Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.318
Peer-review started: October 2, 2023
First decision: December 6, 2023
Revised: December 17, 2023
Accepted: January 25, 2024
Article in press: January 25, 2024
Published online: February 27, 2024
Processing time: 146 Days and 2.5 Hours
Partial splenic embolization (PSE) has been suggested as an alternative to splenectomy in the treatment of hypersplenism. However, some patients may experience recurrence of hypersplenism after PSE and require splenectomy. Currently, there is a lack of evidence-based medical support regarding whether preoperative PSE followed by splenectomy can reduce the incidence of complications.
To investigate the safety and therapeutic efficacy of preoperative PSE followed by splenectomy in patients with cirrhosis and hypersplenism.
Between January 2010 and December 2021, 321 consecutive patients with cirrhosis and hypersplenism underwent splenectomy at our department. Based on whether PSE was performed prior to splenectomy, the patients were divided into two groups: PSE group (n = 40) and non-PSE group (n = 281). Patient characteristics, postoperative complications, and follow-up data were compared between groups. Propensity score matching (PSM) was conducted, and univariable and multi
After PSM, the non-PSE group showed significant reductions in hospital stay, intraoperative blood loss, and operation time (all P = 0.00). Multivariate analysis revealed that spleen length, portal vein diameter, splenic vein diameter, and history of PSE were independent predictive factors for IB. A nomogram predictive model of IB was constructed, and DCA demonstrated the clinical utility of this model. Both groups exhibited similar results in terms of overall survival during the follow-up period.
Preoperative PSE followed by splenectomy may increase the incidence of IB and a nomogram-based prediction model can predict the occurrence of IB.
Core Tip: Partial splenic embolization (PSE) has been suggested as an alternative to splenectomy for treating hypersplenism, but some patients may experience recurrence of hypersplenism after PSE and still require splenectomy. Preoperative PSE followed by splenectomy may increase the incidence of intraoperative bleeding (IB), and a nomogram-based prediction model can predict the occurrence of IB.
- Citation: Huang L, Li QL, Yu QS, Peng H, Zhen Z, Shen Y, Zhang Q. Will partial splenic embolization followed by splenectomy increase intraoperative bleeding? World J Gastrointest Surg 2024; 16(2): 318-330
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/318.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.318
Cirrhosis, an end-stage chronic liver injury, is frequently accompanied by severe complications such as portal hypertension (PH), esophageal and gastric variceal bleeding (EGVB), splenomegaly, and hypersplenism[1,2]. Previously, splenectomy has been used to treat patients with cirrhosis and hypersplenism[3]. Furthermore, a combined approach of splenectomy and peri esophagogastric devascularization has been shown to be an effective treatment option for cirrhotic patients with EGVB and PH. However, it is important to note that this procedure carries the risk of postoperative compli
Currently, PSE has emerged as a crucial treatment modality for hypersplenism associated with PH. It effectively treats hypersplenism, lowers portal pressure, and improves liver function[6,7]. The occurrence of acute ischemia of the splenic segment tissue and subsequent splenic infarction were attributed to PSE. The subsequent absorption of necrotic tissue and the development of splenic tissue fibrosis results in spleen atrophy and a reduction in spleen volume. The reduction in spleen volume enhances the space within the splenic pedicle, leading to a more precise disconnection between the perisplenic ligament and the splenic pedicle vessels. Consequently, this reduces the occurrence of complications[8]. Despite the therapeutic effectiveness of PSE in treating hypersplenism and reducing PH, some patients may experience recurrence of hypersplenism following PSE treatment, leading to the need for splenectomy[9].
Previous research has indicated that PSE reduces surgical complexity and lowers the occurrence of postoperative complications[10]. However, some studies suggest that PSE increases perisplenic adhesions and intraoperative bleeding (IB) during splenectomy[11]. Despite these findings, there is insufficient evidence-based medical support to determine whether preoperative PSE followed by splenectomy reduces complications and the optimal timing for performing splenectomy after PSE remains uncertain. Furthermore, research on predictive factors for complications in patients undergoing preoperative PSE followed by splenectomy is limited.
This study aimed to assess the safety and therapeutic efficacy of preoperative PSE followed by splenectomy in patients with cirrhosis and hypersplenism. The aim of this study was to examine the occurrence of these complications. To achieve these objectives, we retrospectively analyzed the clinical data of patients who underwent preoperative PSE followed by splenectomy at our hospital over a period of 6 years. The ultimate goal was to develop a reliable risk model to identify the independent risk factors for IB in patients with cirrhosis.
Prior to commencing this study, the objectives and specific details were comprehensively explained to the participants and informed consent was obtained from each participant or their respective representatives. This study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki, revised in Fortaleza, Brazil, October 2013). The study protocol was approved by the Institutional Review Board of Anhui Provincial Traditional Chinese Hospital (ID: 2020AH-13).
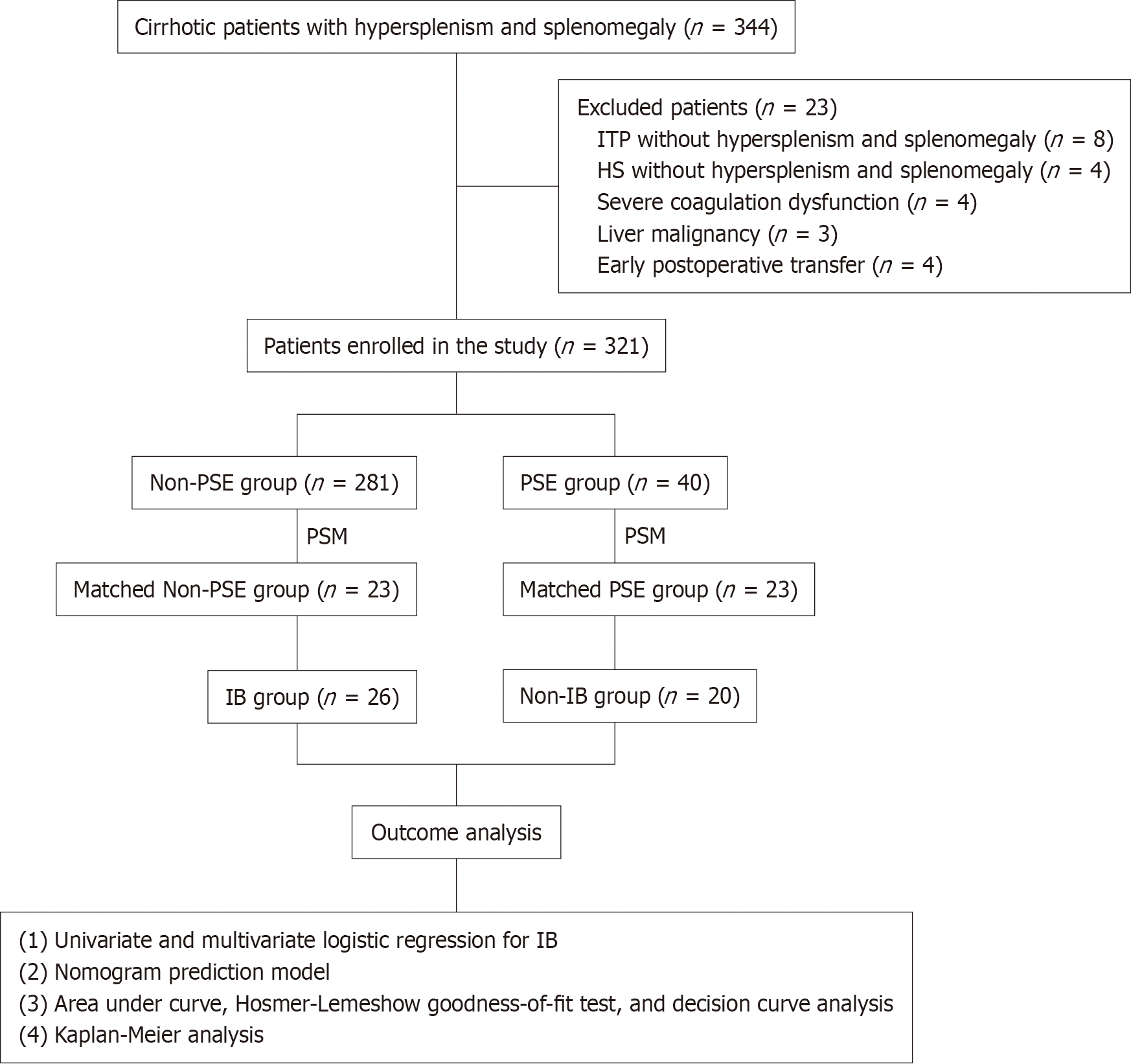
From January 2017 to June 2023, 344 consecutive patients underwent splenectomy at the First Hospital affiliated with Anhui University of Traditional Chinese Medicine. After careful application of the inclusion and exclusion criteria, 321 patients were enrolled in the study. This study employed a retrospective cohort design in which patients were divided into two groups based on whether they underwent preoperative PSE: The PSE group and the non-PSE group. Detailed assessments were conducted to evaluate the clinical characteristics of the patients, including age, sex, etiology of cirrhosis, spleen size, and portal vein system diameter. Additionally, intraoperative outcomes such as blood loss, operation time, and postoperative complications such as PVT and postoperative pancreatic fistula (PPF) were collected and analyzed. Furthermore, the study reviewed the follow-up data on the overall survival (OS) of patients.
