Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3720
Revised: September 25, 2024
Accepted: October 23, 2024
Published online: December 27, 2024
Processing time: 160 Days and 4.7 Hours
Gastric cancer is the leading cause of cancer-related deaths worldwide. Early gastric cancer (EGC) is often associated with the risk of lymph node metastasis, which influences treatment decisions. Despite the use of enhanced computed tomography, the prediction of lymph node involvement remains challenging.
To investigate the risk factors for lymph node metastasis and invasion depth in patients with EGC.
In total, 210 patients with pathologically diagnosed EGC were included in this study. Univariate and multivariate statistical analyses were used to predict risk factors for lymph node metastasis and invasion depth in patients with EGC.
Among the 210 patients, 27 (12.9%) had lymph node metastases. Of the 117 patients with submucosal gastric cancer, 24 (20.5%) had lymph node metastases. Both univariate and multivariate analyses indicated that the depth of invasion in EGC was a risk factor for lymph node metastasis in these patients. Additionally, pathological type was identified as a risk factor for cancer cell invasion in patients with EGC.
EGC invasion depth, not tumor type, size, age, sex, or location, predicts lymph node spread. Tumor type, not size, age, sex, or location, predicts cancer cell invasion.
Core Tip: This study investigates the risk factors for lymph node metastasis and invasion depth in early gastric cancer (EGC) by analyzing 210 cases from Huzhou Central Hospital. Our findings highlight that invasion depth and pathological type are significant predictors of lymph node metastasis in EGC, while other factors such as tumor size, age, gender, and tumor location are not. The study underscores the importance of assessing invasion depth and pathological type in EGC diagnosis and treatment planning, offering valuable insights for improving patient outcomes.
- Citation: Xiang Y, Yao LD. Risk factors for lymph node metastasis and invasion depth in early gastric cancer: Analysis of 210 cases. World J Gastrointest Surg 2024; 16(12): 3720-3728
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3720.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3720
Gastric cancer is the fifth most common cancer worldwide and ranks third among global cancer-related deaths. In China, gastric cancer has an incidence rate of 20.6 per 100000, ranking fifth globally[1]. Early gastric cancer (EGC) refers to tumors confined to the mucosal or submucosal layer, regardless of the tumor size or the presence of lymph node metastasis. This concept was first proposed by Japanese scholar Murakami. The primary responsibility of gastroenterological endoscopists is the timely diagnosis of EGC and correct classification of lesions to choose appropriate treatment options, such as surgery or endoscopy. The choice of treatment primarily depends on the risk of lymph node involvement[2]. However, it is currently believed that imaging examinations have low sensitivity and specificity for the detection of lymph node metastasis in EGC[3]. According to most studies, invasion depth and tumor size are related to lymph node metastasis in EGC[4-8]. Some studies[4-6] suggest that the histological type of the tumor is related to lymph node metastasis in EGC, but this view is not universally accepted[7,8]. Other studies have suggested that the tumor location may be related to lymph node metastasis in EGC[6].
This study provides a deeper understanding of the factors influencing lymph node metastasis in EGC, which is crucial for optimizing treatment strategies and improving patient outcomes. By exploring not only commonly accepted risk factors, such as invasion depth and tumor size, but also less universally agreed factors, such as histological type and tumor location, we aim to contribute to a more comprehensive approach for assessing lymph node metastasis risk. Ad
It is well known that the success rate of enhanced CT scans in predicting lymph node metastasis in EGC patients is currently low, despite numerous studies dedicated to improving the detection rate of lymph node metastasis in EGC through CT scans[9-11]. We found that when radiologists observe enlarged lymph nodes but cannot definitively determine whether there is metastasis, they often make a diagnosis of 'lymph node presentation’. This article also discusses whether the diagnosis of lymph node presentation has any indicative significance for lymph node metastasis in EGC.
This study retrieved data from the medical record system of Huzhou Central Hospital for all patients who underwent radical gastrectomy and were pathologically diagnosed with EGC between December 1, 2017, and August 31, 2021.
Inclusion criteria: All patients who underwent radical gastrectomy and were pathologically confirmed as EGC.
Exclusion criteria: Patients who received preoperative chemotherapy, those with multiple metastatic tumors, and those with incomplete clinical data were excluded.
The variables included age, sex, gastric cancer location, pathological classification, postoperative lymph node metastasis, tumor invasion depth, tumor size, and preoperative enhanced CT findings indicating lymph node metastasis. The data were extracted in February 2023. Two researchers independently reviewed the patients’ data.
