Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1559
Peer-review started: December 23, 2022
First decision: January 9, 2023
Revised: January 25, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: August 27, 2023
Processing time: 244 Days and 21.6 Hours
Tumour rupture of gastrointestinal stromal tumours (GISTs) has been considered to be a remarkable risk factor because of its unfavourable impact on the oncological outcome. Although tumour rupture has not yet been included in the current tumor-node-metastasis classification of GISTs as a prognostic factor, it may change the natural history of a low-risk GIST to a high-risk GIST. Originally, tumour rupture was defined as the spillage or fracture of a tumour into a body cavity, but recently, new definitions have been proposed. These definitions distinguished from the prognostic point of view between the major defects of tumour integrity, which are considered tumour rupture, and the minor defects of tumour integrity, which are not considered tumour rupture. Moreover, it has been demonstrated that the risk of disease recurrence in R1 patients is largely modulated by the presence of tumour rupture. Therefore, after excluding tumour rupture, R1 may not be an unfavourable prognostic factor for GISTs. Additionally, after the standard adjuvant treatment of imatinib for GIST with rupture, a high recurrence rate persists. This review highlights the prognostic value of tumour rupture in GISTs and emphasizes the need to carefully take into account and minimize the risk of tumour rupture when choosing surgical strategies for GISTs.
Core Tip: Tumour rupture is a remarkable risk factor that can change the natural history of low-risk gastrointestinal stromal tumours (GISTs) to a high-risk GIST. This review analyses the concept and prognostic value of tumour rupture in GISTs and highlights the impact of the risk of tumour rupture on the choice of surgical strategy.
- Citation: Peparini N. Impact of tumour rupture risk on the oncological rationale for the surgical treatment choice of gastrointestinal stromal tumours. World J Gastrointest Surg 2023; 15(8): 1559-1563
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1559.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1559
Tumour rupture in gastrointestinal stromal tumours (GISTs) has not been consistently defined in published studies. Although many studies have found an increased risk of recurrence and lower survival rates in patients with tumour rupture, other studies have not found any unfavourable prognostic effect. This is likely due to differences in tumour rupture definitions[1]. Tumour rupture has been considered to be a remarkable (often surgery-related) risk factor that can change the natural history of a low-risk GIST to a high-risk GIST, heavily impacting the long-term outcome[2-5]. However, in addition to tumour rupture, different factors may also impact GIST prognosis. Synchronous GISTs and another primary tumour can significantly increase in the possibility for recurrent disease, resulting in a worse prognosis and a more aggressive course than a single GIST[6].
This review analyses the concept of tumour rupture and its prognostic value in GISTs and highlights the impact of the risk of tumour rupture during surgical treatment for these tumours. Additionally, it emphasize the need to carefully take into account and minimize the risk of tumour rupture when choosing surgical strategies for GISTs.
Originally, tumour rupture was defined as the spillage or fracture of a tumour into a body cavity, but recently, new definitions have been proposed. According to these new definitions, the constant factor of all major defects of tumour integrity that qualify for tumour rupture (i.e., tumour fracture and/or tumour spillage in the abdominal cavity, blood-stained ascites, gastrointestinal perforation at the tumour site, microscopic transperitoneal adjacent organ infiltration, piecemeal resection or intralesional dissection, and incisional biopsy)[7,8] is substantial peritoneal exposure to tumour cells. This should be considered a remarkable risk factor because of potential peritoneal contamination. In contrast, minor defects of tumour integrity (such as those caused by core needle biopsy, microscopic peritoneal tumour penetration, iatrogenic superficial tumour capsule laceration or microscopically positive margins) are not considered tumour rupture[7-9].
The impact of R1 resection on the oncological outcome of resectable gastrointestinal stromal tumours is debated. A systematic review and meta-analysis indicated that a microscopically positive margin could significantly impact disease-free survival but had no influence on overall survival. Moreover, adjuvant imatinib treatment could reduce the risk of recurrence for R1 resected primary GISTs[10].
