Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3843
Revised: October 1, 2024
Accepted: October 28, 2024
Published online: December 27, 2024
Processing time: 92 Days and 23.2 Hours
Intestinal ischemia-reperfusion (I/R) injury (II/RI) is a critical condition that results in oxidative stress, inflammation, and damage to multiple organs. Zinc, an essential trace element, offers protective benefits in several tissues during I/R injury, but its effects on intestinal II/RI remain unclear.
To investigate the effects of zinc pretreatment on II/RI and associated multiorgan damage.
C57BL/6 mice were pretreated with zinc sulfate (ZnSO4, 10 mg/kg) daily for three days before I/R injury was induced via superior mesenteric artery occlusion (SMAO) and abdominal aortic occlusion (AAO) models. Tissue and serum samples were collected to evaluate intestinal, liver, and kidney damage using Chiu’s score, Suzuki score, and histopathological analysis. Caco-2 cells and intestinal organoids were used for in vitro hypoxia-reoxygenation injury models to measure reactive oxygen species (ROS) and superoxide dismutase (SOD) levels.
Zinc pretreatment significantly reduced intestinal damage in the SMAO and AAO models (P < 0.001). The serum levels of liver enzymes (alanine aminotransferase, aspartate aminotransferase) and kidney markers (creatinine and urea) were lower in the zinc-treated mice than in the control mice, indicating reduced hepatic and renal injury. In vitro, zinc decreased ROS levels and increased SOD activity in Caco-2 cells subject to hypoxia-reoxygenation injury. Intestinal organoids pretreated with zinc exhibited enhanced resilience to hypoxic injury compared to controls.
Zinc pretreatment mitigates II/RI and reduces associated multiorgan damage. These findings suggest that zinc has potential clinical applications in protecting against I/R injuries.
Core Tip: This study demonstrated the protective effects of zinc pretreatment on reducing oxidative stress, apoptosis, and multiorgan damage during intestinal ischemia-reperfusion injury (II/RI). Using mouse models and in vitro systems, zinc significantly mitigated damage in the intestine, liver, and kidneys by reducing reactive oxygen species production and enhancing cellular resilience to hypoxic injury. These findings highlight the potential clinical applications of zinc in protecting against II/RI and improving outcomes in patients experiencing ischemic events.
- Citation: Cheng MZ, Luo JH, Li X, Liu FY, Zhou WJ. Zinc pretreatment for protection against intestinal ischemia-reperfusion injury. World J Gastrointest Surg 2024; 16(12): 3843-3856
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3843.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3843
Intestinal ischemia-reperfusion (I/R) injury (II/RI) is a prevalent and life-threatening pathophysiological event[1-3]. II/RI occurs in various clinical scenarios, including severe infections, trauma, burns, shock, intestinal volvulus, and mesenteric thromboembolism[4]. II/RI leads to oxidative stress, bacterial translocation, and inflammation in intestinal epithelial cells, inducing apoptosis, autophagy, and necroptosis[5-7]. This disrupts the integrity of the intestinal mucosal barrier, al
Zinc is ubiquitously present in the human body and is essential for the activity of more than 300 enzymes[12]. It plays crucial roles in growth, immune function, and injury repair. Zinc protects cardiomyocytes from reperfusion injury by inactivating glycogen synthase kinase 3β. In cardiac I/R models, mice lacking the zinc transporter SLC39A2 gene exhibit larger infarct areas[13,14]. In renal ischemia models, presupplementation with zinc chloride significantly reduces tubular injury, inflammation, and apoptosis[15,16]. Zinc is primarily absorbed in the small intestine, and its absorption and homeostasis are regulated by zinc transporters[12,17]. Zinc enters intestinal epithelial cells via transporters on the apical membrane and is transported into the bloodstream via transporters on the basolateral membrane to be distributed throughout the body[18]. However, the role of zinc in II/RI remains unclear.
This study established superior mesenteric artery (SMA) occlusion (SMAO) I/R injury models and abdominal aortic occlusion (AAO) I/R injury models. Our findings indicate that compared with control mice, mice pretreated with ZnSO4 exhibited significantly reduced intestinal tissue damage. These results suggest that zinc pretreatment holds great potential for clinical applications aimed at alleviating II/RI.
Caco-2 cells were procured from the American Type Culture Collection (ATCC, HTB-37). The cells were cultured in Eagle's minimum essential medium supplemented with 20% fetal bovine serum and 1% nonessential amino acids. The culture conditions were maintained at 37 °C with 5% CO2 and a humidity range of 70%-80%.
C57BL/6 mice were obtained from the Guangdong Medical Laboratory Animal Centre, Guangzhou, China. All the animal experiments were performed with male mice, which were housed under specific-pathogen-free (SPF) conditions with a standard 12-hour light/dark cycle and unrestricted access to food and water. All animal research protocols followed the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and were approved by the Ethics Committee on Animal Care and Use at Southern Medical University.
C57BL/6 mice were housed in SPF-grade animal facilities. Eight-week-old male mice were used for the experiments. The mice were fasted for 12 hours before surgery. The abdominal fur was shaved, and an oxygen pump and small animal anesthesia machine were used. The oxygen flow rate was controlled at 0.5 L/min, and the isoflurane flow rate was 1 L/min. The mice were placed in an isoflurane anesthesia chamber for induction, with the isoflurane flow rate maintained at 0.4 L/min during surgery. A 3-cm incision was made along the midline of the abdomen, and the SMA was exposed via saline-soaked cotton swabs. An iris scissor was used to make a small incision around the SMA, and a microvascular clamp was applied to occlude the primary branch of the SMA, creating a 5-7 cm ischemic segment of the ileum near the cecum. The ischemic intestine turned dark red within approximately 30 minutes, indicating successful occlusion. After 1 hour of ischemia, saline was applied to the clamped area. The microvascular clamp was gently removed, and the abdominal muscles and skin were sutured. Tissue and serum samples were collected 6 hours after reperfusion. Zinc ions (10 mg/kg) were administered intraperitoneally daily for three days before surgery.
The AAO model followed a protocol similar to that of the SMAO model in terms of housing, anesthesia, and preparation of C57BL/6 mice. The abdominal aorta was occluded via a microvascular clamp for 20 minutes. Tissue and serum samples were collected at 6 and 24 hours postreperfusion. Zinc ions (10 mg/kg) were administered intraperitoneally daily for three days before surgery.
