Published online Feb 27, 2023. doi: 10.4240/wjgs.v15.i2.273
Peer-review started: October 11, 2022
First decision: November 6, 2022
Revised: November 19, 2022
Accepted: February 1, 2023
Article in press: February 1, 2023
Published online: February 27, 2023
Processing time: 139 Days and 9.5 Hours
Research on long-term survival after resection of giant (≥ 10 cm) and non-giant hepatocellular carcinoma (HCC) (< 10 cm) has produced conflicting results.
This study aimed to investigate whether oncological outcomes and safety profiles of resection differ between giant and non-giant HCC.
PubMed, MEDLINE, EMBASE, and Cochrane databases were searched. Studies designed to investigate the outcomes of giant vs non-giant HCC were included. The primary endpoints were overall survival (OS) and disease-free survival (DFS). The secondary endpoints were postoperative complications and mortality rates. All studies were assessed for bias using the Newcastle–Ottawa Scale.
24 retrospective cohort studies involving 23747 patients (giant = 3326; non-giant = 20421) who underwent HCC resection were included. OS was reported in 24 studies, DFS in 17 studies, 30-d mortality rate in 18 studies, postoperative complications in 15 studies, and post-hepatectomy liver failure (PHLF) in six studies. The HR was significantly lower for non-giant HCC in both OS (HR 0.53, 95%CI: 0.50-0.55, P < 0.001) and DFS (HR 0.62, 95%CI: 0.58-0.84, P < 0.001). No significant difference was found for 30-d mortality rate (OR 0.73, 95%CI: 0.50-1.08, P = 0.116), postoperative complications (OR 0.81, 95%CI: 0.62-1.06, P = 0.140), and PHLF (OR 0.81, 95%CI: 0.62-1.06, P = 0.140).
Resection of giant HCC is associated with poorer long-term outcomes. The safety profile of resection was similar in both groups; however, this may have been confounded by reporting bias. HCC staging systems should account for the size differences.
Core Tip: Resection of giant hepatocellular carcinoma (HCC) is associated with poorer long-term outcomes, with a safety profile similar to that of resection of non-giant HCC. The importance of this is that HCC staging systems should account for the size differences.
- Citation: Lee AJ, Wu AG, Yew KC, Shelat VG. Does size matter for resection of giant versus non-giant hepatocellular carcinoma? A meta-analysis. World J Gastrointest Surg 2023; 15(2): 273-286
- URL: https://www.wjgnet.com/1948-9366/full/v15/i2/273.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i2.273
Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer[1]. It is the third most common cause of cancer-related deaths worldwide and has the fifth-highest incidence rate of cancers[2]. Currently, most HCCs develop secondary to underlying liver disease, often due to chronic hepatitis B or C virus infection[3]. Most developed countries have surveillance programs that identify HCC early, resulting in potentially curative treatment for 40%–50% of patients[4,5]. For patients who do not qualify for curative treatment, locoregional or systemic treatments can be used, depending on the stage of the disease[4]. Despite early detection and advances in management, HCC has a 5-year survival rate of 18%[6].
In cancer management, prognostic factors are used in staging systems to help recommend appropriate treatment strategies and counsel patients on recurrence risk and survival estimates[7]. Key predictors of prognosis in patients with HCC include the extent of liver dysfunction, tumor burden, and patient performance status[8]. Tumor size, one of the determinants of tumor burden, has been identified as an independent predictor of overall survival, with larger tumors generally predicting poorer outcomes[9,10]. Despite this, there is currently no consensus on the inclusion of tumor size in HCC staging systems. Some systems, such as the Barcelona Clinic Liver Cancer (BCLC) system[11] and American Joint Committee on Cancer (AJCC) 8th edition staging system[12], include size, while others, such as the Hong Kong Liver Cancer (HKLC) classification[13], do not. Furthermore, the size cut-off may vary in systems that incorporate tumor size, and when used to guide management, such as in the BCLC system, surgical resection remains the primary treatment modality for patients with a single tumor, regardless of tumor size.
Despite being recommended as the first-line treatment for early-stage tumors, resection is still contentious for giant HCC (≥ 10 cm in diameter). Studies on the long-term survival rates after resection of giant and non-giant HCCs have yielded conflicting results. In studies by Noh et al[14] and Allemann et al[15], no significant difference in survival was found between patients with giant and non-giant HCC. Conversely, studies by Fang et al[16] and Lee et al[17] found poorer survival outcomes in patients with giant HCC. Furthermore, the prognosis after resection of single large HCCs (≥ 5 cm) has been shown to be closer to intermediate-stage tumors than single tumors of smaller size[18,19]. In light of conflicting evidence, this study aimed to investigate whether oncological outcomes and safety profiles of surgical resection differ between giant and non-giant HCC.
