Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3277
Revised: August 28, 2024
Accepted: September 14, 2024
Published online: October 27, 2024
Processing time: 155 Days and 15.5 Hours
At present, immune checkpoint inhibitors (ICIs) remain the 1st-line therapy me
To investigate the effects of ICIs combined with bevacizumab monoclonal anti
A total of 110 MSS/pMMR patients with advanced CRC after first-line treatment failure in the Affiliated Hospital of Qinghai University were enrolled for a ran
The positive expression rates of CD8 (+) T lymphocytes (30% vs 50%), TAMs (23.30% vs 60%), and CAFs (23.30% vs 50%) before and after treatment in both groups exhibited statistical significance (P < 0.05). Additionally, the therapeutic effects of both groups (partial remission: 26.67% vs 10%; objective response rate: 26.70% vs 10%) were significantly different (P < 0.05). Although the experimental group showed a higher progression-free survival, median progression-free survival, and disease control rate than the control group, the difference was not statistically significant. Moreover, no significant difference in the occurrence rate of drug-related adverse reactions after treatment between the two groups was found (P > 0.05).
ICIs in combination with bevacizumab can not only improve the patient’s prognosis but also yield safe and controllable adverse drug reactions in patients suffering from MSS/pMMR advanced CRC after failure to a 1st-line therapy.
Core Tip: In this study, immune checkpoint inhibitors (ICIs) in combination with bevacizumab were applied to microsatellite stable (MSS)/proficient mismatch repair (pMMR) colorectal cancer (CRC) patients with first-line treatment failure. It was found that ICIs combined with bevacizumab treatment significantly changed the tumor immune cells compared with the pre-treatment period. Additionally, ICIs combined with bevacizumab not only further improved their clinical efficacy compared with ordinary chemotherapy combined with anti-angiogenic drugs, but also yielded safe and controllable adverse drug reactions, which provided a new option for MSS/pMMR CRC patients experiencing 1st-line treatment failure.
- Citation: Wang L, Diao YZ, Ma XF, Luo YS, Guo QJ, Chen XQ. Clinical evaluation of sintilimab in conjunction with bevacizumab for advanced colorectal cancer with microsatellite stable-type after failure of first-line therapy. World J Gastrointest Surg 2024; 16(10): 3277-3287
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3277.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3277
Colorectal cancer (CRC), a frequently diagnosed malignancy arising from the digestive tract in China, has been reported with a high incidence and death rate. Over 1.9 million global new cases of CRC and around 935000 CRC-related deaths are estimated by the 2020 GLOBOCAN statistics. CRC ranks 3rd in the morbidity and 2nd in the death rate among mali
At present, immune checkpoint inhibitors (ICIs) represent the 1st-line therapeutic approach for high microsatellite instability/deficient mismatch repair (MSI-H/dMMR) metastatic CRC (mCRC). Yet, ICIs show limited therapeutic efficacy for microsatellite stable (MSS) CRC, which is primarily because MSS CRC is a “cold tumor” with almost no lymphocyte infiltration. However, anti-angiogenic drugs can improve the immune microenvironment by promoting more immune cells to enter the immune microenvironment, thereby exerting anti-tumor effects. The REGONIVO and RE
Cytotoxic cluster of differentiation (CD) 8 (+) T lymphocytes are the main effector cells in tumor immunity. Studies[7] have shown that patients with high-density tumor antigen-specific CD8 (+) T lymphocytes at the invasive tumor edge are more likely to benefit from treatment with programmed death 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) in
In this study, 110 patients with MSS/proficient mismatch repair (MSS/pMMR) advanced CRC who failed first-line treatment were prospectively collected, with 60 patients in the experimental group receiving bevacizumab combined with sintilimab treatment for 4 cycles, and 50 patients in the control group receiving FOLFIRI combined with bevacizumab treatment for 4 cycles. The toxic side effects in both groups were observed, and the changes in the number of tumor CD8 (+) T cells, TAMs, and CAFs before and after treatment were analyzed, as well as their correlation with efficacy. The results found that the combination of anti-PD-1 drugs and anti-VEGF drugs can improve the tumor immune tolerance microenvironment of MSS/pMMR advanced CRC and increase the effectiveness of immunotherapy. This study may provide more clinical evidence for future large-sample clinical studies and lay a theoretical foundation for personalized immunotherapy of MSS/pMMR CRC.