We meticulously collected and analyzed clinical data from a standardized database of patients during the perioperative period. Color Doppler ultrasound was used to measure the diameters of the portal and splenic veins, assess the spleen size, and determine the presence of PVT.
All patients included in the study met the following inclusion criteria: (1) A diagnosis of cirrhosis and PH of any etiology; (2) display secondary splenomegaly and hypersplenism attributed to cirrhosis. Hypersplenism is defined as a leukocyte count less than 3.5 × 109/L and a platelet count less than 7.5 × 109/L[12]; and (3) have not previously undergone splenectomy.
The study excluded participants who had the following conditions: (1) Liver cirrhosis without hypersplenism or splenomegaly; (2) severe coagulation dysfunction; (3) liver or any other malignancy; (4) declined to participate; and (5) underwent early postoperative transfer. All surgical procedures were non-emergency procedures.
The puncture point for the right femoral artery, located under the inguinal ligament, was chosen as the most prominent point of femoral artery pulse. The right femoral artery was punctured using the modified Seldinger technique under digital subtraction angiography guidance. Imaging results were used to super selectively advance the microcatheter into the splenic artery. The extent of splenic artery embolism typically ranges from 50% to 70%.
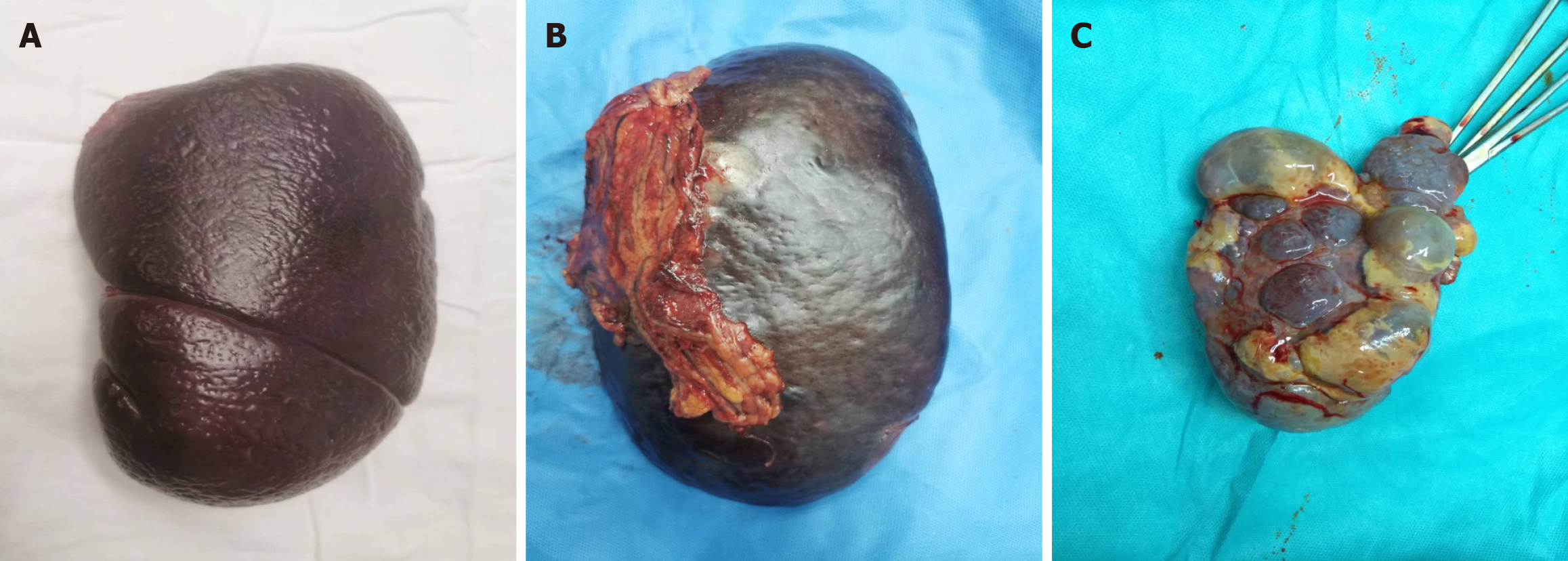
All patients enrolled in the study underwent endotracheal intubation and received intravenous anesthesia for open splenectomy. The splenic artery was fully exposed for ligation, and the serosal tissue in front of the splenic pedicle space was separated and dissected after separating the perisplenic adhesions (Figure 1). To ensure safe dissection, dissection should be performed as close as possible to the splenic parenchyma to avoid the pancreatic tail and stomach. Each secondary splenic pedicle vessel was carefully dissected and ligated under direct visualization. Subsequently, splenic tissue was removed, ligated, and sutured. Finally, a drainage tube was placed in the splenic fossa.
This study collected and analyzed data on postoperative complications following splenectomy during hospitalization. These complications included PVT, postoperative abdominal hemorrhage, abdominal infection, PPF, liver failure, severe ascites, and encephalopathy. The criteria for diagnosing PPF were as follows: (1) Drainage duration exceeding 3 d; (2) amylase level in drainage fluid more than three times the upper limit of normal; and (3) no record of biochemical leakage[13]. PVT was defined as the presence of thrombosis in the portal vein (trunk and intrahepatic branches), mesenteric vein, or splenic vein confirmed by Doppler ultrasound displaying hyperechoic or isoechoic filling in the cavity. Postoperative abdominal hemorrhage was defined as abdominal bleeding exceeding 300 mL within 24 h after surgery. Additionally, clinical details of the surgery, such as operation time, intraoperative blood loss, and length of hospital stay, were recorded.
All patients who underwent splenectomy received routine follow-up assessments either via telephone or at the outpatient department. The follow-up assessment was completed on June 30, 2023. The primary endpoint was OS. OS was defined as the duration from surgery to death from any cause. Patients who survived until the last follow-up were excluded.
Statistical analyses and plotting were conducted using the SPSS Version 24.0 software (IBM Corp., Chicago, IL, United States) and R (Version 4.2.2). Continuous variables following a normal distribution were reported as mean ± SD. Non-normally distributed variables were reported as medians with interquartile ranges (P25 and P75). Group comparisons of measurement data were performed using an independent t-test. Categorical variables were evaluated using the χ² test, with or without Fisher's exact test, as appropriate.
Propensity score matching (PSM) was performed to eliminate confounding variables associated with clinical characteristics between the two groups. PSM was performed using the 1:1 nearest-neighbor caliper matching method with the caliper value set at 0.2[14]. Factors influencing IB were assessed using univariate analysis. Variables that showed statistical significance (P < 0.05) were further evaluated using multivariable logistic analysis to identify the main independent risk factors. A nomogram was constructed using R software (version 4.2.2), and nomogram differentiation was assessed by calculating the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. To evaluate the clinical utility of the nomogram model, a decision curve analysis (DCA) was conducted using the R "rmda" package. Survival curves were plotted using the Kaplan-Meier method, and differences between the two groups were assessed using the log-rank test. Statistical significance was considered at the 5% level, and 2-tailed test were used for the analysis.
