Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1532
Peer-review started: March 8, 2023
First decision: April 2, 2023
Revised: April 17, 2023
Accepted: May 17, 2023
Article in press: May 17, 2023
Published online: July 27, 2023
Processing time: 135 Days and 12.3 Hours
Hypereosinophilic syndrome (HES) is classified as primary, secondary or idiopathic. Idiopathic HES (IHES) has a variable clinical presentation and may involve multiple organs causing severe damage. Hepatic sinusoidal obstruction syndrome (HSOS) is characterized by damage to the endothelial cells of the hepatic sinusoids of the hepatic venules, with occlusion of the hepatic venules, and hepatocyte necrosis. We report a case of IHES with HSOS of uncertain etiology.
A 70-year-old male patient was admitted to our hospital with pruritus and a rash on the extremities for > 5 mo. He had previously undergone antiallergic treatment and herbal therapy in the local hospital, but the symptoms recurred. Relevant examinations were completed after admission. Bone marrow aspiration biopsy showed a significantly higher percentage of eosinophils (23%) with approximately normal morphology. Ultrasound-guided hepatic aspiration biopsy indicated HSOS. Contrast-enhanced computed tomography (CT) of the upper abdomen showed hepatic venule congestion with hydrothorax and ascites. The patient was initially diagnosed with IHES and hepatic venule occlusion. Prednisone, low molecular weight heparin and ursodeoxycholic acid were given for treatment, followed by discontinuation of low molecular weight heparin due to ecchymosis. Routine blood tests, biochemical tests, and imaging such as enhanced CT of the upper abdomen and pelvis were reviewed regularly.
Hypereosinophilia may play a facilitating role in the occurrence and development of HSOS.
Core Tip: Idiopathic hypereosinophilic syndrome (IHES) is characterized by a continuous increase and abnormal accumulation of eosinophils in the peripheral blood. Its clinical manifestations vary, and may involve multiple organs and cause serious damage. Hepatic sinusoidal obstruction syndrome (HSOS) can lead to veno-occlusion and hepatocyte necrosis. We report a case of IHES with HSOS. However, the cause of HSOS was unknown, and we could not determine whether it was caused by herbal medicine or IHES. Prednisone, low molecular weight heparin and ursodeoxycholic acid were given. Hypereosinophilia may play a facilitating role in the occurrence and development of HSOS.
- Citation: Xu XT, Wang BH, Wang Q, Guo YJ, Zhang YN, Chen XL, Fang YF, Wang K, Guo WH, Wen ZZ. Idiopathic hypereosinophilic syndrome with hepatic sinusoidal obstruction syndrome: A case report and literature review. World J Gastrointest Surg 2023; 15(7): 1532-1541
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1532.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1532
Idiopathic hypereosinophilic syndrome (IHES) is characterized by a continuous increase and abnormal accumulation of eosinophils in the peripheral blood[1]. Its clinical manifestations vary from asymptomatic eosinophilia to severe multi-tissue injury and even terminal organ failure, including damage to lungs, heart, digestive tract, skin, and peripheral or central nervous system[1,2].
Hepatic sinusoidal obstruction syndrome (HSOS), namely, hepatic veno-occlusive disease (HVOD), is characterized by injury of the sinusoidal endothelial cells of hepatic venules, which leads to occlusion of venules and necrosis of hepatocytes. Its clinical manifestations are weight gain with or without ascites, hepatogenic right upper abdominal pain, hepatomegaly and jaundice[3].
Other complications of hypereosinophilic syndrome (HES) have been reported previously, including HSOS, but in that case, HES was considered to be involved in the occurrence and development of HSOS[4,5]. Here, we report a case of IHES with unknown etiology of hepatic venule occlusion and review previous literature in PubMed.
A 70-year-old male patient was admitted to our hospital with itchy skin on his extremities for > 5 mo.
Five months before admission, without obvious inducement, the patient showed pruritus and red patches on the palms of both hands and soles of both feet. The patches protruded from the skin. He had no history of insect bites, no food or drug allergies, and no abdominal pain, diarrhea, nausea and vomiting. The patient paid no attention to these symptoms. The itching and rash did not subside for 3 mo and he attended the local hospital for treatment. A diagnosis of urticaria was made, and he was given antiallergic treatment with clavulvoxel gel but the details are unknown; after which, the itching symptoms improved. However, during this period, itching on the palms of both hands and soles of both feet occurred repeatedly, and spread to the limbs and face. The nature of the skin rash on the limbs was the same as before, and his face showed extensive redness and swelling, without itching and discomfort. One month before admission, he attended the local hospital for treatment in the Department of Traditional Chinese Medicine. The ingredients of this treatment were frankincense, Rehmannia glutinosa, Akebia quinata, Paeonia albiflora pall, licorice, Prunella vulgaris, chrysanthemum, Divaricate saposhnikovia, gentian, poria, myrrh, Angelica sinensis, Sophora flavescens, dandelion and Angelica dahurica. After > 1 wk of treatment, the rash dissipated, but there was still itching and discomfort in both hands and feet. Three days before admission, he attended Shaoxing People’s Hospital for treatment. Cetirizine and ebastine were given for antiallergic treatment. The patient then went to the Department of Gastroenterology Outpatient of Run Run Shaw Hospital affiliated to Zhejiang University Medical College for further treatment and was proposed to be admitted to the hospital due to eosinophilia.
The patient was previously healthy and generally in good condition.
The patient denied any family genetic history.
The patient’s vital signs were stable and his spirit was good. The skin and sclera were yellow, and asthma, fever and gastrointestinal symptoms were absent. Urine and stools were normal. The palms of both hands and the soles of both feet had red patches protruding from the skin. There was no enlargement of superficial lymph nodes throughout the body. Pulmonary and cardiac examinations did not show any significant abnormalities. The abdomen was flat and soft without tenderness or rebound pain. There was no percussion pain in the liver area, and the liver and spleen were not felt under the ribs. Murphy sign and mobile dullness were negative, and bowel sounds were 4 times/min. Edema in the lower extremities was not observed and pathological signs were not elicited.