The primary dependent variables were lymph node metastasis and invasion depth. The χ2 test was used to compare categorical variables. Binary logistic regression analysis was used to explore the relationship between lymph node metastasis and the depth of invasion in EGC. In both univariate and multivariate analyses, odds ratios (OR) and 95%CI were calculated to assess risk. Statistical significance was defined as P < 0.05. Statistical analyses were conducted using SPSS software version 27.0, and GraphPad PRISM 9.5.
The age of the 210 patients ranged from 31 to 91 years, with a median age of 66. The study included 156 males (74.3%) and 54 females (25.7%). The tumors were located in the cardia in 18 cases (8.6%), fundus in 2 cases (1.0%), stomach body in 41 cases (19.5%), angular incisure in 21 cases (10.0%), and antrum in 128 cases (60.9%). The pathological types included highly differentiated adenocarcinoma in 14 patients (6.7%), moderately differentiated adenocarcinoma in 84 (40.0%), poorly differentiated adenocarcinoma in 66 (31.4%), mucinous adenocarcinoma in 3 (1.4%), signet ring cell carcinoma in 41 (19.5%), papillary adenocarcinoma in 1 (0.5%), and lymphoepithelioma-like carcinoma in 1 (0.5%). Invasion reached the mucosal layer in 93 patients (44.3%) and the submucosal layer in 117 patients (55.7%). There were 27 cases (12.9%) had lymph node metastasis and 183 (87.1%) did not (Table 1).
| Feature | Value |
| All cases | 210 |
| Age (years), median (years) | (31-91), 65 |
| Sex | |
| Male | 156 (74.3) |
| Female | 54 (25.7) |
| Primary site | |
| Cardia | 18 (8.6) |
| Fundus | 2 (1.0) |
| Body | 41 (19.5) |
| Angle | 21 (10.0) |
| Pylorus | 128 (60.9) |
| Histological differentiation | |
| Well-differentiated adenocarcinoma | 14 (6.7) |
| Moderately differentiated adenocarcinoma | 84 (40.0) |
| Poorly differentiated adenocarcinoma | 66 (31.4) |
| Mucinous adenocarcinoma | 3 (1.4) |