Of the 344 patients who met the initial inclusion criteria, 23 were excluded from the study. Among them, eight patients had idiopathic thrombocytopenic purpura, four patients had hereditary spherocytosis and underwent splenectomy, showing no symptoms of hypersplenism or splenomegaly, four patients developed severe coagulopathy, three patients had liver malignancy, and four patients experienced early postoperative complications. Ultimately, 321 patients who met the inclusion criteria were enrolled in this study. Among the 321 patients, there were 173 men and 148 were female. The PSE group included 40 patients, while the non-PSE group included 281 patients (Figure 2).
When comparing the baseline clinical data between the PSE group and non-PSE group, no significant differences were observed in various indicators, including age, sex, etiology of cirrhosis, and portal and splenic vein diameters (SVDs) (all P > 0.05). Additionally, no significant differences were observed in parameters including white blood cell (WBC), red blood cell (RBC), hemoglobin (HGB), alanine aminotransferase (ALT), prothrombin time (PT), and operation between the two groups (all P > 0.05). In contrast to the PSE group, the non-PSE group demonstrated significantly reduced hospital stays (13.99 ± 3.57d vs 17.10 ± 4.42 d), intraoperative blood loss (267.36 ± 161.07 mL vs 399.50 ± 240.76 mL), and operation time (181.64 ± 36.57 min vs 202.98 ± 36.38 min) (all P = 0.00).
Table 1 presents the results of PSM conducted at a 1:1 ratio, with 23 patients in each group. Following PSM, the clinical data of the matched PSE group were comparable to those of the matched non-PSE group, with all P values exceeding 0.05. Furthermore, the matched non-PSE group demonstrated significantly reduced hospital stays (13.52 ± 2.25 d compared to 16.70 ± 4.18 d), intraoperative blood loss (250.13 ± 129.02 mL compared to 450.00 ± 268.40 mL), and operation time (178.78 ± 37.42 min compared to 220.09 ± 35.41 min) when compared to the matched PSE group.
| Variables | Unmatched | P value | Matched | P value | ||
| PSE group, n = 40 | Non-PSE group, n = 281 | PSE group, n = 23 | Non-PSE group, n = 23 | |||
| Age in yr | 33.95 ± 12.21 | 35.07 ± 14.74 | 0.65 | 33.57 ± 13.56 | 34.04 ± 12.23 | 0.90 |
| Sex, n (%) | 0.12 | 0.57 | ||||
| Male | 17 | 156 | 12 | 10 | ||
| Female | 23 | 125 | 11 | 13 | ||
| Etiology of cirrhosis, n (%) | 0.88 | 0.30 | ||||
| HLD cirrhosis | 34 | 222 | 19 | 17 | ||
| HBV cirrhosis | 4 | 48 | 3 | 4 | ||
| HCV cirrhosis | 1 | 3 | 1 | 1 | ||
| Schistosomiasis cirrhosis | 0 | 1 | 0 | 0 | ||
| Alcoholic cirrhosis | 0 | 2 | 0 | 0 | ||
| Autoimmunity cirrhosis | 1 | 3 | 0 | 1 | ||
| Unknown | 0 | 1 | ||||
| The size of spleen in mm | ||||||
| Length of spleen | 161.85 ± 21.26 | 171.21 ± 26.74 | 0.04 | 170.13 ± 15.64 | 163.00 ± 25.33 | 0.26 |
| Thickness of spleen | 66.45 ± 11.03 | 73.06 ± 16.91 | 0.02 | 69.74 ± 8.77 | 64.43 ± 15.88 | 0.17 |
| The diameter of portal vein system in cm | ||||||
| Portal vein | 1.35 ± 0.16 | 1.41 ± 0.24 | 0.15 | 1.38 ± 0.19 | 1.28 ± 0.17 | 0.50 |
| Splenic vein | 1.24 ± 0.21 | 1.23 ± 0.24 | 0.71 | 1.23 ± 0.23 | 1.13 ± 0.17 | 0.10 |
| Preoperative blood examination | ||||||
| WBC as 109/L | 2.69 ± 1.05 | 3.13 ± 2.08 | 0.19 | 2.51 ± 1.09 | 3.12 ± 2.44 | 0.28 |
| RBC as 1012/L | 3.76 ± 0.37 | 3.91 ± 0.65 | 0.16 | 3.77 ± 0.43 | 3.58 ± 0.54 | 0.18 |
| HGB in g/L | 106.40 ± 18.31 | 109.75 ± 18.71 | 0.29 | 103.61 ± 16.80 | 104.96 ± 15.08 | 0.75 |
| PLT as 109/L | 66.35 ± 30.04 | 50.03 ± 28.68 | 0 | 59.26 ± 29.86 | 59.04 ± 48.48 | 0.99 |
| TBIL in μmol/L | 34.54 ± 25.58 | 22.98 ± 15.61 | 0 | 30.09 ± 26.63 | 28.14 ± 22.13 | 0.79 |
| ALT in U/L | 30.20 ± 13.31 | 29.01 ± 17.01 | 0.67 | 28.74 ± 13.03 | 28.96 ± 21.20 | 0.97 |
| AST in U/L | 38.00 ± 16.13 | 30.00 ± 11.44 | 0 | 33.57 ± 11.18 | 35.48 ± 17.46 | 0.66 |
| ALB in g/L | 35.04 ± 3.42 | 37.60 ± 4.60 | 0 | 34.77 ± 3.07 | 34.98 ± 3.98 | 0.84 |
| PT in s | 14.95 ± 3.44 | 14.70 ± 2.43 | 0.57 | 15.08 ± 3.49 | 14.30 ± 2.26 | 0.37 |
| D-dimer in mg/L | 1.46 ± 4.28 | 0.67 ± 0.83 | 0.01 | 0.47 ± 0.26 | 0.56 ± 0.54 | 0.47 |
| Operation, n (%) | ||||||
| Splenectomy only | 18 | 237 | 0 | 11 | 11 | 1 |
| Concomitant pericardial devascularization | 22 | 44 | 12 | 12 | ||
| Intraoperative outcomes | ||||||
| Intraoperative blood loss in mL | 399.50 ± 240.76 | 267.36 ± 161.07 | 0 | 450.00 ± 268.40 | 250.13 ± 129.02 | 0 |
| Operation time in min | 202.98 ± 36.38 | 181.64 ± 36.57 | 0.01 | 220.09 ± 35.41 | 189.78 ± 37.42 | 0.01 |
| Postoperative complications, n (%) | ||||||
| PVT | 14 | 107 | 0.71 | 8 | 6 | 0.53 |
| PPF | 6 | 27 | 0.30 | 4 | 4 | 1 |
| Abdominal hemorrhage | 3 | 6 | 0.06 | 2 | 3 | 0.65 |
| Hospital stay in d | 17.10 ± 4.42 | 13.99 ± 3.57 | 0 | 16.70 ± 4.18 | 13.52 ± 2.25 | 0 |
Notable variations were observed in multiple indices between pre- and post-PSE, specifically in the diameters of the portal and splenic veins, along with changes in the HGB, platelet, total bilirubin, ALT, aspartate aminotransferase, albumin (ALB), and PT levels (all P < 0.05). No significant differences were observed in spleen length (LS) and thickness as well as WBC, RBC, and D-dimer levels (all P > 0.05) (Table 2).