Auxiliary examination of the hematological system after admission found that the platelet count was 75 × 109/L (normal range, 125–350 × 109/L), percentage of neutrophils was 31.4% (normal range, 40.0%–75.0%), percentage of eosinophils was 34.4% (normal range, 0.4%–8.0%), absolute number of eosinophils was 2.47 × 109/L (normal range, 0.02–0.52 × 109/L), eosinophil count was 2800.0/μL (normal range, 50.0–300.0/μL), and red blood cell count, white blood cell count, hemoglobin, and absolute number of lymphocytes were all within the normal range. Coagulation function tests showed that prothrombin time (PT) was 15.5 s (normal range, 11.5–14.5 s), PT% was 71.0% (normal range, 80.0%–120.0%), PT control was 13.0 s, international normalized ratio was 1.25 (normal range, 0.90–1.10) and D-dimer was 0.86 μg/mL (normal range, 0.0–0.50 μg/mL).
Blood and stools from the digestive system were negative for protozoa, fecal egg accumulation, parasites and fungi. Serum tumor marker tests revealed increased levels of carbohydrate antigen (CA)125 [743.30 U/mL (< 35.0 U/mL)] and ferritin [626.0 μg/L (30.0–400.0 μg/L)], but CA19-9, alpha-fetoprotein, carcinoembryonic antigen, and total prostate-specific antigen were within normal limits. Blood biochemistry showed that alanine transaminase (59 U/L), aspartate transaminase (60 U/L), alkaline phosphatase (445 U/L), glutamyl transpeptidase (931 U/L), total bilirubin (31.5 μmol/L), direct bilirubin (20.6 μmol/L), total bile acid (21.28 μmol/L), and C-reactive protein (12.7 mg/L) were elevated, while albumin (35.1 g/L), albumin and globulin ratio (1.12), high-density lipoprotein cholesterol (0.75 mmol/L), β-hydroxybutyric acid (0.01 mmol/L), retinol binding protein (24 mg/L) and cholinesterase (2951 U/L) were decreased. Lactate dehydrogenase was within the normal range.