| Signet ring cell carcinoma | 41 (19.5) |
| Papillary adenocarcinoma | 1 (0.5) |
| Lymphoepithelioma-like gastric carcinoma | 1 (0.5) |
| Depth of invasion | |
| Mucosa | 93 (44.3) |
| Submucosa | 117 (55.7) |
| Lymph node metastasis | |
| Metastasis | 27 (12.9) |
| No metastasis | 183 (87.1) |
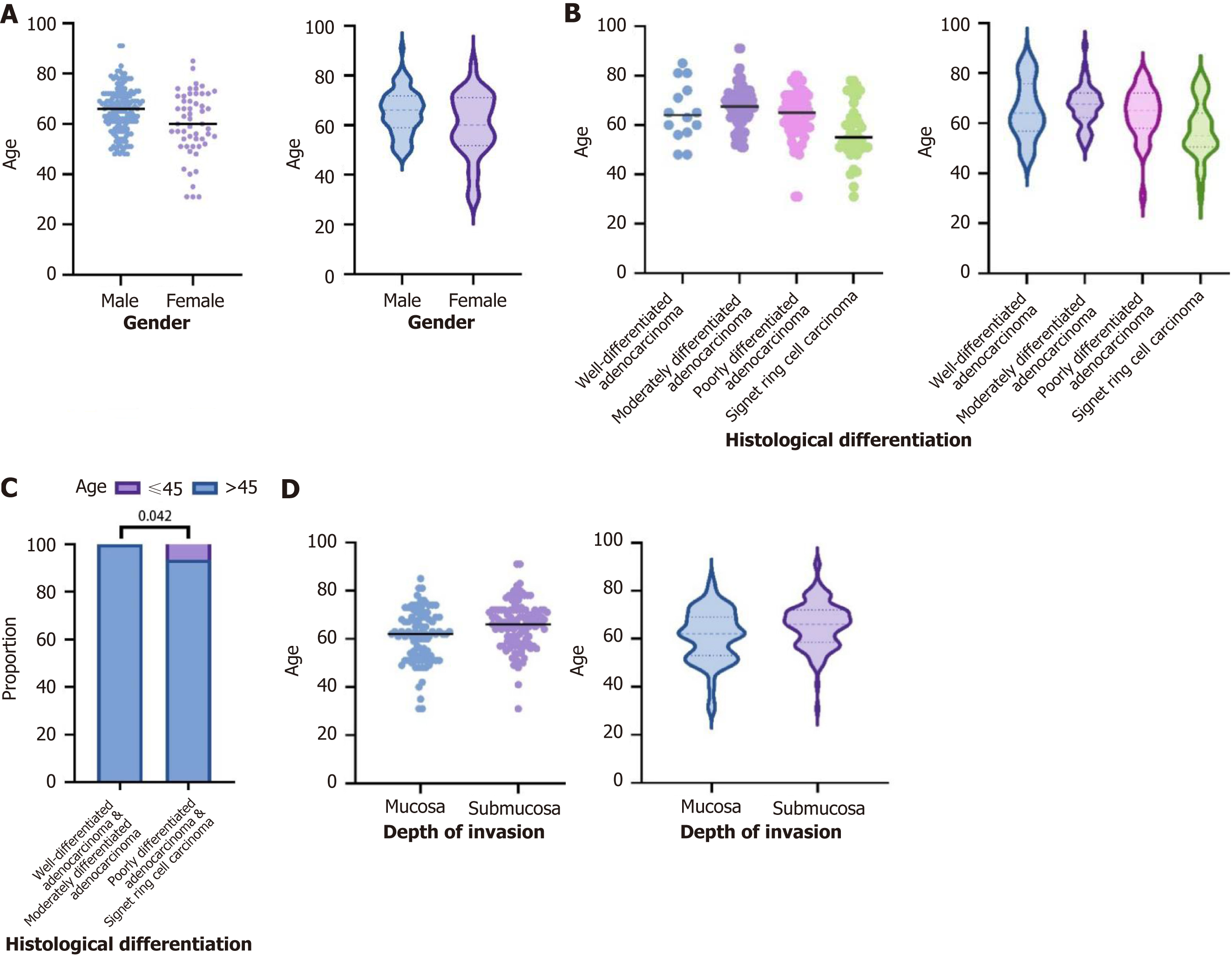
Among all patients, the number of males was significantly higher than that of females. The median age of the female patients (60 years) was lower than that of the male patients (66 years). In younger patients (aged 45 years or below), the proportion of females was higher (seven patients, 100%) (Figure 1A).
The median ages of the patients with highly differentiated adenocarcinoma, moderately differentiated adenocarcinoma it was 67.5 years, poorly differentiated adenocarcinoma it was 65 years, and signet ring cell carcinoma were 64, 67, 65, and 55 years, respectively. Among younger patients (aged 45 years or below), the proportion of poorly differentiated adenocarcinoma (two patients, 28.6%) and signet ring cell carcinoma (five patients, 71.4%) was higher (Figure 1B).
Among patients with highly differentiated and moderately differentiated adenocarcinomas, 100% were older than 45 years and 0% were younger than 45 years. In contrast, among patients with poorly differentiated adenocarcinoma and signet ring cell carcinoma, 93.5% were older than 45 years and 6.5% were younger than 45 years (P < 0.05; Figure 1C).
The median age of patients with gastric cancer invading the mucosal and submucosal layers was 62 and 66 years, respectively (Figure 1D).
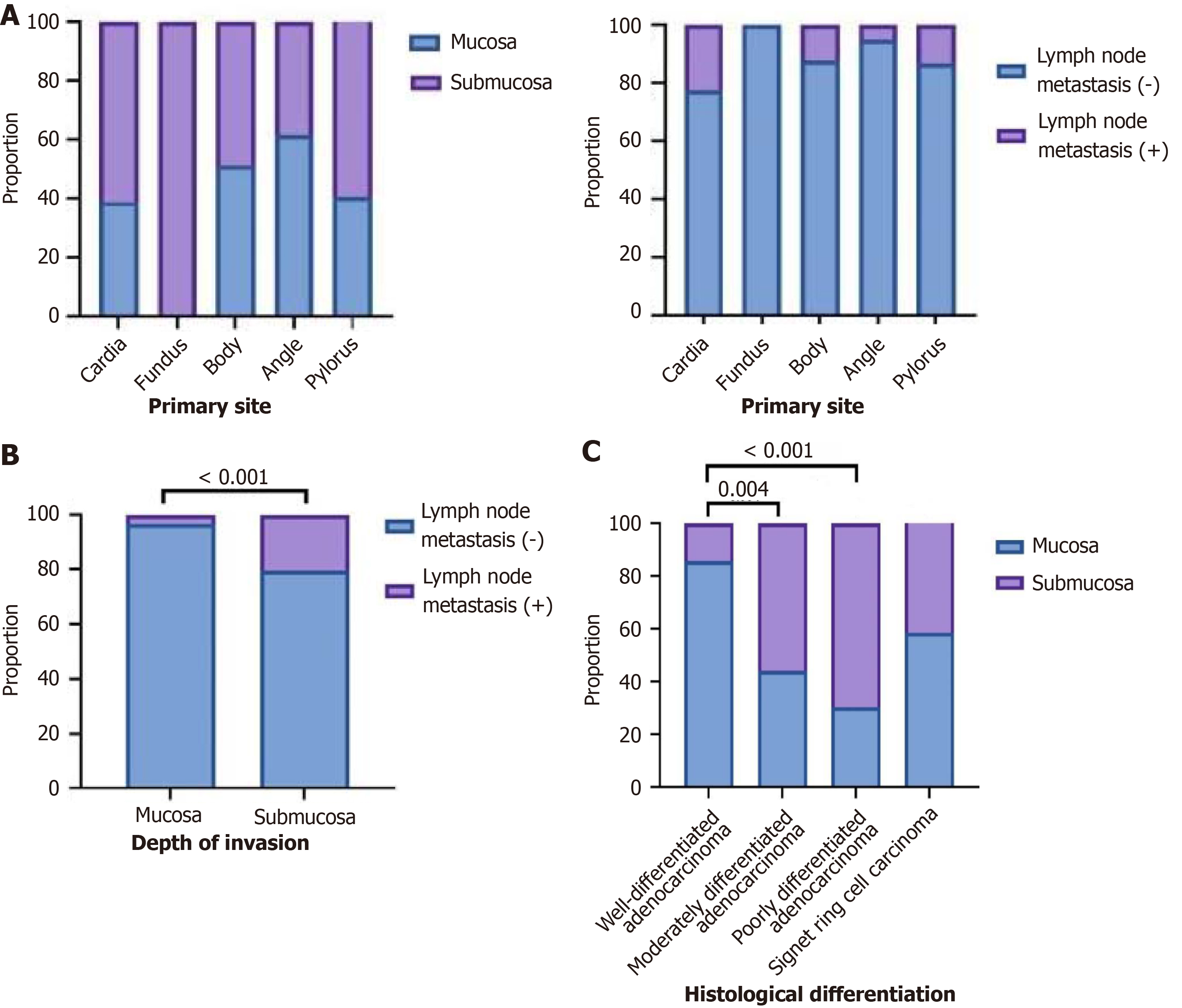