| Variables | Groups | t | P value | |
| Pre-PSE group, n = 40 | Post-PSE group, n = 40 | |||
| Frequency | 1.50 ± 0.82 | |||
| Time from last PSE to surgery in mo | 5.03 ± 9.53 | |||
| The size of spleen in mm | ||||
| Length of spleen | 171.70 ± 25.07 | 161.85 ± 21.26 | 18.28 | 0.06 |
| Thickness of spleen | 71.45 ± 14.07 | 66.45 ± 11.03 | 7.309 | 0.08 |
| The diameter of portal vein system in cm | ||||
| Portal vein | 1.57 ± 0.16 | 1.35 ± 0.16 | 8.953 | 0 |
| Splenic vein | 1.47 ± 0.19 | 1.24 ± 0.21 | 5.956 | 0 |
| Preoperative blood examination | ||||
| WBC as 109/L | 2.87 ± 0.93 | 2.69 ± 1.05 | 3.684 | 0.40 |
| RBC as 1012/L | 3.67 ± 0.23 | 3.76 ± 0.37 | 0.609 | 0.18 |
| HGB in g/L | 119.1 ± 6.30 | 106.40 ± 18.31 | 1.071 | 0 |
| PLT as 109/L | 28.55 ± 14.29 | 66.35 ± 30.04 | 4.15 | 0 |
| TBIL in μmol/L | 54.68 ± 16.26 | 34.54 ± 25.58 | 5.195 | 0 |
| ALT in U/L | 58.0 ± 17.32 | 30.20 ± 13.31 | 3.378 | 0 |
| AST in U/L | 77.15 ± 17.02 | 38.00 ± 16.13 | 8.136 | 0 |
| ALB in g/L | 29.90 ± 1.49 | 35.04 ± 3.42 | 1.844 | 0 |
| PT in s | 17.97 ± 1.09 | 14.95 ± 3.44 | 2.091 | 0 |
| D-dimer in mg/L | 0.33 ± 0.12 | 1.46 ± 4.28 | 3.756 | 0.10 |
IB was defined as a volume exceeding 300 mL, determined by the median IB volume. A total of 26 patients (56.5%) experienced IB during splenectomy. Univariate analysis revealed significant differences in various indicators, including ALB, PT, LS, spleen thickness, portal vein diameter (PVD), SVD, and PSE history (all P < 0.05), when assessing the predictive factors of IB. Multivariable logistic analysis further confirmed that LS, PVD, SVD, and PSE history were independent risk factors for PVT (Table 3).
| Characteristics | Univariate analysis | Multivariate analysis | |||||||
| Non-IB group, n = 20 | IB group, n = 26 | P value | β | S.E. | Wald | Odds ratio | 95%CI | P value | |
| ALB in g/L | 36.34 ± 3.49 | 33.75 ± 3.15 | 0.01 | -0.73 | 0.42 | 2.982 | 0.482 | 0.21-1.104 | 0.08 |
| PT in s | 13.70 ± 2.66 | 15.45 ± 2.95 | 0.04 | 0.336 | 0.34 | 3.724 | 1.94 | 0.99-3.802 | 0.05 |
| LS in mm | 152.25 ± 13.21 | 177.58 ± 19.51 | 0 | 0.123 | 0.06 | 5.003 | 1.131 | 1.015-1.259 | 0.03 |
| Thickness of spleen in mm | 62.10 ± 13.96 | 70.92 ± 10.92 | 0.02 | 0.095 | 0.05 | 3.216 | 1.1 | 0.991-1.221 | 0.07 |
| PVD in cm | 1.25 ± 0.15 | 1.40 ± 0.19 | 0.01 | 18.86 | 8.64 | 4.761 | 2E + 08 | 6.806-3.516E + 15 | 0.03 |
| SVD in cm | 1.11 ± 0.18 | 1.24 ± 0.22 | 0.04 | -20 | 9.26 | 4.658 | 0 | 0-0.16 | 0.03 |
| PSE history, n | 0.8 ± 0.41 | 0.27 ± 0.45 | 0 | -4.44 | 2.01 | 4.863 | 0.012 | 0-0.610 | 0.03 |
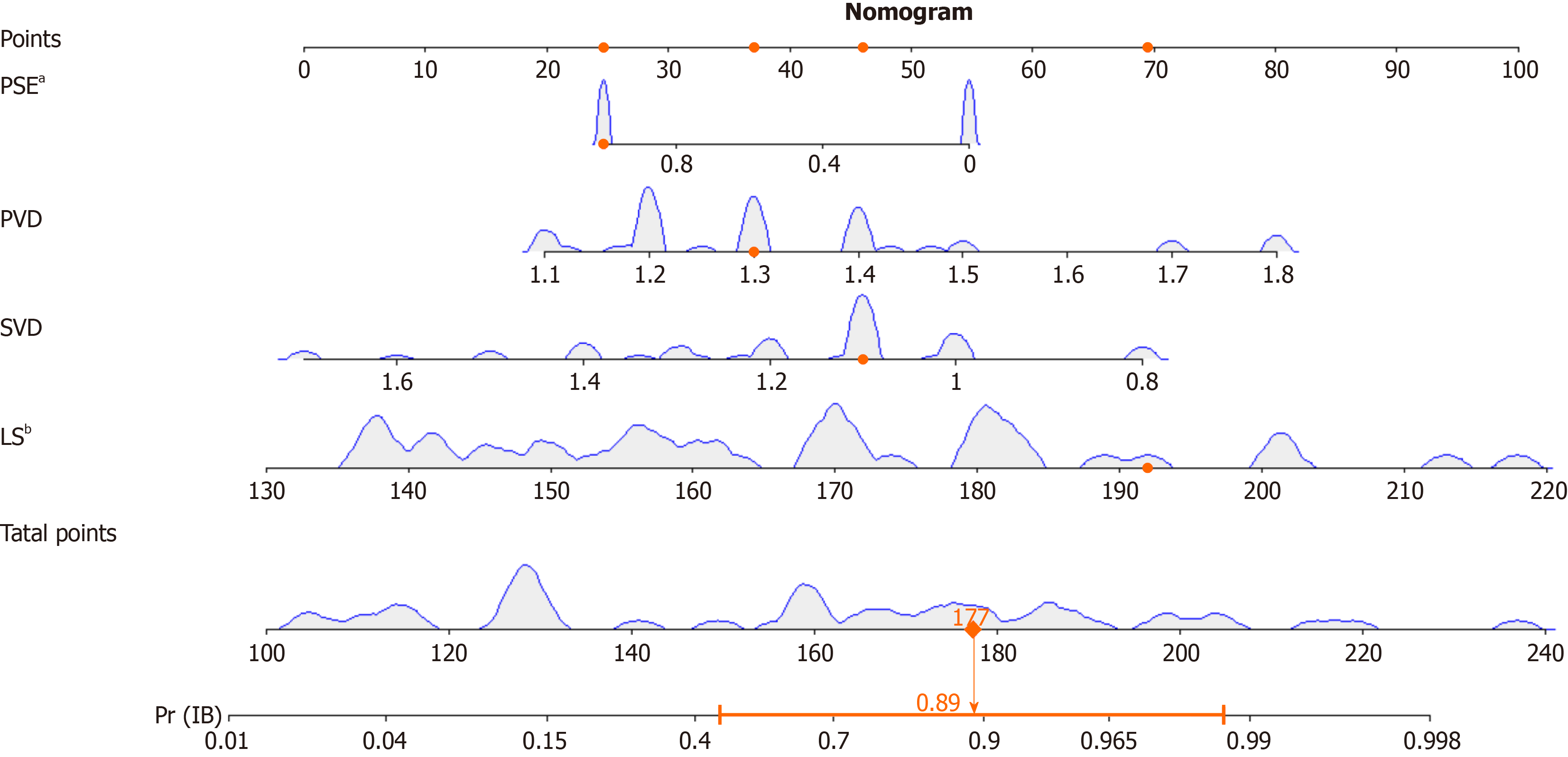
Univariate and multivariate logistic regressions were employed to develop the clinical prediction model, with the occurrence of IB serving as the dependent variable, and incorporating the four selected variables derived from the Least Absolute Shrinkage and Selection Operator (LASSO) regression analysis. The findings revealed that the length of the spleen, PVD, SVD, and PSE history significantly influenced the occurrence of IB (P < 0.05). A nomogram constructed using the predicted variables is shown in Figure 3.