Among the laboratory examination indicators targeting the rheumatic immune system, levels of IgG (17.50 g/L) and IgE (266.0 IU/mL) were increased. IgA, IgM, complement components C3 and C4, rheumatoid factor, erythrocyte sedimentation rate, IgG4, and antinuclear antibody profiles were normal.
Hepatitis B virus (HBV) surface antibody (12.55 IU/L) and HBV core antibody (4.68 S/CO) were increased, while HBV e antibody (0.99S/CO) was decreased. Antibodies against hepatitis C virus (HCV), Treponema pallidum and human immunodeficiency virus (HIV) were negative. Epstein-Barr virus capsid antigen-IgG was high (616.0) while IgM was negative. Tuberculosis-infected T cells were unreactive and Mycobacterium tuberculosis-specific antigen pore was 0. Cytomegalovirus (CMV) IgG antibody was high (> 250.0) while CMV IgM antibody was negative.
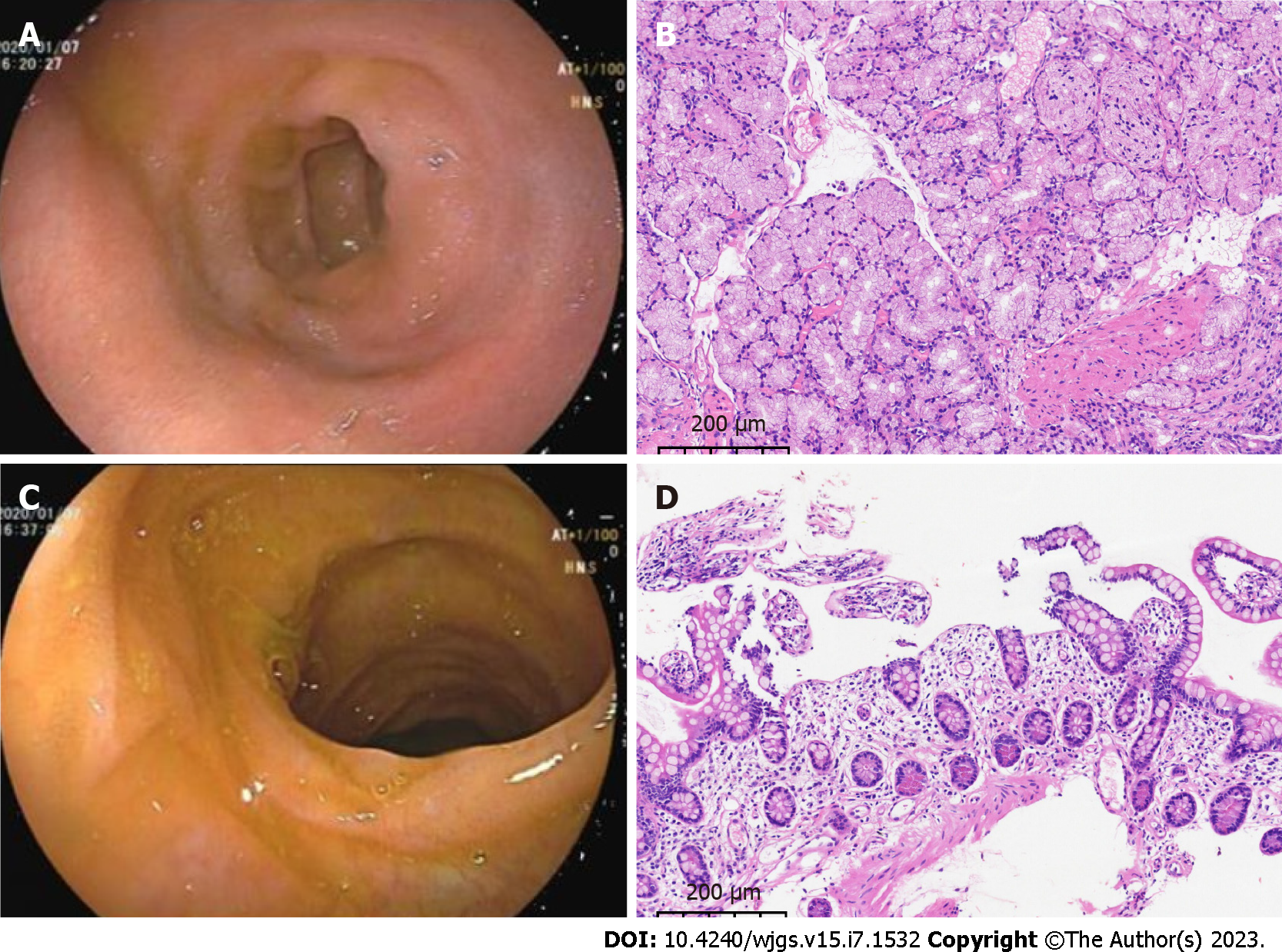
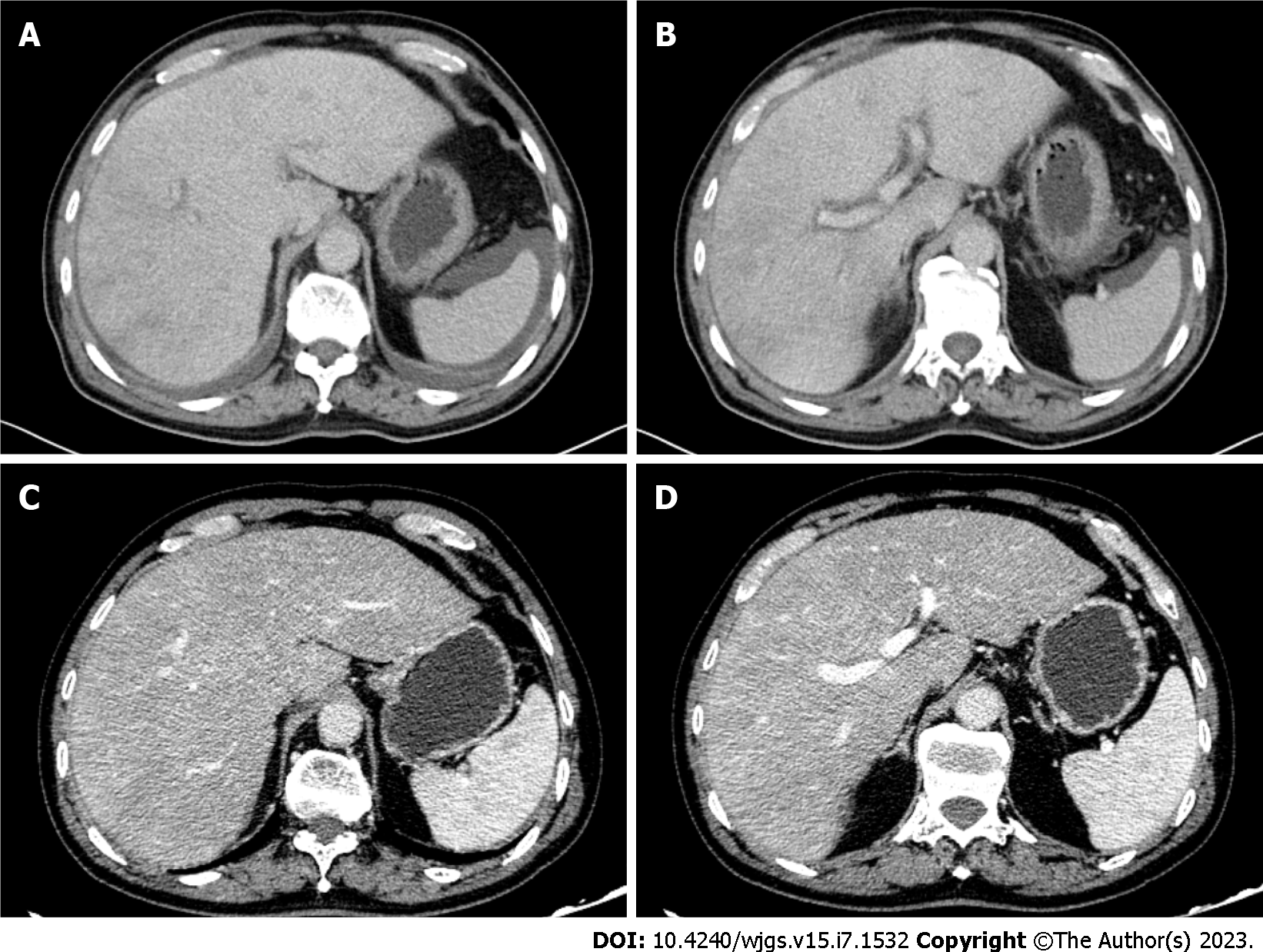
Gastroduodenoscopy showed chronic superficial atrophic gastritis with erosion, predominantly in the gastric sinus and gastric horn and body, and ulceration in the duodenal bulb, with pathology suggesting chronic inflammation of the stomach and duodenum (Figure 1A and B). No significant abnormalities were seen on colonoscopy, and pathology reported chronic inflammation of the mucosa of the terminal ileum, ileocecal region, ascending colon and rectum (Figure 1C and D). Contrast-enhanced computed tomography (CT) of the upper abdomen suggested hepatic venule congestion with hydrothorax and ascites (Figure 2A and B). Chest CT showed nodules beside the right oblique fissure, a few infectious lesions in the left upper lobe, and paraseptal emphysema in both upper lungs. There was a small amount of pleural effusion on both sides accompanied by pulmonary tissue distension, and inflammatory fibrous lesions scattered in both lungs. The apical pleura of the lungs was thickened. Cardiac ultrasound suggested mild aortic regurgitation.
