Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.600
Peer-review started: October 30, 2022
First decision: January 3, 2023
Revised: January 5, 2023
Accepted: March 8, 2023
Article in press: March 8, 2023
Published online: April 27, 2023
Processing time: 175 Days and 4.6 Hours
The incidence rate of acute pancreatitis (AP), which is a pathophysiological process with complex etiology, is increasing globally. miR-125b-5p, a bidirectional regulatory miRNA, is speculated to exhibit anti-tumor activity. However, exosome-derived miR-125b-5p in AP has not been reported.
To elucidate the molecular mechanism of exosome-derived miR-125b-5p promoting AP exacerbation from the perspective of the interaction between immune cells and acinar cells.
Exosomes derived from AR42J cells were isolated and extracted in active and inactive states by an exosome extraction kit, and were verified via transmission electron microscopy, nanoparticle tracking analysis, and western blotting. RNA sequencing assay technology was used to screen differentially expressed miRNAs in active and inactive AR42J cell lines, and bioinformatics analysis was used to predict downstream target genes of miR-125b-5p. The expression level of miR-125b-5p and insulin-like growth factor 2 (IGF2) in the activated AR42J cell line and AP pancreatic tissue were detected by quantitative real-time polymerase chain reaction and western blots. The changes in the pancreatic inflammatory response in a rat AP model were detected by histopathological methods. Western Blot was used to detect the expression of IGF2, PI3K/AKT signaling pathway proteins, and apoptosis and necrosis related proteins.
miR-125b-5p expression was upregulated in the activated AR42J cell line and AP pancreatic tissue, while that of IGF2 was downregulated. In vitro experiments confirmed that miR-125b-5p could promote the death of activated AR42J cells by inducing cell cycle arrest and apoptosis. In addition, miR-125b-5p was found to act on macrophages to promote M1 type polarization and inhibit M2 type polarization, resulting in a massive release of inflammatory factors and reactive oxygen species accumulation. Further research found that miR-125b-5p could inhibit the expression of IGF2 in the PI3K/AKT signaling pathway. Additionally, in vivo experiments revealed that miR-125b-5p can promote the progression of AP in a rat model.
miR-125b-5p acts on IGF2 in the PI3K/AKT signaling pathway and promotes M1 type polarization and inhibits M2 type polarization of macrophage by inhibiting IGF2 expression, resulting in a large release of pro-inflammatory factors and an inflammatory cascade amplification effect, thus aggravating AP.
Core Tip: Our study confirmed that miR-125b-5p can promote the inflammatory response of acute pancreatitis (AP), and its potential targets were found via RNA sequencing assay. However, the likely mechanism remains unclear. Consequently, our study intends to elucidate the molecular mechanism of exosome-derived miR-125b-5p in promoting AP exacerbation from the perspective of the interaction between immune cells and acinar cells.
- Citation: Zheng Z, Cao F, Ding YX, Lu JD, Fu YQ, Liu L, Guo YL, Liu S, Sun HC, Cui YQ, Li F. Acinous cell AR42J-derived exosome miR125b-5p promotes acute pancreatitis exacerbation by inhibiting M2 macrophage polarization via PI3K/AKT signaling pathway. World J Gastrointest Surg 2023; 15(4): 600-620
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/600.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.600
Acute pancreatitis (AP) is a common clinical inflammatory disease of the digestive system, with an increasing trend worldwide[1,2], which is a pathophysiological process with complex etiology. At present, there are no consistent and effective therapies for treatment of AP, resulting in a high mortality rate. The fundamental reason is that the underlying mechanism of AP pathogenesis is not been fully understood[3]. During severe AP, inflammatory mediators released from the pancreas enter the liver. Then, inflammatory mediators produced by the liver spread to the lungs, activate alveolar macrophages, release monocyte chemoattractant protein-1, platelet-activating factor, and reactive oxygen species (ROS), causing lung parenchyma injury[4]. However, previous research has mainly focused on the influence of macrophages on remote organ injury of AP. Few studies have been conducted on the intrinsic macrophages of the pancreas, and a majority of them have focused on the changes of the AP phenotype. Furthermore, there is lack of research on the potential molecular biological mechanisms causing the phenotypic changes.
Previous studies have established that exosomes, as a form of extracellular vesicles, are involved in the pathophysiological process of various diseases and playing a biological regulatory role[5]. Bonjoch et al[6] demonstrated that in the rat model of AP, plasma exosomes can effectively activate alveolar macrophages, promote M1 polarization, and secrete a large number of proinflammatory factors such as interleukin (IL)-1β and IL-6 that participate in AP-related acute lung injury (ALI)[6]. In addition, studies by Jiménez-Alesanco et al[7] also found that upregulation of miR-155 and decreased expression of miR-21 and miR-122 in plasma-derived exosomes can activate M1-type polarization and promote the release of inflammatory factors, thereby aggravating the progression of AP[7]. This suggests that in the course of the pathogenesis of AP, exosomes derived from acinar cells may participate in the regulation of local pancreatic inflammatory injury, macrophage activation, and extra-pancreatic organ injury via their intrinsic proinflammatory miRNAs.
Additionally, miR-125b-5p is a bidirectional regulatory miRNA, which has been found to have low expression in bladder cancer and high expression in stage I lung cancer[8-10]. Therefore, researchers speculate that it can be used as a potential means of diagnosis and treatment in the future. At present, miR-125b-5p inhibits the proliferation and migration of bladder tumor cells by inhibiting the HK2 gene, suggesting that it can exhibit anti-tumor activity[10]. Additionally, miR-125b-5p can promote cardiomyocyte self-remodeling after ischemia by improving cardiomyocyte apoptosis[11]. Previous studies have shown that miR-125b-5p is highly expressed in exosomes secreted by acinar cells, proposing that it might play a role in the pathogenesis of AP[12]. Therefore, miRNAs may play a biological regulatory role in local pancreatic inflammatory injury, macrophage activation, and extra-pancreatic organ injury in the course of AP through a certain mechanism. However, exosome-derived miR-125b-5p in AP has not been reported.
Therefore, we aimed to elucidate the molecular mechanism of exosome-derived miR-125b-5p promoting AP exacerbation from the perspective of the interaction between immune cells and acinar cells.
AR42J and RAW264.7 cell lines were purchased from the Shanghai Institutes for Biological Science (Shanghai, China). The AR42J and RAW264.7 cell lines were cultured in RPIM-1640 and DMEM high glucose media, respectively. Ten percent fetal bovine serum and 1% penicillin/streptomycin double antibody solution were added to the medium. The cells were cultured in a 37 ℃, 5% CO2 incubator to promote stable cell growth.
Culture medium was collected when AR42J cells reached 70%–80% confluency in the culture dish. The collected culture medium was centrifuged at 2000 × g with a centrifugation radius of 11 cm for 30 min, thereby removing cell debris and apoptotic bodies. The supernatant was retained and 0.5 volume of exosome isolation reagent (Invitrogen, CA) was added, and the samples were incubated at 4 ℃ overnight. The samples were centrifuged at 10000 × g and 4 ℃ at with a radius of 11 cm for 60 min, and the supernatant was discarded. The samples were resuspended in PBS and stored in separate package at –80 ℃. According to the operation instructions, the particle size distribution and concentration of AR42J exosomes were measured using a Zeta View instrument.
Transmission electron microscope was used to observe the morphology of exosomes and determine damage of the exosome membrane during the extraction process. Exosomes were fixed and purified with 2% paraformaldehyde (Electron Microscopy Science, United States). Then, the fixed exosomes were dropped onto a carbon-coated copper grid and fixed with 2% paraformaldehyde for 30 s. Finally, the carbon-coated copper grid was examined using transmission electron microscope (JEM-1400 plus, Japan).
AR42J cells were cultured to approximately 50% in 6-well plates, and the medium was changed into serum-free medium. Exosomes were isolated from the culture medium supernatant and resuspended in 500 mL of sterile 1 × PBS for precipitation. miR-125b-5p mimic and negative control mimic were transferred into exosomes by Exo-FectTM Exosome Transfection Reagent (System Biosciences, United States). First, a 150-mL transfection reaction system was configured, which included 10 mL Exo-Fect solution, 20 pmol miRNA, 70 mL sterile 1 × PBS, and 50 mL purified exosomes. Second, the reaction system was placed on a shaker at 37 ℃ for 10 min, then 30 mL ExoQuick-TC was added and placed on ice for 30 min to terminate the reaction. Third, the samples were centrifuged at 13000–14000 rpm for 30 min. The supernatant was discarded, and the transfected exosome precipitates were resuspended in 300 mL of 1 × PBS. The transfected exosomes were added to the AR42J cell line, at least 150 mL of transfected exosomes should be added to each well, and cultured in 6-well plates to approximately 1 × 105 cells.
The RNAprep Prue Cell/Bacteria Kit and miRelute miRNA isolation kit (TIANGEN, China) were used for the extraction of total RNA and miRNA from AR42J cells and pancreatic tissue according to the protocol. The FastKing RT Kit and miRcute Plus miRNA First-strand cDNA Synthesis Kit (TIANGEN, China) were used to reverse transcribe RNA to cDNA. The LightCycler 480 system (Roche Molecular Diagnostics, Pleasanton, United States) was used to perform quantitative real-time polymerase chain reaction (RT-qPCR). The expression of miRNA and mRNA was evaluated by the 2-∆∆Ct method and normalized to GAPDH or U6, respectively.
The density of AR42J cells was adjusted to 3 × 103 cells/mL, and the cell suspension was seeded in 96-well plates with 100 mL per well in six replicates. The cells were cultured in a 37 ℃, 5% CO2 incubator to promote stable cell growth. The experiment was divided into four groups: Control group, exosome-only group, negative control (NC)-exo group, and 125-exo group. At 0, 4, 6, 8, 10, 12, and 24 h after cell adhesion, liquid was removed per well. Ten microliters of RPPI-1640 medium with 10% CCK-8 reagent was added to 100 mL per well, and the 96-well plates were incubated for 1–2 h. Then, the absorbance at 450 nm was measured by using a microplate reader to calculate the cell survival rate.