Rutkowski et al[11] noted that GIST is a tumour growing under the mucosa and may be often ulcerated. Consequently, the mucosal margin from the gastrointestinal lumen is not clinically meaningful. The authors indicated that the margins of clinical importance that are relevant to assess R status (i.e., R0, R1 or R2) are the peritoneal cavity side, which disruption entails tumour rupture, lateral margins or proximal and distal resection margins of the stomach/intestine wall, whose excision should be verified[11].
However, regarding the residual tumour classification of GISTs, it should be considered that not all tumour ruptures are classified as R1 or R2 resection. Nishida highlighted that peritoneum involvement is unrelated to R status; thus, a GIST disrupted in terms of peritoneal penetration otherwise resected with negative margins is still considered an R0 resection[8]
In their systematic review and meta-analysis, Kong et al[12] analysed the impact of R1 resection on the survival outcome of resectable GISTs with and without tumour rupture. They found that when tumour rupture cases were included, R1 resection resulted in a significantly shorter recurrence-free survival or disease-free survival than R0 resection, but the differences in recurrence-free survival and disease-free survival between R0 and R1 resection vanished when tumour rupture cases were excluded[12]. The results of most recent studies suggest that R1 resection does not influence the oncological outcome of resectable GIST compared with R0 resection; consequently, reresection may not be necessary when a positive microscopic margin exists. Moreover, R1 resection would not be considered an indication for adjuvant imatinib treatment in the absence of other high-risk factors as well as tumour rupture[12-17]. However, tumour rupture is significantly associated with the occurrence of R1 resection[12]. Mc Carter and colleagues noted that the significant risk factors associated with a positive microscopic resection margin are tumour size ≥ 10 cm, location and intraperitoneal rupture, and found that the risk of disease recurrence in R1 patients was driven largely by the presence of tumour rupture[18].
In the tumor-node-metastasis (TNM) classification of GISTs T (tumour) staging is dependent on the size of the tumour (T1: ≤ 2 cm; T2: > 2 cm and ≤ 5 cm; T3: > 5 and ≤ 10 cm; T4: >10 cm) and not on the depth of local invasion. TNM staging is dependent on the site (gastric and omental GISTs have a better prognosis than small bowel GISTs or other less common intestinal GISTs), size (T), regional lymph node (N) status and mitotic rate (low mitotic rate: 5 or fewer per 50 high power fields; high mitotic rate: over 5 per 50 high power fields).
In contrast to the TNM classification of gastrointestinal carcinomas, in the TNM classification of GISTs: (1) Involvement of the peritoneum is not prognostically graded as an unfavourable T (tumour) factor, i.e., T4a; and (2) after excluding tumour rupture, R1 may not be an unfavourable prognostic factor for GISTs. Moreover, tumour rupture, which may be the true unfavourable prognostic factor instead of R1, has not yet been included in the current TNM Classification of GISTs[19]. From a prognostic point of view macroscopic injuries to the pseudocapsule (which are considered tumour rupture) should be distinguished from microscopic breaks of the pseudocapsule on pathological examination (that are not considered to be tumour rupture)[20]. However, the choice of surgical strategy should consider the unfavourable impact of an eventual tumour rupture on prognosis and the risk of tumour rupture when performing a dissection on the tumour surface (pseudocapsule), i.e., without clearance distance[21].
Everett and colleagues emphasised that tumour enucleation is considered insufficient because it may leave behind a tumour-seeded pseudocapsule. Moreover, enucleation is associated with tumour rupture[22] and should not be performed even if it is useful to preserve a vital structure. Interruption of the pseudocapsule or incidental peritumoral disruption can change a curable disease to a poor prognostic tumour. Accurate handling is very important to avoid tumour rupture because GISTs are soft and fragile. This can be a problem in laparoscopic and endoscopic treatment of GIST because of the instrumental manipulation of the tumours. Small low-grade GISTs are often treated by endoscopic resection. However, Song and colleagues argued that in the case of smaller tumours (median tumour size of all patients in their study was 1.5 cm; range 0.3-5 cm), the predictive value of tumour rupture and mitotic index diminished, and the risk of peritoneal metastasis may not be increased, even in tumours ruptured during endoscopic resection[23]. Due to the risks of tumour rupture, tumour remnants, perforation and bleeding, endoscopic resection is not currently recommended as a routine treatment for GISTs of the upper or lower gastrointestinal tract. However, it might be comparable to surgical resection for selected smaller tumours (< 3 cm in size). Surgical resection is still considered the standard treatment for tumours ≥ 2 cm or if the tumour has a high mitotic index or mucosal ulceration[24]. However, a high mitotic index is mostly unknown before resection.