In cardiac cancer, 38.9% of cases had invasion to the mucosal layer, 61.1% to the submucosal layer, 77.8% had no lymph node metastasis, and 22.2% had lymph node metastasis. In fundus cancer, 0% of cases had invasion into the mucosal layer and 100% into the submucosal layer; 100% had no lymph node metastasis, and 0% had lymph node metastasis. In gastric body cancer, 51.2% of cases had invasion into the mucosal layer, 48.8% into the submucosal layer, 87.8% had no lymph node metastasis, and 12.2% had lymph node metastasis. In angular incisure cancer, 61.9% of the cases showed invasion into the mucosal layer, 38.1% into the submucosal layer, 95.2% had no lymph node metastasis, and 4.8% had lymph node metastasis. In antral cancer, 40.6% of the cases had invasion into the mucosal layer, 59.4% into the submucosal layer, 86.7% had no lymph node metastasis, and 13.3% had lymph node metastasis (P > 0.05; Figure 2A).
Among gastric cancers with invasion into the mucosal layer, 96.8% had no lymph node metastasis, and 3.2% had lymph node metastasis. Among gastric cancers with invasion into the submucosal layer, 79.5% had no lymph node metastasis and 20.5% had lymph node metastasis (P < 0.05; Figure 2B).
In highly differentiated adenocarcinoma, 85.7% of cases had invasion of the mucosal layer and 14.3% had invasion of the submucosal layer. In moderately differentiated adenocarcinomas, 44% had invasion of the mucosal layer, and 56% had invasion of the submucosal layer. In poorly differentiated adenocarcinomas, 30.3% had invasion of the mucosal layer and 69.7% of the submucosal layer. In signet ring cell carcinoma, 30.3% showed invasion of the mucosal layer, and 69.7% showed invasion of the submucosal layer. There were significant differences between highly differentiated adenocarcinoma, moderately differentiated adenocarcinoma, and poorly differentiated adenocarcinoma (P < 0.05). There was no significant difference between moderately and poorly differentiated adenocarcinomas (P > 0.05). No significant difference was observed between poorly differentiated adenocarcinomas and signet ring cell carcinomas (P > 0.05; Figure 2C).