Totally, one hundred and ten patients suffering from MSS/pMMR advanced CRC who had a failure of 1st-line therapy were randomly chosen based on a random number table between October 2021 and June 2023 at the Affiliated Hospital of Qinghai University. Specifically, sixty subjects (36 males and 24 females; aged 50-75 years, mean age = 62.23 ± 7.49 years) were allocated to the experimental group, involving 8 subjects with stage III CRC and 52 subjects with stage IV CRC; 30 subjects with low differentiation tumors, 22 patients with moderate differentiation tumors, and 8 subjects with high differentiation tumors; 36 subjects with colon tumor and 24 subjects with rectal tumor. On the other hand, 50 participants (30 males and 20 females; aged 50-75 years, mean age = 61.20 ± 7.74 years) were allocated to the control group, involving 4 participants with stage III CRC and 46 participants with stage IV CRC; 35 participants with low differentiation tumors, 10 participants with moderate differentiation tumors, and 5 participants with high differentiation tumors; 25 participants with colon tumor and 25 participants with rectal tumor. No statistical difference was found in baseline data between the two groups (P > 0.05) (Table 1). This study has been approved by the hospital’s Ethics Committee and informed consent has been obtained from the patients.
| Characteristic | Experimental group (n = 60) | Control group (n = 50) | P value |
| Gender | 1.000 | ||
| Male | 36 (60.00) | 30 (60.00) | |
| Female | 24 (40.00) | 20 (40.00) | |
| Age (years) | 62.23 ± 7.49 | 61.20 ± 7.74 | 0.480 |
| Clinical stage | 0.366 | ||
| Stage III | 8 (13.30) | 4 (8.00) | |
| Stage IV | 52 (86.70) | 46 (92.00) | |
| Stage IV | 0.532 | ||
| Hepatic metastases | 48 (80.00) | 43 (86.00) | |
| Bone metastases | 3 (5.00) | 1 (1.66) | |
| Pulmonary metastasis | 1 (1.66) | 1 (1.66) | |
| Brain metastases | 0 (0.00) | 1 (1.66) | |
| Degree of differentiation | 0.091 | ||
| Low differentiation | 30 (50.00) | 35 (70.00) | |
| Moderate differentiation | 22 (36.70) | 10 (20.00) | |
| High differentiation | 8 (13.30) | 5 (10.00) | |
| BMI (kg/m2) | 20.58 ± 2.03 | 20.17 ± 1.65 | 0.256 |
| Colon tumor | 36 (60.00) | 25 (50.00) | 0.293 |
| Rectal tumor | 24 (40.00) | 25 (50.00) |
Inclusion criteria: (1) Patients pathologically confirmed as CRC[10], including signet ring cell carcinoma and mucinous adenocarcinoma meeting the inclusion criteria; (2) Unresectable and regionally advanced or metastatic disease, mutated RAS or BRAF gene, participants who had experienced 1st-line oxaliplatin plus Bevacizumab and failed 1st-line therapy, no drug history of PD-L1/PD-1 inhibitors; (3) Participants of age between 18-80 years; (4) At least one measurable lesion for imaging evaluation, according to response evaluation criteria in solid tumors[11] criteria; (5) Eastern cooperative on
Exclusion criteria: (1) Participants previously exposed to any antibody or agents targeting PD-1, PD-L1, PD-L2, CD137, cytotoxic T lymphocyte associate protein-4, or any other antibody or agents specifically targeting T cell co-stimulation or checkpoint pathway; Participants with bone metastasis who are at risk for paraplegia; Participants with wild-type RAS/BRAF gene; (2) Patients with manifestations of active hemorrhage in known lesions (like hematemesis and melena in the past 2 weeks at random); A gastrointestinal bleeding event of grade 3 or above (national cancer institute common ter
Criteria for withdrawal: (1) Severe toxicity or intolerance to treatment (adverse events)[13]; Participants who self-requested to withdraw from the research; or (2) Participants whom the researcher deemed medically necessary to withdraw from this study.