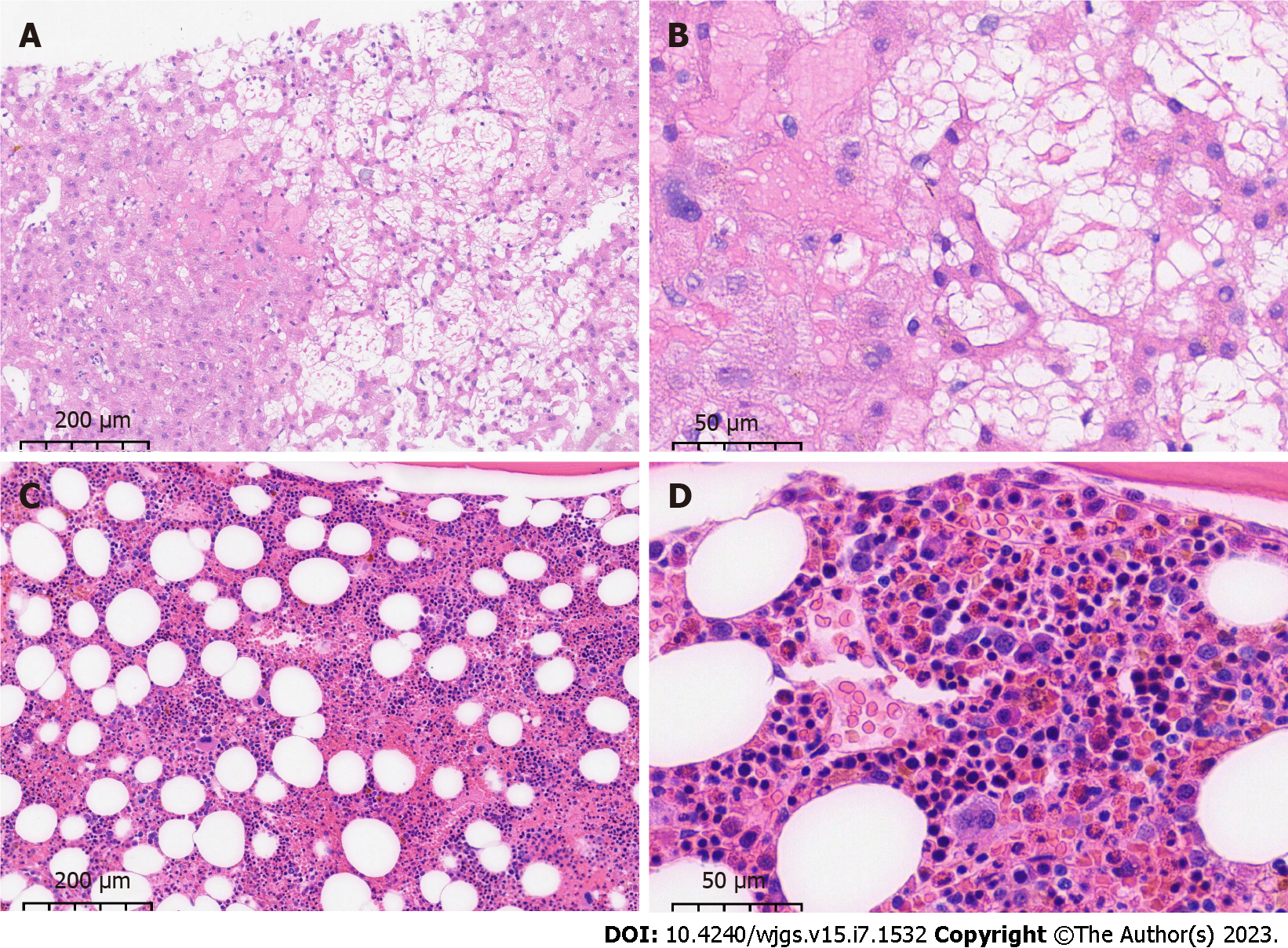
Pathological findings on ultrasound-guided liver puncture biopsy supported the diagnosis of HSOS. Lamellar hepatic sinusoidal dilatation and congestion with loss of hepatocytes and a residual reticulofibrous stent were seen (Figure 3A and B). Bone marrow biopsy revealed active proliferation of bone marrow tissue but a significantly higher percentage of eosinophils (23%) with approximately normal morphology (Figure 3C and D). PDGFR gene rearrangement and BCR/ABL fusion gene were negative. Combined with the above laboratory test indicators, imaging examinations and pathological findings, the patient was diagnosed with IHES and hepatic venule occlusion, after excluding tumors of the hematological and digestive systems, parasite, fungus and other infections causing eosinophilia, hereditary metabolic liver diseases such as alcoholic liver disease and Wilson’s disease, connective tissue diseases such as systemic lupus erythematosus and IgG4-related diseases, and infections with hepatitis A virus, HBV, HCV, HDV, HEV, HIV, T. pallidum, Epstein–Barr virus, tuberculosis, and CMV.
On the tenth day after admission, the patient was treated with prednisone 25 mg orally three times daily (1 mg/kg) and the absolute eosinophil count started to decrease. Subsequently, anticoagulation therapy with low molecular weight heparin 0.4 mL subcutaneously once daily was added. In addition, the patient was treated with ursodeoxycholic acid at 250 mg orally three times daily. The patient was discharged from the hospital on January 17, 2022, in good general condition with symptom improvement after treatment.