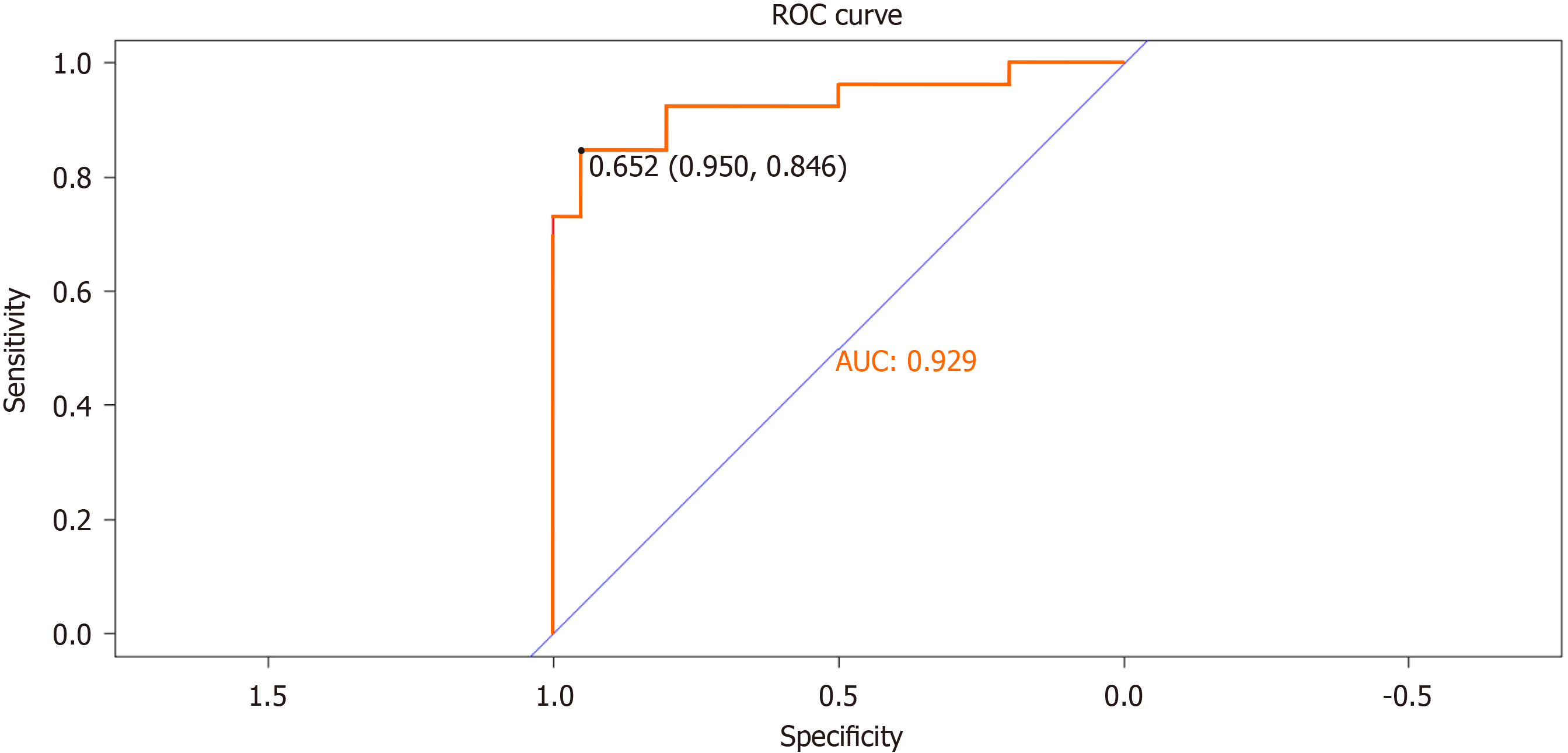
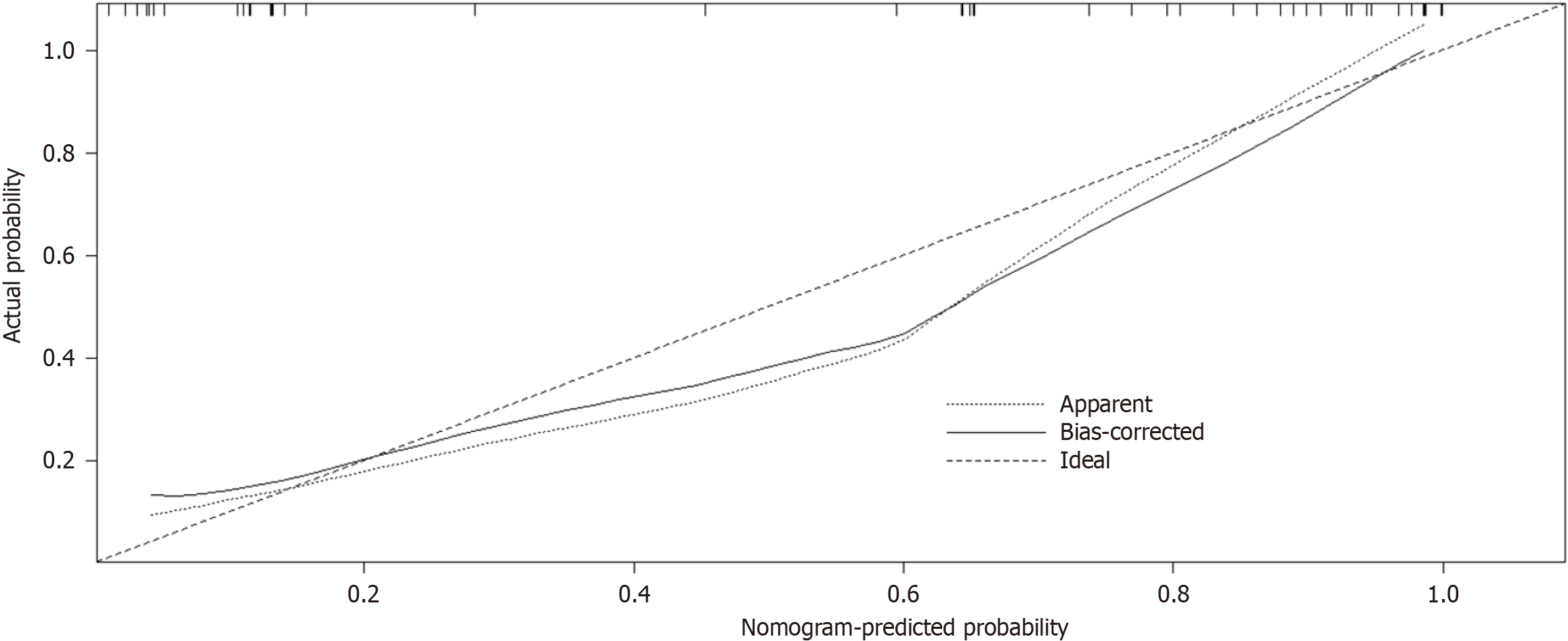
The predictive performance of the differentiation model was assessed by generating a ROC curve and calculating the AUC to predict IB (Figure 4). The AUC value was 0.929 (95%CI: 0.950-0.846), indicating a strong discriminatory ability of the prediction model. Furthermore, the Hosmer-Lemeshow goodness-of-fit test (P = 0.89) demonstrated that the predicted probability of the model closely matched the actual probability, indicating a high degree of calibration. In summary, the nomogram models displayed superior predictive performance (Figure 5).
