Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.480
Peer-review started: November 22, 2022
First decision: January 11, 2023
Revised: January 31, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 27, 2023
Processing time: 125 Days and 1.1 Hours
Peutz-Jeghers syndrome (PJS) is a rare autosomal dominant disorder, and female patients may develop gynecologic tumours. The prognosis for such patients is poor and the specific pathogenesis remains uncertain. Therefore, there are currently no uniform treatment options.
Herein, we introduce the case of a 45-year-old female who was diagnosed with PJS for 45 years and cervical cancer for 3 years. Postoperative pathological examination showed metastases in the right external iliac lymph nodes. The patient was initially treated with a combination of doxorubicin and carboplatin chemotherapy and pelvic magnetic resonance showed that the metastases had grown. Subsequently, we performed whole exome sequencing in this patient and identified the relevant causative gene. In addition to the chemotherapy regimen, sindilizumab was administered and the patient was followed up. After 4 cycles of treatment, the metastases were substantially reduced and were not enlarged after six months of follow-up. This case report suggests that patients with PJS combined with cervical cancer may have a sustained response to immune-combination chemotherapy regimens.
Clinicians should be aware of the importance of immunotherapy in patients with PJS combined with advanced cervical cancer.
Core Tip: Peutz-Jeghers syndrome (PJS) is a rare genetic disease with cancerous potential. In this case, the patient was diagnosed with PJS combined with progressive cervical cancer and she initially received doxorubicin and carboplatin; however, the right parietal iliac vessel metastases did not shrink. This case suggests that the use of programmed cell death protein 1 (PD-1) inhibitors was helpful in this patient and that PD-1 inhibitors combined with chemotherapy may be a good choice for treating this disease.
- Citation: Hu XC, Gan CX, Zheng HM, Wu XP, Pan WS. Immunotherapy in combination with chemotherapy for Peutz-Jeghers syndrome with advanced cervical cancer: A case report. World J Gastrointest Surg 2023; 15(3): 480-487
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/480.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.480
Peutz-Jeghers syndrome (PJS) is a rare autosomal dominant-inherited disorder. The incidence of this syndrome ranges from 1 in 25000 to 1 in 28000 people per year[1]. Current research indicates that this disease is caused by a mutation of the STK11 gene on chromosome 19p13.3(1). Patients with PJS are characterized by dark spots on the skin mucosa and hamartomatous polyps in the digestive tract[2]. At the same time, studies have indicated a higher incidence of malignancy in patients with PJS than in the general population. The cumulative incidence of gastrointestinal cancers is 55%, with colorectal cancer at 39%, pancreatic cancer at 36%-40%, and small bowel cancer at 13%[3,4]. The risk of cancers of the nongastrointestinal tract is also increased, with a cumulative incidence of 32%-54% for breast cancer, 21% for ovarian cancer, and 7% for lung cancer at age 60[5-8]. Cisplatin has been the standard chemotherapy for cervical cancer with distant metastases, and recent evidence supports the use of platinum-based dual therapy rather than cisplatin alone[9-11]. However, in patients with PJS combined with uterine malignancy, the pathogenesis may be more complex and unclear, and there is no standard treatment protocol. We report a patient with PJS combined with progressive cervical cancer. A regimen using a programmed cell death protein 1 (PD-1) inhibitor in combination with chemotherapy may be a good option for treating this disease.
A 45-year-old Chinese female was admitted to the hospital with mucous membrane black spots on the lips, intermittent abdominal pain for more than forty years and recurrent cervical cancer for three years.
