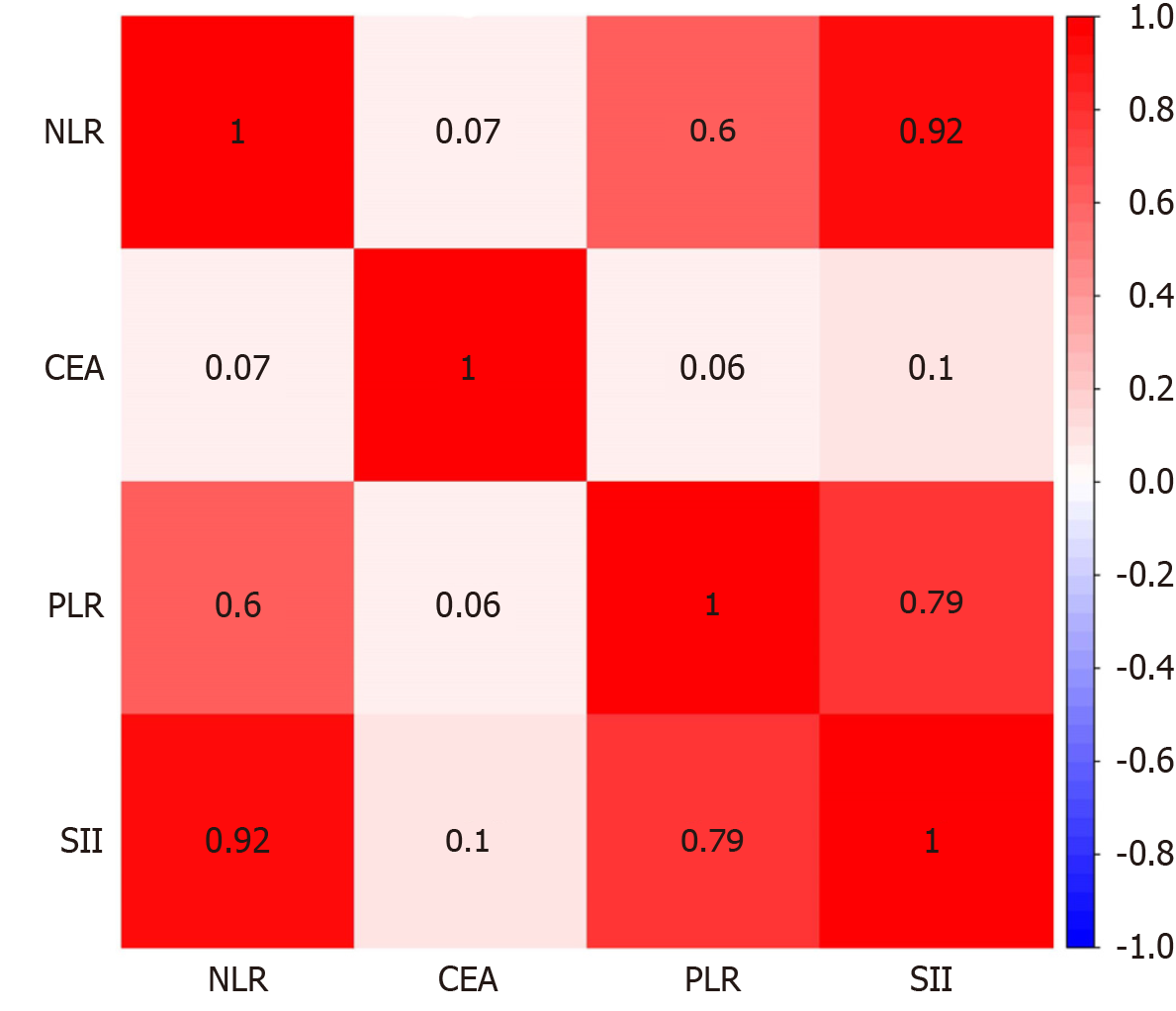
A Spearman correlation analysis was performed to assess the relevance of the NLR, PLR, SII, and CEA. The results revealed that NLR was significantly correlated with PLR and SII (r = 0.604, 0.925, and 0.305, respectively). PLR was significantly correlated with the SII (r = 0.787). The relationships between other variables are shown in Figure 3.