Mouse intestinal, liver, and kidney tissues were fixed in formalin for 24 hours, dehydrated via an automatic tissue processor, and embedded in paraffin. Sections 2.5 µm thick were obtained and baked at 68 °C for 4 hours. Hematoxylin and eosin staining was performed, and the sections were imaged via a light microscope. Tissue damage was scored on the basis of the staining results.
Periodic acid-Schiff staining is predominantly used in histology to detect carbohydrates in tissues. Periodic acid oxidizes hydroxyl groups on adjacent carbon atoms of sugars to aldehyde groups, which then react with Schiff reagent, producing a purple–red color.
Intestinal tissue was scored via Chiu’s criteria[19], a common method for assessing intestinal ischemia–reperfusion injury. The scoring corresponds to intestinal morphology as follows: 0, normal villi; 1, twisted villus tips; 2, goblet cell and crypt disappearance; 3, patchy epithelial cell sloughing; 4, Exposed villi, intact lamina propria, and epithelial cell shedding; 5, exposed lamina propria; and 6, villous hemorrhage or sloughing. Sixty villi per group were scored, and the average score was used for statistical analysis.
Liver tissue was scored via Suzuki’s criteria[20], a common method for assessing hepatic ischemia–reperfusion injury. The scoring corresponds to hepatic morphology as Table 1.
| Score | Sinusoidal congestion | Cytoplasmic vacuolization | Parenchymal necrosis |
| 0 | None | None | None |
| 1 | Minimal | Minimal | Single-cell necrosis |
| 2 | Mild | Mild | < 30% |
| 3 | Moderate | Moderate | < 60% |
| 4 | Severe | Severe | > 60% |
Renal tubular injury is evaluated by observing several specific histopathological characteristics, including loss of the brush border, intratubular cast formation, tubular dilation, and vacuolar degeneration. The extent of renal tubular damage was then quantified using a scoring system based on the affected area of the renal tubules as follows: 0, no injury; 1, injury affecting 1%-25% of the area; 2, injury affecting 26%-50% of the area; 3, injury affecting 51%-75% of the area; and 4, injury affecting 76%-100% of the area[21].
Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and blood urea nitrogen were measured via an automated biochemical analyzer (Mindray, BS-240 VET).
Apoptosis in the tissue sections was detected using a TdT-mediated dUTP nick-end labeling (TUNEL) assay kit (Beyotime, C1098), and the staining was visualized under a light microscope. The number of apoptotic cells in each group was quantified.
The cells were deprived of complete medium and incubated with PBS under hypoxic conditions for 12 hours. After reoxygenation for 3 hours in complete medium, the reactive oxygen species (ROS) and superoxide dismutase (SOD) levels were measured. For the ZnSO4 group, ZnSO4 was added to the complete medium at a concentration of 50 μM during reoxygenation.
ROS detection was performed via an ROS assay kit (Beyotime, S0033S). DCFH-DA, a nonfluorescent probe, permeates cells and is hydrolyzed by intracellular esterases to form DCFH. This nonfluorescent compound is then oxidized by ROS to form fluorescent DCF. The fluorescence was measured with excitation at 488 nm and emission at 525 nm. The fluorescence intensity of the control group was normalized to 1, and differences in fluorescence intensity between groups were calculated.
SOD activity was assessed via a SOD activity detection kit (Beyotime, S0101S), which employs the WST-8 method because of its superior stability and sensitivity compared with those of other techniques. WST-8 reacts with superoxide anions produced by xanthine oxidase (XO), resulting in the formation of a water-soluble formazan dye. SOD activity inhibits this reaction; thus, the level of formazan dye produced is inversely proportional to SOD activity. The absorbance of the reaction mixture was measured at 450 nm postincubation, following the manufacturer's protocol.
Segments of approximately 15 cm of the small intestine were harvested from 6-8-week-old mice and subsequently cut into 0.3-0.5 mm fragments. The intestinal fragments were rinsed three times with a PBS solution containing antibiotics (penicillin-streptomycin, 1:100). The segments were then placed in a chelation solution (PBS-antibiotic mixture supplemented with 900 μL of 0.5 M EDTA) and incubated on a rotator at 4 °C for 30 minutes. After incubation, the supernatant was discarded, and 30 mL of PBS-antibiotic mixture was added. The suspension was vortexed for 2 minutes, filtered through a 70 μm mesh into a new centrifuge tube, and centrifuged at 200 × g for 10 minutes at 4 °C. The supernatant was then discarded, and 1 mL of organoid culture medium was added, consisting of DMEM/F12 basal medium supplemented with 10 μM HEPES, 0.1 μg/mL recombinant human Noggin protein, 0.5 μg/mL recombinant Human R-spondin 1 protein, 0.1 μg/mL recombinant human EGF Protein, B-27 supplement without vitamin A (50 ×, serum-free), N-2 supplement (100 ×, serum-free), and 2 mmol/L L-glutamine, was added. The organoids were counted, and their concentration was adjusted to 2 organoids per microliter. Subsequently, 20 μL of the organoids were mixed with 80 μL of matrix gel, and 40-50 μL of this mixture was plated per well in a 24-well plate. The plate was incubated for 1 hour, followed by the addition of 650 μL of culture medium to each well. The plate was then placed in a 37 °C incubator with 5% CO2.
For the hypoxia model, small intestinal organoids were cultured for five days, followed by the addition of ZnSO4 or saline. After 24 or 48 hours of treatment, the culture medium was removed from the organoids, and 650 μL of PBS was added. The organoids were then placed in a 2.5 L sealed culture jar along with a corresponding 2.5 L anaerobic gas pack and an anaerobic indicator (manufactured by Mitsubishi Gas Chemical Co., Inc.). Timing started upon sealing the jar. After two hours of hypoxia, the jar was opened. Then, the PBS was removed, and culture medium was added. Organoid images were captured at designated time points.