After discharge, the patient underwent regular routine blood tests, eosinophil count, liver and coagulation function tests, and contrast-enhanced CT of the upper abdomen and pelvis to adjust the drug dose (Figure 2C and D). On January 21, 2020, 4 d after discharge, the absolute number of eosinophils decreased to 0.01 × 109/L, and the dose of prednisone was adjusted to 15 mg orally twice daily, which was reduced to 10 mg twice daily 1 mo later. On April 13, 2020, the absolute number of eosinophils increased to 0.55 × 109/L, and has been in the normal range since then. The dose of prednisone was reduced to 5 mg in July 2020, and the drug was discontinued in December 2020 when the absolute number of eosinophils was normal. However, the dose was increased to 5 mg per day as the absolute eosinophil count increased to 2.21 × 109/L. The dose was maintained for 1 year and then changed to 4 mg daily until now. In addition, anticoagulation therapy with low molecular weight heparin was discontinued 4 mo after discharge due to petechiae on the patient’s body. To date, under treatment with prednisone and ursodeoxycholic acid, the results of liver function tests, eosinophil count, and CT scanning have all indicated that the patient is in good condition.
HES is defined as an absolute eosinophil count in the peripheral blood > 1.5 × 109/L after two examinations (interval > 1 mo) and (or) a bone marrow nucleated cell count eosinophil percentage ≥ 20% and (or) pathologically confirmed extensive tissue eosinophil infiltration, and (or) significant eosinophil granular protein deposition (in the presence or absence of obvious infiltration of eosinophils in tissues)[6]. The most important characteristic of HES is hypereosinophilia accompanied by eosinophil-mediated organ damage and (or) dysfunction, while excluding other potential causes.
HES is divided into primary, reactive or idiopathic HES. The proportion of eosinophils in the bone marrow nucleated cell count of our patient was ≥ 20%, and the absolute count of eosinophils in peripheral blood was ≥ 1.5 × 109/L. PDGFR gene rearrangement and BCR/ABL fusion gene were negative, which excluded clonal HES. The patient had no previous allergic disease, parasitic infection, connective tissue disease, splenomegaly, endocardial disease or severe mucosal ulcer disease, and no increase in CD25+ atypical spindle-shaped mast cells on bone marrow aspiration. Because the patient had no inflammatory reaction, tumors of the hematological and digestive systems were excluded. The patient was diagnosed with IHES as the cause of HES was unknown.
The onset of IHES is usually insidious, with large differences in clinical manifestations and lack of specificity, which may involve damage to the lungs, heart, digestive tract, skin, or peripheral or central nervous system. Thrombosis is a more common manifestation, and it has been documented that thromboembolic complications may occur in a quarter of HES patients[7]. Some reports have pointed out that HES can cause portal or hepatic vein thrombosis or Budd–Chiari syndrome, which are summarized in Table 1[4,5,8-26]. This patient presented with maculopapular rash and pruritus as the first manifestations, consistent with the diversity of clinical manifestations of IHES.
| Author | Complication | Affected area | Treatment | Prognosis |
| Kojima et al[4] | SOS | Hepatic veins, portal vein | Prednisolone | Eosinophilia developed again after corticosteroid therapy was discontinued |
| Yamaga et al[5] | SOS | Hepatic veins, portal vein | Prednisolone and ursodeoxycholic acid | Remains in clinical remission despite a reduction in prednisone dosage |
| Lim et al[8] | Hepatic lobar or segmental involvement | Portal vein | NA | NA |
| Kikuchi et al[9] | Thrombosis | Portal area of the liver, pulmonary artery, myocardium, and other organs | NA | Died due to hemorrhagic shock |
| Monterrubio Villar et al[10] | Portal vein and mesenteric, splenic, inferior cava, iliac and femoral veins, colon | Acenocoumarol, methylprednisolone, prednisone, streptokinase | Recovered, no evidence of recurrent thrombosis | |
| Yamada et al[11] | Portal vein, lobules of the liver, spleen, small intestine | Thrombectomy, heparin, methylprednisolone, prednisolone | Remains in clinical remission on prednisolone at 0.3 mg/kg/d | |
| Ames[12] | Portal and splenic vein | Tinzaparin | Recovered | |
| Sui et al[13] | Portal vein, deep vein of lower extremities, mesenteric vein, lungs | Methylprednisolone, piperacillin, partial excision of the small intestine, prednisone, coumadin | Eosinophil and platelet counts were within normal limits and no recurrence of thrombosis | |
| Todd et al[14] | Portal vein, suprahepatic inferior vena cava, bilateral deep vein from the superficial femoral vein to the tibial veins, pulmonary artery | Platelet transfusions, unfractionated heparin, prednisolone | Successfully warfarinized and remains thrombus-free | |
| Ames[15] | Superior and inferior vena cava, pulmonary arteries, right atrium, both femoral veins and right hepatic and portal vein | Methylprednisolone | Recovered | |
| Lin et al[16] | Superior mesenteric, splenic, hepatic, and portal veins, renal artery, mesentery | Methylprednisolone, plasma exchange/hemofiltration, single or combined use of unfractionated heparin and argatroban, alteplase, urokinase, excision of necrotic intestinal canal | Recovered and remains in clinical remission | |
| L'Ollivier et al[17] | Right lobe of the liver, right branch of the portal vein | Rivaroxaban, triclabendazole | Recovered, the abscesses and lesions in the liver were reduced in number and size on CT scan, the portal thrombosis had almost disappeared | |
| Moon et al[18] | The umbilico-portal confluence of the left portal vein contiguous with the umbilical vein | Total parenteral nutrition | Thrombus completely resolved. The infant showed good weight gain with well-tolerated formula feeds. | |
| Alshurafa et al[19] | Portal vein, transverse colon | IV hydration, anticoagulation, H. pylori quadruple eradication regimen | Significant improvement in eosinophilia and thrombocytopenia after H. pylori treatment | |
| Zemleduch et al[20] | Portal vein, ascending and transverse colon, greater curvature of the stomach, deep vein of lower extremities, left occipital and right frontal lobes | Heparin, corticosteroid therapy | Remains in good health with no hematological or thrombotic sequels | |