Cells were seeded on 6-well plates at a concentration of 1 × 106 cells/per well and treated with NC-exo or 125-exo for 8 h. Subsequently, cells were digested and collected, and the supernatant was discarded. Cells were washed once with pre-cooled PBS, digested with 0.25% trypsin, centrifuged at 500 × g for 5 min, then resuspended in 400 mL of 1 × binding buffer. A cell cycle staining kit (Yeasen, China, Cat No. 4040301) was used to detect the cell cycle. Cycle apoptosis was detected by Annexin V-APC/7-AAD apoptosis detection kit (Nanjing KeyGen Biotech, China). The percentage of cell cycles and cell apoptosis was evaluated by Guava easyCyte HT system (Millipore). The experiment was repeated in triplicate.
The contents of tumor necrosis factor-alpha (TNF-α), IL-6, and C-reactive protein (CRP) in the supernatant of the different groups were detected by enzyme linked immunosorbent assay (ELISA) kits (R&D Systems, MN, United States). The standard and sample were diluted in proportion, and an appropriate amount of each was added to the corresponding reaction well and incubated for 2 h at
To lyse the cells and pancreatic tissue, RIPA lysis buffer (Beyotime Biotechnology, Jiangsu, China) was used. This solution contains a protease inhibitor cocktail (Roche, United States). To detect the protein amount present in the lysates, Bradford reagent (Sigma, United States) was used, then 30 μg of protein per row was run on 10% SDS-PAGE gels and immunoblotted onto a 0.22 m polyvinylidene fluoride membrane (Merck Millipore, Darmstadt, Germany). The membranes were incubated for 1 h with 5% non-fat milk solution (BD Biosciences, United States) dissolved in TBST. After adding primary antibodies against CD9 (Abcam, Cambridge, United Kingdom), CD81 (Abcam, Cambridge, United Kingdom), CD63 (Abcam, Cambridge, United Kingdom), TSG101 (Protein Tech Group, IL, United States), CD206 (Protein Tech Group, IL, United States), inducible nitric oxide synthase (iNOS) (Abcam, Cambridge, United Kingdom), insulin-like growth factor 2 (IGF2) (Protein Tech Group, IL, United States), Bax (Protein Tech Group, IL, United States), Bcl-2 (Protein Tech Group, IL, United States), HMGB1 (Protein Tech Group, IL, United States), PI3K (Protein Tech Group, IL, United States), p-PI3K (Protein Tech Group, IL, United States), AKT (Protein Tech Group, IL, United States), p-AKT (Protein Tech Group, IL, United States), β-tubulin (Protein Tech Group, IL, United States), β-actin (Protein Tech Group, IL, United States), and GAPDH (Abcam, ab8245, 1:1000), the membrane was incubated at 4 °C overnight. The secondary antibody specific to primary antibody was then added. An Odessey CLx system showed the presence of protein bands (LI-GOR, United States).
RNA sequencing assay (RNA-seq) was performed by a service provider (HWayen Biotechnologies Company, Shanghai, China). Total RNA from AP model cells and normal cells was extracted using TRIzol reagent (Sigma-Aldrich, MO, United States) and quantified with a NanoDrop ND-2000 (Thermo Fisher Scientific, Inc., MA, United States). RNA integrity was evaluated by an Agilent Bioanalyzer 2100 (Agilent Technologies, CA, United States). A TruSeq RNA Sample Prep Kit v2 (Illumina Inc, CA, United States) was used to generate RNA-seq cDNA libraries. The workflow of sample preparation included isolation of polyadenylated RNA, RNA fragmentation, synthesis of cDNA, ligation of barcoded adapters, and PCR amplification. After the DNA size and purity of the cDNA library were checked and qualified, clusters of cDNA libraries were generated and sequenced on the Illumina platform at Shanghai HWayen Biotechnologies Company (Shanghai, China). The raw sequencing reads were processed by removing failed reads, low-quality reads, and those with joint contamination, finally retaining only the high-quality read results. The raw reads of each sample were mapped to the rat reference genome to generate the RNA-seq data.
Differentially expressed genes screened by RNA-seq were identified, for which, P < 0.05 was considered statistically significant. In order to further clarify the function of differentially expressed RNAs, Kyoto Encyclopedia of Genes and Genomes databases (KEGG, http://www.genome.jp/kegg) was used for enrichment and screening of related signaling pathways.
The 3¢-untranslated regions (UTR) of IGF2 constructs containing the predicted miR-125b-5p seed-matching sites from the cDNA library was amplified by PCR, then cloned into pmiR-RB-ReportTM-3'-UTR [wild type and mutant (MUT) type] and 50 nM miR-125b-5p mimics, 100 nM miR-125b-5p inhibitor, and negative control miRNA (Ribobio, Guangzhou, China) using Lipofectamine 3000 (Invitrogen, United States) according to the manufacturer’s protocol. After 48 h, cells were lysed with a dual luciferase assay kit (Ribobio, Guangzhou, China), and luciferase activities were calculated and normalized to the control.
All experimental procedures and feeding management methods involving animals were reviewed and approved by the ethics committee of XuanWu Hospital, Capital Medical University (No. 2020-158). The international guidelines for the use and management of experimental animals were strictly followed. Wistar rats were purchased at least 1 wk prior to the experiment to fully adapt to the environment (23 ℃, 50% humidity, 12 h/12 h light/dark, ad libitum food and water), and they were starved, with access to only water, 12 h prior to AP modeling. Anesthesia was induced by intraperitoneal injection of pentobarbital sodium solution (40 mg/kg). The AP model was induced by retrograde injection of 3.5% sodium taurocholate solution (0.15 mL/100 g) into the biliopancreatic duct. The rats were sacrificed 24 h after the establishment of the AP model. The blood samples were centrifuged at 876 × g for 15 min at 4 ℃, and the upper serum was stored at –80 ℃. Some pancreatic tissues were washed with saline and frozen in liquid nitrogen for tissue protein extraction. The remaining pancreatic tissues were fixed in 4% paraformaldehyde and embedded in paraffin after 48 h for hematoxylin and eosin staining and immunohistochemical staining.
The pancreatic tissue samples of rats were processed for immunohistochemical analysis. Pancreatic tissue was fixed in 10% formalin solution. Then, tissue paraffin was embedded. The embedded tissue wax blocks were cut into 5–8 μm slices and attached to glass slides and baked in an oven at 67 ℃ for 48 h. The tissue sections were dewaxed and rehydrated. The endogenous peroxidase activity was blocked by adding methanol containing 3% H2O2 and soaked for 15 min, and antigen repair. The primary antibody was diluted with antibody diluent at a ratio of 1:100 and dropped on the slide at 4 ℃ overnight. Then, approximately 100 μL of the working solution of the secondary antibody was added and incubated for 15 min at 25 ℃. Finally, DAB color development, hematoxylin counterstained and dehydrated and transparent were performed.
Immunohistochemical analysis and imaging software were used to determine and analyze the range and intensity of immunohistochemical staining. When the number of positive cells was 0%, it was marked as 0; marked as 1 at less than 25%; marked as 2 at 25%–50%; marked as 3 at 50%–75%; and marked as 4 at 75%–100%. When the intensity of staining was weakly positive, it was marked as 1; marked as 2 at moderately positive; and marked as 3 at intensity positive. The final immune score was the product of the staining intensity value and the positive cell range value (range 0–12 points).
GraphPad Prism 8.0 software (Graph Pad Software, La Jolla, CA, United States) was used for statistical analysis, each experiment was repeated at least three times, and data were calculated as mean ± SD. Measurement data were analyzed by the student’s t test or one-way analysis of variance. Linear correlation was used to analyze the expression correlation between miR-125b-5p and IGF2. P < 0.05 was considered statistically significant.
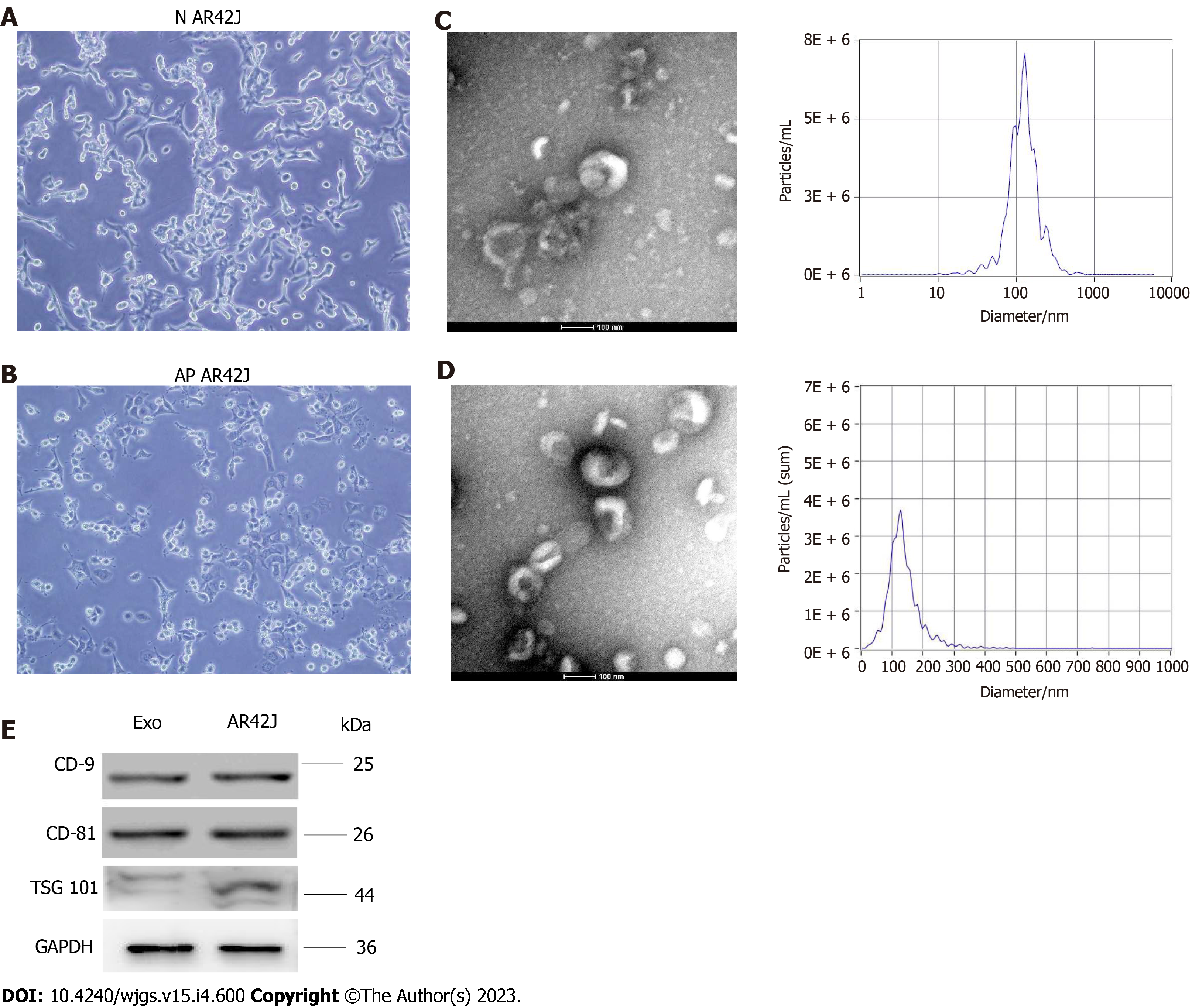
Exosomes were isolated and extracted from the supernatant of the AR42J cell line in non-activated and activated states, respectively (Figure 1A and B). Transmission electron microscopy was used to observe the exosomes in two different states, which both had a typical “dish” shape. Particle size analysis showed that the diameter of AR42J exosomes in the inactive state ranged from 68.3 to 181.9 nm, with an average of 121.4 nm, and the concentration of exosomes was 3.3 × 210 particles/mL. In the activated state, the diameter of AR42J exosomes ranged from 82.1 to 197.5 nm, with an average of 132.8 nm, and the concentration of exosomes was 6.23 × 210 particles/mL (Figure 1C and D). Western blotting confirmed the expression of exosome markers, including CD9, CD81, and TSG101 (Figure 1E).