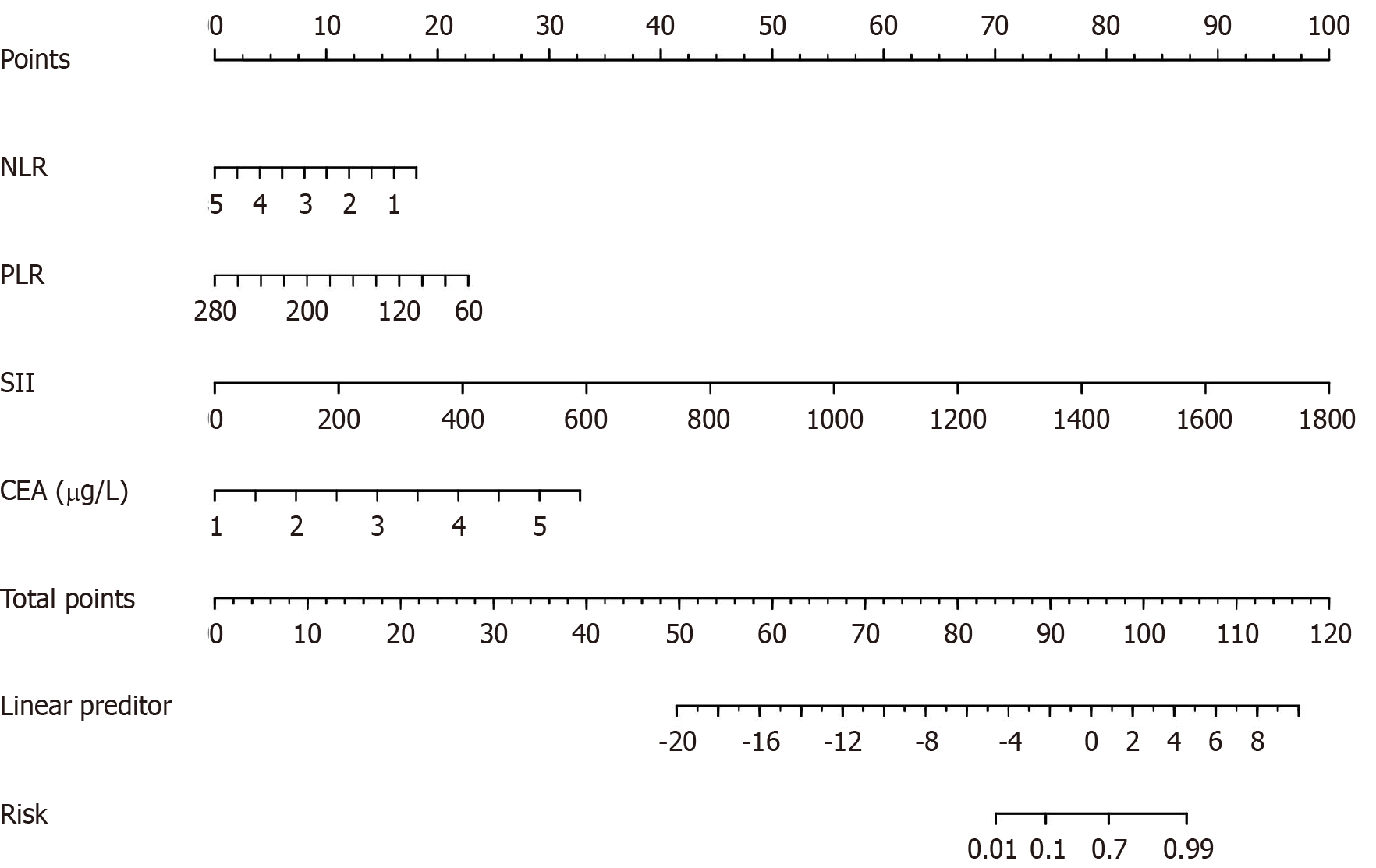
All patients who underwent radical resection of CRC were stochastically divided into training (n = 60) and test (n = 20) sets. A nomogram model was built based on the results of the multifactor analysis, and the scores corresponding to each index were added to obtain the aggregate points. These points were converted into the predicted probability of abdominal infection in patients undergoing radical resection of CRC (Figure 4).