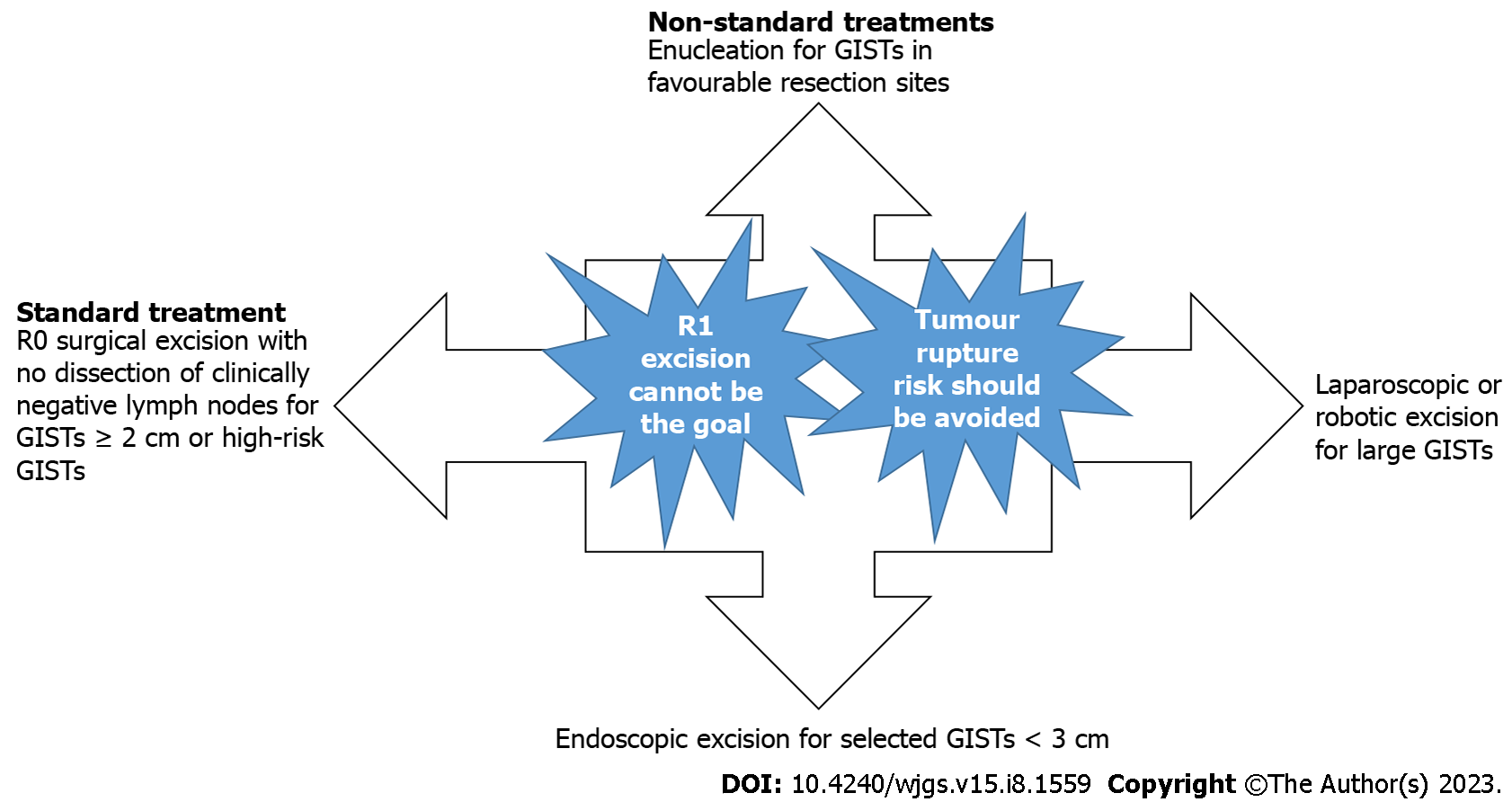
According to the most recent guidelines, the standard treatment for localized GISTs is complete surgical excision of the lesion, with no dissection of clinically negative lymph nodes. The goal is R0 excision, i.e., an excision whose margins are clear of tumour cells at least at the site of origin in the GI tract. In low-risk GISTs located in unfavourable locations, R1 margins can be acceptable, given the lack of evidence that R1 surgery is associated with a worse overall survival.