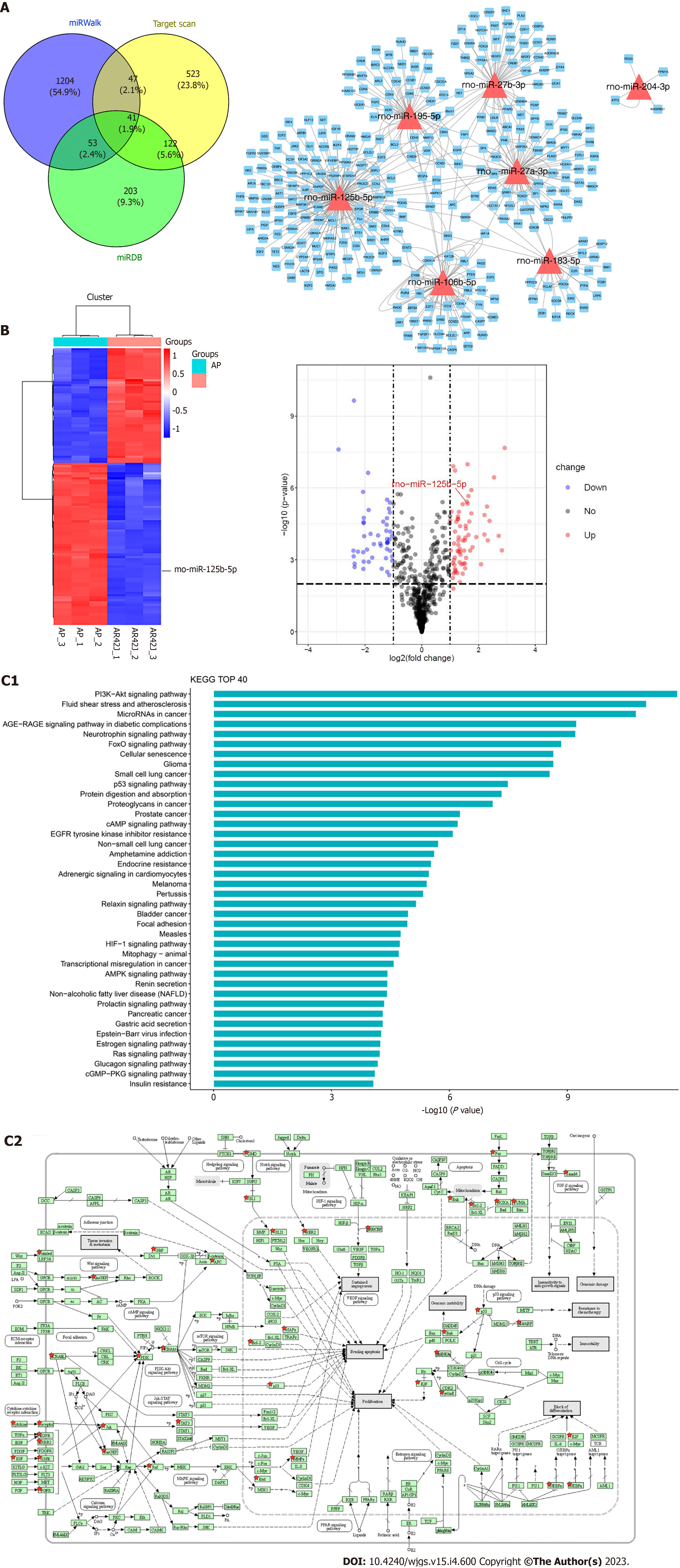
To further identify miRNAs differentially expressed in AR42J cell lines between activated and inactive states, RNA-seq was used for screening. Among them, miR-125b-5p, miR-27b-3p, miR-195-5p, miR-27a-3p, miR-106b-5p and miR-183-5p were all the differentially expressed miRNAs screened using RNA-seq. miRDB, miRWalk, and Targetscan data were used to predict their downstream binding target genes (Figure 2A). Further research revealed that miR-125b-5p was upregulated in the activated state and downregulated in the non-activated state (Figure 2B). KEGG pathway analysis showed that the differentially regulated RNAs mainly functioned in signal transduction. Furthermore, these results showed that the IGF2-PI3K/AKT signaling pathway may be the signaling pathway of miR-125b-5p which promotes the exacerbation of AP (Figure 2C).
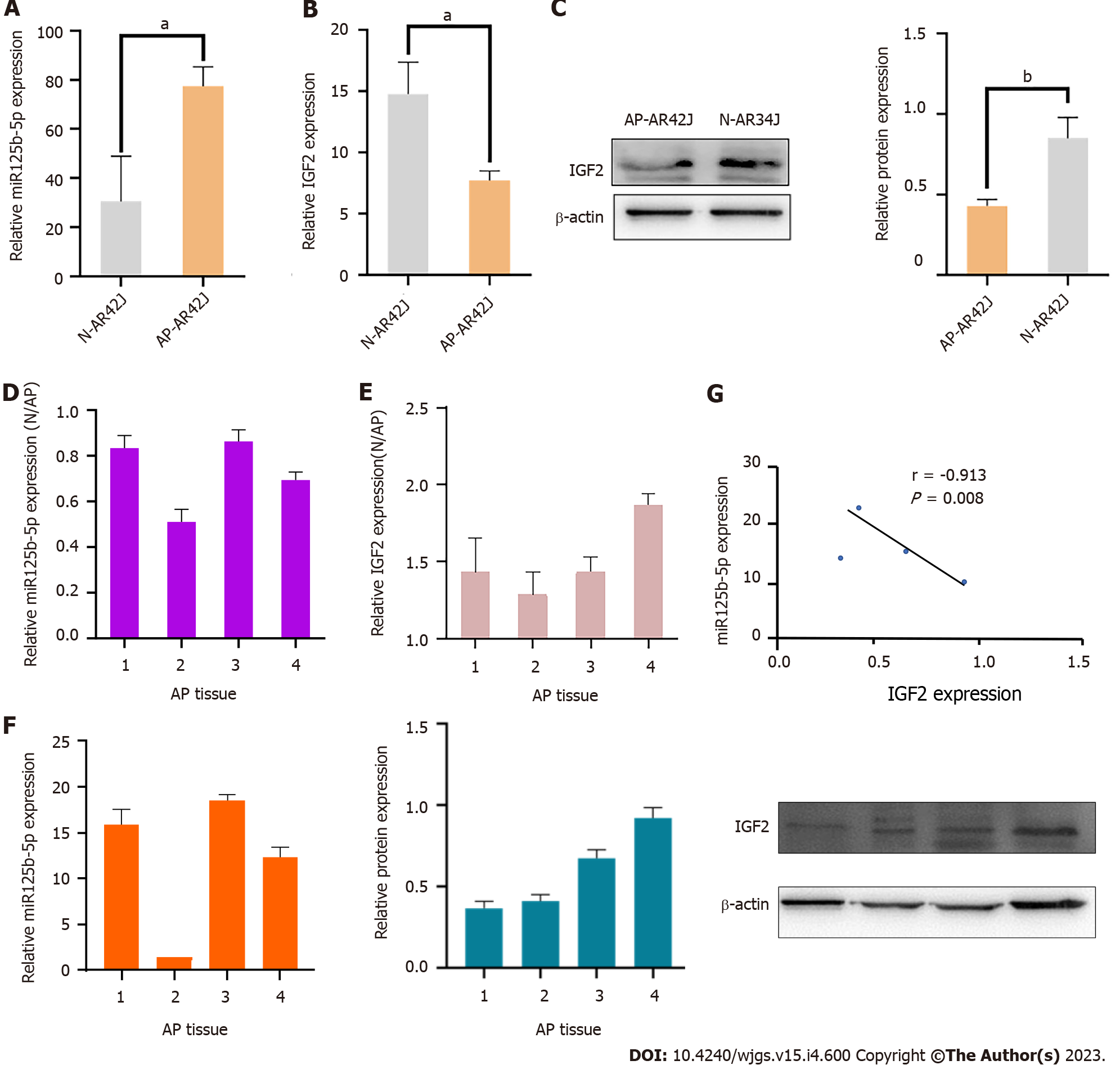
In order to determine whether miR-125b-5p is involved in the regulation of inflammatory progression of AP, the expression of miR-125b-5p and IGF2 in activated and inactivated AR42J cell lines was analyzed using RT-qPCR. Compared with the inactive AR42J cell line, miR-125b-5p expression was significantly increased in the activated AR42J cells, but IGF2 expression was significantly decreased (Figure 3A–C). Meanwhile, RT-qPCR was used to analyze the expression of miR-125b-5p and IGF2 of pancreatic tissue of normal and AP rat models. The experimental results showed that the expression of miR-125b-5p was increased in pancreatitis tissues, while that of IGF2 was decreased (Figure 3D and E). Statistical analysis showed that miR-125b-5p was negatively correlated with IGF2 protein expression (r = –0.913; P = 0.008) (Figure 3F and G). Consequently, above results revealed that miR-125b-5p was upregulated and IGF2 was downregulated in AP in vitro and in vivo experiments.