Total RNA from mouse tissues and organoids was extracted and reverse transcribed. The resulting cDNAs were used for PCR with the SYBR-Green Master PCR Mix (Accurate Biology, Changsha, China). All PCR reactions were conducted in triplicate using the QuantStudio 3 qPCR System (Thermo Scientific, Waltham, MA, United States). The data for each sample were normalized to the endogenous ACTIN controls. Primers for qPCR were as Table 2.
| Gene name | Forward | Reverse |
| IL-1β (mouse) | 5'-GAAATGCCACCTTTTGACAGTG-3' | 5'- TGGATGCTCTCATCAGGACAG-3' |
| IL-6 (mouse) | 5'-TCCAATGCTCTCCTAACAGATAAG-3' | 5'-CAAGATGAATTGGATGGTCTTG-3' |
| TNFα (mouse) | 5'-CGTCAGCCGATTTGCTATCT-3' | 5'-CGGACTCCGCAAAGTCTAAG-3' |
| Lgr5 (mouse) | 5'-CCTACTCGAAGACTTACCCAGT-3' | 5'-GCATTGGGGTGAATGATAGCA-3' |
| NRF2 (mouse) | 5'-CATAGCAGAGCCCAGTGACG-3' | 5'-TTCCATTGTGCCTTCAGCGT-3' |
| ZNT1 (mouse) | 5'-GGAAGCGGAAGACAACAGGG-3' | 5'-CAAGGCATTCACGACCACG-3' |
| ZNT2 (mouse) | 5'-CGGAGCCCGGTCCTTCTTA-3' | 5'-GCATGGCAATAATGGTTGCTCT-3' |
| ZNT3 (mouse) | 5'-GAAGAGTCTTTTCACAGAGCCC-3' | 5'-TGTGTGCTAAATACCCACCAAC-3' |
| ZNT4 (mouse) | 5'-AAGCGCCTCAAATCCCTGC-3' | 5'-CCACCACGACTCGAAGTTTATT-3' |
| ZNT5 (mouse) | 5'-GTTGGTTTTCATACGGCTTCCA-3' | 5'-CTGAAGGCGCTTAGCTCCAC-3' |
| ZNT6 (mouse) | 5'-ATGGGGACGATTCATCTCTTTCG-3' | 5'-CACAGCACGTTGATTGCACC-3' |
| ZNT7 (mouse) | 5'-GGATGATGAATACAAACCACCCA-3' | 5'-AAAGCGAAAGAGAGGTTCAGG-3' |
| ZNT8 (mouse) | 5'-AGCCACCAAGATGTACGCC-3' | 5'-CTTGCTTGCTCGACCTGTT-3' |
| ZNT9 (mouse) | 5'-TATGTGGTTCCCGACATTCACC-3' | 5'-TGATGGGACTTTGTCAGCTTTT-3' |
| ZNT10 (mouse) | 5'-GGCCGTTACTCAGGCAAGAC-3' | 5'-GCATGTTGAACGAGTCCGAGA-3' |
| ZIP1 (mouse) | 5'-ACCGAGTTATCACAGCCACC-3' | 5'-TGGTTAGCCCTGACCTTCG-3' |
| ZIP2 (mouse) | 5'-AGGAGTAAAAATCGGCTGCCT-3' | 5'-TGTAGCTGCATCCATCTGGAAC-3' |
| ZIP3 (mouse) | 5'-TGTAGCCAGGAATACGCCAC-3' | 5'-TGCCTGTGAAGGTCATCGAG-3' |
| ZIP4 (mouse) | 5'-CGGGCCACCAGCGTATTTA-3' | 5'-AGGGACTTGTGTTGGCTCTG-3' |
| ZIP5 (mouse) | 5'-ATGGTCAGCCAACGAATGGT-3' | 5'-TGGACATCTGGGCAGGAATG-3' |
| ZIP6 (mouse) | 5'-TGGGCGAGATCCTTTCCCTA-3' | 5'-CAGTCACACGGTTGCTGGTA-3' |
| ZIP7 (mouse) | 5'-GACAGTGTCCAGGTGGTGTT-3' | 5'-CATGGCCACTCCCACGATAG-3' |
| ZIP8 (mouse) | 5'-AACCAGCTCGAACTTCTCTGC-3' | 5'-GAAAGACTGGGCTTTGCGTTG-3' |
| ZIP9 (mouse) | 5'-GCAGTTCCAAAATCACCACCA-3' | 5'-TTGCCCAAGAGCATTCAGTGT-3' |
| ZIP10 (mouse) | 5'-TCACTGTGAGCAACGGAGTC-3' | 5'-GCTGAACTGCCCATCAAAGC-3' |
| ZIP11 (mouse) | 5'-CTGCCCGCTGGAGAATATGA-3' | 5'-CAAGGTTACAGCTCCGTGGT-3' |