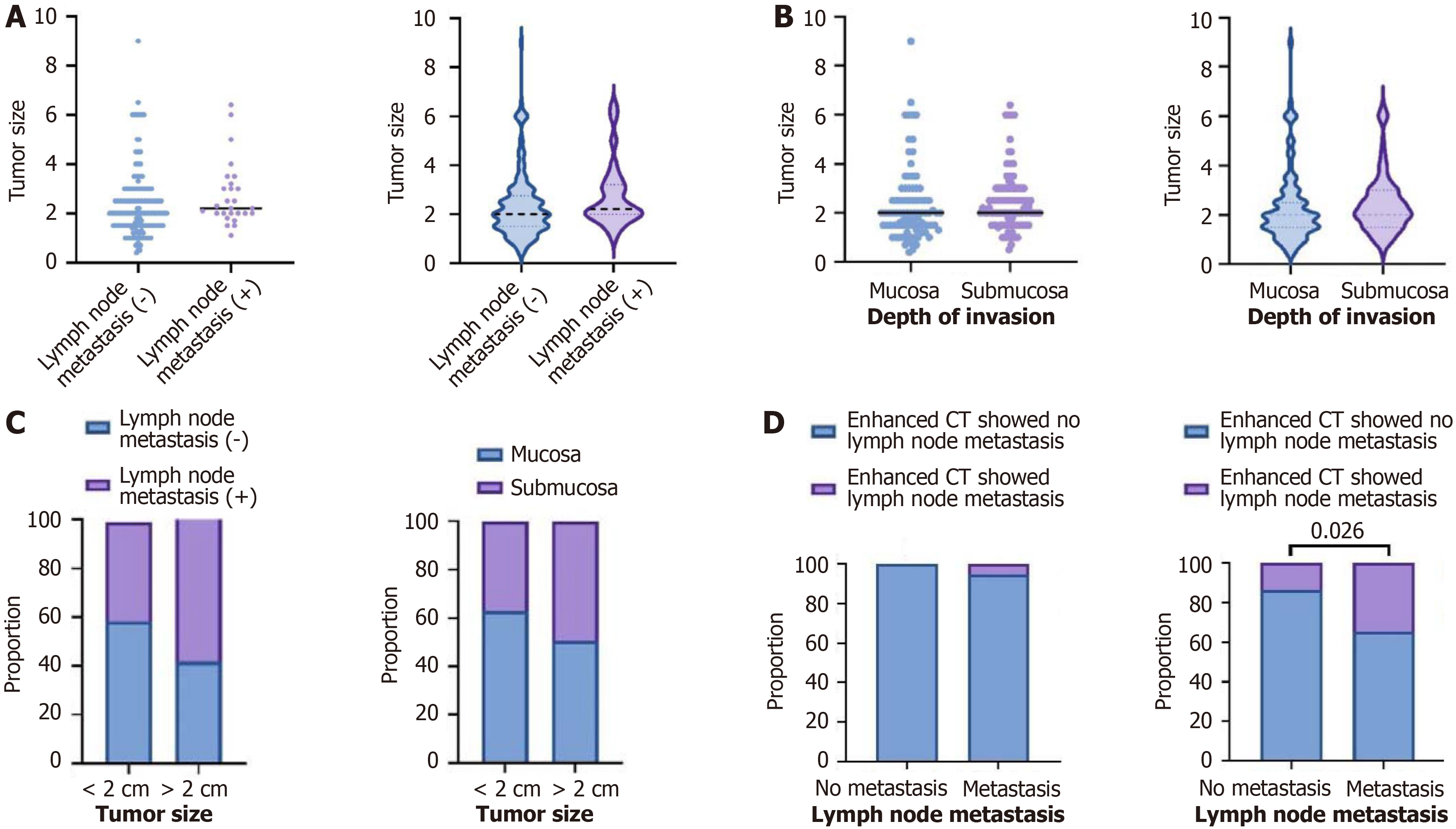
In patients without lymph node metastasis, the median tumor size was 2 cm, whereas in patients with lymph node metastasis, the median tumor size was 2.2 cm (Figure 3A).
In patients with mucosal layer invasion, the median tumor size was 2 cm, and in patients with submucosal layer invasion, the median tumor size was 2 cm (Figure 3B).
Among tumors smaller than 2 cm, 58.19% had no lymph node metastasis and 40.74% had lymph node metastasis. Among tumors larger than 2 cm, 41.81% had no lymph node metastasis and 59.26% had lymph node metastasis (P > 0.05). Among tumors smaller than 2 cm, 62.92% had no lymph node metastasis and 37.08% had lymph node metastasis. Among tumors larger than 2 cm, 50.43% had no lymph node metastasis and 49.57% had lymph node metastasis (P > 0.05; Figure 3C).