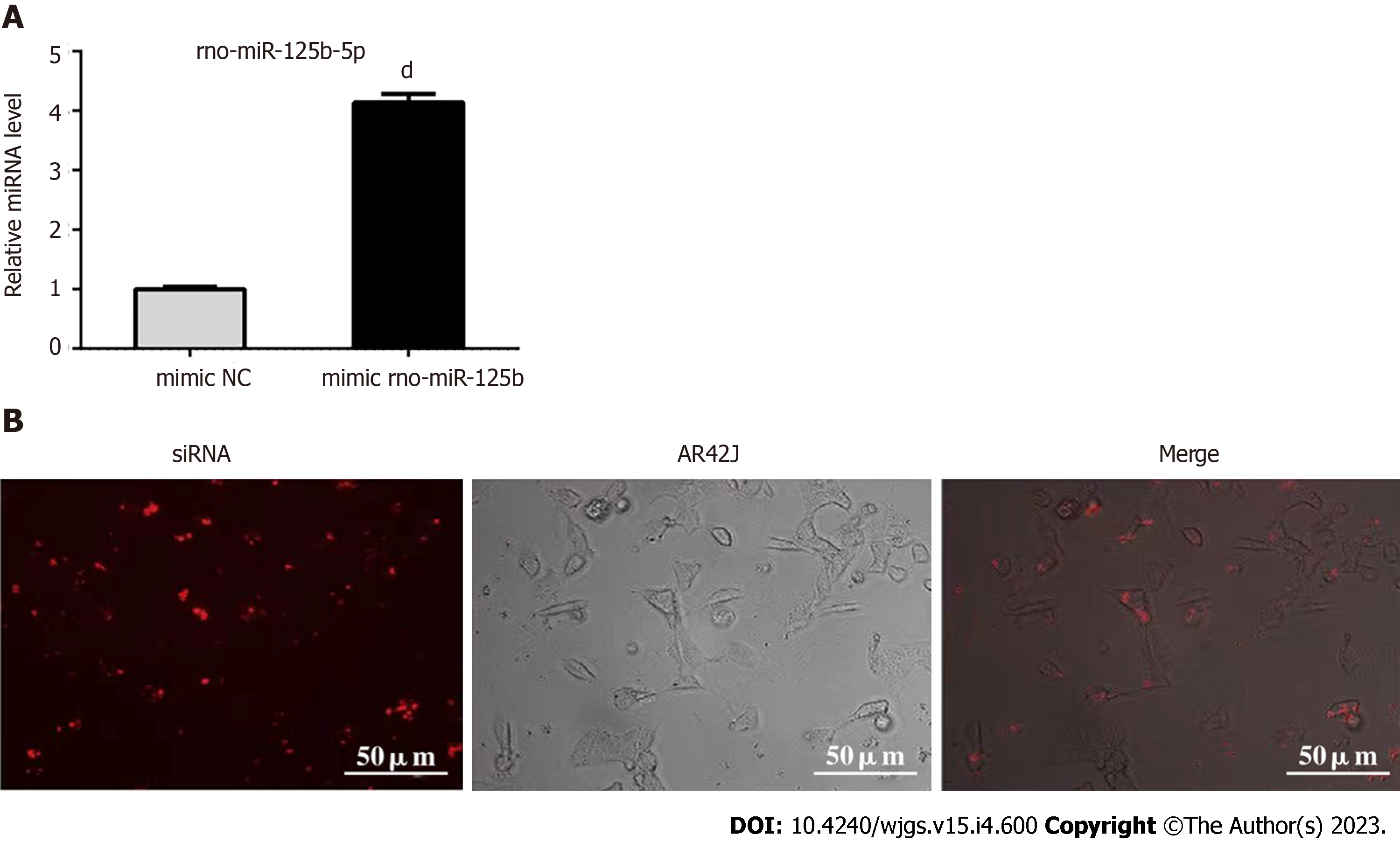
Exosomes transfected with miR-125-5p mimic and negative control miRNA (mimic NC) were added to AR42J cells in order to evaluate the endocytosis efficiency of exosomes transporting miR-125b-5p into AR42J cells. AR42J cells containing miR-125b-5p mimic were detected by RT-qPCR (Figure 4A). Compared with mimic NC, the expression of miR-125b-5p was significantly increased in AR42J cells treated with the miR-125b-5p mimic, indicating that the expression of miR-125b-5p was increased in AR42J cells and achieved through the exosomal transport of AR42J.
To further clarify whether the above changes in miR-125b-5p were achieved through exosomal transport, Texas red-labeled siRNA was transfected into exosomes as the positive control group of the experiment. After 24 h of cell culture, Texas-red labeled siRNA can be seen in the cytoplasm of AR42J cells under an immunofluorescence microscope (Figure 4B). Therefore, the above experiments confirmed that exosomes derived from the AR42J cell line could effectively deliver miR-125b-5p to AR42J cells in vitro.
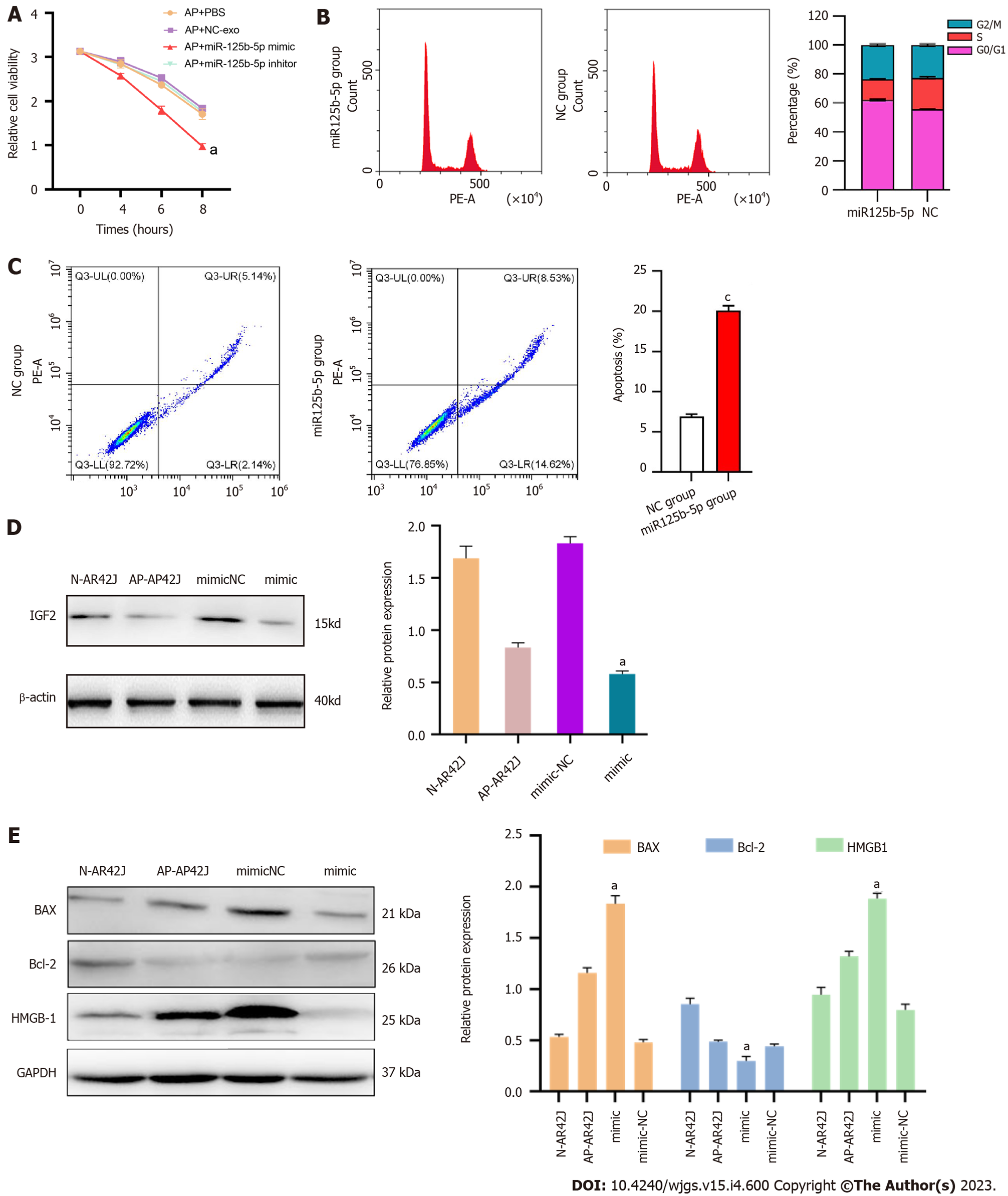
To further explore the function and effect of miR-125b-5p on the AP cell model, the CCK-8 assay was used to evaluate the effect of miR-125b-5p on the viability of AR42J cells in the activated state. The results showed that compared with the PBS group, the exosome group overexpressing miR-125b-5p promoted the necrosis of AR42J cells in the activated state. After 8 h, the cell absorbance value of exosome group overexpressing miR-125b-5p was significantly lower than that of PBS group (PBS group: 1.705 ± 0.120 vs miR-125b-5p mimic group: 0.975 ± 0.064, t = 7.59, P = 0.016) (Figure 5A).
The cell cycle and apoptosis were analyzed by flow cytometry, and the results showed that the proportion of G0/G1 in the overexpression miR-125b-5p group was higher than that in the NC group. In addition, the percentage of cells in G0/G1 phase of the cell cycle decreased from 60.37 ± 1.7% (miR-125b-5p overexpression group) to 51.25 ± 1.4% (NC group) (Figure 5B). Compared with the NC group, the apoptosis of AR42J cell line treated with miR-125b-5p overexpression group was significantly increased in the activated state (Figure 5C).
Meanwhile, western blot showed that overexpression of miR-125b-5p reduced the expression of IGF2 protein in activated AR42J cells (Figure 5D). The expression of apoptosis-related gene, BAX, increased, while the expression of apoptosis-related gene Bcl-2 decreased. In addition, the expression of the necrosis-related gene, HMGB-1, showed an upward trend (Figure 5E). Therefore, based on the above experiments, miR-125b-5p can promote the death of activated AR42J cells by inducing cell cycle arrest and apoptosis.