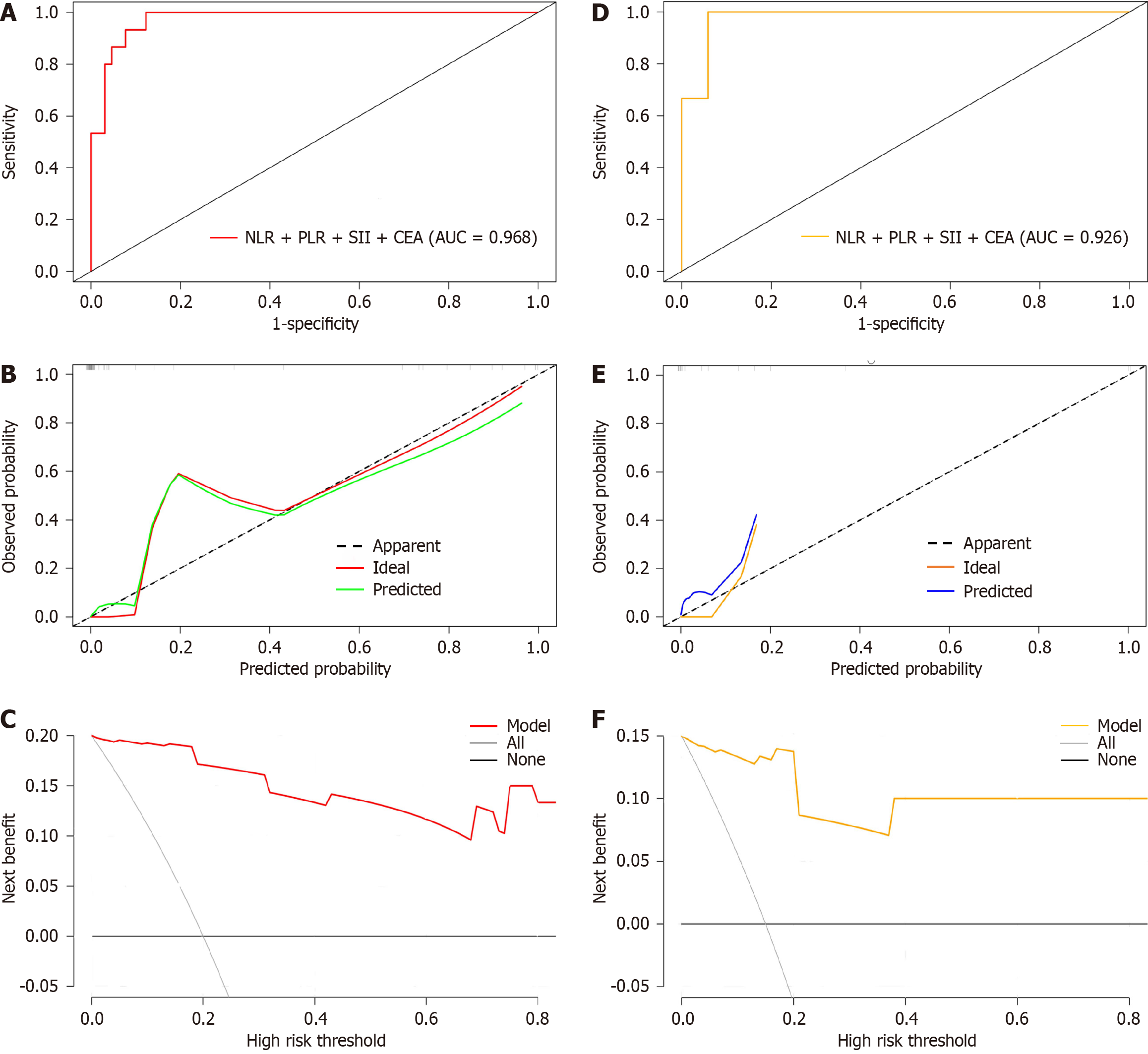
For internal verification, bootstrap was used to sample 1000 times, and the areas under the ROC, DCA, and calibration curves were used to estimate the efficiency of the nomogram. An AUC of 0.968 [95% confidence interval (CI): 0.948-0.988] indicated that the model had a certain estimation efficiency, as shown in Figure 5A. The calibration curve further revealed that the estimated value was consistent with the measured value, and the average absolute error (0.032) was small, indicating that the nomogram model had a good predictive effect (Figure 5B). In addition, the DCA curve indicated that the net benefit of this model was better when the threshold value was > 5%, indicating the clinical practicality of the model (Figure 5C).
Bootstrap was used to sample 1000 times in the test set, and the AUC was 0.926 (95%CI: 0.906-0.980), indicating that the nomogram model had fine prediction efficiency (Figure 5D). The average absolute error of the calibration curve of the test set was 0.048, indicating good agreement between the estimated value of the model and the predicted value of the correction (Figure 5E). The DCA curve indicated that the model was effective (Figure 5F).
IAI is a common complication after radical resection of CRC and is a critical cause of prolonged hospital stay and decreased quality of life in patients with CRC. This study retrospectively analyzed the data of 80 patients who underwent radical resection of CRC and established a nomogram model with good prediction accuracy to confirm that preoperative NLR, PLR, SII, and CEA levels can be used as tools to predict IAI after radical resection of CRC.
Continuous research on malignant tumors has shown that inflammation is a sign of tumor occurrence and development and is linked to disease forecasting in patients. The immune process between inflammation and tumors is highly complex and is affected by many factors[25]. Studies have shown that various inflammation-related indicators are linked to disease forecasting for patients with CRC, and their combined use can further improve the prognosis of these patients[26,27]. Among them, NLR can reflect the degree of neutrophil activation, PLR can reflect the balance between PLTs and LYs, CEA can induce the abnormal division of tumor nuclear DNA in response, and SII can comprehensively reflect immune function and inflammatory response. In this study, the levels of NLR, PLR, SII, and CEA before radical resection of CRC in patients with CRC were markedly higher in the IAI group than in the non-IAI group, demonstrating that inflammatory indicators were positively correlated with postoperative IAI. This finding is consistent with those of previous studies. Guthrie et al[28] retrospectively analyzed 126 patients who underwent radical resection of CRC and found that a high preoperative NLR was associated with decreased survival rates in patients with cancer. Emir et al[29] retrospectively analyzed 113 patients with CRC and found that higher preoperative PLR levels are associated with reduced survival in patients with CRC. In addition, Ma et al[30] found that the SII was a risk factor for postoperative complications in stages II–III CRC and could be used to predict the risk of postoperative complications in these patients. Baqar et al[31] revealed that preoperative CEA levels are related to age, BMI, American Society of Anesthesiologists score, and tumor stage. CEA levels can be used as a reliable predictor of postoperative complications in patients with CRC. Similarly, several investigators have probed the prognostic value of inflammatory indicators in patients with CRC, including the NLR, MLR, PLR, SIS, and SII, to determine the optimum predictor and aid clinical decision-making[17,32-35]. Based on previous studies, the ROC curve method was used in this study to compute the diagnostic value of each indicator; the results suggest that preoperative NLR, PLR, SII, and CEA levels had high diagnostic values, indicating that NLR, PLR, SII, and CEA are effective indicators of postoperative abdominal infection in patients with CRC. However, the prognostic effect of a single factor in predicting the disease has certain limitations. To circumvent this issue, this study used the NLR, PLR, SII, and CEA as predictors to build a risk prediction model and evaluated it to determine the risk value of IAI in patients after radical resection of CRC.
