Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.1000
Peer-review started: January 16, 2023
First decision: February 1, 2023
Revised: February 20, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: May 27, 2023
Processing time: 129 Days and 12.1 Hours
With the recent improvement of endoscopic techniques, endoscopic ultrasound-guided fine needle aspiration and endoscopic submucosal tunnel dissection (ESTD) have been widely used for accurate diagnosis and dissection acceleration of esophageal tumors.
We used a modified submucosal tunnel technique during endoscopic en bloc resection in a 58-year-old man with large esophageal submucosal gland duct adenoma (ESGDA). During modified ESTD, the oral end of the involved mucosa was cut transversely, followed by a submucosal tunnel created from the proximal to the distal end, and the anal end of the involved mucosa blocked by the tumor was incised. As a result of retaining submucosal injection solutions using the submucosal tunnel technique, it was possible to reduce the amount of injection required and increase the efficiency and safety of dissection.
Modified ESTD is an effective treatment strategy for large ESGDAs. Single-tunnel ESTD appears to be a time-saving procedure compared with conventional endoscopic submucosal dissection.
Core Tip: With the recent improvement of endoscopic techniques, endoscopic ultrasound-guided fine needle aspiration and endoscopic submucosal tunnel dissection (ESTD) have been widely used for accurate diagnosis and dissection acceleration. Here we used a modified submucosal tunnel technique during endoscopic en bloc resection in a 58-year-old man with large esophageal submucosal gland duct adenoma (ESGDA) 3.5 cm × 2.2 cm in size with negative margins. Modified ESTD is an effective treatment strategy for large ESGDAs. Single-tunnel ESTD appears to be a time-saving procedure compared with conventional ESD.
- Citation: Chen SY, Xie ZF, Jiang Y, Lin J, Shi H. Modified endoscopic submucosal tunnel dissection for large esophageal submucosal gland duct adenoma: A case report. World J Gastrointest Surg 2023; 15(5): 1000-1006
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/1000.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.1000