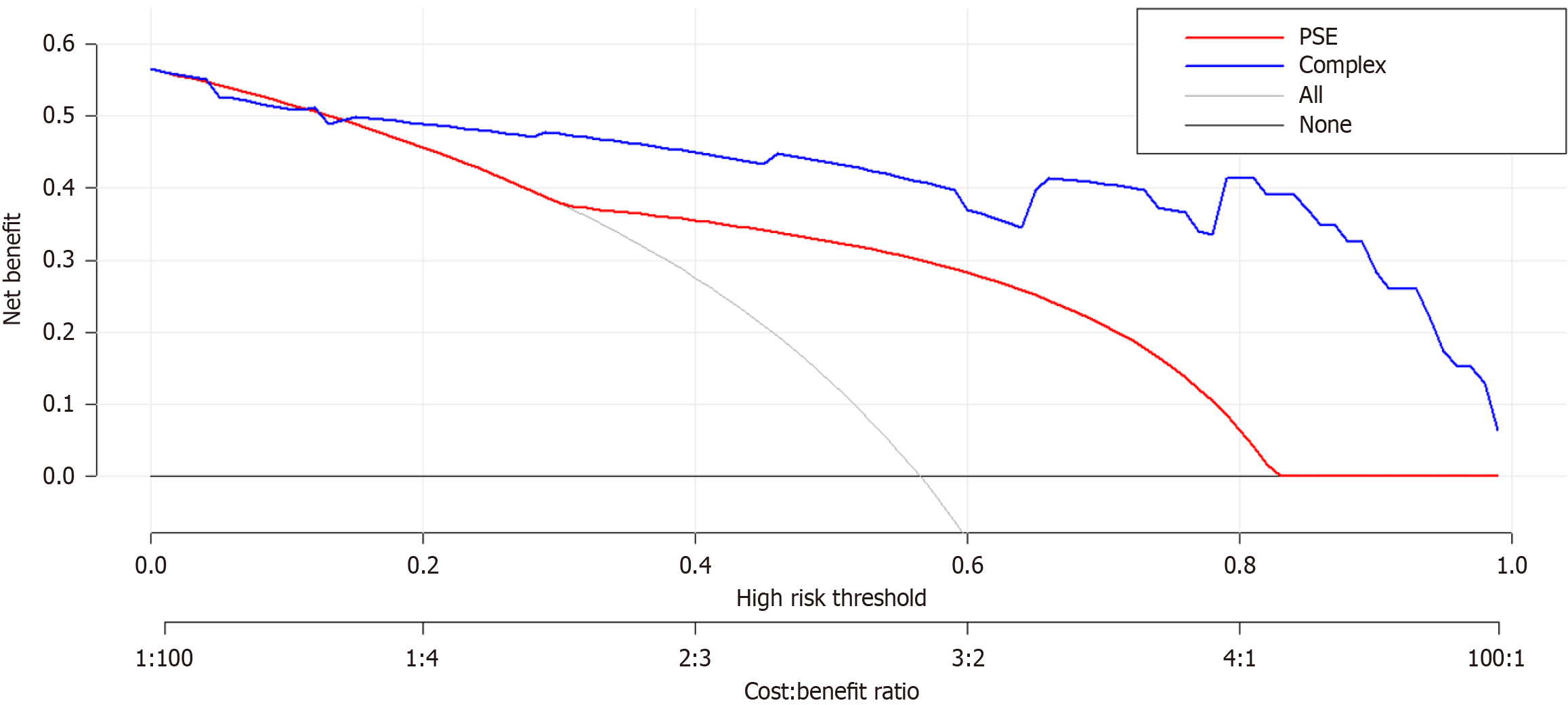
The DCA of the IB risk nomogram is shown in Figure 6. The results demonstrate that the net benefits are greater for threshold probabilities within the range of 20%-99%, suggesting that intervention within this range is more favorable.
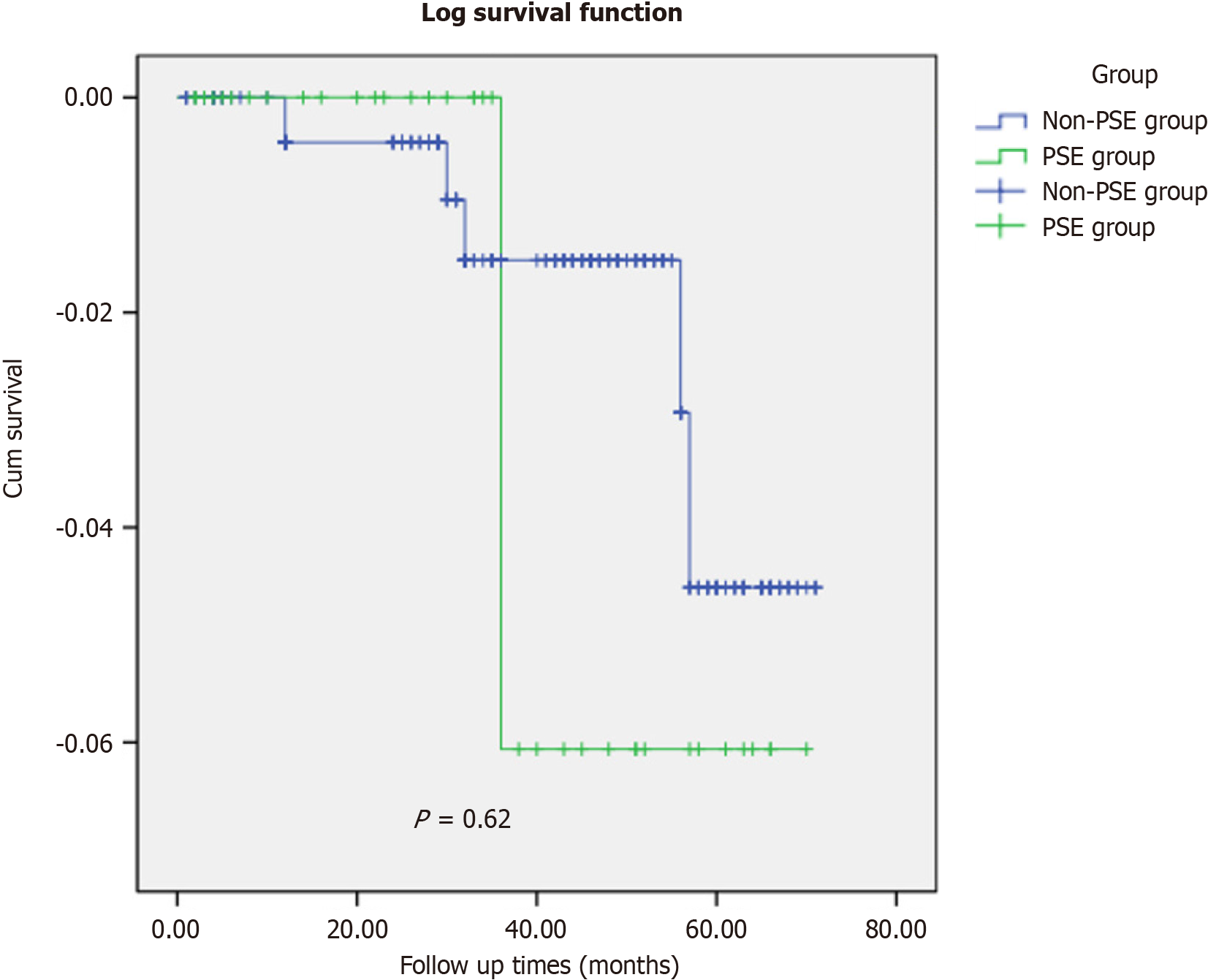
The follow-up rates in the non-PSE group were 92.2% (259/281) and 92.5% (37/40). The median follow-up time in the control group was 43 mo (range: 29-56 mo), while that in the study group was 34 months (range: 15-51 mo). The log-rank test (P = 0.62) indicated no significant differences in the follow-up data between the two groups (Figure 7).
In the non-PSE group, there were five deaths, whereas in the PSE group, there was one death. Three patients in the Non-PSE group died of liver failure during follow-up. One patient in the non-PSE group developed refractory ascites and subsequently died of a spontaneous ascitic fluid infection. In both groups, one patient died of pulmonary infection.
With advancements in interventional radiology, PSE has gained widespread use in clinical practice for treating liver cirrhosis and hypersplenism. PSE is commonly used for patients with liver cirrhosis and hypersplenism who are in poor physical condition and are unable to undergo surgical treatment or preoperative treatment prior to splenectomy[15]. PSE has been demonstrated to reduce splenic volume, thereby improving surgical visibility and intraoperative exposure[16]. However, preoperative PSE prior to splenectomy reduces surgical risk. Some institutions have performed preoperative PSE followed by splenectomy and have arrived at conclusions that differ from ours[17-19].