| Aukstuolis et al[21] | Right and left portal vein, mesenteric, and splenic vein, ascending colon, small bowel | Methylprednisolone, prednisone, warfarin, benralizumab | Remains in clinical remission on benralizumab | |
| Elouaer-Blanc et al[22] | Budd–Chiari syndrome | Hepatic vein | Diuretic therapy, prednisone, hydroxyurea, mesocaval anastomosis | Died due to sepsis and acute renal failure |
| Inoue et al[23] | Hepatic vein, subcutaneous nodule | Interventional therapy, steroid therapy | Liver function tests improved but the findings of the obstruction of hepatic veins were unchanged | |
| Lin et al[24] | Intrahepatic inferior vena cava, hepatic veins, mucosal and submucosal layers of the jejunum | Methylprednisolone, prednisolone, percutaneous transhepatic angioplasty, montelukast | No recurrence of ascites, hepatomegaly, or inferior vena cava stenosis during 1-year follow-up period | |
| Dasari et al[25] | Hepatic vein | Endoscopic variceal ligation, prednisolone | Recovered and currently asymptomatic | |
| Shizuku et al[26] | Hepatic vein | Prednisolone, deceased-donor liver transplantation | Recovered and eosinophil count was maintained at a normal level |
HSOS, also known as HVOD, is an intrahepatic postsinusoidal portal hypertension resulting from stenosis or occlusion of the central and inferior lobular veins of the liver after injury, which, as described above, leads to weight gain with or without ascites, right upper abdominal pain of hepatic origin, hepatomegaly, and jaundice[3]. The occurrence of hepatic venule occlusion is mainly associated with hematopoietic stem cell transplantation (HSCT), while chemotherapeutic agents have also been suggested to cause hepatic venule occlusion, such as inotuzumab ozogamicin[27,28], gemtuzumab ozogamicin[29] and other targeted drugs, as well as oxaliplatin[30,31]. Herbs containing pyrrolizidine alkaloid (PA) have also been reported to cause occlusion of hepatic venules, that is, PA-HSOS, such as Gynura segetum[32-34] and Heliotropium eichwaldii[35]. In this case, the patient presented with jaundice. Imaging showed hepatic venule congestion and ascites, and liver puncture indicated hepatic venule occlusion. However, he had not been treated with HSCT or chemotherapeutic drugs, and it cannot be excluded that the herbs he received caused the hepatic venule occlusion. It is reported that peony may cause adverse reactions in the liver[36], but there is no literature to show that these herbs taken by the patient contain PA, and there is no report indicating that these herbs are associated with hepatic venule occlusion.
In 1995, Kojima and Sasaki[4] reported a case of HES complicated by acute HSOS. The HSOS caused a series of clinical symptoms that returned to normal after the use of corticosteroids to treat HES. In addition, eosinophil granular proteins led to direct tissue damage and a local blood hypercoagulable state. It was considered that hypereosinophilia was involved in the development of hepatic venule occlusion[4]. A case of HSOS with hypereosinophilia reported by Yamaga et al[5] was relieved after treatment with prednisone and ursodeoxycholic acid[5]. In our patient, the severity of hepatic venule occlusion and changes in liver function indicators changed with the indicators of eosinophils. Although no eosinophil infiltration was found in the liver biopsy, the eosinophil and liver function indicators began to return to normal after the patient was treated with prednisone. However, when the dose of prednisone was reduced toward discontinuation, the eosinophils increased again, and the symptoms of hepatic venule occlusion were aggravated, and pelvic effusion increased. With the continued use of prednisone, these indicators returned to normal. Therefore, it cannot be ruled out that the hepatic venule occlusion was caused by IHES.
The pathogenesis of HSOS involves the injury of endothelial cells and hepatocytes in the hepatic sinusoids caused by HSCT, chemotherapeutic agents, PA, etc., as well as locally released cytokines that also induce the activation of cell adhesion molecules on endothelial cells, leading to local cell damage and shedding[37], resulting in activation of the coagulation cascade, the formation of blood clots, and the loss of thrombus-fibrinolytic balance[38]. Not only do eosinophils cause tissue damage[39,40], but they can also be rapidly recruited to the site of injury for platelet adhesion to form thrombus and are activated through direct interaction with platelets. Activated eosinophils contribute to platelet activation, inhibit the function of thrombomodulin[41,42], and promote thrombus formation[43]. It is speculated from the pathogenesis that hypereosinophilia may lead to venous thrombosis and hepatic venule occlusion through an imbalance of the coagulation fibrinolysis balance caused by endothelial cell injury.
Prednisone is preferred for the first-line treatment of IHES, and imatinib, interferon, azathioprine, hydroxyurea, or monoclonal antibodies can be chosen as second-line therapeutic agents[6]. The present case was also treated with prednisone and the dose was reduced or restored according to the condition.
With regard to the pharmacological treatment of hepatic venule occlusion, there is no specific drug available at present, and symptomatic supportive treatment is mostly given, including liver protection, diuresis, and microcirculation improvement. High-dose hormone shock therapy may be effective for HSCT-HSOS, during which the risk of infection needs to be monitored[44], while the therapeutic effect of HSOS caused by other reasons is uncertain. In addition, anticoagulation can be administered, especially in patients in the acute/subacute phase in the presence of manifestations such as ascites and jaundice, and some studies have shown that low molecular weight heparin can play a therapeutic role[45]. Defibrotide is effective for the prevention and treatment of HSCT-HSOS[44], as well as recombinant human soluble thrombomodulin[46,47] and ursodeoxycholic acid[44]. However, most studies have focused on HSCT-HSOS and a small number on PA-HSOS. Therefore, the treatment modality for HSOS remains to be clarified and standardized. In this case, using the new EBMT criteria for HSOS in adults, we judged that the patient had mild HSOS based on the time since first clinical symptoms, bilirubin levels, transaminases, weight increase and renal function[48], and was treated with low molecular weight heparin combined with ursodeoxycholic acid.