The esophageal glands reside in the submucosal layer of the upper and lower segments of the esophagus, opening at the esophageal lumen by tortuous tubules. The glands play a role in protecting the esophagus from damage caused by acid exposure through the secretion of bicarbonate and prostaglandin. Esophageal submucosal gland duct adenoma (ESGDA) is quite rare and usually presents as a submucosal lesion with or without a central depression[1-2]. This is the first report of the successful resection of a large esophageal submucosal tumor (SMT) using endoscopic-related techniques.
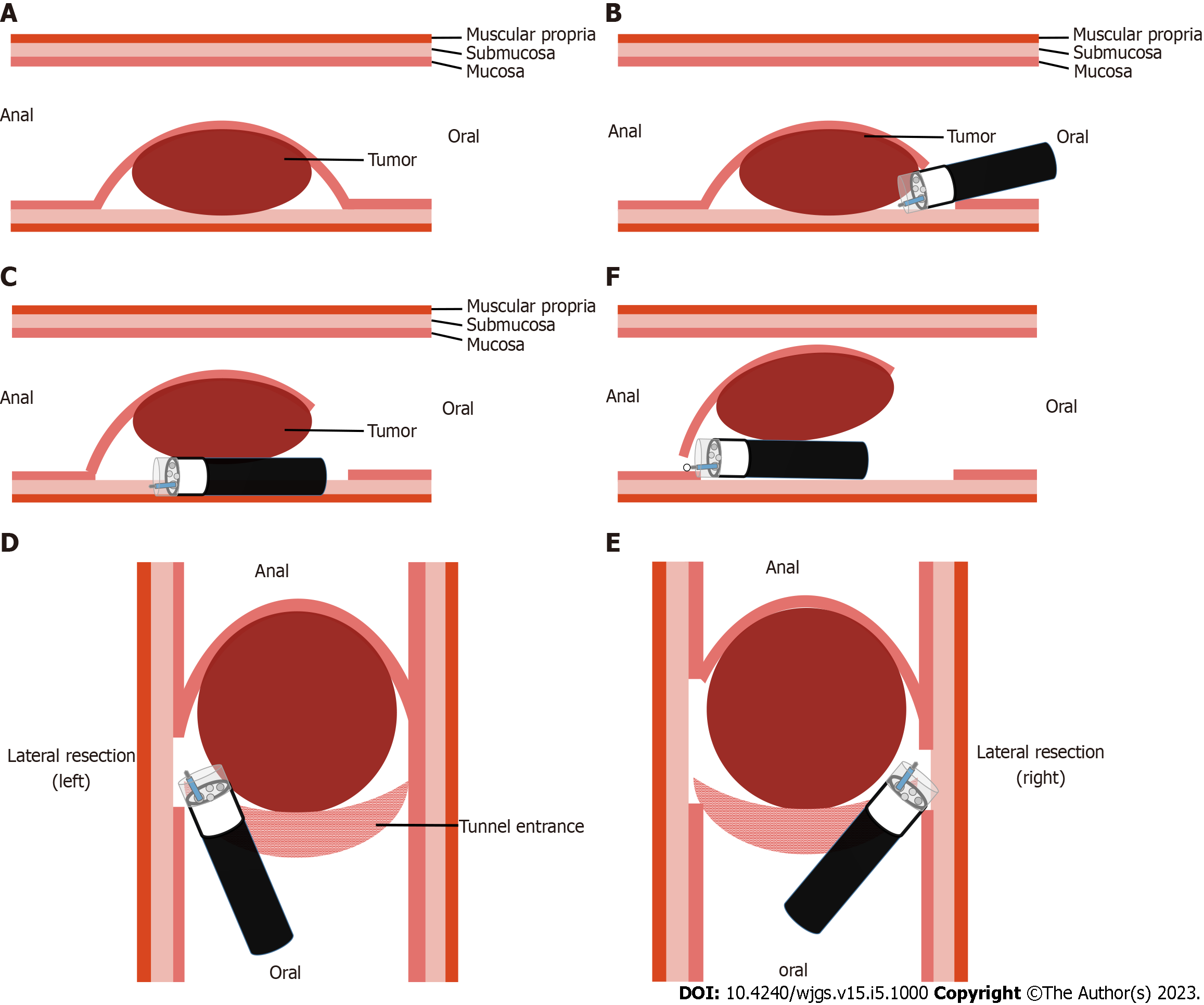
As described in the published literature, single-tunnel ESTD[3] has shown greater efficiency for large superficial esophageal squamous cell neoplasms. In contrast to conventional ESTD, modified ESTD does not begin with cutting the distal end of the involved mucosa, which is occluded by a large esophageal SMT. First, the oral end of the involved mucosa was cut transversely. Subsequently, a submucosal tunnel was created from the proximal to the distal end. After completion of the tunnel, the lateral mucosa was incised, followed by the anal end of the involved mucosa, until complete removal of the tumor was achieved. Modified ESTD can be faster than conventional ESD for large esophageal tumors.
A 58-year-old man was referred to our hospital with a complaint of gastro-esophageal reflux symptoms for one month.
Symptoms started 1 mo before presentation with recurrent retro-sternal heartburn.
He was made a definite diagnosis of hypertension, and his blood pressure control is steady by oral anti-hypertensive drugs.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.5℃; blood pressure, 140/85 mmHg; heart rate, 85 beats per min; respiratory rate, 18 breaths per min. Furthermore, no enlargement was found in superficial lymph nodes. There was no obvious redness and swelling. Digital anal examination was not performed.
Levels of serum tumor markers were normal (carcinoembryonic antigen, 2.8 ng/mL; carbohydrate antigen 12-5, 12 U/mL; carbohydrate antigen 724, 0.33 U/mL), except elevated carbohydrate antigen 19-9 at the level of 45.25 U/mL. No abnormality was found in routine blood, stool and urine analyses.