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A search was conducted using PubMed, MEDLINE (via Ovid), EMBASE, and Cochrane Central databases, from inception to 17 December 2021. A combination of search terms such as “HCC" or "liver cancer", "surgical resection" or “hepatectomy” or “liver resection”, “giant” or “huge” or "10 cm" was used. Only English studies were shortlisted for screening purposes. The articles were first screened by their titles and abstracts. Subsequently, full texts of suitable articles were reviewed for inclusion. The search, article review, quality assessment, and data extraction were conducted independently by two authors (Lee AJ and Wu AG). All disagreements were resolved by consensus or by appeal to a senior author. The study protocol was registered with PROSPERO (Number: CRD42022297772).
Cohort and case-control studies were included. Only studies designed to compare the outcomes of resection of giant vs non-giant HCC and provided Kaplan-Meier curves for overall survival (OS) or disease-free survival (DFS) were included. In duplicate studies, the most recent study was chosen.
Old studies published before 2000 were excluded from the meta-analysis to ensure that this study was relevant to current practice, as surgical techniques have been refined since then. Studies with a high risk of publication bias such as case reports and series were excluded. Reviews, editorials, conference abstracts, and non-human studies were excluded from the meta-analysis.
The quality of all the studies was assessed using the Newcastle – Ottawa scale for cohort studies. Studies that scored 7–9 points, 4–6 points, and 3 or fewer points were considered to have a low, moderate, and high risk of bias, respectively.
Two review authors (Lee AJ and Wu AG) independently extracted the publication details (name of the first author, year of publication, and country) and study characteristics (patient demographics, tumor characteristics, Child Pugh score, OS, DFS, hospital mortality, and postoperative complications) from each study. The Child–Pugh score was dichotomized into Child’s A vs Child’s B or higher. Individual patient data (IPD) were reconstructed from available Kaplan-Meier survival curves using an iterative algorithm initially proposed by Guyot et al[20].
The primary endpoints of this study were OS and DFS, while the secondary endpoints were postoperative complications and mortality. Additionally, we investigated whether non-size tumor and liver characteristics such as vascular invasion, multinodularity and presence of Child’s B or higher cirrhosis in non-giant tumors with respect to giant tumors. After extracting the relevant information on OS and DFS from the published survival curves, a one-stage analysis was performed using Cox proportional hazard models based on the shared frailty model. The frailty model was chosen to account for study heterogeneity by incorporating