PSE has the potential to reduce splenic venous blood flow, lower PH, and suppress splenic hyperfunction by impeding blood flow through the secondary splenic artery[20]. Furthermore, ischemic infarction may occur in the splenic region, and reducing splenic volume can be advantageous for splenectomy[21,22]. In contrast to previous studies, our study indicates that PSE followed by splenectomy may lead to increased perisplenic adhesions, prolonged surgical duration, and increased IB. We used PSM to control for confounding variables related to clinical characteristics between the two groups and performed logistic regression analysis to identify independent risk factors for IB. The results of our study suggest that parameters such as LS, PVD, SVD, and a history of PSE significantly affect the occurrence of IB. Additionally, both AUC and DCA indicated the strong discriminative capabilities of the prediction model. The findings of this study suggest a potential increase in IB associated with preoperative PSE, followed by splenectomy.
A comprehensive analysis of the surgical procedures revealed that the disparity lies in the timing of the preoperative PSE. According to the literature[23], splenectomy within 24 h after PSE may reduce surgical risk. However, our findings indicate that performing splenectomy after a period exceeding 1 mo after PSE increases surgical complexity and the likelihood of IB.
Post-PSE complications can influence therapeutic outcomes. Complications include splenic infarction, splenic abscess, pleural effusion, spontaneous bacterial peritonitis, ectopic embolization, portal and splenic vein thrombosis, ascites, jaundice, and liver failure[24]. Splenic infarction is classified into four stages: Hyperacute, acute, subacute, and chronic[25]. The first stage lasts approximately 1 d, during which the spleen tissue exhibits congestion and edema. The second stage lasts around 1 wk and is characterized by inflammatory exudation in the spleen tissue and the formation of mild perisplenic adhesions. The third stage lasts from 1 wk to 1 mo and is characterized by a reduction in inflammation in the spleen tissue, leading to the development of dense adhesions with the surrounding tissues, such as the omentum. The fourth stage lasts for over a month and is characterized by fibrosis and scar formation in the spleen tissue[26]. Therefore, if splenectomy is performed more than a month after PSE, dense adhesions around the spleen can result in IB. However, only a small number of patients undergo splenectomy within a day after PSE, and the purpose of PSE sequential splenectomy is to reduce the intraoperative splenic volume and facilitate surgery. Therefore, for most patients, splenectomy is performed more than a month after PSE[27].
Unlike previous studies[28], our splenectomy was typically performed 1 mo or more after PSE, which may have contributed to the increased IB. Enlarged spleens can limit surgical space and exposure, and increase surgical risk. PSE can reduce the size of the spleen, enhance operating space, and facilitate exposure. PSE can mitigate PH, improve liver function, and alleviate symptoms of hypersplenism, making it a potential alternative to splenectomy. Preoperative PSE before laparoscopic splenectomy can reduce surgical complexity and postoperative complication rates[29,30]. Some studies suggest that patients with PSE are at risk of massive IB due to splenic infarction, extensive adhesions, and thickened collateral circulation vessels. Patients with PSE who undergo splenectomy may experience prolonged operative times, substantial bleeding, and postoperative complications due to blood protein and coagulation factor loss. They also had longer hospital stays than those who did not undergo PSE. Currently, there is no consensus on the preferred embolization plan or optimal timing for splenectomy after embolization.
The optimal duration between embolization and splenectomy requires further investigation. It has been established that a PSE and splenectomy duration of less than 24 h effectively reduces splenic volume and enhances surgical visibility and exposure, thereby decreasing the risk of surgical complications. However, this study only considered cases with a duration exceeding 1mo. These results suggest that while a duration exceeding 1 mo may increase the risk of splenic adhesion-related complications, it can also improve liver function and facilitate surgical exposure by reducing the size of the spleen.
This study focused on PSE cases with embolization durations exceeding 1 mo. A significant reduction in spleen size was observed when comparing color Doppler ultrasound images before and after embolization. However, during intraoperative exploration, extensive inflammatory exudation and adhesions around the spleen were observed, resulting in a significant increase in IB and prolonged hospital stay. Therefore, based on the findings of this study, it is necessary to adopt precise intraoperative procedures and carefully separate adhesions in patients with embolization duration exceeding 1 mo to minimize IB.
Moreover, LS, PVD, and SVD were significant contributing factors for IB. The elongated shape of the spleen can result in adhesion between its upper end and the stomach, which can further complicate dissection of the short gastric artery and lead to increased surgical complexity and IB. Similarly, an increase in the diameter of the portal and splenic veins can lead to tortuous dilation of vessels within the splenic pedicle, making separation more challenging. Consequently, detaching the splenic pedicle can lead to an increase in IB. This finding suggests that further investigation is needed to evaluate the long-term efficacy of PSE as a treatment for patients with cirrhosis with PH and hypersplenism.
Nomograms, which are graphically intuitive representations of mathematical models, can be used to predict specific end points by integrating multiple influencing factors. This is because of their ability to provide personalized assessments, thereby facilitating disease management and clinical decision making[31]. Our study examined the clinical characteristics, hematology, and complications of patients diagnosed with cirrhosis and PH. Using LASSO and univariate and multivariate regression analyses, we identified several factors influencing IB, including history of LS, PVD, SVD, and PSE. To facilitate the visual analysis of complications, we developed a nomogram. In this study, we identified four factors that influence IB. Subsequently, the established model was validated, both internally and externally. The AUC was 0.929 (95%CI: 0.950-0.846), indicating the robustness of the model, as revealed by the Hosmer-Lemeshow goodness-of-fit test (P = 0.89). Furthermore, the DCA plot indicated a favorable net clinical benefit for the nomogram.
This study has several limitations. First, the retrospective nature of the study and its focus on a single institution limits the generalizability of the findings. Additionally, a small proportion of discharged patients were lost to follow-up and the sample size of patients with preoperative PSE was limited. A prospective, randomized, multicenter study is recom
In summary, preoperative PSE followed by splenectomy may increase the incidence of IB in patients with severe splenic adhesion. This results in significantly prolonged surgery duration and increased surgical risk. Additionally, the nomogram-based prediction model effectively predicted the occurrence of IB.
Partial splenic embolization (PSE) has been suggested as an alternative to splenectomy for the treatment of hypersplenism; however, some patients may experience recurrence of hypersplenism after PSE and still require splenectomy. Studies have demonstrated that PSE can reduce surgical complexity and occurrence of postoperative complications. Currently, there is a lack of evidence-based medical support regarding whether preoperative PSE followed by splenec
There is a lack of clear evidence-based medical support regarding whether preoperative PSE followed by splenectomy can decrease complications and the optimal timing for performing splenectomy after PSE remains uncertain. Addressing these questions is crucial for providing evidence-based guidance for clinicians to decrease perioperative complications.
This study aimed to investigate the safety and therapeutic efficacy of preoperative PSE followed by splenectomy in patients with cirrhosis and hypersplenism.
Between January 2010 and December 2021, 321 consecutive patients with cirrhosis and hypersplenism who underwent splenectomy were enrolled. Based on whether PSE was performed prior to splenectomy, the patients were divided into two groups: PSE group (n = 40) and non-PSE group (n = 281). Patient characteristics, postoperative complications, and follow-up data were compared between groups. Propensity score matching (PSM) was conducted, and univariable and multivariable analyses were used to establish a nomogram predictive model for intraoperative bleeding (IB). The receiver operating characteristic curve, Hosmer-Lemeshow goodness-of-fit test, and decision curve analysis (DCA) were employed to evaluate the differentiation, calibration, and clinical performance of the model.
After PSM, the non-PSE group showed significant reductions in hospital stay, intraoperative blood loss, and operation time (all P = 0.00). Multivariate analysis revealed that spleen length, portal vein diameter, splenic vein diameter, and history of PSE were independent predictive factors for IB. A nomogram predictive model of IB was constructed, and DCA demonstrated the clinical utility of this model. Both groups exhibited similar results in terms of overall survival during the follow-up period.