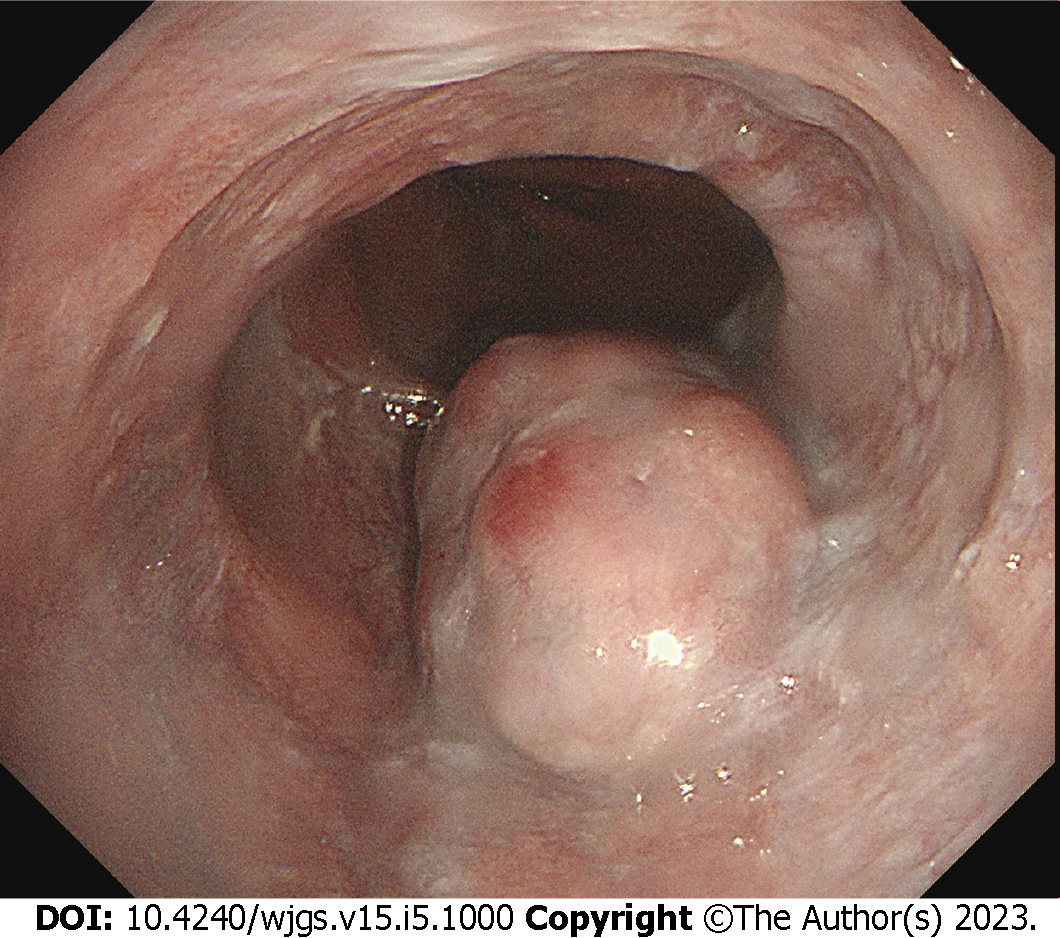
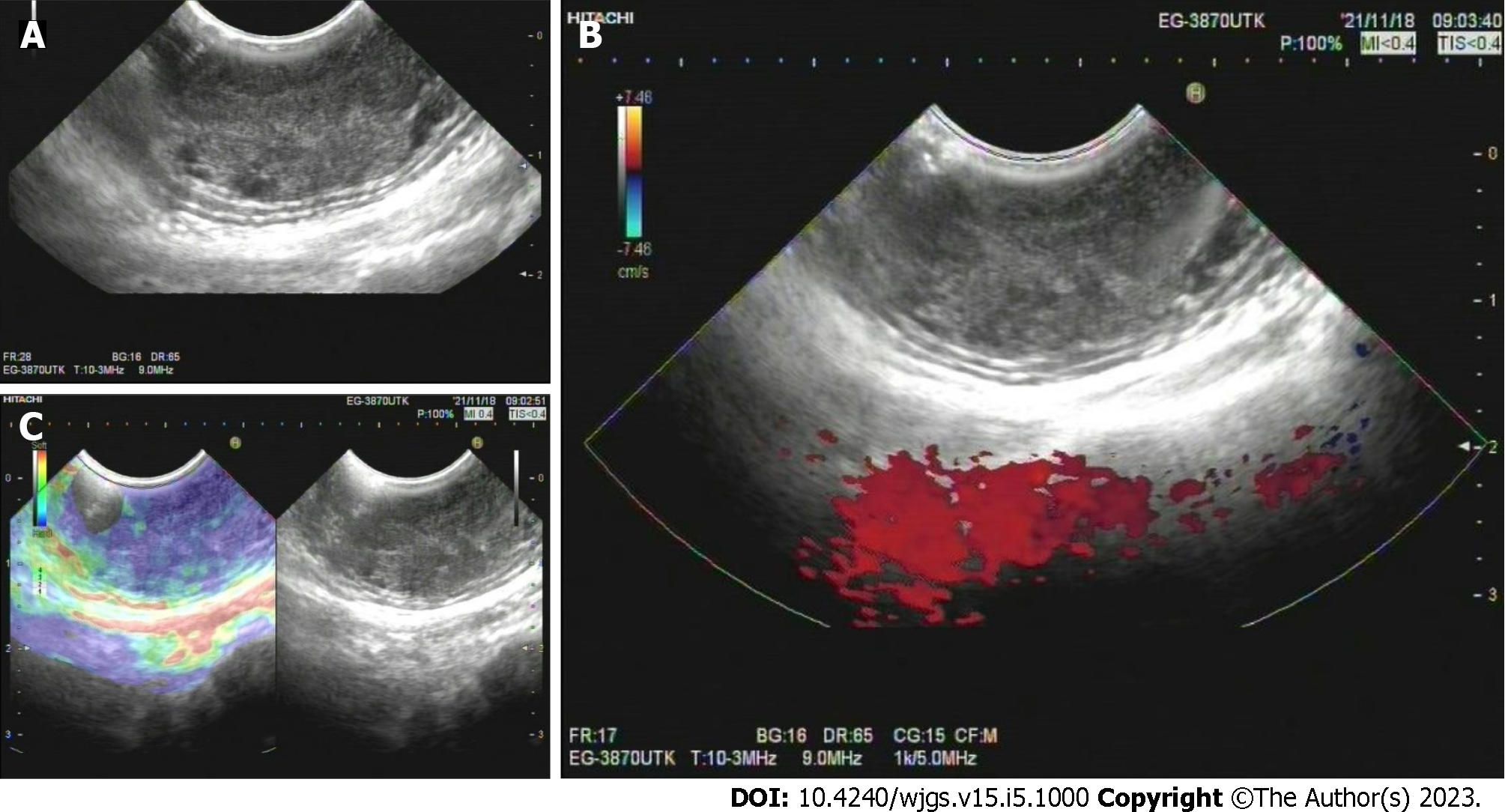
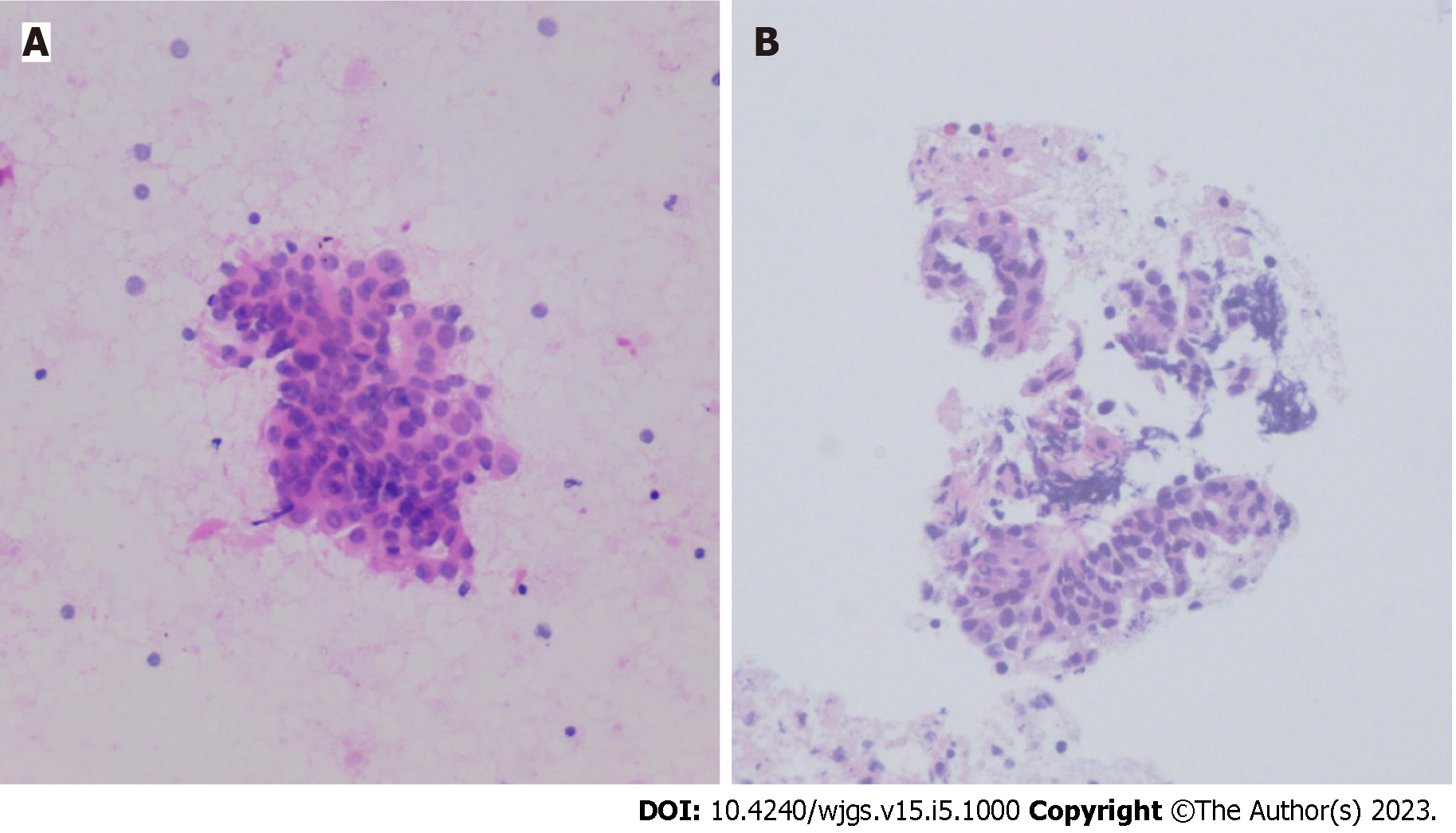
White-light endoscopy showed one 35-mm SMT located in the lower thoracic esophagus, with a central depression with a reddish appearance, just above the squamous-columnar epithelium junction (Figure 1). Endoscopic ultrasound (EUS) showed that the SMT was a well-defined heterogeneous, hypoechoic lesion with scattered small anechoic areas in the third layer (Figure 2A). No vascular flow was noted on color Doppler within the lesion (Figure 2B). Elastography revealed a hard mass (Figure 2C). Both cytological and histological analysis of fine-needle aspiration (FNA) specimens reported atypical glandular epithelial cells, ruling out squamous carcinoma (Figure 3). Thorax computed tomography (CT) revealed an eccentric soft tissue density mass in the esophagus.
Combined with the patient’s medical history, the final diagnosis was esophageal benign submucosal mesenchymal tumor.
Given that the mass did not involve the muscularis propria, modified ESTD was performed using a single-accessory channel endoscope with a transparent cap attached to the front. First, the oral end of the involved mucosa was cut transversely. Subsequently, a submucosal tunnel was created from the proximal end to the distal end. After completion of the tunnel, the lateral mucosa was incised, followed by the anal end of the involved mucosa, until complete removal of the tumor was achieved (Figure 4). The post-ESTD defect was closed using repositionable clips in the distal–proximal sequence.
Subsequently, cefazolin sodium 1 g was administered intravenously twice a day for three days from the day of the resection.