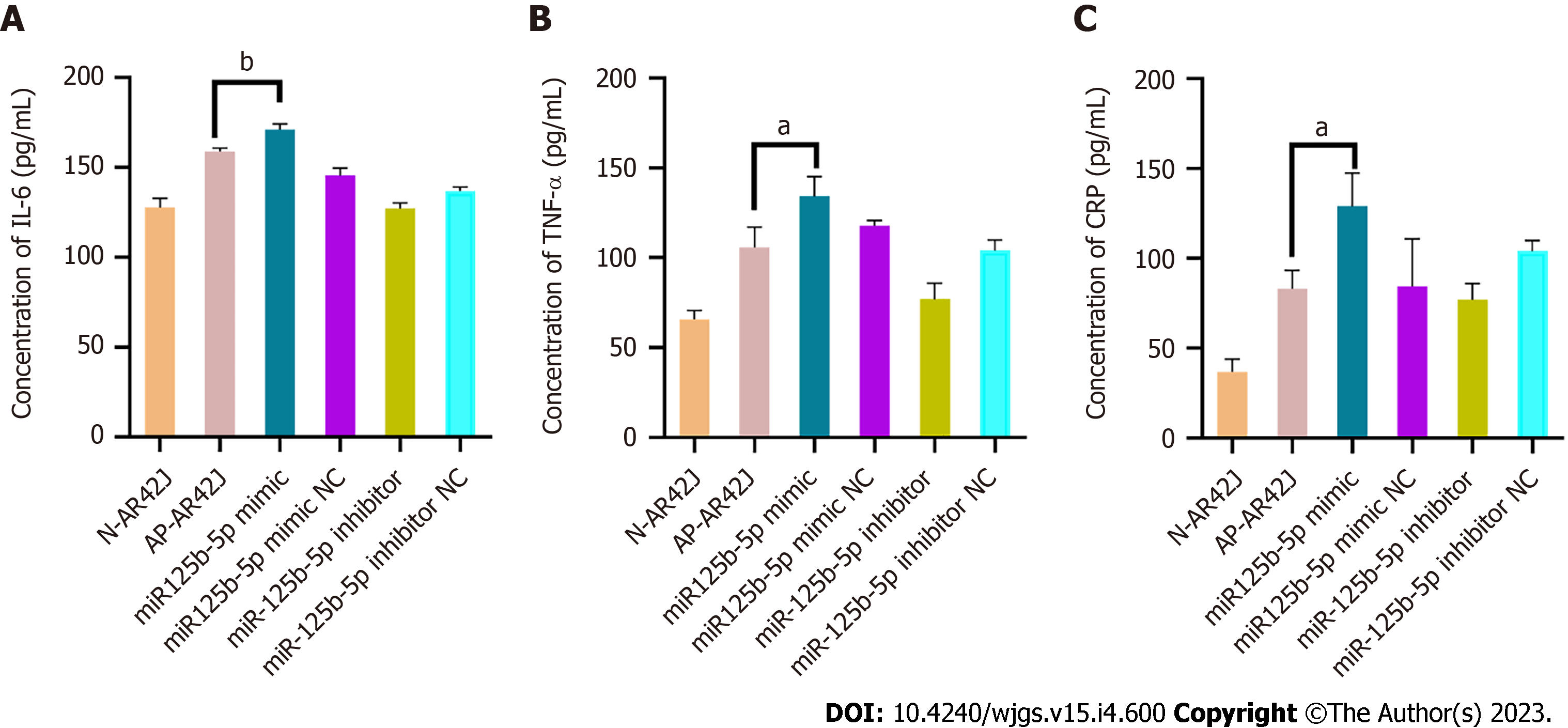
The corresponding ELISA kits were used to detect the inflammatory factors IL-6, TNF-α, and CRP in AR42J cells treated with overexpressing miR-125b-5p exosomes in the activated state. Compared with the AP group, the levels of IL-6 (158.86 ± 1.49 pg/mL vs 171.286 ± 2.36 pg/mL, P = 0.0076), TNF-α (105.86 ± 9.33 pg/mL vs 134.38 ± 8.98 pg/mL, P = 0.0106), and CRP (83.1 ± 8.32 pg/mL vs 129.04 ± 15.16 pg/mL, P = 0.024) in the miR-125b-5p overexpression group were increased, which confirmed that miR-125b-5p can promote cellular inflammatory injury (Figure 6).
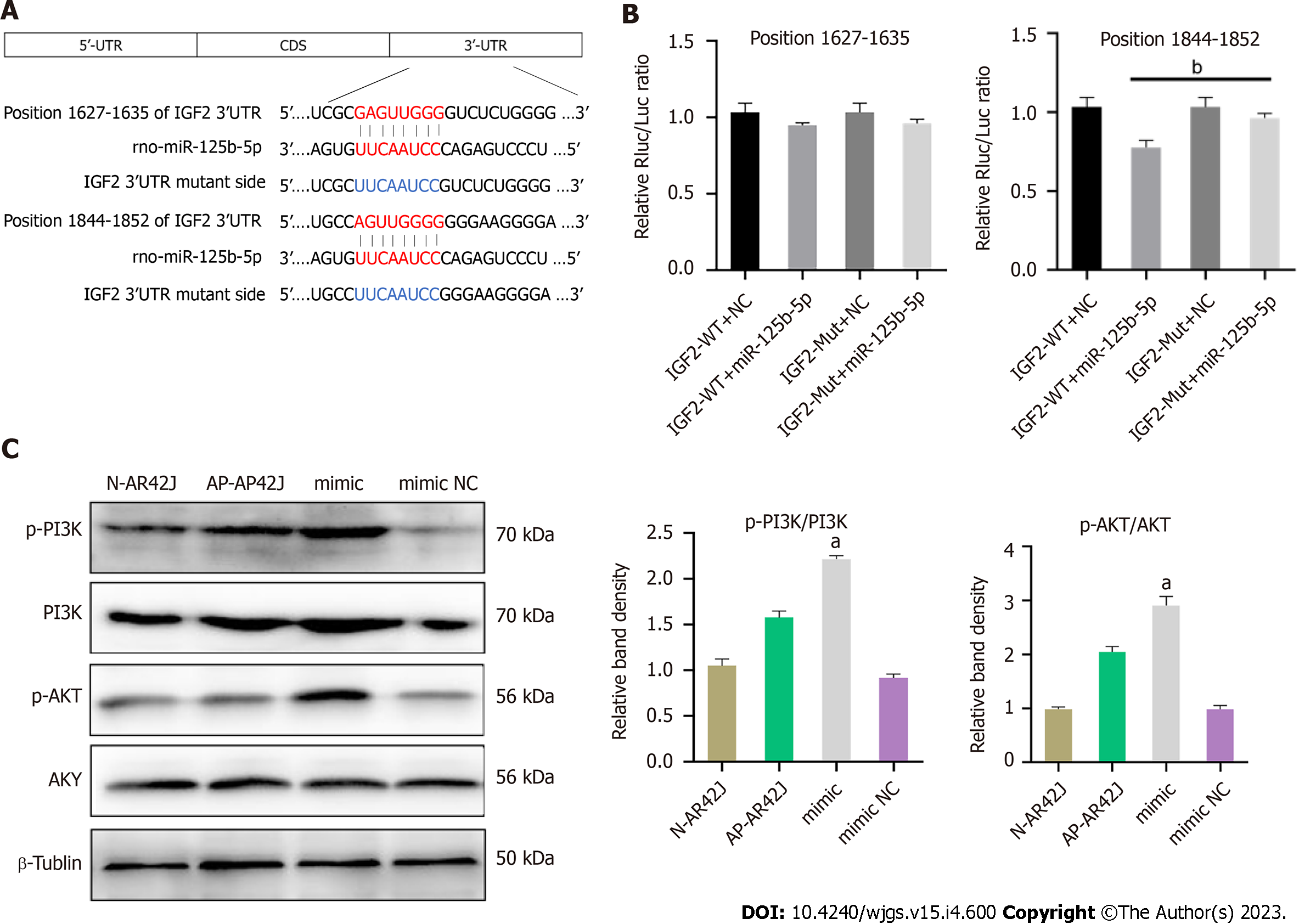
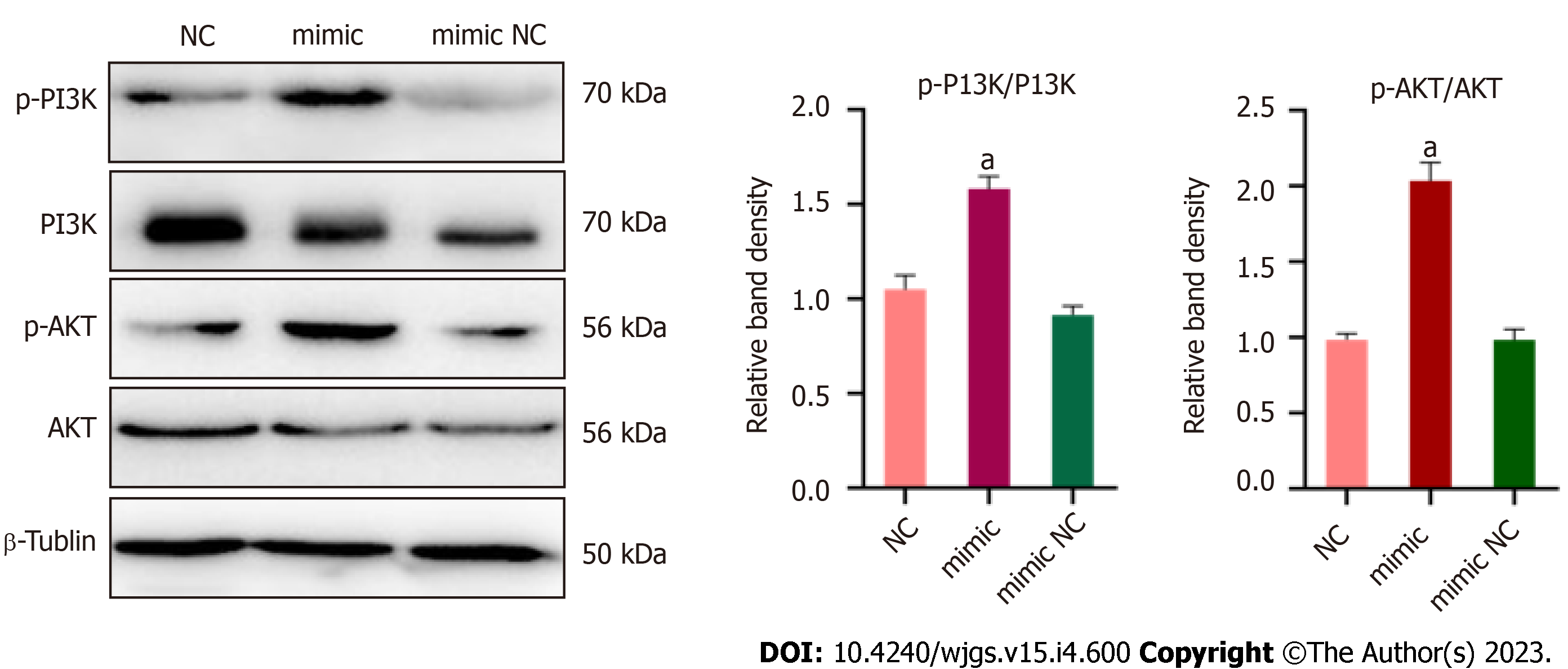
According to the analysis of miRDB, miRWalk, and Targetscan databases, IGF2 may be a potential target of miR-125b-5p. Among them, the 3¢-UTR of IGF2 had highly conserved binding sites at positions 1627–635 and 1844–1852 (Figure 7A). In addition, miRNAs were co-transfected by vectors containing wild-type (WT) or MUT IGF2 3¢-UTR fusion luciferase as well as miR-125b-5p mimics or negative controls. Dual luciferase assay showed that overexpression of miR-125b-5p could reduce WT luciferase activity in AR42J cells. However, the MUT IGF2 3¢-UTR completely restored luciferase activity (Figure 7B). Western blot analysis confirmed that compared with the AP group, the overexpression of miR125b-5p promoted the phosphorylation and activation of PI3K/AKT signaling pathway, thus promoting the aggravation of inflammatory responses (Figure 7C).