| ZIP12 (mouse) | 5'-TCCTCTACTGAGACCGGCAA-3' | 5'-CAGTGGTCACCAGCAGAGAG-3' |
| ZIP13 (mouse) | 5'- CTGCCTGTCGCCTGGATAAT-3' | 5'-CAAGGGGAAAACCCCACTGA-3' |
| ZIP14 (mouse) | 5'-TCTGGTTTTCTGAGGTGCAGG-3' | 5'-GCTCTGGAGACCTCTTTGCG-3' |
| ACTIN (mouse) | 5'-GGCTGTATTCCCCTCCATCG-3' | 5'-CCAGTTGGTAACAATGCCATGT-3' |
Student's t test or paired t test was utilized for analysis. All data are presented as the mean ± SEM, with P < 0.05 con
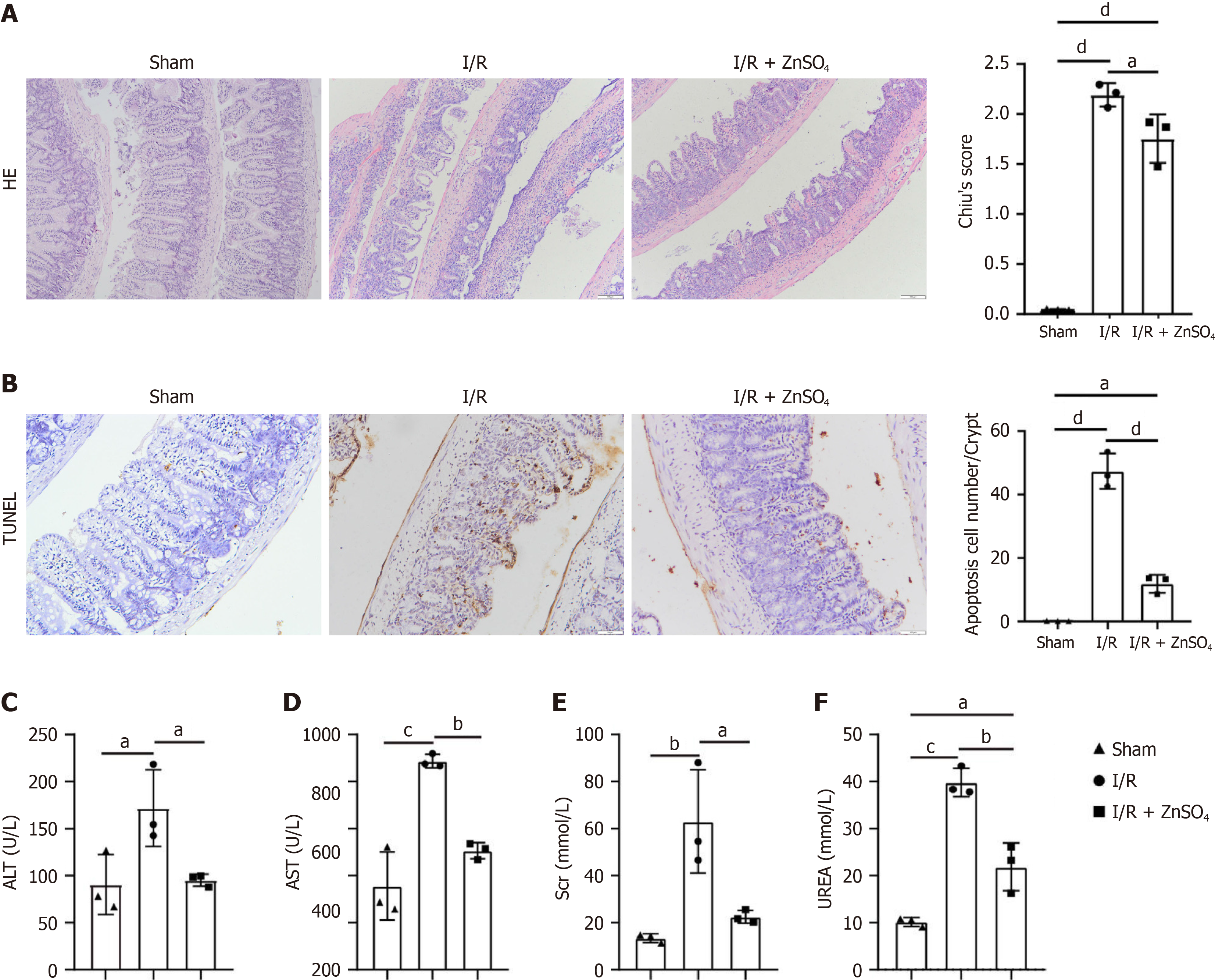
In the SMAO model, a 5-7 cm segment of the ileum near the cecum underwent I/R injury. Zinc sulfate (10 mg/kg) was administered intraperitoneally daily for three days prior to surgery. Following 1 hour of ischemia and 6 hours of reperfusion, intestinal tissue and serum samples were collected. Chiu’s score analysis revealed that the intestinal damage in the zinc pretreatment group was significantly lower than in the control group (Figure 1A). TUNEL staining revealed fewer apoptotic cells in the intestinal tissue of the zinc pretreatment group (Figure 1B).
II/RI caused secondary liver and kidney damage. Compared to the control group, the zinc pretreatment group presented significantly lower serum ALT and AST levels (Figure 1C and D), indicating reduced liver damage. The serum creatinine and urea nitrogen levels were also significantly lower (Figure 1E and F), indicating reduced kidney damage.
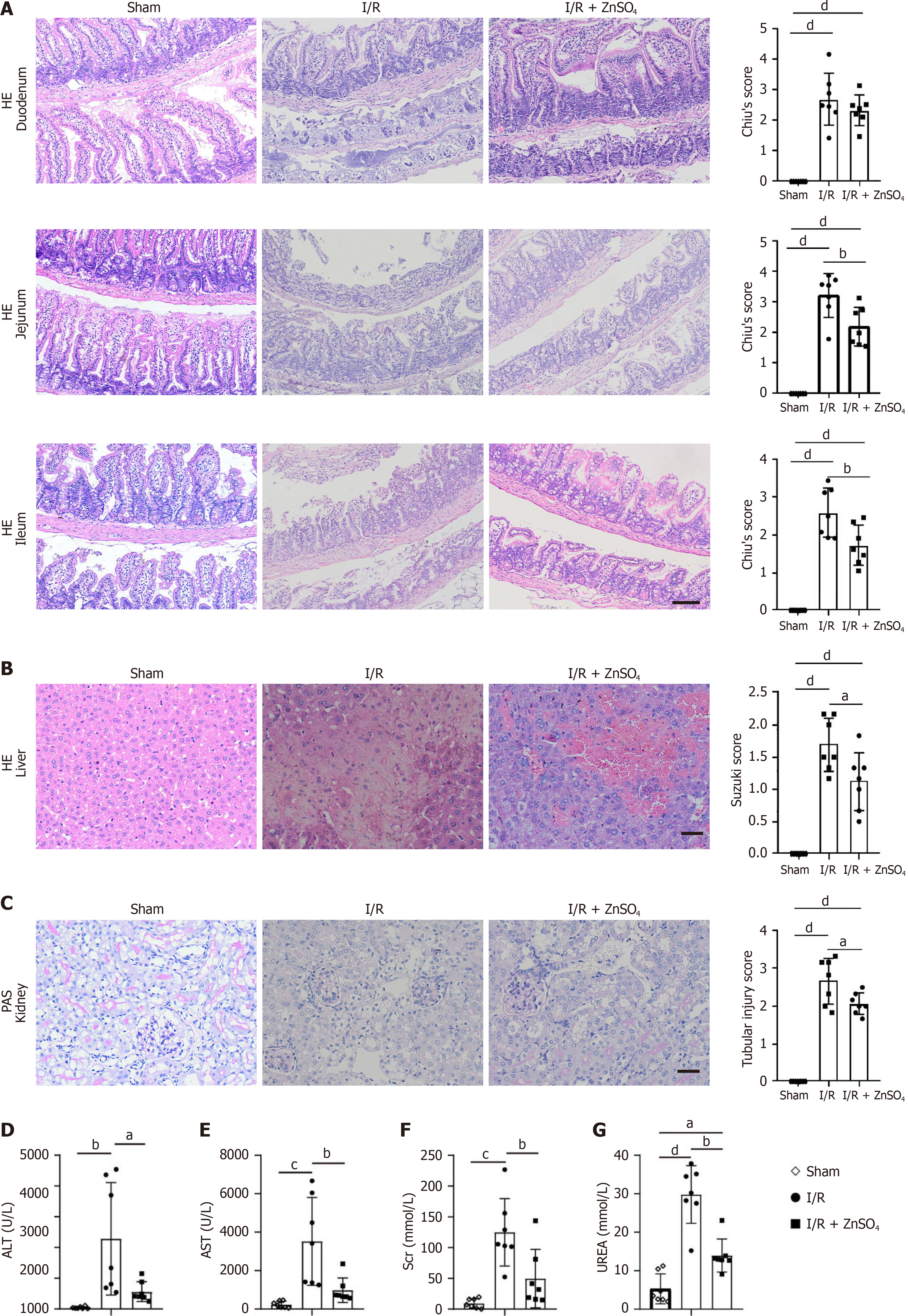
The AAO model results in I/R injury in multiple organs, including the intestine, liver, and kidneys. Zinc sulfate (10 mg/kg) was administered intraperitoneally daily for three days before surgery. After 20 minutes of ischemia, tissue and serum samples were collected at 6 and 24 hours after reperfusion. Chiu’s score analysis revealed that, compared with control treatment, zinc pretreatment significantly reduced damage to the duodenum, jejunum, and ileum (Figure 2A). Suzuki score analysis indicated that liver damage was also significantly lower in the experimental group than in the control group (Figure 2B). Renal tubular injury was less severe in the zinc pretreatment group than in the control group (Figure 2C). In addition, reduced serum ALT and AST levels were noted in the zinc pretreatment group compared to the control group (Figure 2D and E), indicating less liver damage. The serum creatinine and urea levels were also lower than those in the control group (Figure 2F and G), indicating less kidney damage.