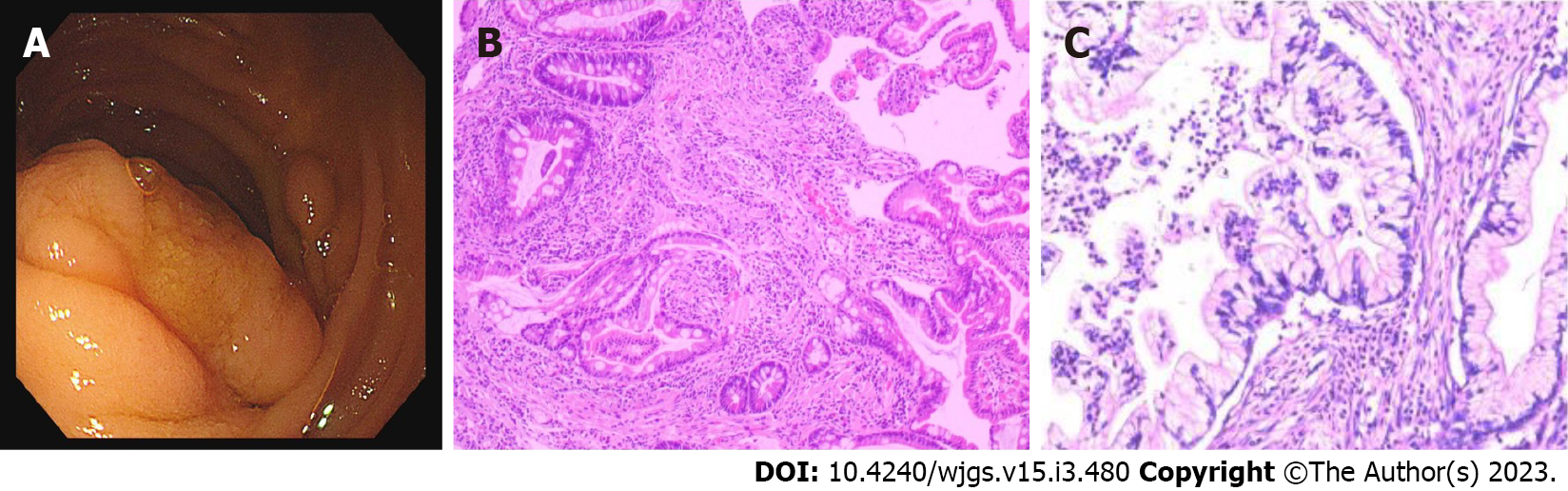
Forty years prior, the patient visited several hospitals for lip pigmentation and abdominal pain and was finally diagnosed with PJS by gastroscopy (Figure 1A and B). The patient later underwent three "partial small bowel resections" and two "intestinal polypectomies" for intestinal obstruction. Three years ago, the patient was diagnosed with cervical cancer and underwent "extensive hysterectomy and bilateral adnexal resection". Postoperative pathology suggested an endogenous cervical (size 5.4 cm × 3.5 cm) highly differentiated adenoma with metastasis to the right external iliac vessels (Figure 1C). The patient was treated with six cycles of chemotherapy with a carboplatin-doxorubicin (CD) (doxorubicin 30 mg/m2 and carboplatin area under the curve = 5) regimen before admission.
The patient reported no remarkable history of past illness.
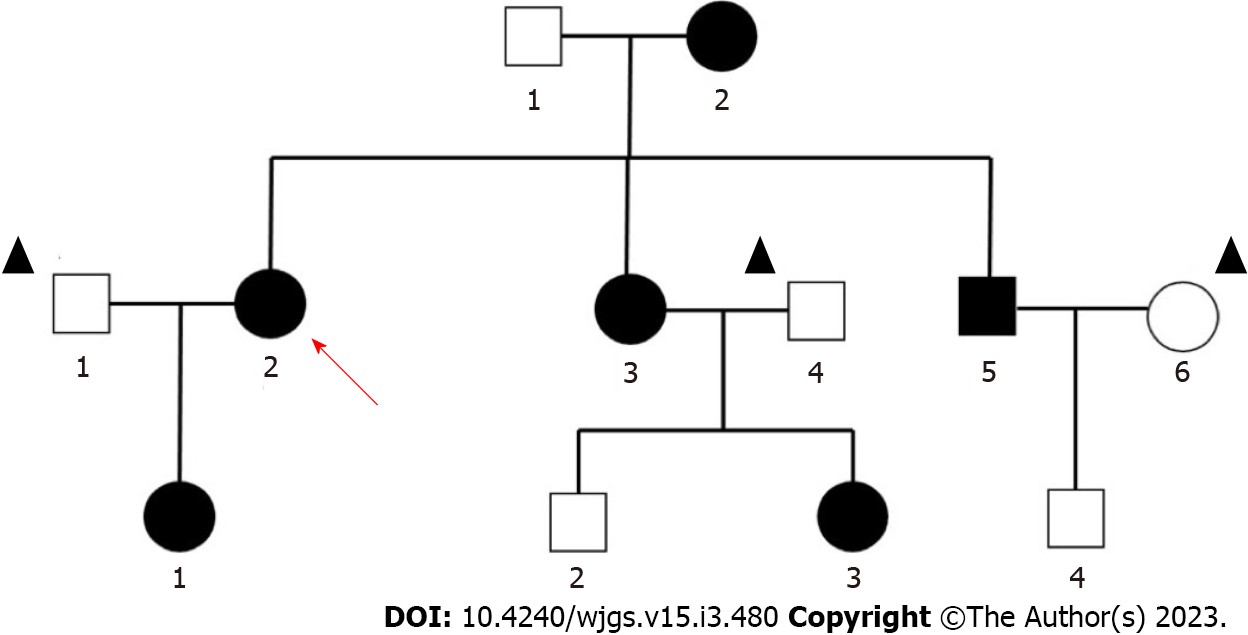
The patient's mother, brother, sister and daughter all had PJS. Her mother died of colon cancer at the age of forty. Genetic mapping was recorded (Figure 2).