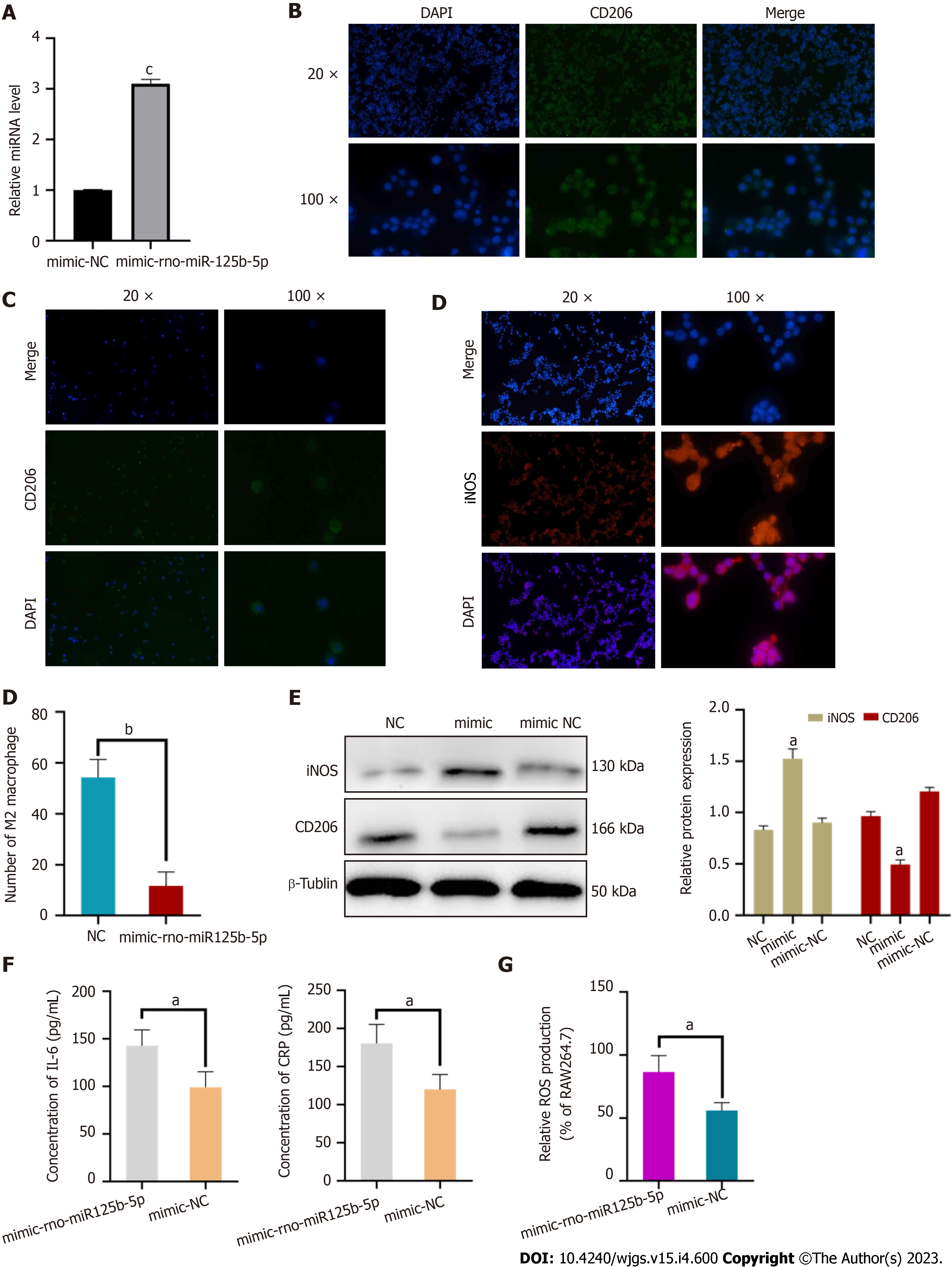
RAW264.7 cells containing miR-125b-5p mimic were detected by RT-qPCR. Compared with mimic NC, the expression of miR-125b-5p was significantly increased in RAW264.7 cells treated with miR-125b-5p mimic, which confirmed the increased expression of miR-125b-5p in RAW264.7 cells (Figure 8A).
In order to further explore the effect of miR-125b-5p on macrophage polarization, we added exosomes containing overexpressed miR-125b-5p to the RAW264.7 cell line stimulated by IL-4, and then determined the polarization of macrophages through in vitro experiments. Among them, the surface marker of M1 macrophages was iNOS, and the surface marker of M2 macrophage was CD206. It was found that overexpression of miR-125b-5p could inhibit M2 type polarization and promote M1 type polarization of macrophages compared with the control group [(11.67 ± 4.49) vs (54.33 ± 5.73), t = 8.280, P = 0.0012)] (Figure 8B–D).
Western blots showed that compared with the NC group, the expression of iNOS was upregulated and the expression of CD206 was downregulated in the overexpression group, which further confirmed that miR-125b-5p could promote M1 polarization and inhibit M2 polarization of macrophages (Figure 8E).
ELISA kits were used to detect IL-6 and CRP inflammatory factors in the RAW264.7 cell line which was treated with overexpressed miR-125b-5p exosomes. Compared with the mimic-NC group, the level of IL-6 (99.106 ± 13.29 pg/mL vs 142.778 ± 13.58 pg/mL, P = 0.0314) and CRP (120.181 ± 20.41 pg/mL vs 180.557 ± 15.98 pg/mL, P = 0.0301) were increased in the miR-125b-5p overexpression group, which confirmed that miR-125b-5p could promote the release of inflammatory factors from macrophages (Figure 8F). Meanwhile, compared with the mimic-NC group, overexpression of miR-125b-5p exosomes can promote the ROS production in the RAW264.7 cell line (55.964 ± 5.03 vs 86.375 ± 10.76, P = 0.0224), thereby improving the level of cellular oxidative stress (Figure 8G).
The experiments were divided into three group, which included NC group (RAW264.7 cell line treated with IL-4 for 24 h), mimic group (exosomes overexpressing miR-125b-5p were added to RAW264.7 cell line treated with IL-4) and mimic-NC group (mimic-NC exosomes were added to RAW264.7 cell line treated with IL-4). It was found that compared with the NC group, overexpression of miR-125b-5p could activate the PI3K/AKT signaling pathway, which further aggravated the inflammatory response (Figure 9).