In patients with negative lymph nodes postoperatively, 100% had no indication of metastasis on preoperative enhanced CT and 0% had an indication of metastasis. In patients with positive lymph node dissection postoperatively, 96.3% had no indication of metastasis on preoperative enhanced CT and 3.7% had an indication of metastasis (P > 0.05). In patients with negative lymph nodes postoperatively, 86.1% had no indication of metastasis in preoperative enhanced CT, and 13.9% had lymph node presentation indicated; in patients with positive lymph nodes postoperatively, 65.4% had no indication of metastasis in preoperative enhanced CT, and 34.6% had lymph node presentation indicated (P < 0.05; Figure 3D).
Logistic regression models were used to explore the risk factors for lymph node metastasis in EGC. Multivariate analysis showed that invasion depth was associated with adverse outcomes, whereas sex, age, pathological type, and tumor size were not (Table 2).
| Trait | Numerical value | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age, year | |||||
| ≤ 45 | 7 | 1.00 | - | 1.00 | - |
| 46-65 | 102 | 0.212 (0.043-1.050) | 0.057 | 0.116 (0.011-1.179) | 0.069 |
| > 65 | 101 | 0.147 (0.029-0.750) | 0.021 | 0.049 (0.004-0.549) | 0.014 |
| Sex | |||||
| Male | 156 | 1.00 | - | 1.00 | - |
| Female | 54 | 1.533 (0.644-3.653) | 0.334 | 1.359 (0.437-4.219 | 0.596 |
| Depth of infiltration | |||||
| Infiltrate into the mucosal layer | 93 | 1.00 | - | 1.00 | - |
| Infiltrate into the submucosa | 117 | 7.742 (2.252-26.212) | < 0.001 | 14.346 (2.973-69.234) | < 0.001 |
| Position | |||||
| Preventriculus | 18 | 1.00 | - | 1.00 | - |
| Pylorus | 2 | 0.00 (0.00) | 0.999 | 0.00 (0.00) | 0.999 |
| Gastric body | 41 | 0.486 (0.114-2.078) | 0.330 | 0.405 (0.076-2.167) | 0.291 |
| Gastric angle | 21 | 0.175 (0.018-1.737) | 0.137 | 0.206 (0.017-2.496) | 0.214 |
| Sinuses ventriculi | 128 | 0.536 (0.158-1.821) | 0.318 | 0.250 (0.056-1.115) | 0.069 |
| Pathological type | |||||
| Highly differentiated adenocarcinoma | 14 | 1.00 | - | 1.00 | - |
| Moderately differentiated adenocarcinoma | 84 | 170048308.00 (0.00) | 0.999 | 49334064.34 (0.00) | 0.999 |
| Poorly differentiated adenocarcinoma | 66 | 323091785.20 (0.00) | 0.999 | 81279052.88 (0.00) | 0.999 |
| Mucinous adenocarcinoma | 3 | 1.00 (0.00) | 1.00 | 0.89 (0.00 | 1.00 |
| Signet-ring cell carcinoma | 41 | 391626406.30 (0.00) | 0.999 | 122520894.2 (0.00) | 0.999 |
| Tumor size, cm | |||||
| ≤ 2 | 120 | 1.00 | - | 1.00 | - |
| > 2 | 90 | 2.143 (0.941-4.877) | 0.069 | 2.668 (0.984-7.238) | 0.054 |
Logistic regression models were used to explore the risk factors for the invasion in EGC. Multivariate analysis showed that the pathological type was associated with adverse outcomes, while sex, age, and tumor size were not associated with adverse outcomes (Table 3).