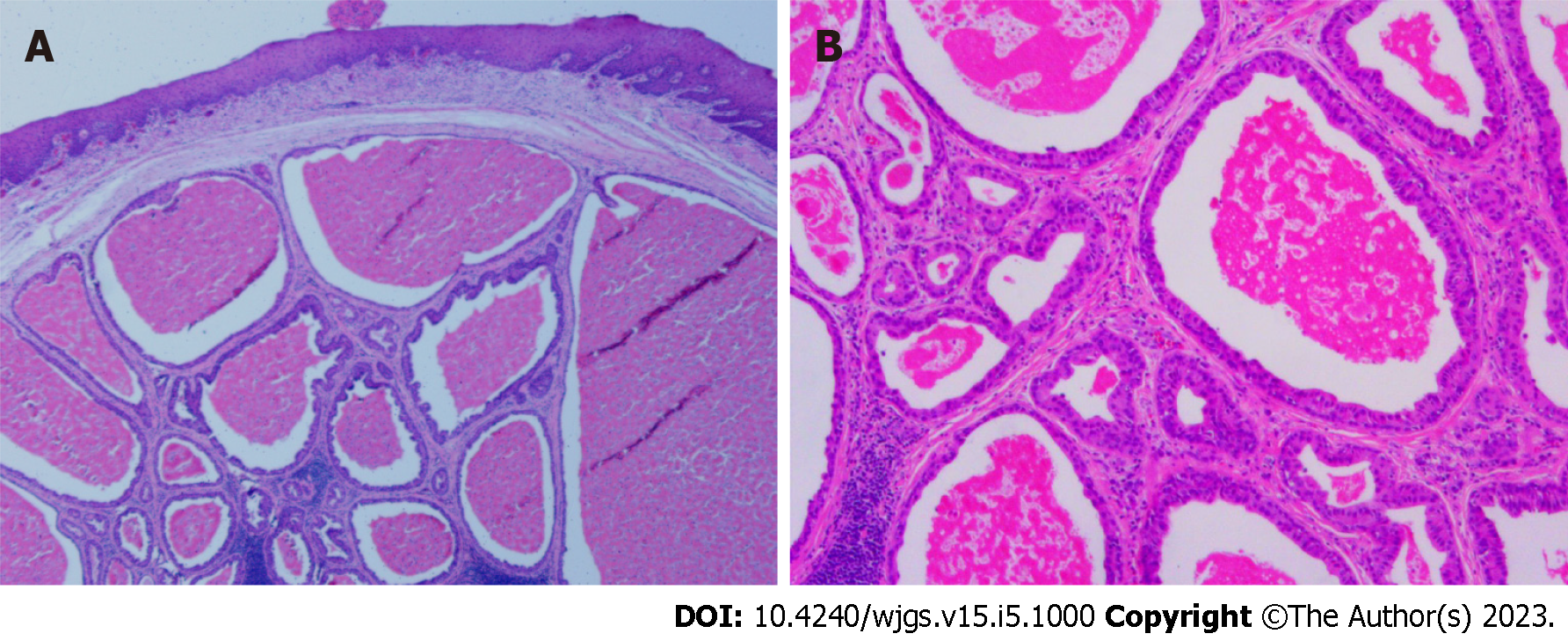
The procedure was completed without any adverse events, and the patient was discharged on postoperative day 7. Post-ESTD pathology confirmed an ESGDA 3.5 cm × 2.2 cm in size with negative margins. A cystic pattern with distinct 2-cell layers was observed, and the inner luminal cells were eosinophilic (Figure 5). Immunohistochemical staining showed the positive expression of P63, CK7, CK8/18, and Ki-67.
At 12 mo postoperatively, the patient received gastroscopy showing the esophageal wound healed well.
To the best of our knowledge, this present study represents the largest ESGDA treated by modified ESTD to be reported in the literature. Published literature reports suggest a potential biological pathway for ESGDA to progress to malignancy; thus, resection of these tumors is recommended if possible.