We report a case of IHES with hepatic venule occlusion. The cause of hepatic venule occlusion was unclear, and it cannot be excluded that it was caused by herbal medicine, nor can it be ruled out that it was caused by IHES, which suggests the possibility of combined hepatic venule occlusion existing in IHES and the potential promoting role of hypereosinophilia in the development of HSOS. For reference, prednisone, low molecular weight heparin, and ursodeoxycholic acid were administered.
| 1. | Curtis C, Ogbogu P. Hypereosinophilic Syndrome. Clin Rev Allergy Immunol. 2016;50:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, Hellmann A, Metzgeroth G, Leiferman KM, Arock M, Butterfield JH, Sperr WR, Sotlar K, Vandenberghe P, Haferlach T, Simon HU, Reiter A, Gleich GJ. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 526] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 3. | DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 669] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 4. | Kojima K, Sasaki T. Veno-occlusive disease in hypereosinophilic syndrome. Intern Med. 1995;34:1194-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Yamaga Y, Tsugihashi Y, Nakamura T, Taniguchi T, Honjou G, Kage M. Sinusoidal obstructive syndrome with hypereosinophilia successfully treated with prednisolone. Clin J Gastroenterol. 2012;5:24-30. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Leukemia and Lymphoma Group; Chinese Society of Hematology; Chinese Medical Association. [Chinese expert consensus on the diagnosis and treatment of eosinophilia (2017)]. Zhonghua Xueyexue Zazhi. 2017;38:561-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Ogbogu PU, Rosing DR, Horne MK 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:457-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 274] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Lim JH, Lee WJ, Lee DH, Nam KJ. Hypereosinophilic syndrome: CT findings in patients with hepatic lobar or segmental involvement. Korean J Radiol. 2000;1:98-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kikuchi K, Minami K, Miyakawa H, Ishibashi M. Portal vein thrombosis in hypereosinophilic syndrome. Am J Gastroenterol. 2002;97:1274-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Monterrubio Villar J, Córdoba López A, Macayo Sánchez AJ. Idiopathic eosinophilia associated with portal vein and massive thrombosis: successful thrombolysis with streptokinase. Med Sci Monit. 2006;12:CS53-CS56. [PubMed] |
| 11. | Yamada Y, Hoshino K, Shimojima N, Shinoda M, Obara H, Kawachi S, Fuchimoto Y, Tanabe M, Kitagawa Y, Morikawa Y. Idiopathic hypereosinophilic syndrome in a case with ABO-incompatible liver transplantation for biliary atresia complicated by portal vein thrombosis. Pediatr Transplant. 2010;14:e49-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ames PR. Recurrent abdominal thrombosis despite heparin thromboprophylaxis in a patient with transient eosinophilia. Clin Appl Thromb Hemost. 2011;17:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Sui T, Li Q, Geng L, Xu X, Li Y. A case of hypereosinophilic syndrome presenting with multiorgan thromboses associated with intestinal obstruction. Turk J Haematol. 2013;30:311-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Todd S, Hemmaway C, Nagy Z. Catastrophic thrombosis in idiopathic hypereosinophilic syndrome. Br J Haematol. 2014;165:425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Ames PR. Transient Eosinophilia with Catastrophic Vessel Occlusion. Intern Med. 2015;54:2095. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Lin J, Huang X, Zhou W, Zhang S, Sun W, Wang Y, Ren K, Tian L, Xu J, Cao Z, Pu Z, Han X. Thrombosis in the portal venous system caused by hypereosinophilic syndrome: A case report. Medicine (Baltimore). 2018;97:e13425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | L'Ollivier C, Eldin C, Lambourg E, Brouqui P, Lagier JC. Case Report: First Molecular Diagnosis of Liver Abscesses Due to Fasciola hepatica Acute Infection Imported from Vietnam. Am J Trop Med Hyg. 2020;102:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Moon CJ, Kwon TH, Lee HS. Portal vein thrombosis and food protein-induced allergic proctocolitis in a premature newborn with hypereosinophilia: a case report. BMC Pediatr. 2021;21:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Alshurafa A, Sied M, Elkhdier M, Abdalhadi AM, Yassin MA. Helicobacter pylori infection manifesting as Hypereosinophilic syndrome and immune thrombocytopenia complicated by portal vein thrombosis and ischemic colitis. IDCases. 2022;27:e01451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 20. | Zemleduch T, Czapla A, Kimla P, Kudliński B. Rare Case of a Young Male Presented with Abdominal Pain, Solid Colon Tumors, and Eosinophilia, Followed by Tremendous Thromboembolic Complications and Eventually Diagnosed with Idiopathic Hypereosinophilic Syndrome. Case Rep Med. 2022;2022:1424749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Aukstuolis K, Cooper JJ, Altman K, Lang A, Ayars AG. Hypereosinophilic syndrome presenting as coagulopathy. Allergy Asthma Clin Immunol. 2022;18:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Elouaer-Blanc L, Zafrani ES, Farcet JP, Saint-Marc Girardin MF, Mathieu D, Dhumeaux D. Hepatic vein obstruction in idiopathic hypereosinophilic syndrome. Arch Intern Med. 1985;145:751-753. [PubMed] |