A surgical scar of approximately 5 cm was visible below the umbilicus. Other physical examinations showed no important abnormalities.
The leukocytes, bilirubin, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, infectious disease screening and serum tumour markers were within normal limits, but the carbohydrate antigen-199 (CA199) level was elevated (71.2).
After admission, 18F-fluorodeoxyglucose positron emission tomography (PET)/computed tomography (CT) indicated multiple small metastases next to the right iliac vessels [the largest was approximately 0.5 cm × 0.5 cm; the regular scan maximum standardized uptake value (SUVmax) = 1.4; delayed scan SUVmax = 3.2].
Based on the patient's transoral single-balloon enteroscopy findings and the patient's surgical report, we diagnosed her with PJS combined with advanced cervical cancer.
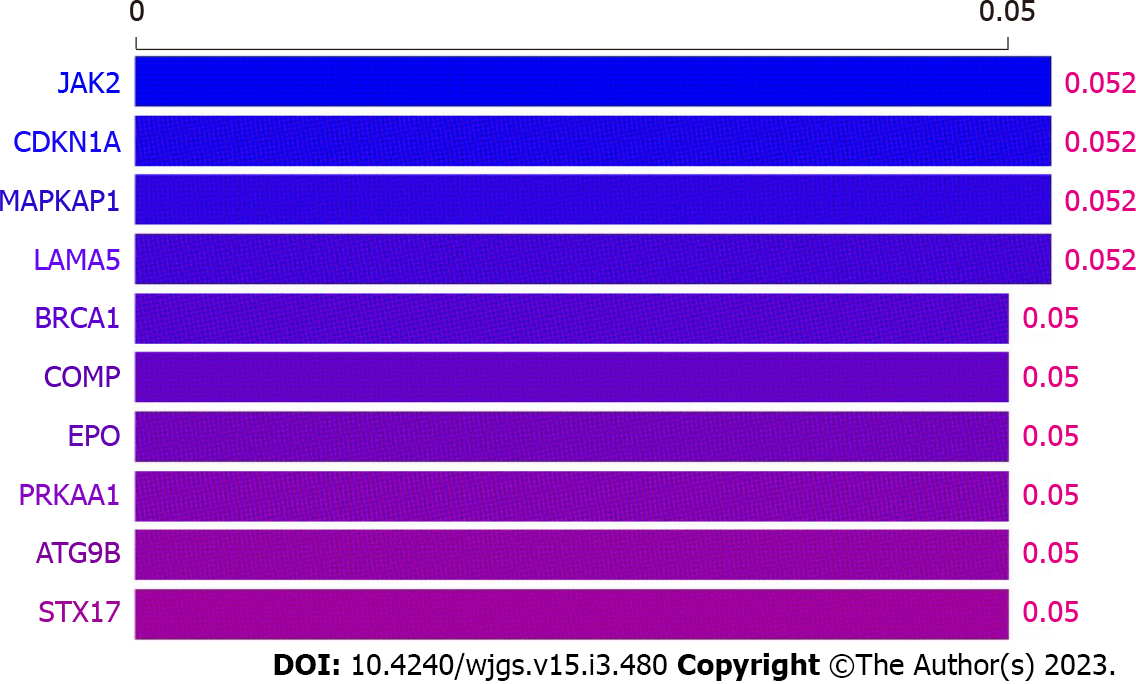
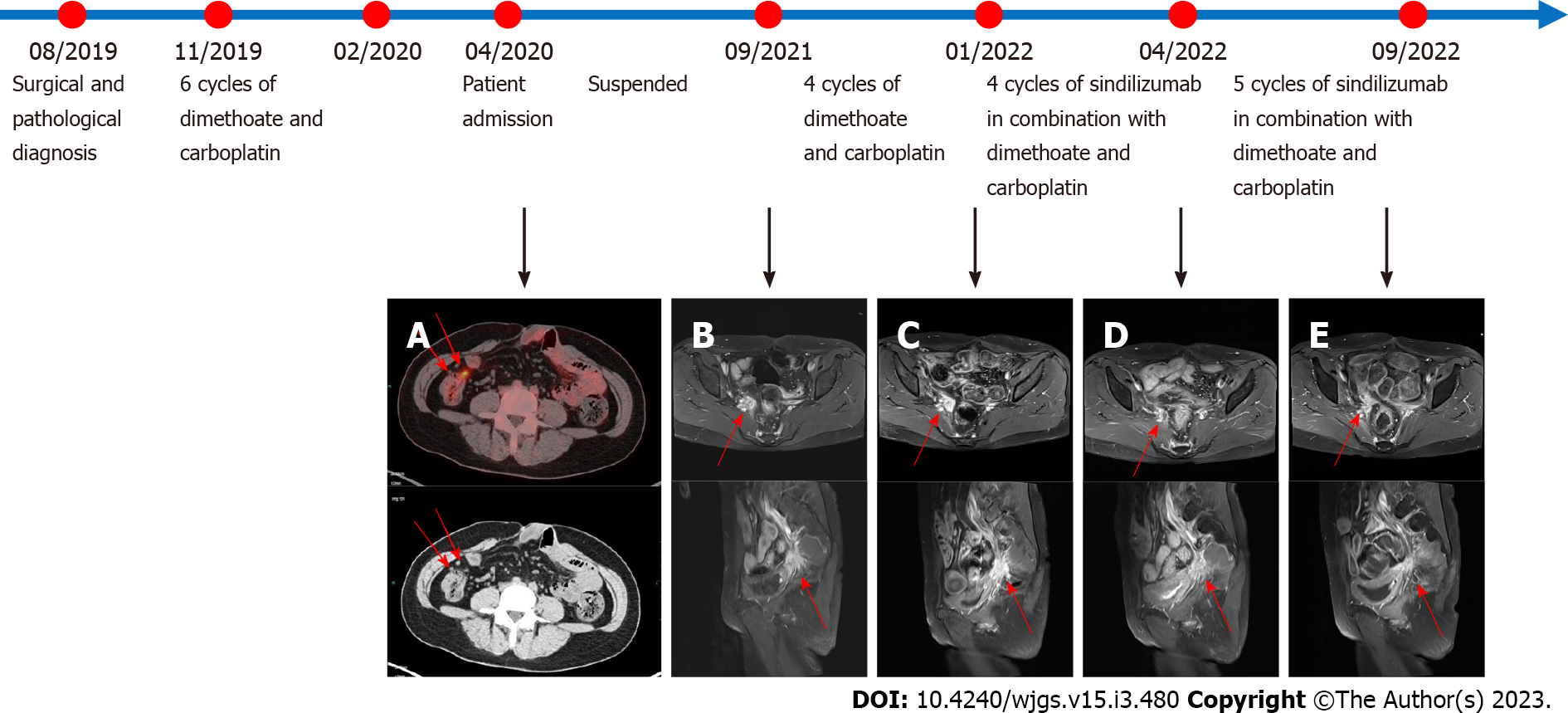
The patient was hospitalized to complete PET-CT and transoral single-balloon enteroscopy and did not continue chemotherapy due to financial reasons. In September 2021, pelvic magnetic resonance (MR) suggested a substantially larger right iliac metastasis than before, with CA199 = 146.8 U/mL. The patient then underwent a four-cycle CD chemotherapy protocol. In January 2022, pelvic MR suggested that the metastases continued to increase in size and CA199 was 160.8 U/mL. The patient's platelet count was 42 × 109/L, suggesting a risk of bleeding, so the patient was not directly punctured to test the expression of PD-L1 on the surface of the tumour cells. In addition, considering that the patient had both PJS and cervical cancer, whole exome sequencing (WES) was performed on the patient's blood and showed a mild correlation between Janus kinase-2 (JAK2) and the development of the disease (Figure 3). The patient was then treated for the first time with a PD-1 inhibitor (sindilizumab 200 mg, 21 d per cycle) in combination with the CD chemotherapy regimen[12]. In April 2022, pelvic MR suggested that the lesion had decreased greatly and CA199 was 29.8 U/mL, so the treatment was continued with the above regimen. In September 2022, pelvic MR showed no progression of metastases and CA199 was 27.1 U/mL. The whole treatment process was documented (Figure 4), and the overall health parameters during treatments were recorded (Table 1).
| Time | April, 2020 | September, 2021 | January, 2021 | November, 2021 | December, 2021 | January, 2022 | February, 2022 | March, 2022 | April, 2022 | May, 2022 | June, 2022 | July, 2022 | August, 2022 | September, 2022 | |
| Treatment | Admission | Suspend | 4 cycles of doxorubicin and carboplatin | 9 cycles of sindilizumb in combination with doxorubicin and carboplatin | |||||||||||
| Temp/°C | 37.1 | NA | 36.2 | 37 | 36.5 | 36.8 | 36.5 | 36.3 | 37.1 | 36.8 | NA | 36.8 | 36.9 | 37.2 | 36.9 |
| HR/min | 86 | NA | 76 | 84 | 75 | 79 | 82 | 78 | 82 | 81 | NA | 79 | 75 | 79 | 77 |
| RR/min | 19 | NA | 19 | 18 | 19 | 19 | 19 | 18 | 19 | 17 | NA | 18 | 19 | 18 | 19 |
| BP/kPa | 16.1/7.5 | NA | 15.4/8.5 | 18.7/9.7 | 18.4/11.1 | 16.9/11.9 | 16.5/9.3 | 16.5/9.7 | 16.0/9.5 | 14.0/8.4 | NA | 12.0/8.0 | 12.5/8.7 | 13.2/9.6 | 13.1/8.0 |
| Weight/kg | 56 | NA | 49 | 51 | 51.5 | 52 | 53.8 | 52 | 51.3 | 50.9 | NA | 50.5 | 51 | 52.3 | 53 |
| WBC/109/L | 5.11 | NA | 5.4 | 5.91 | 5.03 | 3.6 | 3.34 | 2.91 | 3.23 | 4.02 | NA | 4.42 | 4.51 | 4.43 | 4.52 |
| Hb/g/L | 133 | NA | 124 | 114 | 105 | 96 | 102 | 102 | 106 | 109 | NA | 111 | 112 | 112 | 113 |
| Alb/g/L | 39.9 | NA | 38.5 | 42 | 40.3 | 36.9 | 45.6 | NA | 44.2 | 43.4 | NA | 43.1 | 40.7 | 37.6 | 35.3 |
| ALT/U/L | 34 | NA | 20 | 17 | 17 | 16 | 20 | 21 | 22 | 23 | NA | 24 | 36 | 47 | 54 |
| AST/U/L | 29 | NA | 25 | 24 | 17 | 8.3 | 26 | NA | 27 | 28 | NA | 28 | 37 | 43 | 47 |
| CEA/µg/L | 1 | NA | 0.8 | 1.2 | 1.1 | 0.9 | 1 | 0.8 | 0.9 | 0.8 | NA | 0.8 | 0.9 | 0.8 | 0.8 |
| CA125/U/mL | 11.1 | NA | 14.4 | 19.3 | 14.6 | 8.9 | 10.2 | 10.3 | 9.7 | 9.1 | NA | 9 | 9.3 | 9.7 | 10.7 |
| CA199/U/mL | 71.2 | NA | 146.8 | 151.5 | 153.6 | 154.3 | 160.8 | 37.8 | 32.6 | 29.8 | NA | 22.3 | 24.3 | 25.6 | 27.1 |
| CA153/U/mL | 4.9 | NA | 5.6 | 11.1 | 9.4 | 7.3 | 8.4 | 9 | 6.1 | 5.2 | NA | 4.6 | 4.6 | 4.5 | 4.5 |
| CA724/U/mL | 2.3 | NA | 2.7 | 5.7 | 8.5 | 14.3 | 1.5 | 5.6 | 3.4 | 3.8 | NA | 2.4 | 2.3 | 2.2 | 2.1 |
Studies have found that sindilizumab-treated patients often suffer from adverse effects such as fever (38%), anaemia (74.1%) and elevated aspartate aminotransferase (41%) and alanine aminotransferase (40.6%)[13,14]. At the same time, carcinoma embryonic antigen (CEA), CA199 and cancer antigen-125 (CA125) are useful markers for detecting cervical cancer and monitoring the clinical course. In particular, CA199 and CA125 have been shown to be particularly useful in patients with adenocarcinoma[15]. In our case, after two months of follow-up, the patient's temperature, heart rate, respiratory rate, blood pressure, white blood cell, haemoglobin concentration, liver function and tumour markers (including CA199, CA125 and CEA) were within the normal ranges (Table 2).
| Time | Temp/°C | HR/min | RR/min | BP/kPa | Weight/kg | WBC/109/L | Hb/g/L | Alb/g/L | ALT/U/L | AST/U/L | CEA/µg/L | CA125/U/mL | CA199/U/mL | CA153/U/mL | CA724/U/mL |
| January, 2022 | 36.5 | 92 | 17 | 14.2/9.6 | 44.7 | 5.99 | 118 | 43.1 | 19 | 17 | 2.8 | 32 | 23 | 13.6 | 18.3 |
| November, 2022 | 37 | 87 | 19 | 14.7/10.4 | 45.2 | 6.45 | 115 | 35.8 | 18 | 23 | 2.3 | 31.7 | 24.6 | 15.6 | 19.3 |
We introduced a case of PJS combined with advanced cervical cancer. After the use of a PD-1 inhibitor combined with a CD chemotherapy regimen, the patient's right iliac metastases were markedly reduced in volume to a stable state, and a more satisfactory result was achieved.
PJS is a rare autosomal dominant disorder characterized by gastrointestinal malformations and skin pigmentation. Mutations in the STK11 gene can be detected in 50% to 80% of patients. Other related causative genes include the possible gene in the 19q13.4 region, the Brg1 gene and the IFTTM1 gene[16]. Patients with PJS are more prone to various malignancies, among which gastrointestinal and reproductive tract and endocrine tumours are the most common. Cervical adenocarcinoma is a common malignancy among patients with PJS (46.8%)[17]. Patients with PJS combined with cervical cancer often present with symptoms such as menstrual irregularities, endocrine disorders and abnormal vaginal bleeding, but there is no uniform treatment protocol. In 2008, Li et al[18] first reported a case of PJS complicated by cervical adenocarcinoma and small bowel malignancy in which the patient underwent total hysterectomy after neoadjuvant chemotherapy (paclitaxel 120 mg/dL and carboplatin 350 mg/dL 1 d per week for 6 wk, and the tumour markers returned to normal after three months. However, the patient was not followed up. In 2008, Kilic-Okman et al[19] reported a case of PJS combined with stage IIIB cervical cancer in which the patient received six cycles of combination chemotherapy (5-fluorouracil, adriamycin, and cyclophosphamide) and radiotherapy. That patient died of cervical adenocarcinoma progression within one month after the completion of radiotherapy. In 2019, Kim et al[20] reported a case of PJS combined with gastric-type mucinous cervical adenocarcinoma. As the mass was confined to the cervix and no peripheral lymph node metastasis was present in this patient, the patient achieved recovery after radical cervical surgery followed by adjuvant radiotherapy. In 2021, Vu Dinh et al[21] reported a case of PJS combined with stage IIIC gastric cervical mucinous adenocarcinoma. After adjuvant radiotherapy, the disease was stable with no recurrence at one year of follow-up.
PD-1 (also known as CD279) is a coreceptor expressed on the surface of antigen-stimulated T cells. PD-1 and its ligand (PD-L1) belong to the immune checkpoint pathway. Cervical cancer patients can exhibit PD-L1 expression. In 2018, Feng et al[22] reported that 59.1% of cervical cancer patients exhibited PD-L1 expression. Additionally, increased incidents of abortion and childbearing can also enhance PD-L1 expression in tumour cells. The study confirmed that PD-1 in tumour-invasive lymphocytes (TILs) and PD-L1 and TILs in cancer cells together constitute the PD-1/PD-L1 pathway, and the imbalance of this pathway is one of the mechanisms of tumour development and cellular immune escape. In addition, Meng et al[23] revealed that 60.82% of patients had PD-L1 expression, and PD-L1 overexpression was associated with vascular invasion and lymph node metastasis in cervical cancer.
JAK2 is a nonreceptor tyrosine kinase that plays key roles as the intracellular signalling effector of the cytokine receptor[24]. JAK2 was also found to regulate the expression of PD-L1. In 2016, Ikeda et al[25] found that the PD-L1 protein is upregulated by the simultaneous amplification of the PD-L1 and JAK2 genes through JAK-STAT signalling in non-small cell lung cancer. In 2017, Garcia-Diaz et al[12] discovered that interferon-γ could induce PD-L1 expression via the interferon-γ-JAK1/JAK2-STAT1/STAT2/STAT3-IRF1 axis in tumour cells, leading to immune escape and cancer induction.
Based on the above studies and the WES in this case, JAK2 was mildly associated with the development of the disease, and we used sindilizumab in combination with carboplatin and doxorubicin for the treatment of PJS combined with advanced cervical carcinoma[12,22-25]. A more satisfactory result was achieved based on the comparison of the right parietal iliac metastases on pelvic MR before and after drug administration, which showed a substantial reduction.
The unique feature of this case is that PJS is a rare disease about which there are only a few international reports, and there is no uniform treatment protocol. We performed WES, identified the relevant causative genes, and treated the patient with sindilizumab in combination with chemotherapy for the first time. The metastases were substantially reduced and the CA199 was greatly decreased after the treatment, which may suggest that immune-combination chemotherapy may be one of the future treatment directions for PJS combined with progressive cervical cancer.
We reported a case of PJS combined with advanced cervical cancer. Protein 1 inhibitor combined with a CD chemotherapy regimen substantially decreased the size of the patient’s metastases showing that the aforementioned protocol could be a good choice for such patients.
| 1. | Sandru F, Petca A, Dumitrascu MC, Petca RC, Carsote M. Peutz-Jeghers syndrome: Skin manifestations and endocrine anomalies (Review). Exp Ther Med. 2021;22:1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Tacheci I, Kopacova M, Bures J. Peutz-Jeghers syndrome. Curr Opin Gastroenterol. 2021;37:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, Trimbath JD, Giardiello FM, Gruber SB, Offerhaus GJ, de Rooij FW, Wilson JH, Hansmann A, Möslein G, Royer-Pokora B, Vogel T, Phillips RK, Spigelman AD, Houlston RS. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209-3215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 4. | Signoretti M, Bruno MJ, Zerboni G, Poley JW, Delle Fave G, Capurso G. Results of surveillance in individuals at high-risk of pancreatic cancer: A systematic review and meta-analysis. United European Gastroenterol J. 2018;6:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Turpin A, Cattan S, Leclerc J, Wacrenier A, Manouvrier-Hanu S, Buisine MP, Lejeune-Dumoulin S. [Hereditary predisposition to cancers of the digestive tract, breast, gynecological and gonadal: focus on the Peutz-Jeghers]. Bull Cancer. 2014;101:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Klimkowski S, Ibrahim M, Ibarra Rovira JJ, Elshikh M, Javadi S, Klekers AR, Abusaif AA, Moawad AW, Ali K, Elsayes KM. Peutz-Jeghers Syndrome and the Role of Imaging: Pathophysiology, Diagnosis, and Associated Cancers. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 7. | Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 874] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 8. | Lim W, Olschwang S, Keller JJ, Westerman AM, Menko FH, Boardman LA, Scott RJ, Trimbath J, Giardiello FM, Gruber SB, Gille JJ, Offerhaus GJ, de Rooij FW, Wilson JH, Spigelman AD, Phillips RK, Houlston RS. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology. 2004;126:1788-1794. [PubMed] [DOI] [Full Text] |
| 9. | Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, Miller DS, Olt G, King S, Boggess JF, Rocereto TF. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004;22:3113-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 431] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649-4655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 485] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 11. | Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1:28-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 308] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 12. | Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017;19:1189-1201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1222] [Cited by in RCA: 1403] [Article Influence: 155.9] [Reference Citation Analysis (4)] |
| 13. | Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, Zhang W, Gao Y, Jin Z, Zhou J, Jin C, Zou L, Qiu L, Li W, Yang J, Hou M, Zeng S, Zhang Q, Hu J, Zhou H, Xiong Y, Liu P. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6:e12-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, Chen G, Mei X, Yang Z, Ma R, Bi M, Ren X, Zhou J, Li B, Song Y, Feng J, Li J, He Z, Zhou R, Li W, Lu Y, Wang Y, Wang L, Yang N, Zhang Y, Yu Z, Zhao Y, Xie C, Cheng Y, Zhou H, Wang S, Zhu D, Zhang W, Zhang L. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol. 2020;15:1636-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 283] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 15. | Borras G, Molina R, Xercavins J, Ballesta A, Iglesias J. Tumor antigens CA 19.9, CA 125, and CEA in carcinoma of the uterine cervix. Gynecol Oncol. 1995;57:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kopacova M, Tacheci I, Rejchrt S, Bures J. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 17. | Ishida H, Tajima Y, Gonda T, Kumamoto K, Ishibashi K, Iwama T. Update on our investigation of malignant tumors associated with Peutz-Jeghers syndrome in Japan. Surg Today. 2016;46:1231-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Li LJ, Wang ZQ, Wu BP. Peutz-Jeghers syndrome with small intestinal malignancy and cervical carcinoma. World J Gastroenterol. 2008;14:7397-7399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 19. | Kilic-Okman T, Yardim T, Gücer F, Altaner S, Yuce MA. Breast cancer, ovarian gonadoblastoma and cervical cancer in a patient with Peutz-Jeghers Syndrome. Arch Gynecol Obstet. 2008;278:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Kim Y, Kim EY, Kim TJ, Lim KT, Lee KH, Chun Y, So KA. A rare case of gastric-type mucinous adenocarcinoma in a woman with Peutz-Jeghers syndrome. Obstet Gynecol Sci. 2019;62:474-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Vu Dinh G, Doan Thi Hong N, Vo Ngoc T, Nguyen Thanh L, Hoang Thi H, Phung Thi H. Peuzt - Jeghers syndrome with gastric type mucinous endocervical adenocarcinoma in a young woman: A case report. Ann Med Surg (Lond). 2021;69:102700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Feng YC, Ji WL, Yue N, Huang YC, Ma XM. The relationship between the PD-1/PD-L1 pathway and DNA mismatch repair in cervical cancer and its clinical significance. Cancer Manag Res. 2018;10:105-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Meng Y, Liang H, Hu J, Liu S, Hao X, Wong MSK, Li X, Hu L. PD-L1 Expression Correlates With Tumor Infiltrating Lymphocytes And Response To Neoadjuvant Chemotherapy In Cervical Cancer. J Cancer. 2018;9:2938-2945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Sopjani M, Morina R, Uka V, Xuan NT, Dërmaku-Sopjani M. JAK2-mediated Intracellular Signaling. Curr Mol Med. 2021;21:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Ikeda S, Okamoto T, Okano S, Umemoto Y, Tagawa T, Morodomi Y, Kohno M, Shimamatsu S, Kitahara H, Suzuki Y, Fujishita T, Maehara Y. PD-L1 Is Upregulated by Simultaneous Amplification of the PD-L1 and JAK2 Genes in Non-Small Cell Lung Cancer. J Thorac Oncol. 2016;11:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https: //creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oon C, Malaysia; Song Q, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H