The AUC of the model for predicting abdominal infection after radical CRC surgery was 0.968 (95%CI: 0.948-0.988) in the training set and 0.926 (95%CI: 0.906-0.980) in the validation set, demonstrating the high accuracy of the model. The calibration curves of the training and validation sets indicate that the predicted values of the model closely align with the observed values. The DCA curves also indicate that the model has a good net benefit when the threshold value is above 5%, indicating that the model has high clinical functionality. These results indicate that the model has a certain value in predicting the incidence of abdominal infection after radical resection of CRC. According to previous studies, NLR, PLR, SII, and CEA, as predictive indicators, have good forecasting practicability in different models. Yan et al[36] built a nomogram model for forecasting the survival rate after radical CRC surgery based on NLR, CEA, CA125, and clinical staging. The C-index of the model was 0.918 (95%CI: 0.885-0.952), indicating the fine correctness of the model. Ma et al[30] established a nomogram prediction model that unites inflammatory factors and conducted internal verification. The AUC of the model was 0.825 (95%CI: 0.764-0.886), indicating an advantage in predicting postoperative complications in patients with stage II–III CRC. Liu et al[37] identified a series of factors related to the survival of patients with distant metastatic CRC and constructed a prognostic histogram for patients with stage IV CRC. The C-index of the model was 0.742 (95%CI: 0.726-0.758), which proved that the model had good calibration and discriminating power.
This study has some limitations. First, the data were limited, and the selected case samples were from a single center with a small number of cases, which may have introduced bias to the results. The sample size should be broadened to ensure the accuracy of the results. Second, because this was a retrospective study, selected patients could not represent the overall population, which is prone to selection bias and recall bias, and we could not calculate the incidence rate or directly analyze the relative risk. The scope of research objects should be expanded.
This study found that NLR, PLR, SII, and CEA levels may be risk factors for IAI in patients with CRC after radical resection. The nomogram model built based on the above indices can be used as a prediction tool for abdominal infection after radical resection of CRC, aiding individualized treatment decisions. Simultaneously, it holds clinical significance in improving patient prognosis.
Postoperative complications are important clinical outcomes in patients with colorectal cancer (CRC). Abdominal infection is a serious complication of radical resection of CRC. Currently, the risk factors for abdominal infections after CRC surgery are unclear.
To explore the correlation between inflammatory indicators and abdominal infection in patients after radical resection of CRC and construct a risk prediction model to provide a theoretical basis for prevention and intervention.
To explore the predictive value of preoperative neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and carcinoembryonic antigen (CEA) levels for intra-abdominal infection (IAI) in patients after radical resection of CRC and provide an evaluation tool for individualized treatment of patients.
This study was based on a retrospective analysis of the preoperative NLR, PLR, SII, and CEA levels in 80 patients who were admitted and underwent radical resection of CRC. A risk prediction model was constructed and verified.
Studies have shown that the NLR, PLR, SII, and CEA are risk factors for IAI in patients with CRC after radical surgery. The area under the curve of the training set (n = 60) for the nomogram prediction model was 0.968 [95% confidence interval (CI): 0.948-0.988], and that of the validation set (n = 20) was 0.926 (95%CI: 0.906-0.980). Calibration curves of the training and validation sets showed that the predicted results corresponded with the observed results. decision curve analysis curve analysis showed that patients with CRC could benefit from the prediction model.
A nomogram combining the NLR, PLR, SII, and CEA levels was established. It has good accuracy and reliability in predicting abdominal infections in patients after radical resection of CRC, which is helpful in clinical treatment decision-making and has clinical significance in improving the prognosis of patients.