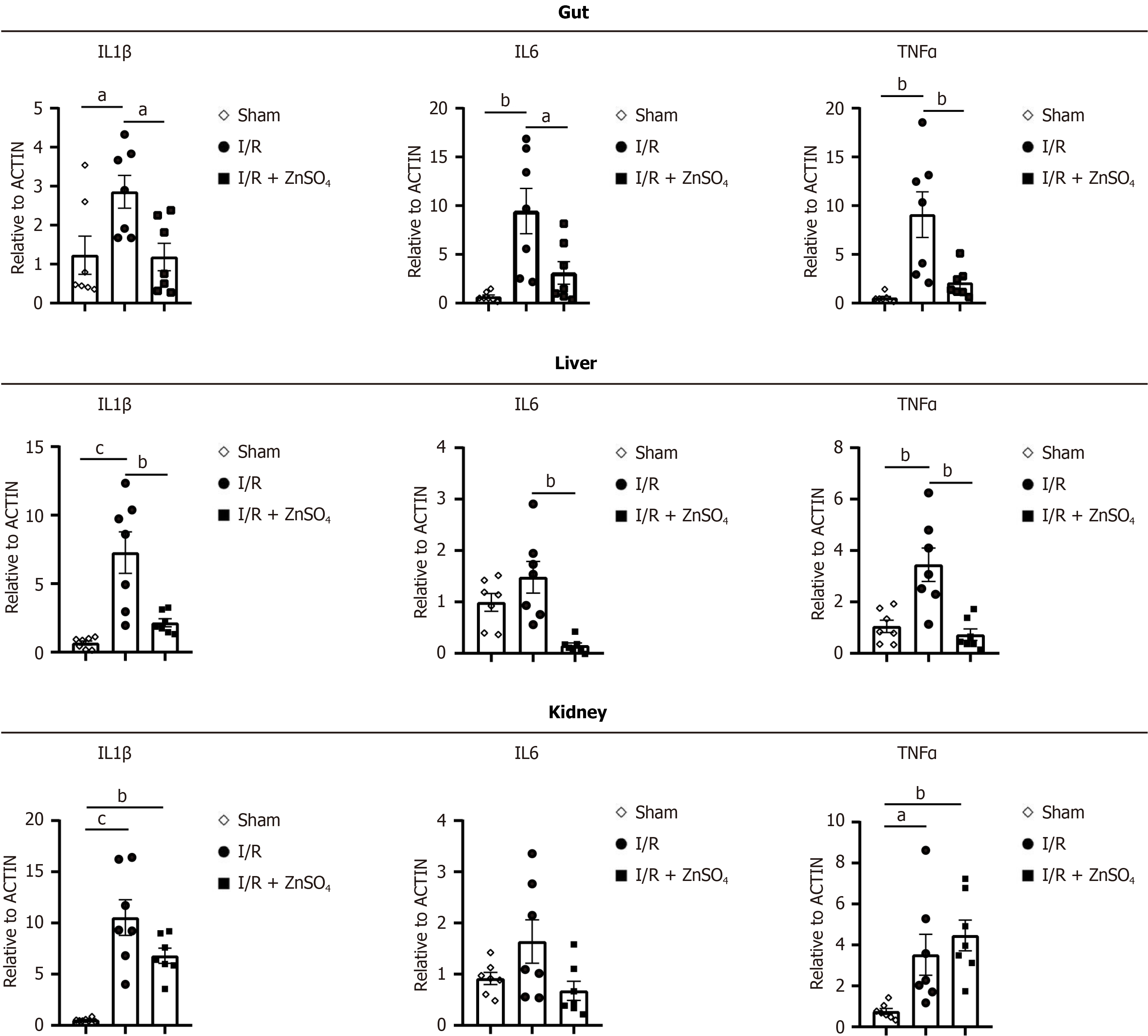
We further evaluated the expression of inflammatory biomarkers in the AAO model. Il1β, Il6, and TNFα levels were elevated in the small intestine, liver, and kidneys. Zinc pretreatment significantly reduced the expression of Il1β, Il6, and TNFα in the small intestine and liver. However, no significant changes in the expression of these inflammatory markers were observed in the kidneys (Figure 3).