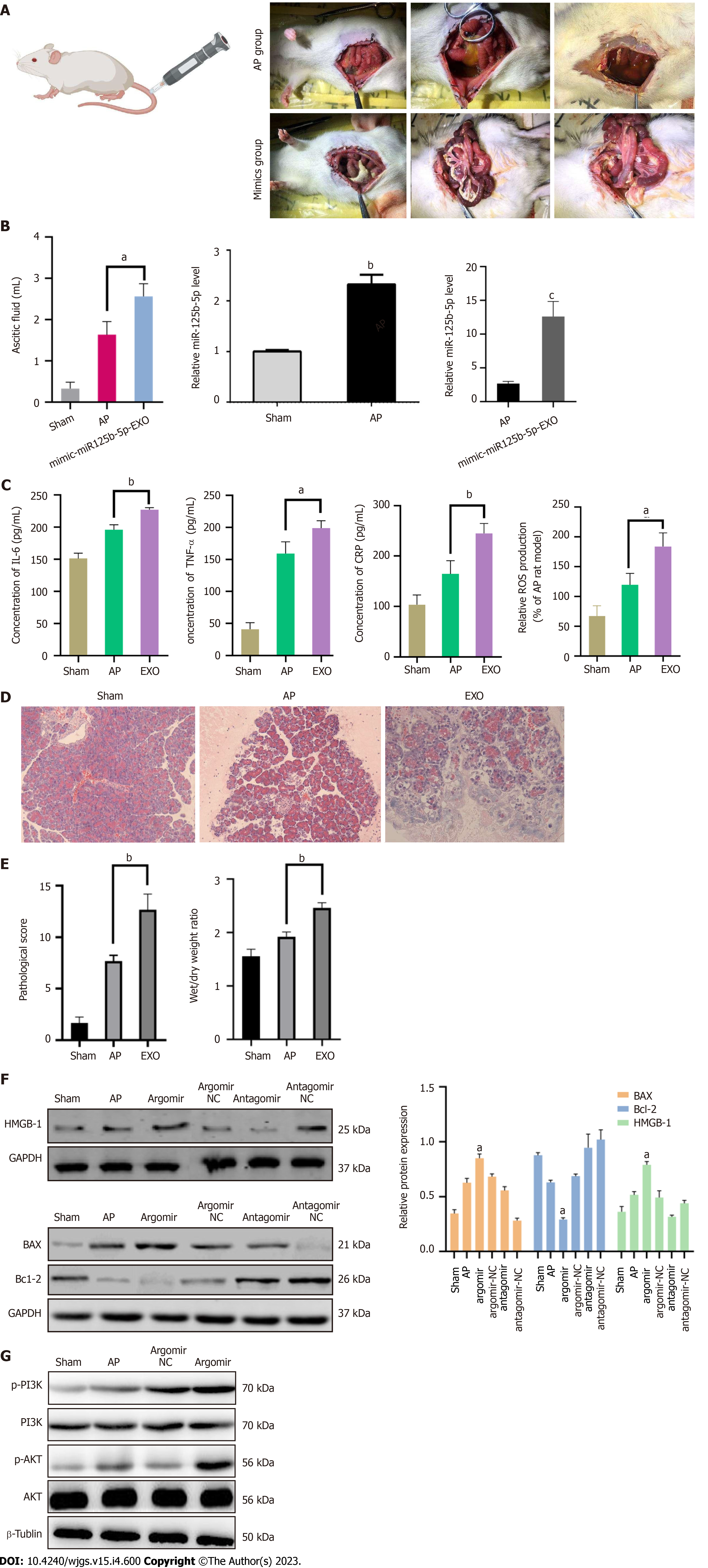
To further investigate whether miR-125b-5p could promote the aggravation of AP in vivo, the AP model was induced by retrograde and slow injection of 3.5% sodium taucholate solution (0.15 mL/100 g) into the biliopanatic duct. The experiment was divided into sham group, AP group, and miR-125b-5p overexpression group (EXO group), with 5 rats in each group. In the sham group, the rats were operated on the abdomen without other drugs. In the AP group, normal saline was injected into Wistar rats via the tail vein. In the overexpression group, exosomes overexpressing miR-125b-5p were injected into Wistar rat via the tail vein. After 24 h, the rats were sacrificed, and the changes of inflammatory indices in the three groups were observed. The results showed that compared with the AP group, calcium foci were observed in the abdominal cavity of rats in the overexpression group, and the amount of ascites was significantly increased (1.63 ± 0.261 mL vs 2.56 ± 0.249 mL, P = 0.0131). Meanwhile, the expression of miR-125b-5p increased in AP and EXO group (Figure 10A and B). The levels of IL-6, TNF-α, CRP, and ROS in the serum of the three group were detected by ELISA. Compared with the AP group, the levels of IL-6 (195.86 ± 6.28 pg/mL vs 227.14 ± 2.54 pg/mL, P = 0.0033), TNF-α (159.19 ± 15.09 pg/mL vs 198.81 ± 9.35 pg/mL, P = 0.0301), CRP (164.52 ± 21.51 pg/mL vs 245.14 ± 15.83 pg/mL, P = 0.0095) inflammatory factors, and ROS (119.69 ± 15.59 vs 184 ± 18.83, P = 0.0181) were increased in the miR-125b-5p overexpression group (Figure 10C). Histopathology confirmed that compared with the AP group, the degree of pancreatic tissue edema and necrosis was severe in the miR-125b-5p overexpression group. The pathological score and dry/wet ratio of pancreatic tissue were 12.6 ± 1.2 (P = 0.0021) and 2.46 ± 0.07 (P = 0.0019), respectively (Figure 10D and E).
Western blot analysis showed that, compared with the AP group, the expression of BAX and HMGB-1 was increased, while the expression of Bcl-2 was decreased in the miR-125b-5p overexpression group (Figure 10F). In addition, the expression of IGF2 protein in the miR-125b-5p overexpression group was decreased, and the activation of PI3K/AKT signaling pathway was promoted (Figure 10G). Therefore, the above evidence indicates that miR-125b-5p can promote the aggravation of AP in vivo.
AP is a common digestive system disease, which seriously affects the short and long-term life quality and prognosis of patients[13]. Therefore, it is necessary to understand the pathogenesis of AP in order to finding the best treatment strategy. In the pathogenesis of AP, the inflammatory cascade caused by acinar cell injury and immune system activation is an important factor for the occurrence and progression of the disease. In recent years, exosomes, immune cells, and immune microenvironment changes are hot spots in the research direction of inflammatory immunity. In addition to participating in the regulation of AP acinar cell injury, exosomes may play a regulatory role in macrophage polarization[14]. However, this mechanism in AP is still unclear; furthermore, there is lack of research on pancreatic resident macrophages[15]. Therefore, the inflammation caused by AP acinar cell injury is combined with the imbalance of the immune microenvironment to explain the potential pathogenic mechanism of AP evolution from the perspective of inflammatory immunity, which makes up for the limitation of existing research that only explores from a single perspective. This study establishes the connection between immune cells and pancreatic acinar cells, and reveals the characteristics of the pathogenesis from the perspective of immune cell and acinar cell interaction, which can better elucidate the molecular mechanism of miR-125b-5p promoting AP progression.
In recent years, exosomes have been proven to be an important biological information transmitter, participating in intercellular communication by transporting their internal active substances. Studies have shown that exosomes are closely related to the development of various inflammatory disease and are widely involved in the pathophysiological process of disease[16]. Some studies have also found that exosomes play a significant role in the process of macrophage activation, and the genetic material carried in them can play a biological regulatory role in macrophage activation[14]. In addition, macrophage activation also affects the development of AP to a certain extent, and its activation degree is also considered to be closely related to the severity of AP and the occurrence of local complications[15]. Some studies have found that plasma derived exosomes can effectively reach alveolar macrophages and promote the M1 type polarization of macrophages during AP, secrete a large amount of IL-1β, IL-6, CCL-2 and can also trigger NOD-like receptor protein 3-dependent pyrophosphorylation, release inflammatory factors, and jointly induce the occurrence of ALI[6,17]. However, exosomes derived from different cells may play different regulatory roles on inflammatory response. Studies have shown that exosomes derived from bone marrow mesenchymal stem cells promote the anti-inflammatory phenotype M1 polarization of macrophages by negatively regulating CysLT2R, thus achieving the effect of reducing brain injury[18]. Therefore, in order to exclude the influence of exosomes from different sources in the inflammatory response, this study selected exosomes from rat pancreatic exocrine cell line (AR42J cell line) and used their homology to better explain the influence of exosomes derived from pancreatic cells on the course of AP. In order to further explore how exosomes cause inflammatory injury, some scholars have found that in the rat model of AP, the expression of miR-155 in plasma-derived exosomes is up-regulated, while the expression of miR-21 and miR-122 is down regulated, which can activate pancreatic and alveolar macrophages M1-type polarization to release inflammatory factors that promote the progression of AP[7]. Therefore, we believe that miRNAs in exosomes may act as intercellular communication mediators and play a regulatory role in local pancreatic inflammatory injury, macrophage activation and extra-pancreatic organ injury by acting on their downstream signaling pathways. In the future, differentially expressed miRNAs will be further applied to the exploration of AP treatment. Our previous studies have confirmed that exosomes derived from AR42J cells in the non-activated state can reduce the level of apoptosis and oxidative stress of acinar cells, reduce the level of inflammation, and reduce pancreatic tissue damage during the AP[12]. The mechanism may be related to the inhibition of downstream MAPK and nuclear factor-kappaB signaling pathways, so as to achieve an anti-inflammatory purpose. In this study, we found that exosomes derived from AR42J in the activated state can promote inflammatory injury of acinar cells and aggravate AP progression[12]. RNA-seq results showed that miR-125b-5p was a differentially expressed miRNA in AR42J cell lines between the two different states. RT-qPCR analysis confirmed that the expression of miR-125b-5p was significantly increased in the activated AR42J cell line and AP tissues. In addition, studies have shown that overexpression of miR-125b-5p can cause cell cycle arrest and apoptosis, thus promoting the death of AR42J cells in the activated state. CCK-8 results showed that overexpression of miR-125b-5p could inhibit the proliferation of AR42J cells. The experimental results showed that the cell absorbance value of the exosome group which overexpressed miR-125b-5p was significantly lower than that of the PBS group after 8 h of treatment (1.705 ± 0.120 vs 0.975 ± 0.064, t = 7.590, P = 0.016). Cell cycle results showed that G0/G1 ratio in miR-125b-5p overexpression group was higher than that of NC group. The percentage of cells in G0/G1 phase decreased from 60.37 ± 1.7% (miR-125b-5p overexpression group) to 51.25 ± 1.4% (NC group). These results showed that the apoptosis of AR42J cells in the activated state was significantly increased in the miR-125b-5p overexpression group. ELISA tests confirmed that overexpression of miR-125b-5p could aggravate cellular inflammatory injury, and the results showed that the level of IL-6, TNF-α and CRP in the overexpression group were higher than those in the control group (IL-6: 158.86 ± 1.49 pg/mL vs 171.286 ± 2.36 pg/mL, P = 0.0076; TNF-α: 105.86 ± 9.33 pg/mL vs 134.38 ± 8.98 pg/mL, P = 0.0106; CRP: 83.1 ± 8.32 pg/mL vs 129.04 ± 15.16 pg/mL, P = 0.024).
The pathogenesis of AP initiates the innate immune system of the body and plays an important role in promoting the evolution of its disease course, of which the monocyte/macrophage system is the most important effector cell[19-21]. Monocytes originate from bone marrow stem cells, reach various tissues of the body with blood, and differentiate into macrophages of different tissues. Macrophages are important components of the innate immune system of the body. They have the function of antigen presentation and secretion of various cytokines, play an important role in the pathophysiological process such as inflammation and metabolism, and are also the key factors for the body to maintain its own stability. Macrophages are highly heterogeneous, plastic and diverse, and polarize into two activation states with different phenotypes and biological function depending on their microenvironments and exogenous stimuli. When stimulated by interferon-αand lipopolysaccharide, macrophages often undergo M1 type polarization and play a proinflammatory function. When stimulated by IL-4, macrophages often undergo M2 polarization, thus exerting anti-inflammatory and injury repair functions[22]. It has been found that macrophages are mainly M1 polarized in the course of AP, and play an important role in the continuous development and amplification of the inflammatory response. It is an important determinant of the severity of AP and an effective therapeutic target to control inflammatory damage[19,23]. At the early stage of AP, necrotic acinar cells release a large number of damage-associated molecular patterns to induce monocytes in the blood to migrate widely, recruit them into pancreatic tissues and become macrophages. Under the stimulation of various factors, M1 polarization occurs, releasing a large number of proinflammatory factors and inflammatory mediators, thus forming the interaction between acinar cells and macrophages in the pancreas. In this study, we found that overexpression of miR-125b-5p can inhibit M2 type polarization of macrophages and promote M1 type polarization, and trigger inflammatory cascade amplification effect by releasing inflammatory factors, thus leading to the progression of AP. The results of cell fluorescence test showed that the number of M2 macrophages in miR-125b-5p overexpression group was less than that in the control group [(54.33 ± 5.73) vs (11.67 ± 4.49), t = 8.280, P = 0.0012)]. Among them, the expression of iNOS protein, the surface marker of M1 macrophages, was increased. While the expression of CD206 protein, the surface marker of M2 macrophages, was decreased. In addition, the experimental results also found that miR-125b-5p can promote macrophages to secrete inflammatory factors such as IL-6 and CRP, and aggravate inflammatory responses (IL-6: 99.106 ± 13.29 pg/mL vs 142.778 ± 13.58 pg/mL, P = 0.0314; CRP: 120.181 ± 20.41 pg/mL vs 180.557 ± 15.98 pg/mL, P = 0.0301).