A laparoscopic/robotic approach is clearly discouraged in patients who have large tumours because of the risk of tumour rupture, which is associated with a very high risk of relapse. For selected patients with small tumours in the upper or lower GI tract, endoscopic excision is an acceptable treatment strategy[25]. Three years of adjuvant imatinib is the standard treatment for resected ruptured GISTs, although the recurrence rate is prominently high[26], and five years of adjuvant imatinib treatment in patients with ruptured GISTs seems to be promising[27,28].
In the choice of a surgical strategy for GISTs, key points should be considered. First, R1 resection cannot be a standard treatment for GISTs, and second, the risk of tumour rupture should be carefully evaluated and avoided. According to these key points: (1) Enucleation cannot be considered a standard treatment for GISTs localized in favourable resection sites; (2) laparoscopic/robotic excisions cannot be the standard treatments for large GISTs; and (3) endoscopic treatment cannot be considered a routine procedure for smaller GISTs (Figure 1).
| 1. | Asare EA, Feig BW. Raining Frogs, Flying Horses, and Defining Tumor Rupture in GIST. Ann Surg Oncol. 2019;26:1601-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 897] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 3. | Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, Switaj T, Michej W, Wroński M, Głuszek S, Kroc J, Nasierowska-Guttmejer A, Joensuu H. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011;37:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Qu H, Xu Z, Ren Y, Gong Z, Ju RH, Zhang F, Shao S, Chen X. The analysis of prognostic factors of primary small intestinal gastrointestinal stromal tumors with R0 resection: A single-center retrospective study. Medicine (Baltimore). 2022;101:e29487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Hølmebakk T, Wiedswang AM, Meza-Zepeda LA, Hompland I, Lobmaier IVK, Berner JM, Stoldt S, Boye K. Integrating Anatomical, Molecular and Clinical Risk Factors in Gastrointestinal Stromal Tumor of the Stomach. Ann Surg Oncol. 2021;28:6837-6845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Diamantis A, Samara AA, Symeonidis D, Baloyiannis I, Vasdeki D, Tolia M, Volakakis G, Mavrovounis G, Tepetes K. Gastrointestinal stromal tumors (GISTs) and synchronous intra-abdominal malignancies: case series of a single institution's experience. Oncotarget. 2020;11:4813-4821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Hølmebakk T, Bjerkehagen B, Boye K, Bruland Ø, Stoldt S, Sundby Hall K. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Nishida T, Hølmebakk T, Raut CP, Rutkowski P. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2019;26:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 9. | Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brodowicz T, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dufresne A, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Miah AB, Mir O, Montemurro M, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss SJ, Hall KS, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Gronchi A, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 387] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 10. | Zhi X, Jiang B, Yu J, Røe OD, Qin J, Ni Q, Sun L, Xu M, Zhu J, Ma L. Prognostic role of microscopically positive margins for primary gastrointestinal stromal tumors: a systematic review and meta-analysis. Sci Rep. 2016;6:21541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Rutkowski P, Skoczylas J, Wisniewski P. Is the Surgical Margin in Gastrointestinal Stromal Tumors Different? Visc Med. 2018;34:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Kong M, Liu G, Zhuo H, Xin Y, Chen H, Sheng H, Li L. Association between R1 resection and oncological outcome in resectable gastrointestinal stromal tumors without tumor rupture: A systematic review and meta-analysis. Eur J Surg Oncol. 