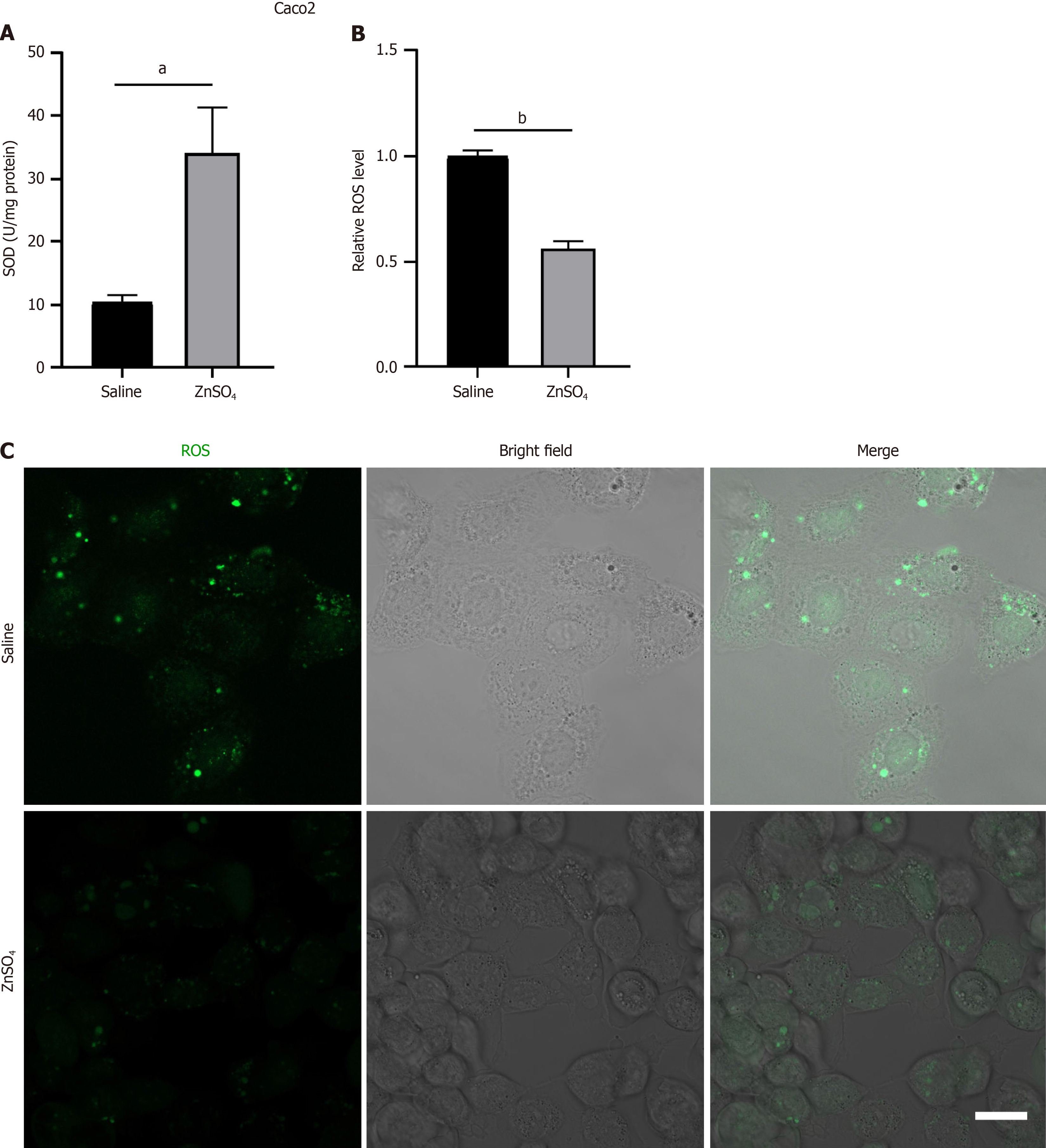
Oxidative stress and ROS production are major contributors to I/R injury. Using a hypoxia–reoxygenation model with Caco-2 cells, we found that, compared with the control treatment, zinc treatment significantly increased SOD activity (Figure 4A). To quantify ROS levels, Caco-2 cells were incubated with DCFH-DA under hypoxia–reoxygenation con
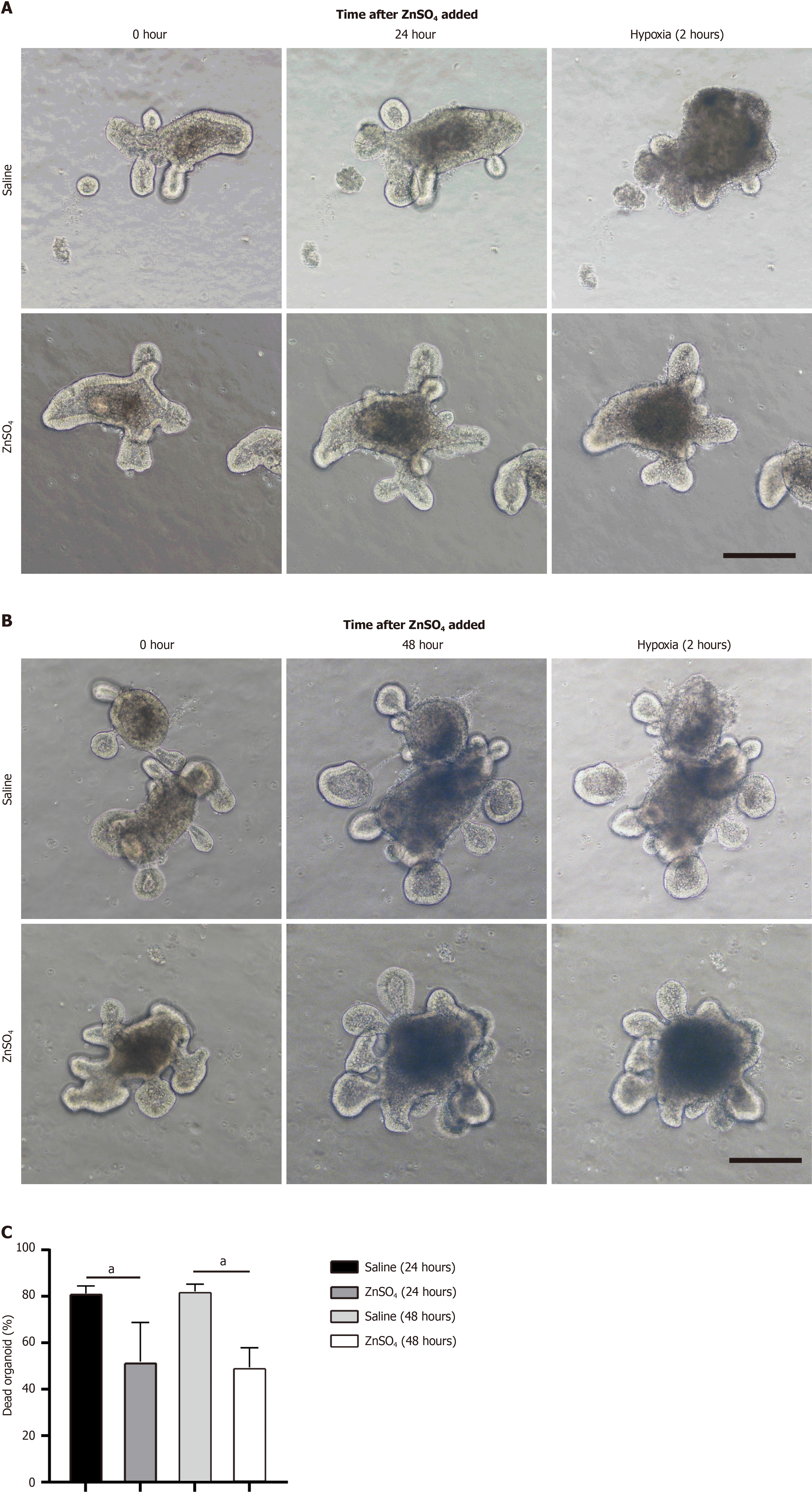
We cultured small intestinal organoids derived from intestinal crypts. After five days of culture, ZnSO4 was added. After 24 or 48 hours of incubation, the organoids were subjected to 2 hours of hypoxia followed by reoxygenation. The control organoids exhibited severe structural disintegration following reoxygenation, whereas the zinc-treated organoids demonstrated significant protection. However, no significant difference in protective effects was observed between the 24-hour and 48-hour zinc treatments (Figure 5).