Based on the general data and laboratory indicators of patients undergoing radical resection of CRC, we observed whether IAI occurred and focused on the analysis of preoperative NLR, PLR, SII, and CEA levels and the construction of relevant prediction models.
| 1. | Ponnala S, Chaudhary S, González-Sarrias A, Seeram NP, Harding WW. Cytotoxicity of aporphines in human colon cancer cell lines HCT-116 and Caco-2: an SAR study. Bioorg Med Chem Lett. 2011;21:4462-4464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56668] [Article Influence: 7083.5] [Reference Citation Analysis (135)] |
| 3. | Salem JF, Gummadi S, Marks JH. Minimally Invasive Surgical Approaches to Colon Cancer. Surg Oncol Clin N Am. 2018;27:303-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Bektas M, Atiq M, Bhutani MS. First report of celiac plexus block for refractory abdominal pain secondary to peripancreatic colon cancer metastasis. Gastrointest Endosc. 2012;76:692-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Guo YC, Sun R, Wu B, Lin GL, Qiu HZ, Li KX, Hou WY, Sun XY, Niu BZ, Zhou JL, Lu JY, Cong L, Xu L, Xiao Y. [Risk factors of postoperative surgical site infection in colon cancer based on a single center database]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 948] [Article Influence: 86.2] [Reference Citation Analysis (1)] |
| 7. | Lian L, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, Wu MY, Chen K, Tao M, Li W. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015;15:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | He K, Si L, Pan X, Sun L, Wang Y, Lu J, Wang X. Preoperative Systemic Immune-Inflammation Index (SII) as a Superior Predictor of Long-Term Survival Outcome in Patients With Stage I-II Gastric Cancer After Radical Surgery. Front Oncol. 2022;12:829689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Zhao N, Xu H, Zhou D, Xu X, Ge W, Cao D. The prognostic role of neutrophil-to-lymphocyte ratio and C-reactive protein in metastatic colorectal cancer using regorafenib: a systematic review and meta-analysis. J Gastrointest Oncol. 2022;13:1772-1781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Zhao QT, Yang Y, Xu S, Zhang XP, Wang HE, Zhang H, Wang ZK, Yuan Z, Duan GC. Prognostic role of neutrophil to lymphocyte ratio in lung cancers: a meta-analysis including 7,054 patients. Onco Targets Ther. 2015;8:2731-2738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Ethier JL, Desautels DN, Templeton AJ, Oza A, Amir E, Lheureux S. Is the neutrophil-to-lymphocyte ratio prognostic of survival outcomes in gynecologic cancers? A systematic review and meta-analysis. Gynecol Oncol. 2017;145:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, Uchida E. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2016;23:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 14. | Bong TSH, Tan GHC, Chia C, Soo KC, Teo MCC. Preoperative platelet-lymphocyte ratio is an independent prognostic marker and superior to carcinoembryonic antigen in colorectal peritoneal carcinomatosis patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Clin Oncol. 2017;22:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Deng J, Zhang P, Sun Y, Peng P, Huang Y. Prognostic and clinicopathological significance of platelet to lymphocyte ratio in esophageal cancer: a meta-analysis. J Thorac Dis. 2018;10:1522-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 16. | Wang BL, Tian L, Gao XH, Ma XL, Wu J, Zhang CY, Zhou Y, Guo W, Yang XR. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med. 2016;54:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 250] [Cited by in RCA: 531] [Article Influence: 59.0] [Reference Citation Analysis (12)] |