Bioinformatics analysis and dual luciferase assays confirmed that IGF2 is a target gene of miR-125b-5p, which inhibits M2 polarization of macrophages through PI3K/AKT signaling pathway, thus promoting the exacerbation of AP. In vitro experiments confirmed that overexpression of miR-125b-5p could reduce the expression of IGF2 in activated AR42J cell line, and could also cause the upregulation of apoptosis-related genes, BAX, and necrosis-related genes, HMGB-1, and the downregulation of Bcl-2. In addition, we also found that miR-125b-5p can promote the phosphorylation of PI3K/AKT signal pathway in the activated AR42J cell line and RAW264.7 cell line, thus activating this pathway to promote the exacerbation of inflammatory response. In vivo experiments found that the degree of abdominal inflammation was more obvious in rats of the overexpression group, with a large number of calcium foci and ascites, accompanied by pancreatic tissue edema and necrosis, which further confirmed that miR-125b-5p could promote the aggravation of AP in vivo.
Although this study revealed, to a certain extent, that the inflammatory cascade response caused by acinar cells injury and immune system activation is an important factor in the occurrence and progression of the disease, there are still some limitations that need to be further explored in the future. First, although this study confirmed that miR-125b-5p can inhibit M2 polarization of macrophages and promote M1 polarization, and thus trigger the massive release of inflammatory factors, thereby aggravating the inflammatory response, the results of this study were only obtained through in vitro experiments, and were not verified in vivo experiments. The results of macrophage polarization of pancreatic tissue in animal models were lacking. Therefore, subsequent experiments need to carry out fluorescent staining and western blotting on pancreatic tissue of rat AP models in the miR-125b-5p overexpression group to determine the recruitment and polarization of macrophages in the pancreatic tissue. In addition, it is also necessary to flow separate macrophages in serum to determine the change of M1/M2 ratio, to further clarify the effect of miR-125b-5p on macrophage polarization. Second, this study only explored the molecular mechanism of AP aggravation from the perspective of miR-125b-5p overexpression, and did not further verify whether silencing miR-125b-5p could reduce the severity of AP. Therefore, it is necessary to further confirm the role of miR-125b-5p in the course of AP through in vivo and in vitro experiments.
Based on the above experimental results, it was confirmed that miR-125b-5p can inhibit M2 polarization of macrophages and promote M1 polarization by regulating the IGF2 expression, thus aggravating the inflammatory response of AP. Among them, PI3K/AKT signaling pathway may be one of the important mechanisms leading to the progression of AP. Therefore, it has potential clinical value to control the pathogenesis of excessive inflammatory responses in AP by inhibiting the inflammatory cascade between acinar cells and macrophages mediated by miR-125b-5p in the future, which can effectively reduce inflammatory damage and improve the prognosis of AP.
Acute pancreatitis (AP) is a common clinical inflammatory disease of the digestive system, with an increasing trend worldwide, which is a pathophysiological process with complex etiology. At present, there are no consistent and effective therapies for treatment of AP, resulting in a high mortality rate.
miR-125b-5p, a bidirectional regulatory miRNA, is speculated to exhibit anti-tumor activity. However, exosome-derived miR-125b-5p in AP has not been reported.
We aimed to elucidate the molecular mechanism of exosome-derived miR-125b-5p promoting AP exacerbation from the perspective of the interaction between immune cells and acinar cells.
RNA-seq technology was used to screen differentially expressed miRNAs in AR42J cell lines, and bioinformatics analysis was used to predict downstream target genes of miR-125b-5p. The expression level of miR-125b-5p and insulin-like growth factor 2 (IGF2) in the activated AR42J cell line and AP pancreatic tissue were detected by quantitative real-time polymerase chain reaction and western blots. The changes in the pancreatic inflammatory response in a rat AP model were detected by histopathological methods. Western Blot was used to detect the expression of IGF2, PI3K/AKT signaling pathway proteins, and apoptosis and necrosis related proteins.
miR-125b-5p expression was upregulated in the activated AR42J cell line and AP pancreatic tissue, while that of IGF2 was downregulated. In addition, miR-125b-5p was found to act on macrophages to promote M1 type polarization and inhibit M2 type polarization, resulting in a massive release of inflammatory factors and reactive oxygen species accumulation. Further research found that miR-125b-5p could inhibit the expression of IGF2 in the PI3K/AKT signaling pathway. In vivo experiments revealed that miR-125b-5p can promote the progression of AP in a rat model.
miR-125b-5p acts on IGF2 in the PI3K/AKT signaling pathway and promotes M1 type polarization and inhibits M2 type polarization of macrophage by inhibiting IGF2 expression, resulting in a large release of pro-inflammatory factors and an inflammatory cascade amplification effect, thus aggravating AP.
It has potential clinical value to control the pathogenesis of excessive inflammatory responses in AP by inhibiting the inflammatory cascade between acinar cells and macrophages mediated by miR-125b-5p in the future, which can effectively reduce inflammatory damage and improve the prognosis of AP.
| 1. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 607] [Article Influence: 121.4] [Reference Citation Analysis (1)] |
| 2. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 583] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 3. | Zheng Z, Ding YX, Qu YX, Cao F, Li F. A narrative review of acute pancreatitis and its diagnosis, pathogenetic mechanism, and management. Ann Transl Med. 2021;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 4. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 583] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 5. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 7486] [Article Influence: 1247.7] [Reference Citation Analysis (4)] |
| 6. | Bonjoch L, Casas V, Carrascal M, Closa D. Involvement of exosomes in lung inflammation associated with experimental acute pancreatitis. J Pathol. 2016;240:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Jiménez-Alesanco A, Marcuello M, Pastor-Jiménez M, López-Puerto L, Bonjoch L, Gironella M, Carrascal M, Abian J, de-Madaria E, Closa D. Acute pancreatitis promotes the generation of two different exosome populations. Sci Rep. 2019;9:19887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Mohapatra S, Pioppini C, Ozpolat B, Calin GA. Non-coding RNAs regulation of macrophage polarization in cancer. Mol Cancer. 2021;20:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 9. | Zeybek A, Öz N, Kalemci S, Edgünlü T, Kızıltuğ MT, Tosun K, Tunç M, Tekin L, Erdal ME. Diagnostic Value of MiR-125b as a Potential Biomarker for Stage I Lung Adenocarcinoma. Curr Mol Med. 2019;19:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Liu S, Chen Q, Wang Y. MiR-125b-5p suppresses the bladder cancer progression via targeting HK2 and suppressing PI3K/AKT pathway. Hum Cell. 2020;33:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, Wang KK, Shen H, Zhang GG, Bai YP. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163-6177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 432] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 12. | Guo Y, Cao F, Ding Y, Lu J, Liu S, Li F. Acinar Cells Derived Exosomes Alleviate the Severity of Acute Pancreatitis. Discov Med. 2021;31:95-105. [PubMed] |
| 13. | Gardner TB. Acute Pancreatitis. Ann Intern Med. 2021;174:ITC17-ITC32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 14. | Zhao Y, Wang H, Lu M, Qiao X, Sun B, Zhang W, Xue D. Pancreatic Acinar Cells Employ miRNAs as Mediators of Intercellular Communication to Participate in the Regulation of Pancreatitis-Associated Macrophage Activation. Mediators Inflamm. 2016;2016:6340457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Hu F, Lou N, Jiao J, Guo F, Xiang H, Shang D. Macrophages in pancreatitis: Mechanisms and therapeutic potential. Biomed Pharmacother. 2020;131:110693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Dai YD, Dias P. Exosomes or Microvesicles, a Secreted Subcellular Organelle Contributing to Inflammation and Diabetes. Diabetes. 2018;67:2154-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Wu XB, Sun HY, Luo ZL, Cheng L, Duan XM, Ren JD. Plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Zhao Y, Gan Y, Xu G, Hua K, Liu D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020;260:118403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 19. | Vrolyk V, Schneberger D, Le K, Wobeser BK, Singh B. Mouse model to study pulmonary intravascular macrophage recruitment and lung inflammation in acute necrotizing pancreatitis. Cell Tissue Res. 2019;378:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Zhao Q, Wei Y, Pandol SJ, Li L, Habtezion A. STING Signaling Promotes Inflammation in Experimental Acute Pancreatitis. Gastroenterology. 2018;154:1822-1835.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 21. | Pan LL, Deng YY, Wang R, Wu C, Li J, Niu W, Yang Q, Bhatia M, Gudmundsson GH, Agerberth B, Diana J, Sun J. Lactose Induces Phenotypic and Functional Changes of Neutrophils and Macrophages to Alleviate Acute Pancreatitis in Mice. Front Immunol. 2018;9:751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Xu X, Xu J, Wu J, Hu Y, Han Y, Gu Y, Zhao K, Zhang Q, Liu X, Liu J, Liu B, Cao X. Phosphorylation-Mediated IFN-γR2 Membrane Translocation Is Required to Activate Macrophage Innate Response. Cell. 2018;175:1336-1351.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Han X, Ni J, Wu Z, Wu J, Li B, Ye X, Dai J, Chen C, Xue J, Wan R, Wen L, Wang X, Hu G. Myeloid-specific dopamine D(2) receptor signalling controls inflammation in acute pancreatitis via inhibiting M1 macrophage. Br J Pharmacol. 2020;177:2991-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tian L, China; Uhlmann D, Germany S-Editor: Fan JR L-Editor: A P-Editor: Wu RR