Preoperative PSE followed by splenectomy may increase the incidence of IB and a nomogram-based prediction model can predict the occurrence of IB. Meticulous separation of splenic adhesions is imperative for achieving safe and efficacious surgical outcomes.
Future research should focus on the duration between embolization and splenectomy to enhance the benefits of this approach and reduce the surgical risks. Additional prospective randomized controlled trials are necessary to expand the findings of this study.
We would like to acknowledge the support of the Department of No. 1 Surgery, the First Affiliated Hospital of Anhui Chinese Medical University, Hefei, China.
| 1. | Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37:778-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (1)] |
| 2. | Chikamori F, Inoue A, Okamoto H, Kuniyoshi N, Kawashima T, Takase Y. Relationships between types of esophagogastric varices and systemic hemodynamics in patients with liver cirrhosis. Hepatogastroenterology. 2011;58:909-915. [PubMed] |
| 3. | Ushitora Y, Tashiro H, Takahashi S, Amano H, Oshita A, Kobayashi T, Chayama K, Ohdan H. Splenectomy in chronic hepatic disorders: portal vein thrombosis and improvement of liver function. Dig Surg. 2011;28:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Du Z, Dong J, Zhang J, Bi J, Wu Z, Lv Y, Zhang X, Wu R. Incidence and risk factors associated with a high comprehensive complication index score after splenectomy in cirrhotic patients with hypersplenism. J Surg Res. 2018;222:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Koconis KG, Singh H, Soares G. Partial splenic embolization in the treatment of patients with portal hypertension: a review of the english language literature. J Vasc Interv Radiol. 2007;18:463-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Ueda J, Mamada Y, Taniai N, Yoshioka M, Matsushita A, Mizutani S, Kawano Y, Shimizu T, Kanda T, Takata H, Furuki H, Aoki Y, Kawashima M, Irie T, Ohno T, Haruna T, Yoshida H. Evaluation of splenic infarction ratio and platelet increase ratio after partial splenic artery embolization. J Int Med Res. 2023;51:3000605231190967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Ishikawa T, Sasaki R, Nishimura T, Matsuda T, Iwamoto T, Saeki I, Hidaka I, Takami T, Sakaida I. Splenic non-infarction volume determines a clinically significant hepatic venous pressure gradient response to partial splenic embolization in patients with cirrhosis and hypersplenism. J Gastroenterol. 2021;56:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Van Der Veken E, Laureys M, Rodesch G, Steyaert H. Perioperative spleen embolization as a useful tool in laparoscopic splenectomy for simple and massive splenomegaly in children: a prospective study. Surg Endosc. 2016;30:4962-4967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Tan Y, Wang J, Sun L, Ye Y. Repeated partial splenic artery embolization for hypersplenism improves platelet count. Open Med (Wars). 2022;17:808-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Naoum JJ, Silberfein EJ, Zhou W, Sweeney JF, Albo D, Brunicardi FC, Kougias P, El Sayed HF, Lin PH. Concomitant intraoperative splenic artery embolization and laparoscopic splenectomy versus laparoscopic splenectomy: comparison of treatment outcome. Am J Surg. 2007;193:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Ekeh AP, Khalaf S, Ilyas S, Kauffman S, Walusimbi M, McCarthy MC. Complications arising from splenic artery embolization: a review of an 11-year experience. Am J Surg. 2013;205:250-4; discussion 254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Ikegami T, Soejima Y, Taketomi A, Kawanaka H, Yoshizumi T, Shimada M, Maehara Y. Hypersplenism after living donor liver transplantation. Hepatogastroenterology. 2009;56:778-782. [PubMed] |
| 13. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 3226] [Article Influence: 358.4] [Reference Citation Analysis (35)] |
| 14. | Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 575] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 15. | Hadduck TA, McWilliams JP. Partial splenic artery embolization in cirrhotic patients. World J Radiol. 2014;6:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (3)] |
| 16. | Wu Z, Zhou J, Pankaj P, Peng B. Comparative treatment and literature review for laparoscopic splenectomy alone versus preoperative splenic artery embolization splenectomy. Surg Endosc. 2012;26:2758-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Rollins Z, Rehman R, Al-Hadidi A, Lapkus M, Novotny N, Brahmamdam P, Metz T, Akay B, Stallion A. Preoperative Splenic Artery Embolization for Massive Splenomegaly in Children: A Single Center Experience. J Laparoendosc Adv Surg Tech A. 2022;32:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Reso A, Brar MS, Church N, Mitchell P, Dixon E, Debru E. Outcome of laparoscopic splenectomy with preoperative splenic artery embolization for massive splenomegaly. Surg Endosc. 2010;24:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Iwase K, Higaki J, Yoon HE, Mikata S, Miyazaki M, Nishitani A, Hori S, Kamiike W. Splenic artery embolization using contour emboli before laparoscopic or laparoscopically assisted splenectomy. Surg Laparosc Endosc Percutan Tech. 2002;12:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Helaly AZ, Al-Warraky MS, El-Azab GI, Kohla MA, Abdelaal EE. Portal and splanchnic hemodynamics after partial splenic embolization in cirrhotic patients with hypersplenism. APMIS. 2015;123:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Zhu S, Chang S. Clinical application value of preoperative selective partial splenic embolization before splenectomy plus portal-azygous disconnection. Int J Clin Exp Pathol. 2015;8:9574-9579. [PubMed] |
| 22. | Zheng L, Deng C, Li J, Wang L, You N, Wu K, Wang W. Treatment of hemangioma of the spleen by preoperative partial splenic embolization plus laparoscopic partial splenectomy: A case report. Medicine (Baltimore). 2018;97:e0498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Nitta T, Fujii K, Kawasaki H, Takasaka I, Kawata S, Onaka M, Ishibashi T. Efficacy and surgical procedures of preoperative splenic artery embolization for laparoscopic splenectomy of a massive splenomegaly: A case report. Int J Surg Case Rep. 2015;16:174-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Talwar A, Gabr A, Riaz A, Desai K, Thornburg B, Mouli S, Lewandowski RJ, Salem R. Adverse Events Related to Partial Splenic Embolization for the Treatment of Hypersplenism: A Systematic Review. J Vasc Interv Radiol. 2020;31:1118-1131.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Jaroch MT, Broughan TA, Hermann RE. The natural history of splenic infarction. Surgery. 1986;100:743-750. [PubMed] |
| 26. | Chapman J, Helm TA, Kahwaji CI. Splenic Infarcts. 2023 Jul 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 27. | Jiao S, Chen H, Wang Y, Zhu J, Tan J, Gao J. Splenectomy versus Partial Splenic Embolization for Massive Splenomegaly Secondary to Hepatitis B-Related Liver Cirrhosis: A Case-Control Study. Gastroenterol Res Pract. 2016;2016:3471626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Baú PC, Cavazolla SA, Souza HP, Garicochea B. Preoperative embolization of the splenic artery in patients that underwent splenectomy for immune thrombocytopenic purpura. Acta Cir Bras. 2007;22:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Li J, You N, Deng C, Wu K, Wang L, Huang X, Wang W, Fan J, Zheng L. Use of Iodized Oil and Gelatin Sponge Embolization in Splenic Artery Coiling Reduces Bleeding from Laparoscopic Splenectomy for Cirrhotic Portal Hypertension Patients with Complicating Hypersplenic Splenomegaly: A Comparative Study. J Laparoendosc Adv Surg Tech A. 2018;28:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Ransom KJ, Kavic MS. Laparoscopic splenectomy following embolization for blunt trauma. JSLS. 2008;12:202-205. [PubMed] |
| 31. | Alhulaili ZM, Linnemann RJ, Dascau L, Pleijhuis RG, Klaase JM. A Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis analysis to evaluate the quality of reporting of postoperative pancreatic fistula prediction models after pancreatoduodenectomy: A systematic review. Surgery. 2023;174:684-691. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Joshi MK, India S-Editor: Qu XL L-Editor: Filipodia P-Editor: Zheng XM