2021;47:1526-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Pantuso G, Macaione I, Taverna A, Guercio G, Incorvaia L, Di Piazza M, Di Grado F, Cilluffo G, Badalamenti G, Cipolla C. Surgical treatment of primary gastrointestinal stromal tumors (GISTs): Management and prognostic role of R1 resections. Am J Surg. 2020;220:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Shannon AB, Song Y, Fraker DL, Roses RE, DeMatteo RP, Miura JT, Karakousis GC. Do microscopic surgical margins matter for primary gastric gastrointestinal stromal tumor? Surgery. 2021;169:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Patel DJ, Kulshrestha S, Bunn C, Littau M, Agnew S, Baker MS. Positive microscopic surgical margins: Is there an association with survival in resected small gastrointestinal stromal tumors? Am J Surg. 2021;221:549-553. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Hølmebakk T, Bjerkehagen B, Hompland I, Stoldt S, Boye K. Relationship between R1 resection, tumour rupture and recurrence in resected gastrointestinal stromal tumour. Br J Surg. 2019;106:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 17. | Hølmebakk T, Hompland I, Bjerkehagen B, Stoldt S, Bruland ØS, Hall KS, Boye K. Recurrence-Free Survival After Resection of Gastric Gastrointestinal Stromal Tumors Classified According to a Strict Definition of Tumor Rupture: A Population-Based Study. Ann Surg Oncol. 2018;25:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PW, Demetri GD, Blanke CD, von Mehren M, Brennan MF, McCall L, Ota DM, DeMatteo RP; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant Gist Study Team. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012;215:53-9; discussion 59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th edition. Oxford: John Wiley &Sons, Inc.,2017. |
| 20. | Nishida T, Cho H, Hirota S, Masuzawa T, Chiguchi G, Tsujinaka T; Kinki GIST Study Group. Clinicopathological Features and Prognosis of Primary GISTs with Tumor Rupture in the Real World. Ann Surg Oncol. 2018;25:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Peparini N, Chirletti P. Tumor rupture during surgery for gastrointestinal stromal tumors: pay attention! World J Gastroenterol. 2013;19:2009-2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Everett M, Gutman H. Surgical management of gastrointestinal stromal tumors: analysis of outcome with respect to surgical margins and technique. J Surg Oncol. 2008;98:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Song S, Ren W, Wang Y, Zhang S, Liu F, Cai Q, Xu G, Zou X, Wang L. Tumor rupture of gastric gastrointestinal stromal tumors during endoscopic resection: a risk factor for peritoneal metastasis? Endosc Int Open. 2018;6:E950-E956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Wu H, Li H, Xu Q, Shang L, Zhang R, Li C, Fu M, Xu W, Chen J, Liu J, Li L. Surgical Resection Is Still Better Than Endoscopic Resection for Patients With 2-5 cm Gastric Gastrointestinal Stromal Tumours: A Propensity Score Matching Analysis. Front Oncol. 2021;11:737885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Zhu H, Zhao S, Jiao R, Zhou J, Zhang C, Miao L. Comparison of endoscopic versus laparoscopic resection for gastric gastrointestinal stromal tumors: A preliminary meta-analysis. J Gastroenterol Hepatol. 2020;35:1858-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Bang YH, Ryu MH, Kim HD, Lee HE, Kang YK. Clinical outcomes and prognostic factors for patients with high-risk gastrointestinal stromal tumors treated with 3-year adjuvant imatinib. Int J Cancer. 2022;151:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Kang S, Ryu MH, Bang YH, Kim HD, Lee HE, Kang YK. Adjuvant Imatinib Treatment for 5 Years versus 3 Years in Patients with Ruptured Localized Gastrointestinal Stromal Tumor: A Retrospective Analysis. Cancer Res Treat. 2022;54:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Liu R, Wu Y, Gong J, Zhao R, Li L, Wan Q, Lian N, Shen X, Xia L, Shen Y, Xiao H, Wu X, Chen Y, Cen Y, Xu X. Development and external validation of a nomogram for individualized adjuvant imatinib duration for high-risk gastrointestinal stromal tumors: A multicenter retrospective cohort study. Cancer Med. 2022;11:3093-3105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y, Japan; Samara AA, Greece S-Editor: Gong ZM L-Editor: A P-Editor: Wu RR