We further investigated the potential mechanism by which zinc protects organoids from hypoxia-induced damage. The mRNA level of the intestinal stem cell marker Lgr5 was reduced in intestinal organoids following hypoxia; however, zinc pretreatment did not affect Lgr5 expression (Supplementary Figure 1A). In contrast, zinc pretreatment enhanced the expression of the antioxidant transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2; Supplementary Figure 1B). Zinc ion homeostasis is tightly regulated by two main families of transport proteins: The Zrt- and Irt-like protein (ZIP) family and the zinc transporter (ZnT) family[22]. We analyzed the expression profiles of zinc transporter family members ZIP1-14 and ZNT1-10, revealing significant alterations in their expression in response to hypoxia in intestinal organoids. Zinc pretreatment resulted in the downregulation of ZIP2, ZIP3, ZIP4, ZIP6, ZIP7, ZIP8, ZIP9, ZIP10 and ZNT3, ZNT6, ZNT7, ZNT8, while ZNT1, ZNT2, and ZNT10 were upregulated (Supplementary Figure 1C-Z). These findings indicate that I/R leads to dysregulation of zinc transporter expression. Zinc may protect intestinal organoids by activating the Nrf2 antioxidant signaling pathway.
This study demonstrated that zinc pretreatment significantly attenuated II/RI and mitigated associated hepatic and renal damage in the SMAO model and AAO model. In vitro experiments further revealed that zinc reduces oxidative stress in Caco-2 cells under hypoxia–reoxygenation conditions and protects small intestinal organoids from hypoxia-induced damage. These findings suggest that zinc protects intestinal stem cells, decreases oxidative stress in intestinal epithelial cells, and reduces the severity of intestinal I/R injury. Zinc pretreatment shows significant potential for clinical app
Severe II/RI is life-threatening and occurs in various clinical conditions, including severe infections, trauma, burns, shock, intestinal volvulus, and mesenteric thromboembolism. II/RI is also a severe secondary event associated with diseases such as atherosclerosis, obesity, and diabetes[23,24]. Furthermore, pneumoperitoneum, which is used to enhance visualization during laparoscopic surgery, increases intra-abdominal pressure, thereby reducing cardiac output and mean arterial pressure. Mesenteric ischemia is one of the most critical complications during laparoscopic surgery, particularly in patients with cardiovascular, hepatic, or renal diseases. These circulatory changes lead to reduced blood flow in the portal vein, SMA, liver, spleen, pancreas, and intestines, ultimately resulting in oxidative stress in these organs[25-27].
ROS are key mediators in the pathogenesis of II/RI. ROS cause initial endothelial barrier damage, promote com
Zinc plays essential roles in physiological processes and is primarily absorbed in the small intestine. Zinc absorption and homeostasis are regulated by zinc transporters[12,17]. Zinc is transported into intestinal epithelial cells via apical membrane transporters and into the bloodstream via basolateral membrane transporters for systemic distribution[18]. Zinc plays a crucial role in mitigating ischemia–reperfusion injury across various organs[32]. Bulbuloglu et al[33] de
In this study, we employed an intestinal organoid model. Intestinal stem cells, which are the primary source of intestinal epithelial cells, are critical for maintaining organoid integrity and facilitating tissue repair. Compared to traditional cell culture systems, organoid models more accurately replicate the in vivo intestinal environment while offering greater practicality than in vivo mouse experiments. This makes organoid models particularly advantageous for investigating stem cell function and elucidating underlying mechanisms[34,35]. Our findings demonstrate that zinc enhances the resilience of intestinal organoids to hypoxic injury; however, zinc treatment did not affect the expression of the intestinal stem cell marker Lgr5. Further investigation is needed to understand the mechanisms by which zinc modulates intestinal stem cell function.
Our study found that zinc pretreatment significantly reduces ROS production in intestinal epithelial cells and de
This study demonstrated that zinc pretreatment significantly reduces the severity of II/RI and associated multiorgan damage, including hepatic and renal injury, in mouse models. The protective effects of zinc are largely attributed to its ability to decrease oxidative stress, reduce ROS production, and enhance cellular resistance to hypoxic damage. These findings highlight the therapeutic potential of zinc as a preconditioning treatment for reducing I/R injury, particularly in clinical settings involving intestinal, hepatic, and renal ischemic events. Future research should explore the underlying mechanisms and evaluate the clinical applicability of zinc in human patients.