| Trait | Numerical value | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age, year | |||||
| ≤ 45 | 7 | 1.00 | - | 1.00 | - |
| 46-65 | 102 | 2.222 (0.412-11.987) | 0.353 | 1.867 (0.294-11.835) | 0.508 |
| > 65 | 101 | 4.926 (0.908-26.725) | 0.065 | 4.812 (0.733-31.586) | 0.102 |
| Sex | |||||
| Male | 156 | 1.00 | - | 1.00 | - |
| Female | 54 | 0.896 (0.481-1.669) | 0.730 | 1.208 (0.560-2.603 | 0.630 |
| Position | |||||
| Preventriculus | 18 | 1.00 | - | 1.00 | - |
| Pylorus | 2 | 1028029445 (0.00) | 0.999 | 1387506094 (0.00) | 0.999 |
| Gastric body | 41 | 0.606 (0.196-1.873) | 0.384 | 0.613 (0.183-2.053) | 0.428 |
| Gastric angle | 21 | 0.392 (0.107-1.428) | 0.156 | 0.472 (0.117-1.898) | 0.290 |
| Sinuses ventriculi | 128 | 0.930 (0.338-2.557) | 0.888 | 1.227 (0.408-3.688) | 0.716 |
| Pathological type | |||||
| Highly differentiated adenocarcinoma | 14 | 1.00 | - | 1.00 | - |
| Moderately differentiated adenocarcinoma | 84 | 7.622 (1.605-36.186) | 0.011 | 9.854 (1.865-52.077) | 0.007 |
| Poorly differentiated adenocarcinoma | 66 | 13.800 (2.825-67.424) | 0.001 | 19.437 (3.534-106.905) | < 0.001 |
| Mucinous adenocarcinoma | 3 | 9692849057 (0.00) | 0.999 | 2.116E+10 (0.00) | 0.999 |
| Signet-ring cell carcinoma | 41 | 4.250 (0.840-21.492) | 0.080 | 8.277 (1.441-46.973) | 0.018 |
| Tumor size, cm | |||||
| ≤ 2 | 120 | 1.00 | - | 1.00 | - |
| > 2 | 90 | 1.727 (0.988-3.019) | 0.055 | 1.677 (0.883-3.187) | 0.114 |
In this retrospective study of 210 patients who underwent radical gastrectomy for EGC, we found a significant sex disparity with more male than female patients. This could be associated with unhealthy lifestyle habits among men such as smoking and alcohol consumption. Although gastric cancer predominantly affects middle-aged and elderly individuals, we observed that younger patients were more likely to present with poorly differentiated gastric cancers, including poorly differentiated adenocarcinoma and signet ring cell carcinoma. Consistent with previous studies, we found that the antrum, particularly the lesser curvature, was the most common site of gastric cancer[12,13]. The lack of parietal cells in this region may render it more susceptible to cancer, as atrophy and intestinal metaplasia-conditions linked to intestinal-type gastric cancer-are more prevalent along the lesser curvature[14].
Our results suggest that lymph node metastasis in EGC is not associated with sex or age. However, we found that the risk of lymph node metastasis was closely related to invasion depth, which is consistent with prior research[4-8,15]. Moreover, our findings did not show a significant association between tumor size and lymph node metastasis[16]. Additionally, tumor location did not appear to influence the likelihood of lymph node metastasis.
Tumors with poorer differentiation are more aggressive and associated with worse prognoses. In our study, three cases of intramucosal cancer with lymph node metastasis were identified as signet ring cell carcinomas. Nevertheless, our analysis suggests that the risk of lymph node metastasis in EGC is not significantly associated with the tumor cell type, which contradicts the existing literature[17]. Some studies have shown that signet ring cell carcinoma has a higher rate of distant metastasis than non-signet ring cell carcinoma[18]. This discrepancy may be attributed to the relatively small sample size. Because the invasion depth is a key factor in EGC, it is critical to understand the factors that influence this invasion. Our data suggests that tumor differentiation plays a role: Poorly differentiated adenocarcinomas and signet ring cell carcinomas are more prone to invading the submucosal layer than well-differentiated adenocarcinomas. Thus, histopathology may indirectly impact lymph node metastasis, a relationship that warrants further investigation using larger datasets.
Regarding preoperative detection of lymph node metastasis, we found that enhanced abdominal CT did not provide a reliable predictive value. While imaging can suggest lymph node involvement, its specificity remains low, indicating that clinicians must be cautious when deciding on treatment strategies. Our study indicated that enhanced CT could benefit from more sensitive contrast agents and higher resolution to improve the detection of small metastatic lymph nodes. Regular follow-up with gastroscopy and imaging remains critical for postoperative management, especially for patients undergoing endoscopic submucosal dissection (ESD), to monitor potential lymph node metastasis.
An innovative aspect of our study lies in the investigation of both lymph node metastasis and invasion risk factors for EGC. We found no significant association between pathological type and lymph node metastasis risk; however, we observed a strong correlation between pathological type and invasion risk. Furthermore, we highlight the limitations of using preoperative CT for lymph node metastasis detection, suggesting that intermediate lymph nodes identified on imaging should not be overlooked.
Our study has certain limitations. As this was a single-center retrospective analysis, the data are inherently limited in scope and generalizability. As in any retrospective study, there was some degree of data loss. Moreover, we excluded patients who underwent ESD or had distant metastases, which may have introduced a selection bias. Pathology reports from multiple sources also pose challenges in standardizing tumor size measurements. Future studies should include a larger sample size and consider multi-center collaboration to validate these findings.