Patients in the control group received a treatment regimen of FOLFIRI combined with bevacizumab. On day 1 of each cycle, bevacizumab (4 mL: 100mg; Xinda Biopharmaceutical Company Limited, Suzhou, Zhejiang Province, China) (National drug approval, No. S20200013) was intravenously infused at a daily dose of 5 mg/kg; Irinotecan (5 mL: 0.1 g; Qilu Pharmaceutical Company Limited, Haikou, Hainan Province, China) (National Drug Approval, No. H20084572) was administered at a dose of 180 mg/m2; Calcium folinate (3 mL: 30 mg, Jiangsu Dahongying Hengshun Pharmaceutical Company Limited) (National Drug Approval, No. H20020609) was administered at a daily dose of 400 mg/m2; Flu
Patients in the experimental group received a treatment regimen of bevacizumab combined with sintilimab. Sintilimab (10 mL: 100 mg; Xinda Biopharmaceutical) (National Drug Approval, No. S20180016) was intravenously infused at a daily dose of 200 mg; bevacizumab (4 mL: 100 mg; Xinda Biopharmaceutical) (National Drug Approval, No. S20200013) was intravenously infused at a daily dose of 7.5 mg/kg. The infusion time on the first day was controlled at 60-90 minutes, and the subsequent infusions could be controlled at 30-45 minutes. Each cycle lasted for 3 weeks, with 4 cycles of treatment in total[16].
(1) Main endpoint indicators: Progression-free survival (PFS) and median PFS (mPFS); (2) Secondary endpoint indicators: Indicators for evaluating the efficacy of treatments in two groups according to the relevant efficacy evaluation criteria, specifically including complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD), objective response rate (ORR), and disease control rate (DCR); (3) Exploratory endpoint indicators: Indicators for ana
Immunohistochemistry results interpretation: (1) CD8 positive expression localization was analyzed. It’s expressed on the membrane of lymphocytes. Observation and scoring of staining intensity: 0 point (no staining), 1 point (light yellow), 2 points (brown-yellow), and 3 points (brown); Observation and scoring of the staining area (counting the stained lymphocytes in the field of vision): 0 point (< 5% lymphocytes stained), 1 point (5%-25% lymphocytes stained), 2 points (25%-50% lymphocytes stained), and 3 points (> 50% lymphocytes stained). Multiplication of the aforementioned two scores: A product < 2 points was indicative of negative expression; A product ≥ 2 points was indicative of positive ex
Statistical product and service solutions 25.0 was employed for data analysis. The measurement data conformed to a normal distribution were represented as the mean ± SD, with comparisons realized using the t or t/ test of two in