We thank Central Laboratory, Southern Medical University, for providing facilities and technical support.
| 1. | Wu MC, Brennan FH, Lynch JP, Mantovani S, Phipps S, Wetsel RA, Ruitenberg MJ, Taylor SM, Woodruff TM. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc Natl Acad Sci U S A. 2013;110:9439-9444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem. 2018;46:1650-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 969] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 3. | Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1886] [Cited by in RCA: 2622] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 4. | Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, Ward PA. Ischemia/reperfusion injury. J Surg Res. 2002;105:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Wang Z, Sun R, Wang G, Chen Z, Li Y, Zhao Y, Liu D, Zhao H, Zhang F, Yao J, Tian X. SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020;28:101343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 6. | Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Li Z, Wang G, Feng D, Zu G, Li Y, Shi X, Zhao Y, Jing H, Ning S, Le W, Yao J, Tian X. Targeting the miR-665-3p-ATG4B-autophagy axis relieves inflammation and apoptosis in intestinal ischemia/reperfusion. Cell Death Dis. 2018;9:483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Jia Y, Cui R, Wang C, Feng Y, Li Z, Tong Y, Qu K, Liu C, Zhang J. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020;32:101534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 10. | Tilsed JV, Casamassima A, Kurihara H, Mariani D, Martinez I, Pereira J, Ponchietti L, Shamiyeh A, Al-Ayoubi F, Barco LA, Ceolin M, D'Almeida AJ, Hilario S, Olavarria AL, Ozmen MM, Pinheiro LF, Poeze M, Triantos G, Fuentes FT, Sierra SU, Soreide K, Yanar H. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42:253-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 11. | Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Chanoit G, Lee S, Xi J, Zhu M, McIntosh RA, Mueller RA, Norfleet EA, Xu Z. Exogenous zinc protects cardiac cells from reperfusion injury by targeting mitochondrial permeability transition pore through inactivation of glycogen synthase kinase-3beta. Am J Physiol Heart Circ Physiol. 2008;295:H1227-H1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Du L, Zhang H, Zhao H, Cheng X, Qin J, Teng T, Yang Q, Xu Z. The critical role of the zinc transporter Zip2 (SLC39A2) in ischemia/reperfusion injury in mouse hearts. J Mol Cell Cardiol. 2019;132:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Rao K, Sethi K, Ischia J, Gibson L, Galea L, Xiao L, Yim M, Chang M, Papa N, Bolton D, Shulkes A, Baldwin GS, Patel O. Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS One. 2017;12:e0180028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Hadj Abdallah N, Baulies A, Bouhlel A, Bejaoui M, Zaouali MA, Ben Mimouna S, Messaoudi I, Fernandez-Checa JC, García Ruiz C, Ben Abdennebi H. Zinc mitigates renal ischemia-reperfusion injury in rats by modulating oxidative stress, endoplasmic reticulum stress, and autophagy. J Cell Physiol. 2018;233:8677-8690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Baltaci AK, Yuce K. Zinc Transporter Proteins. Neurochem Res. 2018;43:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Maares M, Haase H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 19. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1450] [Article Influence: 25.9] [Reference Citation Analysis (6)] |
| 20. | Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 729] [Article Influence: 22.1] [Reference Citation Analysis (5)] |
| 21. | McLarnon SR, Wilson K, Patel B, Sun J, Sartain CL, Mejias CD, Musall JB, Sullivan JC, Wei Q, Chen JK, Hyndman KA, Marshall B, Yang H, Fogo AB, O'Connor PM. Lipopolysaccharide Pretreatment Prevents Medullary Vascular Congestion following Renal Ischemia by Limiting Early Reperfusion of the Medullary Circulation. J Am Soc Nephrol. 2022;33:769-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Hara T, Takeda TA, Takagishi T, Fukue K, Kambe T, Fukada T. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiol Sci. 2017;67:283-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 23. | Stallion A, Kou TD, Latifi SQ, Miller KA, Dahms BB, Dudgeon DL, Levine AD. Ischemia/reperfusion: a clinically relevant model of intestinal injury yielding systemic inflammation. J Pediatr Surg. 2005;40:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Panés J, Kurose I, Rodriguez-Vaca D, Anderson DC, Miyasaka M, Tso P, Granger DN. Diabetes exacerbates inflammatory responses to ischemia-reperfusion. Circulation. 1996;93:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Unsal MA, Imamoglu M, Kadioglu M, Aydin S, Ulku C, Kesim M, Alver A, Kalyoncu NI, Yaris E, Bozkaya H. The acute alterations in biochemistry, morphology, and contractility of rat-isolated terminal ileum via increased intra-abdominal pressure. Pharmacol Res. 2006;53:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Schietroma M, Carlei F, Cappelli S, Amicucci G. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg. 2006;243:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Yilmaz S, Polat C, Kahraman A, Koken T, Arikan Y, Dilek ON, Gökçe O. The comparison of the oxidative stress effects of different gases and intra-abdominal pressures in an experimental rat model. J Laparoendosc Adv Surg Tech A. 2004;14:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Chen LW, Chang WJ, Chen PH, Liu WC, Hsu CM. TLR ligand decreases mesenteric ischemia and reperfusion injury-induced gut damage through TNF-alpha signaling. Shock. 2008;30:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 30. | Günel E, Cağlayan F, Cağlayan O, Dilsiz A, Duman S, Aktan M. Treatment of intestinal reperfusion injury using antioxidative agents. J Pediatr Surg. 1998;33:1536-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Gutiérrez-Sánchez G, García-Alonso I, Gutiérrez Sáenz de Santa María J, Alonso-Varona A, Herrero de la Parte B. Antioxidant-Based Therapy Reduces Early-Stage Intestinal Ischemia-Reperfusion Injury in Rats. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Akbari G. Role of Zinc Supplementation on Ischemia/Reperfusion Injury in Various Organs. Biol Trace Elem Res. 2020;196:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Bulbuloglu E, Yildiz H, Senoglu N, Coskuner I, Yuzbasioglu MF, Kilinc M, Dogan Z, Deniz C, Oksuz H, Kantarçeken B, Atli Y. Protective effects of zinc, pentoxifylline, and N-acetylcysteine in an animal model of laparoscopy-induced ischemia/reperfusion injury of the small intestine. J Laparoendosc Adv Surg Tech A. 2011;21:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Zhou WJ, Geng ZH, Spence JR, Geng JG. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature. 2013;501:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Grabinger T, Luks L, Kostadinova F, Zimberlin C, Medema JP, Leist M, Brunner T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 2014;5:e1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 36. | Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2965] [Cited by in RCA: 2928] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 37. | Dai H, Wang L, Li L, Huang Z, Ye L. Metallothionein 1: A New Spotlight on Inflammatory Diseases. Front Immunol. 2021;12:739918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/