| 23. | Inoue A, Michitaka K, Shigematsu S, Konishi I, Hirooka M, Hiasa Y, Matsui H, Matsuura B, Horiike N, Hato T, Miyaoka H, Onji M. Budd-Chiari syndrome associated with hypereosinophilic syndrome; a case report. Intern Med. 2007;46:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Lin HK, Lin CC, Tsai IC, Wang JD, Lin WY. Successful treatment of eosinophilia-associated Budd-Chiari syndrome in a child. J Pediatr Gastroenterol Nutr. 2011;53:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Dasari S, Naha K, Hande M, Vivek G. A novel subtype of myeloproliferative disorder? JAK2V617F-associated hypereosinophilia with hepatic venous thrombosis. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Shizuku M, Kurata N, Jobara K, Yoshizawa A, Kamei H, Amano N, Genda T, Ogura Y. Budd-Chiari Syndrome Associated With Hypereosinophilic Syndrome Treated by Deceased-Donor Liver Transplantation: A Case Report. Transplant Proc. 2019;51:3140-3146. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Kantarjian HM, DeAngelo DJ, Advani AS, Stelljes M, Kebriaei P, Cassaday RD, Merchant AA, Fujishima N, Uchida T, Calbacho M, Ejduk AA, O'Brien SM, Jabbour EJ, Zhang H, Sleight BJ, Vandendries ER, Marks DI. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4:e387-e398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 28. | Kebriaei P, Cutler C, de Lima M, Giralt S, Lee SJ, Marks D, Merchant A, Stock W, van Besien K, Stelljes M. Management of important adverse events associated with inotuzumab ozogamicin: expert panel review. Bone Marrow Transplant. 2018;53:449-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Magwood-Golston JS, Kessler S, Bennett CL. Evaluation of gemtuzumab ozogamycin associated sinusoidal obstructive syndrome: Findings from an academic pharmacovigilance program review and a pharmaceutical sponsored registry. Leuk Res. 2016;44:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, Mentha G, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 772] [Article Influence: 35.1] [Reference Citation Analysis (1)] |
| 31. | Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287-4299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 32. | Lin G, Wang JY, Li N, Li M, Gao H, Ji Y, Zhang F, Wang H, Zhou Y, Ye Y, Xu HX, Zheng J. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J Hepatol. 2011;54:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Zhu L, Zhang CY, Li DP, Chen HB, Ma J, Gao H, Ye Y, Wang JY, Fu PP, Lin G. Tu-San-Qi (Gynura japonica): the culprit behind pyrrolizidine alkaloid-induced liver injury in China. Acta Pharmacol Sin. 2021;42:1212-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Cen P, Ding J, Jin J. Hepatic sinusoidal obstruction syndrome caused by the ingestion of Gynura segetum in a patient with alcoholic cirrhosis: a case report. J Int Med Res. 2021;49:300060520980649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Datta DV, Khuroo MS, Mattocks AR, Aikat BK, Chhuttani PN. Herbal medicines and veno-occlusive disease in India. Postgrad Med J. 1978;54:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Dalle JH, Giralt SA. Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation: Risk Factors and Stratification, Prophylaxis, and Treatment. Biol Blood Marrow Transplant. 2016;22:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 38. | Corbacioglu S, Jabbour EJ, Mohty M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol Blood Marrow Transplant. 2019;25:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 39. | Slungaard A, Vercellotti GM, Walker G, Nelson RD, Jacob HS. Tumor necrosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. J Exp Med. 1990;171:2025-2041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Wang JG, Mahmud SA, Thompson JA, Geng JG, Key NS, Slungaard A. The principal eosinophil peroxidase product, HOSCN, is a uniquely potent phagocyte oxidant inducer of endothelial cell tissue factor activity: a potential mechanism for thrombosis in eosinophilic inflammatory states. Blood. 2006;107:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Mukai HY, Ninomiya H, Ohtani K, Nagasawa T, Abe T. Major basic protein binding to thrombomodulin potentially contributes to the thrombosis in patients with eosinophilia. Br J Haematol. 1995;90:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Slungaard A, Vercellotti GM, Tran T, Gleich GJ, Key NS. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J Clin Invest. 1993;91:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Marx C, Novotny J, Salbeck D, Zellner KR, Nicolai L, Pekayvaz K, Kilani B, Stockhausen S, Bürgener N, Kupka D, Stocker TJ, Weckbach LT, Pircher J, Moser M, Joner M, Desmet W, Adriaenssens T, Neumann FJ, Gerschlick AH, Ten Berg JM, Lorenz M, Stark K. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134:1859-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 44. | Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, Veys P, Potter MN; Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 45. | Zhuge YZ, Wang Y, Zhang F, Zhu CK, Zhang W, Zhang M, He Q, Yang J, He J, Chen J, Zou XP. Clinical characteristics and treatment of pyrrolizidine alkaloid-related hepatic vein occlusive disease. Liver Int. 2018;38:1867-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Nakamura D, Yoshimitsu M, Kawada H, Inoue H, Kuroki T, Kaieda T, Fujino S, Hamada H, Suzuki S, Matsushita K, Uozumi K, Arima N. Recombinant human soluble thrombomodulin for the treatment of hepatic sinusoidal obstructive syndrome post allogeneic hematopoietic SCT. Bone Marrow Transplant. 2012;47:463-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Yakushijin K, Ikezoe T, Ohwada C, Kudo K, Okamura H, Goto H, Yabe H, Yasumoto A, Kuwabara H, Fujii S, Kagawa K, Ogata M, Onishi Y, Kohno A, Watamoto K, Uoshima N, Nakamura D, Ota S, Ueda Y, Oyake T, Koike K, Mizuno I, Iida H, Katayama Y, Ago H, Kato K, Okamura A, Kikuta A, Fukuda T. Clinical effects of recombinant thrombomodulin and defibrotide on sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54:674-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Ciceri F, Corbacioglu S, Dalle JH, Dignan F, Fukuda T, Huynh A, Masszi T, Michallet M, Nagler A, NiChonghaile M, Okamoto S, Pagliuca A, Peters C, Petersen FB, Richardson PG, Ruutu T, Savani BN, Wallhult E, Yakoub-Agha I, Duarte RF, Carreras E. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kupeli S, Turkey; Suda T, Japan S-Editor: Li L L-Editor: A P-Editor: Li L