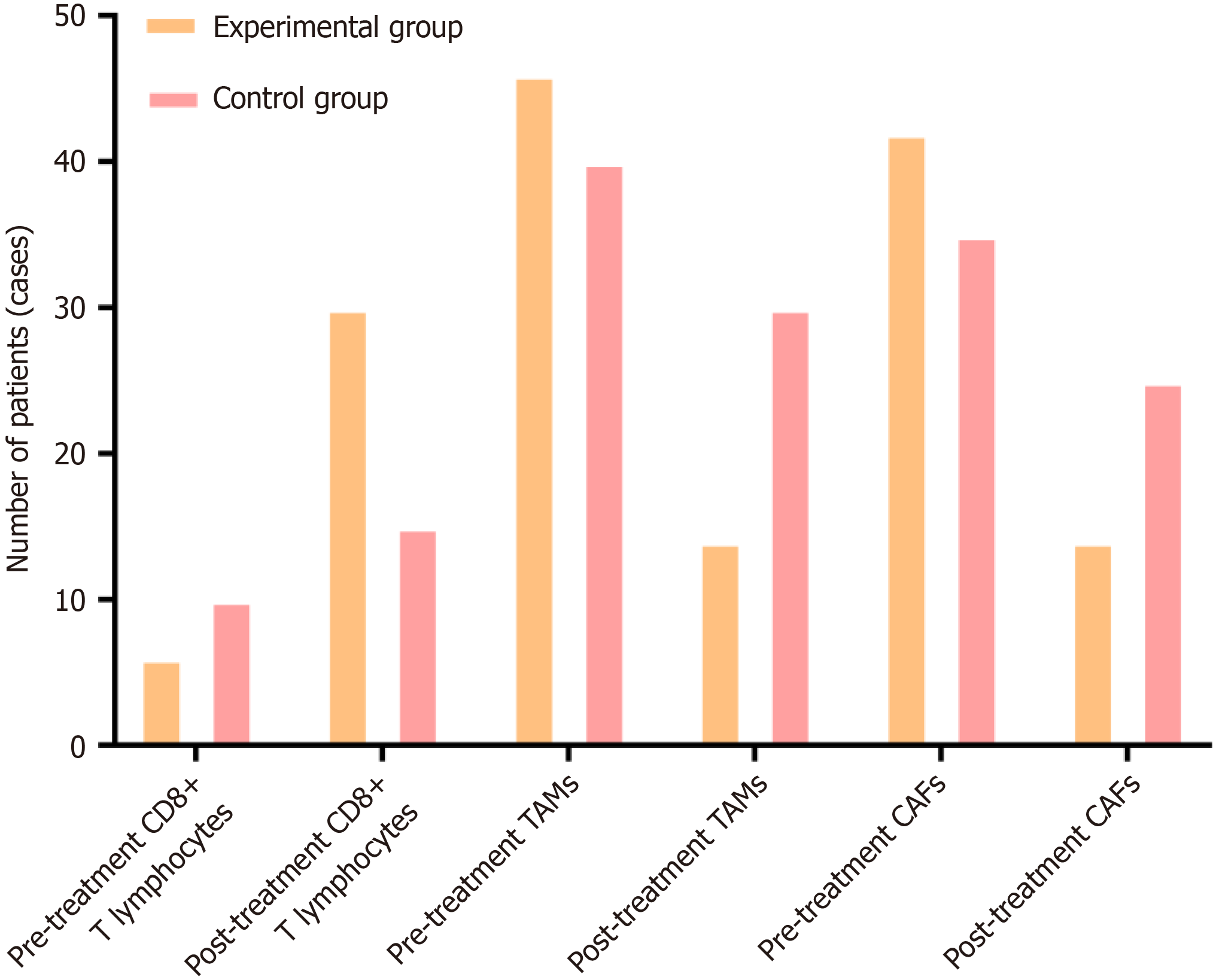
In the experimental group, positive expression rate of CD8 (+) T lymphocytes was markedly raised following treatment, with remarkably decreased positive rates of TAMs and CAFs observed simultaneously (Figure 1 and Table 2).
| Characteristic | Experimental group (n = 60) | Control group (n = 50) | P value |
| CD8 (+) T lymphocytes | |||
| Pre-treatment | 6 (10.00) | 10 (20.00) | 0.139 |
| Post-treatment | 30 (50.00) | 15 (30.00) | 0.033 |
| TAMs | |||
| Pre-treatment | 46 (76.70) | 40 (80.00) | 0.673 |
| Post-treatment | 14 (23.30) | 30 (60.00) | 0.001 |
| CAFs | |||
| Pre-treatment | 42 (70.00) | 35 (70.00) | 1.000 |
| Post-treatment | 14 (23.30) | 25 (50.00) | 0.003 |
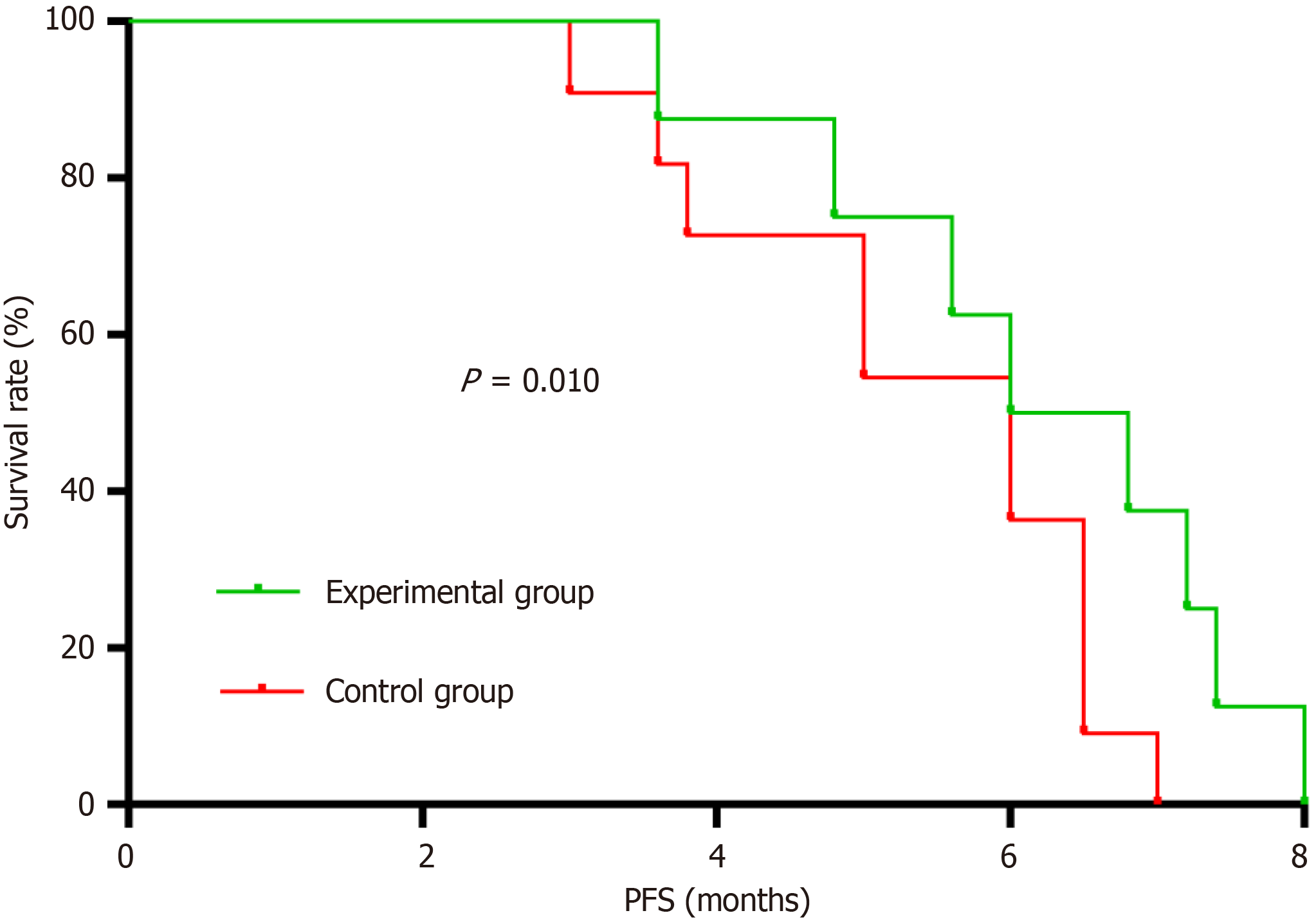
Post-treatment outcomes were evaluated (Figure 2 and Table 3). No case of CR was found in all participants. Statistically significant differences were noted regarding post-treatment outcome PR between the two groups, with 16 cases in the experimental group (ORR = 26.70%) and 5 cases in the control group (ORR = 10.00%) (P < 0.05). Although several patients had SD and PD in both groups, this discrepancy was statistically insignificant (P > 0.05). Higher ORR and DCR were detectable in the experimental participants as compared to the controls, yet the DCR-based difference was statistically insignificant (P > 0.05). PFS insignificantly differed between the experimental and control groups with a P value of > 0.05. The participants in the experimental group had an mPFS of 5 months while that of the controls was 4 months.
| Characteristic | Experimental group (n = 60) | Control group (n = 50) | P value |
| CR | 0 | 0 | - |
| PR | 16 | 5 | 0.023 |
| SD | 12 | 10 | 1.000 |
| PD | 32 | 35 | 0.073 |
| ORR | 16 (26.70) | 5 (10.00) | 0.023 |
| DCR | 28 (46.70) | 15 (30.00) | 0.073 |
| PFS (months, mean ± SD) | 5.04 ± 1.83 | 4.69 ± 1.30 | 0.247 |
| mPFS (months) | 5 | 4 | - |
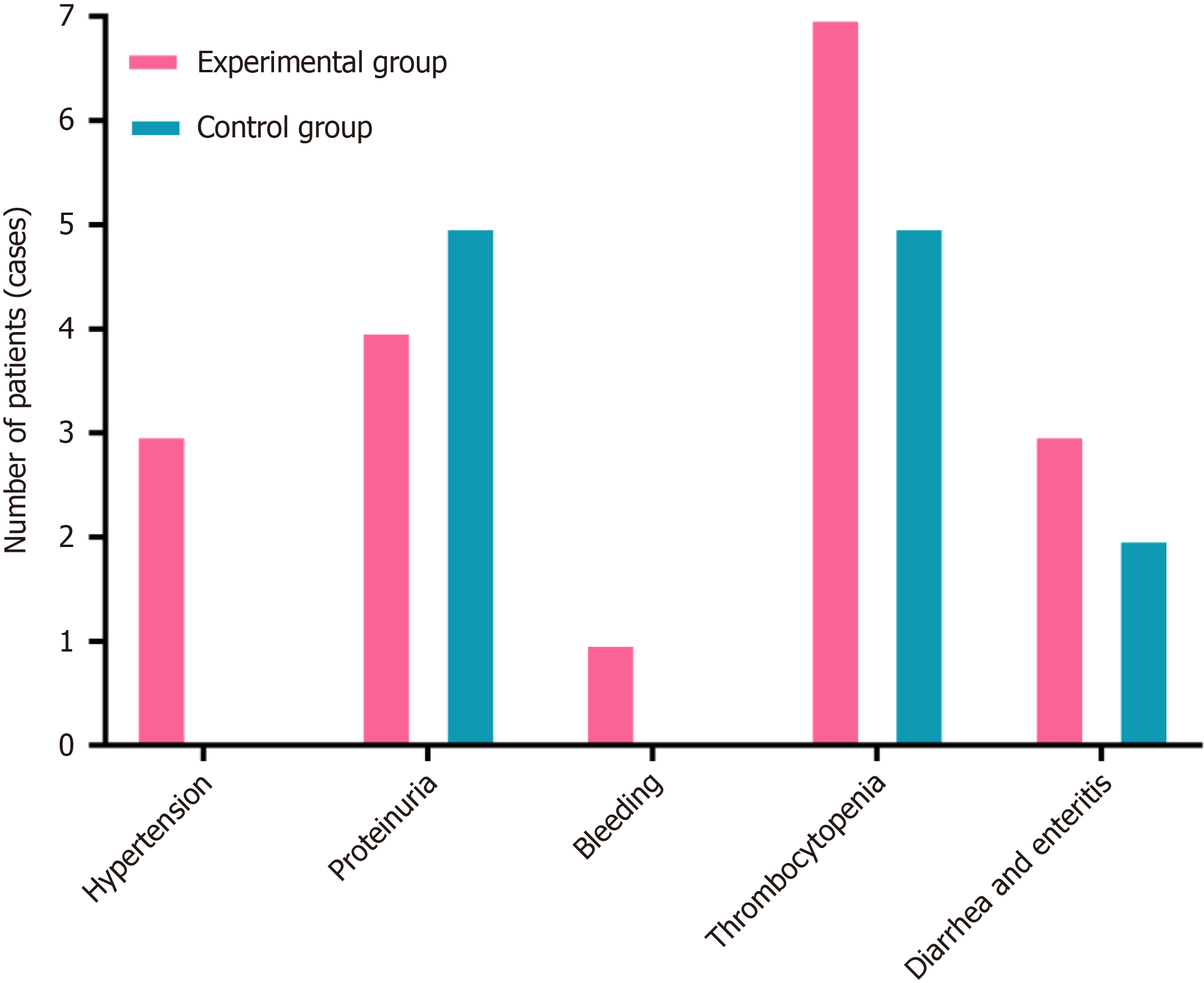
The post-treatment outcomes (drug-associated adverse events) were evaluated. As depicted in Figure 3 and Table 4, there was no statistically significant difference in the incidence of drug-related adverse reactions (such as hypertension, proteinuria, gastrointestinal perforation, bleeding, arterial thrombosis, thrombocytopenia, pneumonia, nephritis, he
| Characteristic | Experimental group (n = 60) | Control group (n = 50) | P value |
| Hypertension | 3 (5.00) | 0 (0.00) | 0.054 |
| I | 2 (3.30) | 0 (0.00) | |
| II | 1 (1.70) | 0 (0.00) | |
| Proteinuria | 4 (6.70) | 5 (10.00) | 0.526 |
| I | 2 (3.35) | 3 (6.00) | |
| II | 2 (3.35) | 2 (4.00) | |
| Gastrointestinal perforation | 0 (0.00) | 0 (0.00) | - |
| I | 0 (0.00) | 0 (0.00) | |
| II | 0 (0.00) | 0 (0.00) | |
| Bleeding | 1 (1.70) | 0 (0.00) | 0.269 |
| I | 1 (1.70) | 0 (0.00) | |
| II | 0 (0.00) | 0 (0.00) | |
| Arterial thrombosis | 0 (0.00) | 0 (0.00) | - |
| I | 0 (0.00) | 0 (0.00) | |
| II | 0 (0.00) | 0 (0.00) | |
| Thrombocytopenia | 7 (11.70) | 5 (10.00) | 0.780 |
| I | 3 (5.00) | 3 (6.00) | |
| II | 4 (6.70) | 2 (4.00) | |
| Pneumonia, nephritis, hepatitis, and endocrine disorders | 0 (0.00) | 0 (0.00) | - |
| I | 0 (0.00) | 0 (0.00) | |
| II | 0 (0.00) | 0 (0.00) | |
| Diarrhea and enteritis | 3 (5.00) | 2 (4.00) | 0.801 |
| I | 2 (3.30) | 2 (4.00) | |
| II | 1 (1.70) | 0 (0.00) | |
| Number of cases | 18 | 12 | 0.525 |
| I | 10 | 8 | |
| II | 8 | 4 |
For the time being, over 50% of CRC cases are diagnosed at the later stage or with distant metastasis. For these patients, chemotherapy alone or in conjunction with molecule-targeted therapy remain the main clinical treatments. Despite the clinical benefits of these treatments for patients[3,4,17-19], considerable limitations exist in the survival improvement. For instance, the patients who suffer from advanced CRC with a previous history of 2nd-line therapy have varying extents of decreased tolerance to chemotherapy, especially in the elderly and frail, with a more obvious accumulation of chemo
Breakthrough progress has been made in the first-line treatment of MSI-H/dMMR mCRC through the application of ICIs. The KEYNOTE-177 study[21] has evaluated the efficacy of pembrolizumab compared to standard treatment (che
Additionally, drug-associated adverse events were accurately assessed. The severity was graded following the national cancer institute common terminology criteria for adverse events v5.0 criteria. Among all the observed adverse reactions, adverse events in both groups were graded I or II. After symptomatic or supportive treatment, the patient’s conditions were significantly improved, with no cases of discontinuation, withdrawal from the study, or death. Statistical analysis demonstrated no significant difference between the two groups of patients in the overall drug reaction rate and the proportions of drug reactions (like hypertension) (P > 0.05). These findings indicated that the combination of ICIs and anti-angiogenic drugs can achieve the same clinical safety as conventional chemotherapy combined with anti-angiogenic drugs. These results may provide more options for advanced CRC patients with varying degrees of chemotherapy tolerance, especially for the elderly and frail, with significant chemotherapy drug toxicity accumulation and complications.
It was found from the short-term perspective that deaths occurred in both groups (8 in the experimental group and 11 in the control group). The experimental participants had a survival rate of 86.70%, where 5 deaths were caused by cachexia and 3 deaths were attributed to hepatic failure owing to hepatic metastasis. On the other hand, the controls (a survival rate of 78.00%) contained 6 deaths resulting from cachexia, 3 deaths ascribed to hepatic metastasis-associated hepatic failure, 1 death owing to severe infection because of delayed therapy for secondary complete intestinal ob
The conjunction of ICIs with anti-angiogenic agents improves the TIM of MSS/pMMR advanced CRC patients with failure of 1st-line therapy and meanwhile accelerate the transformation of “cold tumor” immune-suppression condition to a “hot tumor” immune-supportive condition. In consideration of ensured safety of drug adverse reactions, ICIs in conjunction with anti-angiogenic drugs enhance the anti-tumor ability of patients and clinical treatment effects, further increasing its clinical application value. Despite the good results of our research, future combination therapy for tumors should focus more on individualization, multidisciplinary collaboration, and dynamic monitoring. The integration of mu
We acknowledge and appreciate our colleagues for their valuable suggestions and technical assistance with this study.
| 1. | Sutton RM, McDonald EL, Shakked RJ, Fuchs D, Raikin SM. Determination of Minimum Clinically Important Difference (MCID) in Visual Analog Scale (VAS) Pain and Foot and Ankle Ability Measure (FAAM) Scores After Hallux Valgus Surgery. Foot Ankle Int. 2019;40:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Rakinic J. Benign Anorectal Surgery: Management. Adv Surg. 2018;52:179-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 824] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 4. | Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol. 2016;27:1539-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (4)] |
| 5. | Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 589] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 6. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring A, Azad NS, Laheru D, Donehower RS, Crocenzi T, Goldberg RM, Fisher GA, Lee JJ, Greten T, Koshiji M, Kang SP, Anders RA, Eshleman JR, Vogelstein B, Diaz L. Programmed death-1 blockade in mismatch repair deficient colorectal cancer. J Clin Oncol. 2016;34:103. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Vilain RE, Menzies AM, Wilmott JS, Kakavand H, Madore J, Guminski A, Liniker E, Kong BY, Cooper AJ, Howle JR, Saw RPM, Jakrot V, Lo S, Thompson JF, Carlino MS, Kefford RF, Long GV, Scolyer RA. Dynamic Changes in PD-L1 Expression and Immune Infiltrates Early During Treatment Predict Response to PD-1 Blockade in Melanoma. Clin Cancer Res. 2017;23:5024-5033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 8. | Guo CH, Chen XJ, Wang ZC, Wei WF, Huang XF, Wang W, Li QX. [A study on the joint prediction of lymphatic metastasis in cervical cancer by TAMs and CAFs]. Zhongguo Shiyongfuke Yu Chanke Zazhi. 2021;37:478-481. [DOI] [Full Text] |
| 9. | Sun Y. [Role and Mechanism of Cancer-Associated Fibroblasts in the Classification of Gastric Cancer]. Ph.D Thesis, Shanghai Jiao Tong University. 2019. Available from: https://kns.cnki.net/kcms2/article/abstract?v=-4s28oSk479Gi5uK0roWeNRCt2bWHI72Dvl5Dxj5cjLO2Zs7_eQEudu5NKCvNowjiwEUE3SWkXjhnmkvJEGflp3Kfp60H5X2oalcrYpvFA5zsJWayYpiaImvloNo9j38cL_fuzfM1e2rgRR4Ld6wgUerU431M0QHrT5I1GjkK8TQOxsiV7A1ARnLREVQvLGfeiat3gHFlVD1WBtrfsr-ejcvRWc_olyd&uniplatform=NZKPT. |
| 10. | Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (5)] |
| 11. | Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 460] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 13. | Inoue N, Ishida H, Sano M, Kishino T, Okada N, Kumamoto K, Ishibashi K. Discrepancy between the NCI-CTCAE and DEB-NTC scales in the evaluation of oxaliplatin-related neurotoxicity in patients with metastatic colorectal cancer. Int J Clin Oncol. 2012;17:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Volkert D, Kruse W, Oster P, Schlierf G. Malnutrition in geriatric patients: diagnostic and prognostic significance of nutritional parameters. Ann Nutr Metab. 1992;36:97-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Wang X, Zhan Z, Liao L. [Clinical efficacy and adverse reactions of bevacizumab combined with FOLFIRI regimen in the treatment of metastatic rectal cancer]. Aizheng Jinzhan. 2019;17:1687-1689, 1696. [DOI] [Full Text] |
| 16. | Ji RJ, Guan K, Zhuang JF, Chen DZ. [Meta-analysis of the efficacy and safety of bevacizumab combined with chemotherapy in the treatment of advanced colorectal cancer]. Haijun Yixue Zazhi. 2018;39:526-532. [DOI] [Full Text] |
| 17. | Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW; CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 604] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 18. | Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, Yang L, Deng Y, Chen ZD, Zhong H, Pan H, Guo W, Shu Y, Yuan Y, Zhou J, Xu N, Liu T, Ma D, Wu C, Cheng Y, Chen D, Li W, Sun S, Yu Z, Cao P, Chen H, Wang J, Wang S, Wang H, Fan S, Hua Y, Su W. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA. 2018;319:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (1)] |
| 19. | Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 20. | de Castro Sant' Anna C, Junior AGF, Soares P, Tuji F, Paschoal E, Chaves LC, Burbano RR. Molecular biology as a tool for the treatment of cancer. Clin Exp Med. 2018;18:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 2062] [Article Influence: 343.7] [Reference Citation Analysis (0)] |
| 22. | Lenz HJ, Lonardi S, Zagonel V, Cutsem EV, Limon ML, Wong M, Hendlisz A, Aglietta M, Garcia-Alfonso P, Neyns B, Gelsomino F, Cardin DB, Dragovich T, Shah U, Yang J, Ledeine JM, Overman MJ. Nivolumab (NIVO) + low-dose ipilimumab (IPI) as first-line (1L) therapy in microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): Two-year clinical update. J Clin Oncol. 2020;38:s15. [DOI] [Full Text] |
| 23. | Bielenberg DR, Zetter BR. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015;21:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 24. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5984] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 25. | Brem S, Cotran R, Folkman J. Tumor Angiogenesis: A Quantitative Method for Histologic Grading. JNCI. 1972;48:347-356. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1536] [Article Influence: 192.0] [Reference Citation Analysis (0)] |
| 27. | Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561-17566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 859] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 28. | Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, Phung TL, Mani SA, Stossi F, Sreekumar A, Mancini MA, Decker WK, Zong C, Lewis MT, Zhang XH. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 667] [Cited by in RCA: 646] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 29. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10724] [Article Influence: 766.0] [Reference Citation Analysis (34)] |
| 30. | Wang L, Liu SS, Zhang SM, Chen XQ, Huang T, Tian R, Zhao YQ, Chen Z, Xianba CR. Gastric cancer liver metastasis will reduce the efficacy of immunotherapy. World J Gastrointest Surg. 2024;16:2760-2764. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/