First, the risk of lymph node metastasis in gastric cancer is mainly related to the depth of tumor invasion, possibly indirectly related to the pathological type, and is not related to sex, age, tumor location, or tumor size. Second, preo
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68462] [Article Influence: 13692.4] [Reference Citation Analysis (201)] |
| 2. | Douda L, Cyrany J, Tachecí I. [Early gastric cancer]. Vnitr Lek. 2022;68:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Paredes O, Baca C, Cruz R, Paredes K, Luque-Vasquez C, Chavez I, Taxa L, Ruiz E, Berrospi F, Payet E. Predictive factors of lymphatic metastasis and evaluation of the Japanese treatment guidelines for endoscopic resection of early gastric cancer in a high-volume center in Perú. Heliyon. 2023;9:e16293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Cai F, Dong Y, Wang P, Zhang L, Yang Y, Liu Y, Wang X, Zhang R, Liang H, Sun Y, Deng J. Risk assessment of lymph node metastasis in early gastric cancer: Establishment and validation of a Seven-point scoring model. Surgery. 2022;171:1273-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Sun F, Zhang S, Wang X, Yao M, Zhang C, Liu Z, Ai S, Guan W, Wang M. Mixed Histologic Type is a Risk Factor for Lymph Node Metastasis in Submucosal Invasive Early Gastric Cancer. J Surg Res. 2023;282:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Yang Q, Feng S, Liu H, Zhang X, Cao J, Zhu Y, Zheng H, Song H. Clinicopathological features and lymph node metastasis risk in early gastric cancer with WHO criteria in China: 304 cases analysis. Ann Diagn Pathol. 2021;50:151652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Zhao B, Mei D, Luo R, Lu H, Bao S, Xu H, Huang B. Clinicopathological features, risk of lymph node metastasis and survival outcome of synchronous multiple early gastric cancer. Clin Res Hepatol Gastroenterol. 2020;44:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Liang XQ, Wang Z, Li HT, Ma G, Yu WW, Zhou HC, Liu HB. Indication for endoscopic treatment based on the risk of lymph node metastasis in patients with undifferentiated early gastric cancer. Asian J Surg. 2020;43:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Liu S, Qiao X, Xu M, Ji C, Li L, Zhou Z. Development and Validation of Multivariate Models Integrating Preoperative Clinicopathological Parameters and Radiographic Findings Based on Late Arterial Phase CT Images for Predicting Lymph Node Metastasis in Gastric Cancer. Acad Radiol. 2021;28 Suppl 1:S167-S178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Gao X, Ma T, Cui J, Zhang Y, Wang L, Li H, Ye Z. A CT-based Radiomics Model for Prediction of Lymph Node Metastasis in Early Stage Gastric Cancer. Acad Radiol. 2021;28:e155-e164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Sun Z, Li J, Wang T, Xie Z, Jin L, Hu S. Predicting perigastric lymph node metastasis in gastric cancer with CT perfusion imaging: A prospective analysis. Eur J Radiol. 2020;122:108753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Park KB, Jeon CH, Seo HS, Jung YJ, Song KY, Park CH, Lee HH. Operative safety of curative gastrectomy after endoscopic submucosal dissection (ESD) for early gastric cancer - 1:2 propensity score matching analysis: A retrospective single-center study (cohort study). Int J Surg. 2020;80:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Lee S, Kim SG, Cho SJ. Decision to perform additional surgery after non-curative endoscopic submucosal dissection for gastric cancer based on the risk of lymph node metastasis: a long-term follow-up study. Surg Endosc. 2023;37:7738-7748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Yang K, Lu L, Liu H, Wang X, Gao Y, Yang L, Li Y, Su M, Jin M, Khan S. A comprehensive update on early gastric cancer: defining terms, etiology, and alarming risk factors. Expert Rev Gastroenterol Hepatol. 2021;15:255-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Nakamura R, Omori T, Mayanagi S, Irino T, Wada N, Kawakubo H, Kameyama K, Kitagawa Y. Risk of lymph node metastasis in undifferentiated-type mucosal gastric carcinoma. World J Surg Oncol. 2019;17:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21:4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 965] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 17. | Li Y, Zhu Z, Ma F, Xue L, Tian Y. Gastric Signet Ring Cell Carcinoma: Current Management and Future Challenges. Cancer Manag Res. 2020;12:7973-7981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Li Y, Zhu Z, Ma F, Xue L, Tian Y. Improving survival of stage II-III primary gastric signet ring cell carcinoma by adjuvant chemoradiotherapy. Cancer Med. 2020;9:6617-6628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/