As an esophageal subepithelial tumor, ESGDA is extremely easily mistaken for esophageal carcinoma. EUS evaluation is essential for evaluating subepithelial lesions. The typical EUS feature of ESGDA is a heterogeneous hypoechoic submucosal tumor located in the third sonographic layer, with a few cystic lesions, considered dilated adenomatous gland ducts[4]. Differentiation through EUS-guided FNA is helpful to judge the origin of pathology and make the decision for conservative management vs endoscopic or thoracoscopic intervention[5]. In our case, both cytological and histological analyses showed atypical glandular epithelial cells, so modified ESTD was selected for resection.
As reported previously[3], the major advantage of ESTD over ESD for large superficial esophageal squamous cell neoplasms is that most submucosal injection solutions can be retained in the submucosal layer, resulting in increased efficiency and safety of dissection. Also tunnel endoscopy can be used to expose the intact submucosal lesion between the mucosa and muscular layer of the esophagus. In view of the aforementioned advantages, we performed ESTD for en bloc resection of the large ESGDA, rather than conventional ESD that was be adopted in the literature on ESGDA[1,2,4,6-8]. During routine ESTD, the distal end of the involved mucosa was first cut transversely. In this case, the obstruction caused by a large esophageal tumor made it difficult to cut the distal end of the involved mucosa at the beginning of the operation. An adjustment of the operative sequence was made in the modified ESTD. The oral end of the involved mucosa was first cut transversely, then the lateral mucosa was incised, followed by the anal end of the involved mucosa, after completion of the tunnel.
With regard to the disadvantages of ESTD, they include visual field loss when arterial bleeding requiring immediate endoscopic hemostasis occurs, due to the limited tunnel space. Furthermore, arterial bleeding in ESTD for lower esophageal tumors is relatively more common, differing from that involving the upper and middle esophagus.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atanasova EG, Bulgaria; Atqiaee K, Iran; Mokhtar MN, Malaysia; Piltcher-da-Silva R, Brazil S-Editor: Ma YJ L-Editor: A P-Editor: Zhao S
| 1. | Yamamoto M, Nishida T, Nakamatsu D, Adachi S, Inada M. Endoscopic findings of esophageal gland duct adenoma resected by endoscopic submucosal dissection. Gastrointest Endosc. 2020;92:961-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 2. | Nie L, Wu HY, Shen YH, Fan XS, Sun Q, Huang Q, Chen J. Esophageal submucosal gland duct adenoma: a clinicopathological and immunohistochemical study with a review of the literature. Dis Esophagus. 2016;29:1048-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 3. | Zhang W, Zhai Y, Chai N, Linghu E, Li H, Feng X. Single- and double-tunnel endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. Endoscopy. 2018;50:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Agawa H, Matsushita M, Kusumi F, Nishio A, Takakuwa H. Esophageal submucosal gland duct adenoma: characteristic EUS and histopathologic features. Gastrointest Endosc. 2003;57:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Nashed B, Ayas MF, Gharib H, Issa M, Fatouh K, Sebastian F, Backer Z, Mahat K, Barawi M. Esophageal Schwannoma: An Important Differential Diagnosis for Esophageal Subepithelial Lesions. Cureus. 2022;14:e27168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Shibata M, Kusafuka K, Ono H. A Rare Submucosal Tumor of the Esophagus. Gastroenterology. 2017;152:e6-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Matsushita M, Okazaki K. Esophageal, submucosal, gland duct adenoma: role of EUS for endoscopic removal. Gastrointest Endosc. 2005;61:790; author reply 790-790; author reply 791. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Chinen T, Misawa T, Yoshida K, Nasu T, Kubo S, Toyoshima S, Yao T, Harada N. Esophageal submucosal gland duct adenoma. Gastrointest Endosc. 2004;60:798-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Fan X, Wu Q, Li R, Chen W, Xie H, Zhao X, Zhu S, Fan C, Li J, Liu M, Liu Z, Han Y. Clinical benefit of tunnel endoscopic submucosal dissection for esophageal squamous cancer: a multicenter, randomized controlled trial. Gastrointest Endosc. 2022;96:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 10. | Zou J, Chai N, Linghu E, Li H, Chai M, Shi Y, Wang Z, Li L. Autologous skin-grafting surgery to prevent esophageal stenosis after complete circular endoscopic submucosal tunnel dissection: a case-matched controlled study. Surg Endosc. 2021;35:5962-5970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |