Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.362
Peer-review started: November 18, 2022
First decision: November 30, 2022
Revised: December 9, 2022
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 27, 2023
Processing time: 129 Days and 1.4 Hours
Enhanced recovery after surgery (ERAS) program has been proved to improve postoperative outcome for many surgical procedures, including liver resection. There was limited evidence regarding the feasibility and benefit of ERAS in patients who underwent liver resection for cholangiocarcinoma.
To evaluate the feasibility of ERAS in patients who underwent liver resection for cholangiocarcinoma and its association with patient outcomes.
We retrospectively analyzed 116 cholangiocarcinoma patients who underwent hepatectomy at Srinagarind Hospital, Khon Kaen University between January 2015 and December 2016. The primary outcome was the compliance with ERAS. To determine the association between ERAS compliance and patient outcomes. the patients were categorized into those adhering more than and equal to 50% (ERAS ≥ 50), and below 50% (ERAS < 50) of all components. Details on type of surgical procedure, preoperative and postoperative care, tumor location, postoperative laboratory results, and survival time were evaluated. The compliance with ERAS was measured by the percentage of ERAS items achieved. The Kaplan-Meier curve was used for survival analysis.
The median percentage of ERAS goals achieved was 40% (± 12%). Fourteen patients (12.1%) were categorized into the ERAS ≥ 50 group, and 102 patients were in the ERAS < 50 group. Postoperative hospital stay was significantly shorter in the ERAS ≥ 50 group [8.9 d, 95% confidence interval (CI): 7.3-10.4 d] than in the ERAS < 50 group (13.7 d, 95%CI: 12.2-15.2 d) (P = 0.0217). No hepatobiliary-related complications or in-hospital mortality occurred in the ERAS ≥ 50 group. Overall survival was significantly higher in the ERAS ≥ 50 group. The median survival of the patients in the ERAS < 50 group was 1257 d (95%CI: 853.2-1660.8 d), whereas that of the patients in the ERAS ≥ 50 group was not reached.
Overall ERAS compliance for patients who underwent liver resection for cholangiocarcinoma is poor. Greater ERAS compliance could predict in-hospital, short-term, and long-term outcomes of the patients.
Core Tip: The present study is the first and the largest study demonstrating the enhanced recovery program after surgery (ERAS) compliance and its association with short-term and long-term outcomes of cholangiocarcinoma patients. This study demonstrated that overall ERAS compliance in patients who underwent liver resection for cholangiocarcinoma was poor. The patients with high ERAS compliance were significantly associated with shorter postoperative hospital stay, and, interestingly, longer overall survival.
- Citation: Jongkatkorn C, Luvira V, Suwanprinya C, Piampatipan K, Leeratanakachorn N, Tipwaratorn T, Titapun A, Srisuk T, Theeragul S, Jarearnrat A, Thanasukarn V, Pugkhem A, Khuntikeo N, Pairojkul C, Kamsa-Ard S, Bhudhisawasdi V. Compliance with enhanced recovery after surgery predicts long-term outcome after hepatectomy for cholangiocarcinoma. World J Gastrointest Surg 2023; 15(3): 362-373
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/362.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.362
Enhanced recovery after surgery (ERAS) program has been proven to be beneficial and become the standard of care in colorectal surgery. Over the years, it gains considerable momentum and has been implemented in other surgical specialties[1], even in emergency settings[2]. Since liver resection is a relatively complex surgery, with unique perioperative procedures and complications[3,4], ERAS in liver resection may be more difficult to implement and has different considerations from other abdominal operations. There are several recommendations and evidence supporting ERAS in liver resection procedures[5-7].
Despite a large amount of evidence supporting using ERAS in liver surgery, most of them did not focus specifically on liver resection for cholangiocarcinoma, which has several unique features including: (1) The requirement of anatomic major liver resection; (2) Being non-cirrhotic but having a tense liver from various degree of biliary obstruction; and (3) The requirement of biliary-enteric anastomosis in selected cases[8,9]. There was limited evidence regarding the feasibility and benefit of ERAS in patients who underwent hepatic resection for cholangiocarcinoma. Although the feasibility of applying ERAS in patients who underwent hepatic resection for cholangiocarcinoma has been demonstrated by Yip et al[10] and Quinn et al[8], the association between ERAS compliance and patient outcomes, both in short and long term, has not been reported. We, therefore, aimed to evaluate the feasibility of ERAS in patients who underwent hepatic resection for cholangiocarcinoma, and determine its association with outcomes of the patients.
All patients undergoing hepatic resection for cholangiocarcinoma at Srinagarind Hospital, Khon Kaen University (Khon Kaen, Thailand) between January 2015 and December 2016 were included in this comparative study. We retrospectively reviewed the prospectively maintained medical and pathological records of 116 patients with histologically-confirmed cholangiocarcinoma. During the study period, our team was aware of ERAS of all abdominal operations but did not fully implement a formal ERAS protocol for hepatobiliary surgery.
All patients with radiologically diagnosed cholangiocarcinoma received a common preoperative protocol, which included: (1) Resectability evaluation by reviewing cross-sectional imaging and patient status. The criteria for resectability included: (a) Good performance status (ECOG 0-1); (b) Absence of distant organ or lymph node metastasis on preoperative imaging; and (c) Sufficient volume of expected future liver remnant; (2) Blood examination: Complete blood count, liver tests, coagulogram, hepatitis panels, and tumor markers; and (3) Preoperative biliary drainage of future liver remnants, either endoscopically or percutaneously, in patients with obstructive jaundice with the aim to reduce serum total bilirubin to below 10 mg/dL. All patients were admitted to the hospital at least one day before the operation. All clinical, laboratory, and radiological data were rechecked at the time of the admission.
During the study period, we performed all liver resection by open surgery. Mirror-L incision was used in all cases. The type of liver resection was determined by the extent of the tumor, with plans to achieve at least all gross tumor removal. To optimize the surgical margin, surgeons preferred major hepatic resection to minor hepatic resection, which was performed only in patients with intraoperatively found limited future liver function. Liver parenchyma transection techniques and method of vascular inflow occlusion depended on the surgeon’s preference. Biliary-enteric anastomosis, if needed, encompassed ante-colic hepatico-jejunostomy in all cases.
After surgery, all patients were admitted to the intensive care unit until their conditions were stable and able to be extubated. Patients were allowed to be discharged from the hospital when they were on a full oral diet, received adequate pain controls, and demonstrated acceptable clinical and laboratory results. All patients were followed up in the hepatobiliary clinic with their respective attending surgeon at 2 wk after discharge.
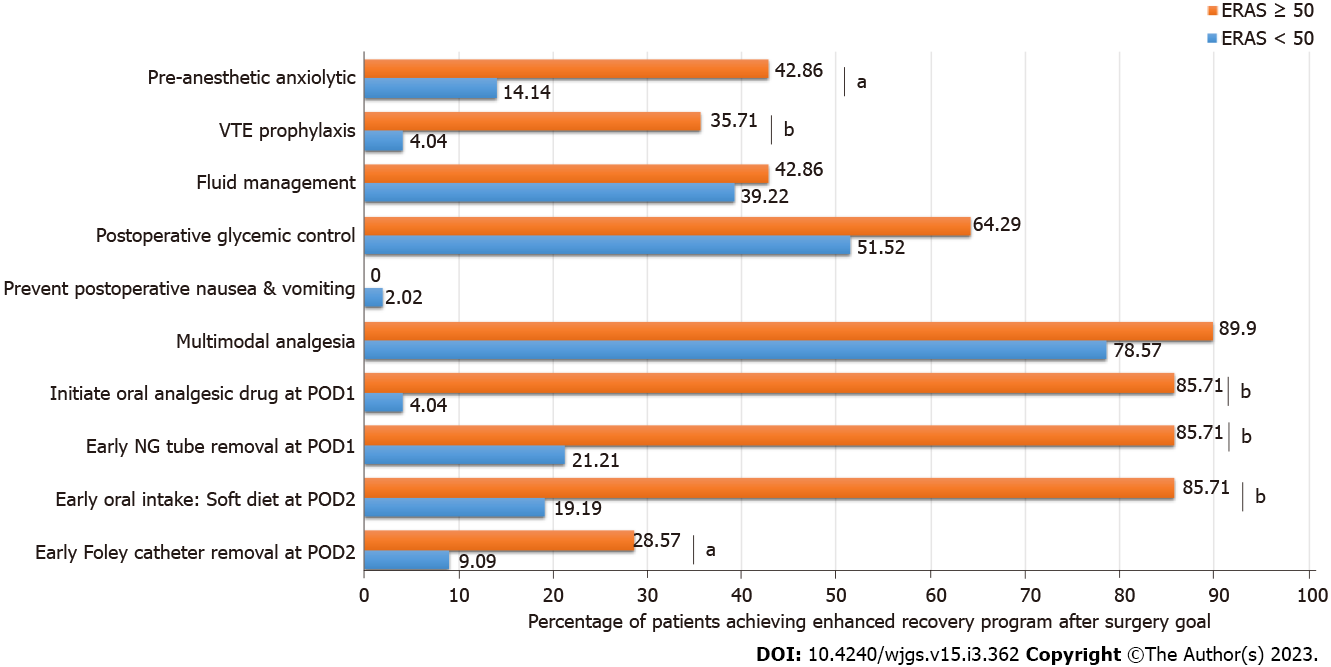
Adherence to ERAS components was recorded. During the study period, our hepatobiliary team had not fully implemented a formal ERAS protocol. Our protocol, as detailed in Table 1, contained 17 components, including preoperative counseling, preoperative fasting and preoperative carbohydrate load, pre-anesthetic anxiolytic, venous thromboembolism (VTE) prophylaxis, antimicrobial prophylaxis and skin preparation, prophylactic nasogastric intubation, preventing intraoperative hypothermia, fluid management, prophylactic abdominal drainage, early mobilization, postoperative glycemic control, preventing postoperative nausea and vomiting (PONV), multimodal analgesia, initial oral analgesic drug at postoperative day 1 (POD1), early nasogastric (NG) tube removal at POD 1, postoperative nutrition and early oral intake, and removal of urinary catheter at POD 2. Patients were then categorized into those who adhered to more than and equal to 50% (ERAS ≥ 50), and below 50% (ERAS < 50) of all ERAS components.
| ERAS item | Goals |
| Preoperative counseling | Patients receive dedicated education, full care pathway, details of operation and associated complication, and estimated length of hospital stay with clear verbal and wriinstruction |
| Preoperative fasting and preoperative carbohydrates load | Preoperative fasting 6 h for solids and 2 h for liquids. Carbohydrate loading evening before the day of surgery and 2 h before induction of anesthesia |
| Pre-anesthetic anxiolytic | Short-acting anxiolytics prior to the induction of anesthesia |
| VTE prophylaxis | Low-molecular weight heparin or unfragmented heparin administration 2-12 h before surgery |
| Antimicrobial prophylaxis and skin preparation | Single dose intravenous antibiotics administration before skin incision and less than 1 h before hepatectomy |
| Prophylactic nasogastric intubation | No use of prophylactic nasogastric intubation |
| Preventing intraoperative hypothermia | Maintenance of perioperative normothermia using forced air blankets and controlling temperature of the operating room |
| Fluid management (CVP monitoring) | The maintenance of low CVP (below 5 cm H2O) with close monitoring during liver transection phase |
| Prophylactic abdominal drainage | None or minimize the use of prophylactic abdominal drainage |
| Early mobilization | Begin to walk around the ward at least 3 times a day |
| Postoperative glycemic control | Insulin therapy to maintain normoglycemia before full oral intake |
| Preventing PONV | Patients should receive PONV prophylaxis with 2 anti-emetic drugs until POD3 |
| Multimodal analgesia | Multimodal analgesia combined with wound infusion analgesia or intrathecal opiates. Removal of epidural analgesia before POD3 |
| Initial oral analgesic drug at POD1 | Initial oral analgesic drug at POD1 |
| Early NG tube removal at POD1 | Removal of NG tube at POD1 unless there was > 400 mL/d drainage |
| Postoperative nutrition and early oral intake | Patients can eat soft diet at POD2 |
| Removal of urinary catheter POD2 | Removal of urinary catheter POD2 |
The primary outcome of this study was the compliance with ERAS, which was measured by the percentage of ERAS items achieved. We also investigated the association between the ERAS compliance and long-term outcomes of the patients. Descriptive analyses were performed and presented as appropriate. Continuous data were analyzed using student’s t-test. Categorical data were compared using the Pearson χ2 test. Survival analysis was presented using the Kaplan-Meier curve. Comparisons amongst groups were analyzed using a log-rank test. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 13 (Lakeway, TX, United States).
The Institutional Review Board, Office of Human Research Ethics, Khon Kaen University reviewed and approved this study (No. HE611590).
There were 116 cholangiocarcinoma patients who underwent hepatic resection during the study period. The median age was 63 ± 9.5 years. Male patients outnumbered female patients (62.1% vs 37.9%). None of the patients achieved ERAS goal of at least 80%. The median percentage of ERAS goals achieved was 40% ± 12%. Only 14 patients (12.1%) achieved at least 50 percent of ERAS goal and were categorized into the ERAS ≥ 50 group. The remaining were categorized into the ERAS < 50 group. All of the patients of this cohort achieved goals in three components, including preoperative counseling, antimicrobial prophylaxis and skin preparation, and preventing intraoperative hypothermia. None of the patients achieved goals in preoperative fasting and preoperative carbohydrate load, avoiding NG intubation, avoiding abdominal drainage, and early mobilization. The ERAS items that had a difference in goal achievement between two groups included: Early removal of Foley catheter, early oral dietary intake, early NG tube removal, initiate oral analgesic drug, postoperative glycemic control, prevention of PONV, multimodal analgesia, VTE prophylaxis, pre-anesthetic anxiolytic, and fluid management, as detailed in Figure 1. There were no differences in patients’ clinical and operative characteristics between groups, except for a higher percentage of male patients in the ERAS < 50 group (65.7% vs 35.7%, P = 0.03), and a higher proportion of intrahepatic tumor location (85.7% vs 39.2%, P = 0.027) and higher preoperative serum cholesterol level (P = 0.0445) in the ERAS ≥ 50 group (Table 2).
| Variable | n (%) or mean (SD) | P value1 | |||
| ERAS < 50 (n = 102) | ERAS ≥ 50 (n = 14) | ||||
| Age | 62.1 | 7.9 | 61.8 | 11.0 | 0.905 |
| Gender (male) | 67 | 65.7 | 5.0 | 35.7 | 0.031 |
| Location | 0.027 | ||||
| Intrahepatic | 40 | 39.2 | 12.0 | 85.7 | |
| Bismuth I | 0 | 0.0 | 0.0 | 0.0 | |
| Bismuth II | 2 | 2.0 | 0.0 | 0.0 | |
| Bismuth IIIA | 38 | 37.3 | 1.0 | 7.1 | |
| Bismuth IIIB | 17 | 16.7 | 1.0 | 7.1 | |
| Bismuth IV | 5 | 4.9 | 0.0 | 0.0 | |
| Type of CCA | 0.442 | ||||
| MF | 10 | 9.8 | 2.0 | 14.3 | |
| PI/FN | 31 | 30.4 | 2.0 | 14.3 | |
| IG/PP | 61 | 59.8 | 10.0 | 71.4 | |
| Procedure | 0.285 | ||||
| Right hepatectomy | 38 | 37.3 | 9.0 | 64.3 | |
| Extended right hepatectomy | 18 | 17.7 | 0.0 | 0.0 | |
| Right trisectionectomy | 12 | 11.8 | 0.0 | 0.0 | |
| Left hepatectomy | 25 | 24.5 | 4.0 | 28.6 | |
| Extended left hepatectomy | 3 | 2.9 | 0.0 | 0.0 | |
| Left trisectionectomy | 2 | 2.0 | 0.0 | 0.0 | |
| Other | 4 | 3.9 | 1.0 | 7.1 | |
| Vascular resection | 7 | 6.9 | 1.0 | 7.1 | 0.969 |
| Vascular inflow occlusion | 39 | 38.2 | 7.0 | 50.0 | 0.399 |
| EBL (mL) | 647.1 | 490.5 | 446.4 | 273.5 | 0.138 |
| Preoperative laboratory investigation | |||||
| TB | 2.1 | 2.6 | 0.8 | 0.7 | 0.070 |
| AST | 365.9 | 359.0 | 215.8 | 122.7 | 0.139 |
| ALT | 253.6 | 250.6 | 166.0 | 96.0 | 0.216 |
| ALP | 141.3 | 106.0 | 84.8 | 39.4 | 0.060 |
| Alb | 2.8 | 0.7 | 3.0 | 0.6 | 0.257 |
| Cholesterol | 133.7 | 39.7 | 156.9 | 29.7 | 0.045 |
The postoperative outcomes are shown in Table 3. There were no hepatobiliary related complications in the ERAS ≥ 50 group. Postoperative hospital stay was significantly shorter in the ERAS ≥ 50 group [8.9 d, 95% confidence interval (CI): 7.3-10.4 d] than in the ERAS < 50 group (13.7 d, 95%CI: 12.2-15.2 d) (P = 0.0217). There were no differences in postoperative laboratory results between the two groups, except for serum cholesterol level at POD3 and POD5.
| Variable | n (%) or mean (95%CI) | P value1 | |||
| ERAS < 50 (n = 102) | ERAS ≥ 50 (n = 14) | ||||
| Overall morbidity | 51 | 50.0% | 4 | 28.6% | 0.132 |
| Hepatobiliary complications | 0.281 | ||||
| Post-hepatectomy liver failure | 14 | 13.7% | 0 | 0% | |
| Bile leakage | 4 | 3.9% | 0 | 0% | |
| Stricture/cholangitis | 1 | 0.9% | 0 | 0% | |
| Transient hyperbilirubinemia | 9 | 8.8% | 0 | 0% | |
| General complications | |||||
| Wound complications | 18 | 18.8% | 0 | 0% | 0.076 |
| Pulmonary complications | 9 | 8.8% | 2 | 14.3% | 0.513 |
| Cardiac complication | 5 | 4.9% | 0 | 0% | 0.397 |
| Acute kidney injury | 2 | 1.9% | 0 | 0 | 0.597 |
| Post-operative stay (d) | 13.7 | 12.2-15.2 | 8.9 | 7.3-10.4 | 0.022 |
| Cholesterol | |||||
| Postoperative day 1 | 131.5 | 123.9-138.9 | 151.1 | 141.2-160.9 | 0.057 |
| Postoperative day 3 | 107.3 | 101.5-113.1 | 127.7 | 116.7-138.7 | 0.013 |
| Postoperative day 5 | 96.6 | 90.8-102.5 | 118.1 | 109.1-127.2 | 0.009 |
| Serum albumin | |||||
| Postoperative day 1 | 3.0 | 2.9-3.1 | 3.1 | 2.9-3.3 | 0.271 |
| Postoperative day 3 | 2.9 | 2.8-2.9 | 3.0 | 2.9-3.2 | 0.224 |
| Postoperative day 5 | 2.8 | 2.7-2.9 | 2.9 | 2.8-3.1 | 0.425 |
| Total bilirubin | |||||
| Postoperative day 1 | 3.2 | 2.4-3.9 | 1.6 | 1.1-2.2 | 0.142 |
| Postoperative day 3 | 2.7 | 2.1-3.4 | 1.4 | 0.9-2.0 | 0.171 |
| Postoperative day 5 | 2.8 | 2.0-3.5 | 1.3 | 0.9-1.6 | 0.157 |
| Alanine aminotransferase | |||||
| Postoperative day 1 | 294.9 | 242.6-347.2 | 231.1 | 166.9-295.3 | 0.376 |
| Postoperative day 3 | 169.4 | 142.6-196.3 | 177.6 | 124.1-231.1 | 0.829 |
| Postoperative day 5 | 89.7 | 74.5-104.9 | 97.1 | 72.5-121.8 | 0.726 |
| Aspartate aminotransferase | |||||
| Postoperative day 1 | 386.5 | 323.4-449.7 | 285.2 | 196.4-373.9 | 0.247 |
| Postoperative day 3 | 169.4 | 142.6-196.3 | 177.6 | 124.1-231.1 | 0.829 |
| Postoperative day 5 | 89.7 | 74.5-104.9 | 97.1 | 72.5-121.8 | 0.726 |
| International normalized ratio (PT/INR) | |||||
| Postoperative day 1 | 1.27 | 1.2-1.35 | 1.27 | 1.18-1.35 | 0.939 |
| Postoperative day 3 | 1.42 | 1.37-1.47 | 1.34 | 1.24-1.45 | 0.293 |
| Postoperative day 5 | 1.39 | 1.3-1.49 | 1.26 | 1.17 -1.35 | 0.295 |
| Postoperative mortality | |||||
| 30 d | 0 | 0% | 0 | 0% | |
| 60 d | 3 | 2.9% | 0 | 0% | |
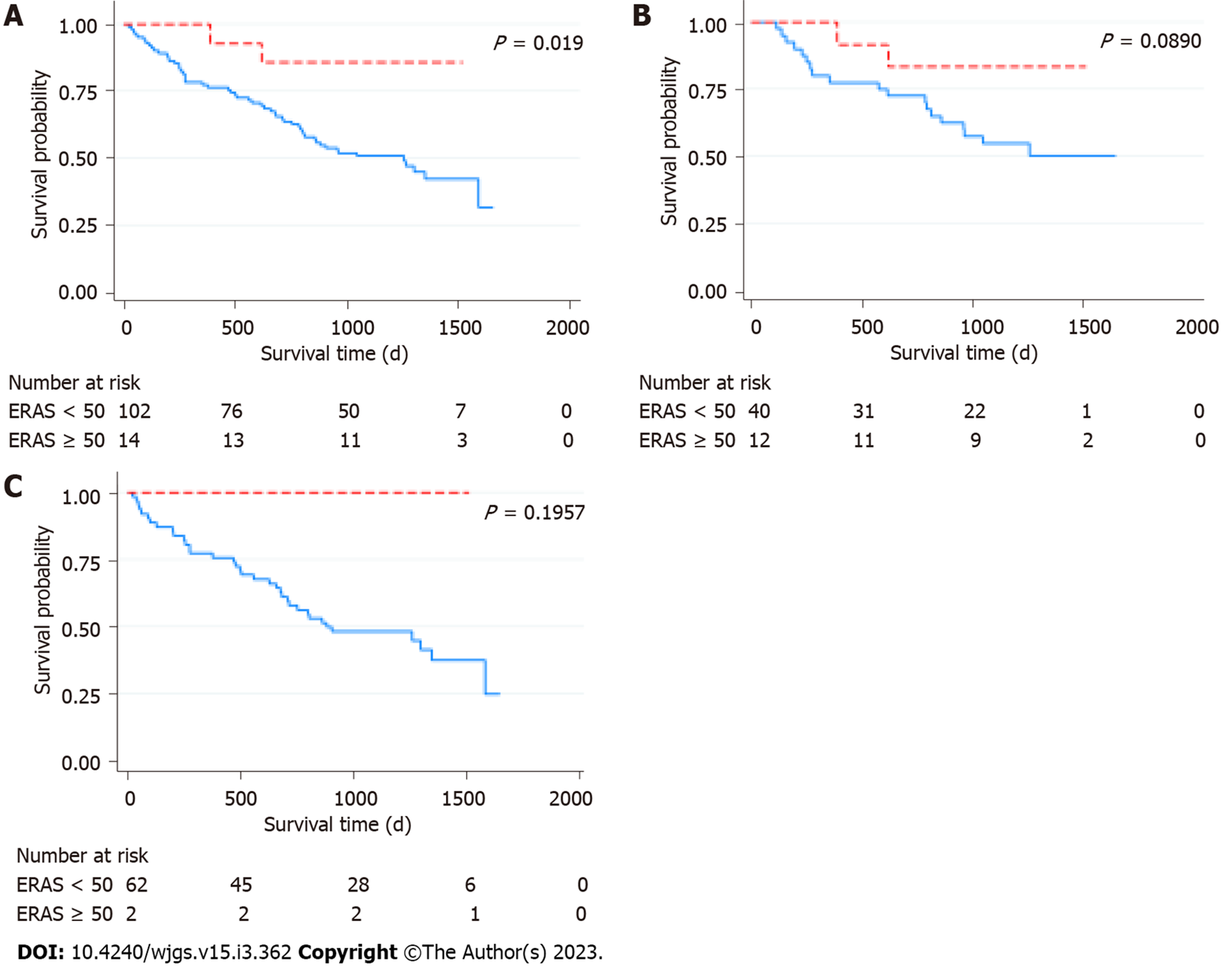
| Survival (95%CI) | 0.019 | ||||
| Median (d) | 1257 | 853.2-1660.8 | Not reached | ||
| 1-yr survival | 77.5% | 63.1-89.1 | 100% | ||
| 3-yr survival | 50.9% | 37.1-67.9 | 85.7 | 53.9-96.2 | |
There was no 30-d mortality in this cohort. There were three patients with 60-d mortality, all of which were in the ERAS < 50 group. The patients died on POD 21, 37, and 45 from bleeding aneurysm of right hepatic artery stump, severe pneumonia, and postoperative liver failure, respectively. With a median follow-time of 1241 d, the median survival of this cohort was 1302 d (95%CI: 1130.6-1473.4 d). There was a statistically significant difference in overall survival between the two groups (P = 0.0187) (Figure 2A). The median survival of the patients in the ERAS < 50 group was 1257 d (95%CI: 853.2-1660.8 d), whereas that of the patients in the ERAS ≥ 50 group was not reached - more than 50% of the patient with ERAS ≥ 50 were still alive at the time of the last follow-up. The respective 1- and 3-year survival rate of the patients in the ERAS < 50 was 77.5% (95%CI: 63.1-89.1) and 50.9% (95%CI: 37.1-67.9), and that of the patients in the ERAS ≥ 50 group was 100% and 85.7% (95%CI: 53.9-96.2). The survival between the groups seem to differ in both intrahepatic (Figure 2B) and extrahepatic tumors (Figure 2C), but the difference was not statistically significant.
This study demonstrated that overall ERAS compliance in patients who underwent liver resection for cholangiocarcinoma was poor. The patients with ERAS ≥ 50 were significantly associated with shorter postoperative hospital stay, and, interestingly, longer overall survival.
Postoperative care for liver resection has many unique challenges that have a large impact on the physiologic outcomes, such as having a large abdominal incision that requires the use of spinal anesthesia, significant intraoperative hemodynamic disturbance, and having a decreased liver volume postoperatively. These factors explain why overall ERAS compliance is lower compared to other abdominal operations, despite the fact that this group of patients might gain the most benefit from ERAS implementation. We initially intended to use 80% ERAS adherence as the cut point for categorizing the patients. However, at the time of the study, there was poor compliance to the ERAS protocol and none of the cases were able to achieve more than 80% of ERAS components. Consequently, a cut point at 50% ERAS was used instead. In the future, when ERAS is more routinely adopted, a higher cut point for components achieved may result in more tiers and more pronounced difference in patient outcomes. It should be noted that some ERAS components might not be suitable for cholangiocarcinoma resection, including the omission of nasogastric tube and abdominal drainage[8]. In our study, none of the patients achieved these component goals. Gastric dilation during the operation would preclude a good exposure of the operative field. Liver transection created a large raw surface of the liver that could cause postoperative bleeding and bile collection, therefore placement of abdominal drainage is almost unavoidable. Instead, several intraoperative manners should be further evaluated and considered to be ERAS components, such as intraoperative vascular inflow occlusion, controlling of central venous pressure, and inferior vena cava clamping[9,11]. These make liver transection safer, and would enhance patient recovery. We found that ERAS components that showed difference in compliance between the groups were mostly related to analgesic and dietary-related components. This finding is compatible with a previous study[12]. These components could be modified easily without any additional costs, and should be prioritized for implementation. Effective pain management might be a key to successfully enhancing recovery after liver resection. Lower postoperative pain, incorporated with early removal of Foley catheter, leads to early mobilization and, subsequently, early returns of bowel movement[8]. The delayed oral intake in the patients with extrahepatic cholangiocarcinoma, who require biliary-enteric anastomosis, preclude enhanced recovery. This leads to several delays, including oral analgesia, NG tube removal, mobilization, and, ultimately, recovery. This explains why we found a higher proportion of extrahepatic cholangiocarcinoma in the ERAS < 50 group. Improvement of ERAS for liver resection is crucial. Since a number of cases are required for achieving the optimal recovery and compliance[13], the large center with a high number of cholangiocarcinoma cases should be the initiator of ERAS development. Since 2016, we have been able to consistently apply these ERAS components: Pre-anesthetic anxiolytic, VTE prophylaxis, preventing intraoperative hypothermia, preventing PONV, early NG tube removal at POD1, and early oral intake. Moreover, we started to perform minimally invasive surgery for liver resection procedure.
Another way in which operative outcomes could be improved is through laparoscopic surgery, as previous studies have shown that laparoscopic liver resection is associated with shorter length of stay[14]. Therefore, ERAS in laparoscopic liver resection should be considered separately from open liver resection. Since laparoscopic liver resection is typically performed in selected patients that require less complicate operative procedure, our study was intentionally conducted when all cholangiocarcinoma cases, at our center, received open resection in order to minimize selection bias.
Recent evidence from other randomized controlled trials reaffirmed that the ERAS protocol for patients who underwent liver resection was associated with decreased length of hospital stay and lower overall morbidity[15-17]. Our study confirmed that these findings are also valid in cholangiocarcinoma patients. We found that the patients with higher ERAS compliance had significantly shorter length of hospital stay. This is comparable with a previous report, which stated that patients undergoing major liver resection that were on ERAS protocol experienced the greatest benefit in terms of decreased length of hospital stay and decreased rate of 30-d complications[12]. Alteration of postoperative liver tests could be used as an indicator for liver recovery and risk of postoperative liver failure[4]. In our study, the postoperative serum cholesterol level was significantly higher in the ERAS ≥ 50 group. It might indirectly indicate that liver recovery is faster in this group. Other explanations include: (1) The patients in this group already had higher cholesterol level preoperatively; and (2) Higher proportion of intrahepatic tumors, which require less extensive liver resection. None of our patients in the ERAS ≥ 50 group experienced hepatobiliary-related complications. There might be synergistic effects between absence of complications and achieving ERAS goals. Both of them promote patient recovery and, ultimately, shorten length of hospital stay. One study reported that even in high risk or with major postoperative complications, high ERAS compliance was achievable[8]. However, it is safe to say that achievement of ERAS ≥ 50 can be used to predict in-hospital, postoperative hepatobiliary-related complications, especially postoperative liver failure.
Although ERAS protocol has been proven to be beneficial amongst patients who underwent liver resection in terms of short-term outcomes[6,7,12], there was no study demonstrating these associations with long-term outcomes. We demonstrated the association between higher ERAS achieving and longer survival of the patients. This issue had been addressed in other cancers[18,19]. ERAS improved survival through various ways: (1) Reduction of postoperative stress leads to better immunologic function against the remaining tumor micro-metastases; and (2) Promoting quick recovery prevents the delay of adjuvant treatment. However, since there is no solid evidence of benefit of postoperative adjuvant chemotherapy for resectable cholangiocarcinoma[20-22], and cholangiocarcinoma is a heterogeneous disease with various progression pathways[23,24], it could not be concluded that improvement of ERAS compliance leads to an improvement of overall survival of cholangiocarcinoma patients. Even so, higher ERAS achievement could at least be used as a marker of better survival of cholangiocarcinoma patients.
To the best of our knowledge, our study was the first to demonstrate the association between greater ERAS achievement and long-term outcome of the patients who underwent liver resection. Moreover, this study was the largest study that focused only on cholangiocarcinoma patients who underwent liver resection by various hepatobiliary surgeons. However, there were several limitations that should be acknowledged. Bias might be introduced due to the following: (1) Being retrospective in nature; (2) Having a short interval of study period when a standard, full-ERAS protocol has not completely been developed. Due to the aforementioned limitations, only a correlation between better ERAS compliance and better outcome can be drawn; we were unable to interpret that better ERAS achievement caused better outcome; and (3) The sample size of the ERAS ≥ 50 group is quite small and could cause a significant type 2 error. Future prospective study should be conducted with full implementation of ERAS protocol specifically for the cholangiocarcinoma patients to demonstrate this association.
Overall ERAS compliance for cholangiocarcinoma is poor. There is a room for improvements of ERAS in patients who underwent liver resection for cholangiocarcinoma. Greater ERAS compliance could predict not only in-hospital, short-term outcomes but also long-term outcomes of the patients.
Enhanced recovery after surgery (ERAS) protocol has shown to be beneficial to patient outcomes in various abdominal surgeries, including hepatectomy. However, no previous study has demonstrated this association for hepatectomy in cholangiocarcinoma patients.
The present study explored the ERAS compliance and its association with outcomes of the patients who underwent open liver resection for cholangiocarcinoma during the first period of ERAS implementation.
To demonstrate the association between good ERAS compliance and short-term and long-term outcomes in cholangiocarcinoma patients.
Cholangiocarcinoma patients who underwent open hepatectomy between January 2015 and December 2016 were retrospectively analyzed. Patient’s compliance to ERAS was measured by the percentage of ERAS items achieved and categorized into more than and equal to 50% (ERAS ≥ 50), and below 50% (ERAS < 50) of of all ERAS components. Details on operative procedure, patient care, and survival were analyzed.
A total of 116 patients were identified - 14 patients (12.1%) were categorized into the ERAS ≥ 50 group, and 102 patients were in the ERAS < 50 group. Postoperative hospital stay was significantly shorter in the ERAS ≥ 50 group [8.9 d, 95% confidence interval (CI): 7.3-10.4 d] than in the ERAS < 50 group (13.7 d, 95%CI: 12.2-15.2 d) (P = 0.0217). No hepatobiliary-related complications or in-hospital mortality occurred in the ERAS ≥ 50 group. Overall survival was significantly higher in the ERAS ≥ 50 group.
Good ERAS compliance is associated with decreased length of hospital stay, decreased morbidity, and better survival.
Current overall ERAS compliance is poor. Future improvements in ERAS compliance could result in better short-term and long-term outcomes.
The authors thank Mr. Ian Thomas for reviewing the English-language presentation of the manuscript.
| 1. | Agarwal V, Divatia JV. Enhanced recovery after surgery in liver resection: current concepts and controversies. Korean J Anesthesiol. 2019;72:119-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Lohsiriwat V. Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol. 2014;20:13950-13955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Benzoni E, Molaro R, Cedolini C, Favero A, Cojutti A, Lorenzin D, Intini S, Adani GL, Baccarani U, Bresadola F, Uzzacu A. Liver resection for HCC: analysis of causes and risk factors linked to postoperative complications. Hepatogastroenterology. 2007;54:186-189. [PubMed] |
| 4. | Sawangkajohn W, Luvria V, Leeratanakachorn N, Tipwaratorn T, Theerakul S, Jarearnrat A, Titapun A, Srisuk T, Pugkhem A, Khuntikeo N, Bhudhisawasdi V, Kamsa-Ard S. Re-Rising of Total Bilirubin Level after Postoperative Day 3 (The V Pattern) Predicting Liver Failure and Survival of Patients who Underwent Hepatectomy for Cholangiocarcinoma. Asian Pac J Cancer Prev. 2020;21:3573-3578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CH, Garden OJ, Farges O, Kokudo N, Vauthey JN, Clavien PA, Demartines N. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40:2425-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 6. | Rouxel P, Beloeil H. Enhanced recovery after hepatectomy: A systematic review. Anaesth Crit Care Pain Med. 2019;38:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Noba L, Rodgers S, Chandler C, Balfour A, Hariharan D, Yip VS. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: a Systematic Review and Meta-Analysis. J Gastrointest Surg. 2020;24:918-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 8. | Quinn LM, Mann K, Jones RP, Bathla S, Stremitzer S, Dunne DF, Lacasia C, Fenwick SW, Malik HZ. Defining enhanced recovery after resection of peri-hilar cholangiocarcinoma. Eur J Surg Oncol. 2019;45:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Leeratanakachorn N, Luvira V, Tipwaratorn T, Theeragul S, Jarearnrat A, Titapun A, Srisuk T, Kamsa-Ard S, Pugkhem A, Khuntikeo N, Pairojkul C, Bhudhisawasdi V. Infrahepatic Inferior Vena Cava Clamping Reduces Blood Loss during Liver Transection for Cholangiocarcinoma. Int J Hepatol. 2021;2021:1625717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Yip VS, Dunne DF, Samuels S, Tan CY, Lacasia C, Tang J, Burston C, Malik HZ, Poston GJ, Fenwick SW. Adherence to early mobilisation: Key for successful enhanced recovery after liver resection. Eur J Surg Oncol. 2016;42:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Zhou J, He X, Wang M, Zhao Y, Zhang N, Wang L, Mao A. Enhanced Recovery After Surgery in Patients With Hepatocellular Carcinoma Undergoing Laparoscopic Hepatectomy. Front Surg. 2021;8:764887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Burchard PR, Dave YA, Loria AP, Parikh NB, Pineda-Solis K, Ruffolo LI, Strawderman M, Schoeniger LO, Galka E, Tomiyama K, Orloff MS, Carpizo DR, Linehan DC, Hernandez-Alejandro R. Early postoperative ERAS compliance predicts decreased length of stay and complications following liver resection. HPB (Oxford). 2022;24:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Lohsiriwat V. Learning curve of enhanced recovery after surgery program in open colorectal surgery. World J Gastrointest Surg. 2019;11:169-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Morise Z. Current status of minimally invasive liver surgery for cancers. World J Gastroenterol. 2022;28:6090-6098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Liang X, Ying H, Wang H, Xu H, Liu M, Zhou H, Ge H, Jiang W, Feng L, Liu H, Zhang Y, Mao Z, Li J, Shen B, Liang Y, Cai X. Enhanced recovery care versus traditional care after laparoscopic liver resections: a randomized controlled trial. Surg Endosc. 2018;32:2746-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Ni CY, Yang Y, Chang YQ, Cai H, Xu B, Yang F, Lau WY, Wang ZH, Zhou WP. Fast-track surgery improves postoperative recovery in patients undergoing partial hepatectomy for primary liver cancer: A prospective randomized controlled trial. Eur J Surg Oncol. 2013;39:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Jones C, Kelliher L, Dickinson M, Riga A, Worthington T, Scott MJ, Vandrevala T, Fry CH, Karanjia N, Quiney N. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg. 2013;100:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Curtis NJ, Taylor M, Fraser L, Salib E, Noble E, Hipkiss R, Allison AS, Dalton R, Ockrim JB, Francis NK. Can the combination of laparoscopy and enhanced recovery improve long-term survival after elective colorectal cancer surgery? Int J Colorectal Dis. 2018;33:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Tian YL, Cao SG, Liu XD, Li ZQ, Liu G, Zhang XQ, Sun YQ, Zhou X, Wang DS, Zhou YB. Short- and long-term outcomes associated with enhanced recovery after surgery protocol vs conventional management in patients undergoing laparoscopic gastrectomy. World J Gastroenterol. 2020;26:5646-5660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 20. | Ma KW, Cheung TT, Leung B, She BWH, Chok KSH, Chan ACY, Dai WC, Lo CM. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore). 2019;98:e14013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Rangarajan K, Simmons G, Manas D, Malik H, Hamady ZZ. Systemic adjuvant chemotherapy for cholangiocarcinoma surgery: A systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Wang ML, Ke ZY, Yin S, Liu CH, Huang Q. The effect of adjuvant chemotherapy in resectable cholangiocarcinoma: A meta-analysis and systematic review. Hepatobiliary Pancreat Dis Int. 2019;18:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 24. | Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Guglielmi A, Itaru E, Pawlik TM. Long-term outcomes of patients with intraductal growth sub-type of intrahepatic cholangiocarcinoma. HPB (Oxford). 2018;20:1189-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cheng KC, China; Li JX, China; Tsoulfas G, Greece S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