| 18. | Wang X, Yang Z, Tian H, Li Y, Li M, Zhao W, Zhang C, Wang T, Liu J, Zhang A, Shen D, Zheng C, Qi J, Zhao D, Shi J, Jin L, Rao J, Zhang W. Circulating MIC-1/GDF15 is a complementary screening biomarker with CEA and correlates with liver metastasis and poor survival in colorectal cancer. Oncotarget. 2017;8:24892-24901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Yan C, Hu Y, Zhang B, Mu L, Huang K, Zhao H, Ma C, Li X, Tao D, Gong J, Qin J. The CEA-/lo colorectal cancer cell population harbors cancer stem cells and metastatic cells. Oncotarget. 2016;7:80700-80715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Zhai H, Huang J, Yang C, Fu Y, Yang B. Serum CEA and CA19-9 Levels are Associated with the Presence and Severity of Colorectal Neoplasia. Clin Lab. 2018;64:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Luo Y, Lai Q, Huang H, Luo J, Miao J, Liao R, Yang Z, Zhang L. Risk factor analysis and nomogram construction for predicting suicidal ideation in patients with cancer. BMC Psychiatry. 2022;22:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Tai Q, Xue W, Li M, Zhuo S, Zhang H, Fang F, Zhang J. Survival Nomogram for Metastasis Colon Cancer Patients Based on SEER Database. Front Genet. 2022;13:832060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Yu C, Zhang Y. Establishment of prognostic nomogram for elderly colorectal cancer patients: a SEER database analysis. BMC Gastroenterol. 2020;20:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Xu J, Dai S, Yuan Y, Xiao Q, Ding K. A Prognostic Model for Colon Cancer Patients Based on Eight Signature Autophagy Genes. Front Cell Dev Biol. 2020;8:602174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Yao D, Dong M, Dai C, Wu S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm Bowel Dis. 2019;25:1595-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 26. | Troncone E, Marafini I, Stolfi C, Monteleone G. Involvement of Smad7 in Inflammatory Diseases of the Gut and Colon Cancer. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Wijnands AM, de Jong ME, Lutgens MWMD, Hoentjen F, Elias SG, Oldenburg B; Dutch Initiative on Crohn and Colitis (ICC). Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology. 2021;160:1584-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 28. | Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109:24-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Emir S, Aydin M, Can G, Bali I, Yildirim O, Öznur M, Yildiz ZD, Sözen S, Gürel A. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur Rev Med Pharmacol Sci. 2015;19:3613-3618. [PubMed] |
| 30. | Ma Z, Liu R, Liu H, Zheng L, Zheng X, Li Y, Cui H, Qin C, Hu J. New scoring system combining computed tomography body composition analysis and inflammatory-nutritional indicators to predict postoperative complications in stage II-III colon cancer. J Gastroenterol Hepatol. 2023;38:1520-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Baqar AR, Wilkins S, Staples M, Angus Lee CH, Oliva K, McMurrick P. The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres. Int J Surg. 2019;64:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Abdallah EA, Souza E Silva V, Braun AC, Gasparini VA, Kupper BEC, Tariki MS, Tarazona JGR, Takahashi RM, Aguiar Júnior S, Chinen LTD. A higher platelet-to-lymphocyte ratio is prevalent in the presence of circulating tumor microemboli and is a potential prognostic factor for non-metastatic colon cancer. Transl Oncol. 2021;14:100932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | McDermott FD. The association of the neutrophil-lymphocyte ratio with the presence of minimal residual disease and outcome in patients with stage II colon cancer treated with surgery alone, by Murray et al. Colorectal Dis. 2021;23:774. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. 2020;20:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Wang SH, Xuan FC, Zheng HS, Lin TY, Zhou W. Glasgow prognostic score is a predictive index for postoperative infectious complications after total proctocolectomy in ulcerative colitis patients. Rev Esp Enferm Dig. 2021;113:418-422. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Yan J, Zhao Z, Wang L, Yu J, Quan F, Duan H. Construction of predictive model for prognosis of patients after radical resection of colon cancer based on nomogram. Am J Transl Res. 2023;15:2783-2792. [PubMed] |
| 37. | Liu Z, Xu Y, Xu G, Baklaushev VP, Chekhonin VP, Peltzer K, Ma W, Wang X, Wang G, Zhang C. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021;21:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: AmeliMojarad M, Iran; Schmidt T, Germany S-Editor: Qu XL L-Editor: A P-Editor: Zheng XM