a random-effects term that modelled patients within each study as failure-prone, similar to other individuals in the same study. Stratified Cox models were generated for sensitivity analysis. The stratified Cox models were adjusted for inter-study heterogeneity by allowing patients from a study to share a baseline hazard unique only to the study while constraining partial likelihood estimates of the Cox coefficients to be equal across strata. As the proportional hazard assumption was not upheld at a longer follow-up duration, the restricted mean survival time (RMST) at various time points was also calculated as an alternative measure of treatment effect that does not require model assumptions. Additionally, a two-stage analysis was performed using inverse-variance weighted random-effects meta-analysis.
HR will be presented for the primary endpoints of DFS and OS, and OR for the secondary dichotomous outcomes with their respective 95%CI. Random-effects models were used for all analyses because of the high heterogeneity among the studies.
All analyses were performed using R (version 4.1.2), with statistical significance set at P < 0.05.
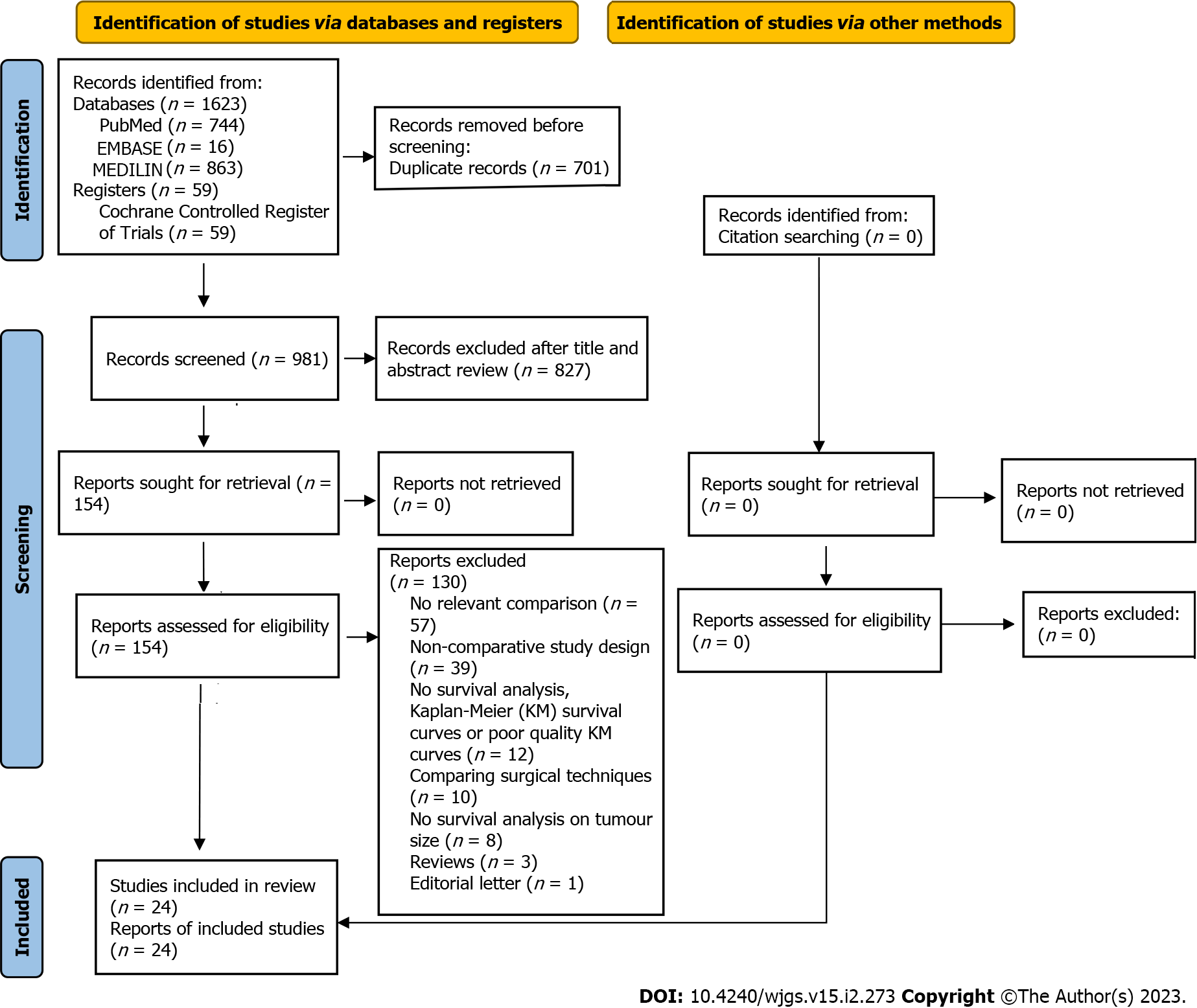
The search yielded 1682 potentially relevant studies. After duplicate removal and abstract screening, 153 full-text articles were reviewed, of which 24 studies[14-17,21-40] were deemed eligible for meta-analysis. All 24 studies obtained a score of 7 or higher on the Newcastle-Ottawa scale, indicating that they were of high quality. In the overall cohort of 23747 patients, there were 3326 patients in the giant HCC (≥ 10 cm) group and 20421 patients in the non-giant HCC (< 10 cm) group (Figure 1). A summary of the study’s characteristics is provided in Table 1 and 2.
| Study | Year | Follow-up, mo | No. | Age, yr | Sex (M/F) | Tumour size, cm | Cirrhosis, n (%) | Child-Pugh class, n (%) | |
| A | B + C | ||||||||
| Allemann et al[15] | 2013 | 25 | 79 | 67 (21-85) | NA | 4.9 (1-9) | 61 (77) | 75 (95) | 4 (5) |
| Chang et al[21] | 2016 | 72.5 | 10167 | NA | 7618/2711 | NA | 1114 (11) | NA | NA |
| Choi et al[22] | 2009 | 36 | 447 | 53.3 (9.7) | 344/103 | NA | 244 (55) | 443 (99) | 4 (1) |
| Fang et al[16] | 2019 | 20 | 104 | NA | 85/19 | NA | 93 (89) | 101 (97) | 3 (3) |
| Giuliante et al[23] | 2013 | NA | 28 | 65.8 (8.8) | 22/6 | 7.9 (7-8.1) | NA | 28 (100) | 0 (0) |
| Huang et al[24] | 2016 | 26 | 272 | NA | 242/30 | NA | 90 (82) | NA | NA |
| Jo et al[25] | 2011 | 30 | 40 | 54.6 (10.5) | 36/4 | 3.81 (2.06) | NA | 35 (88) | 5 (13) |
| Lee et al[17] | 2021 | NA | 3559 | 59.1 (12.1) | 2716/843 | 3.36 (2.14) | NA | NA | NA |
| Lewis et al[26] | 2019 | 22 | 26 | NA | NA | NA | NA | NA | NA |
| Liau et al[27] | 2005 | 27 | 111 | 63.0 (12.0) | 80/31 | 6.1 (2.5) | 40 (36) | 104 (94) | 7 (6) |
| Nagano et al[28] | 2005 | NA | 143 | 62.0 (9.0) | 112/31 | 3.25 (1.2-9.5) | 81 (57) | 101 (71) | NA |
| Noh et al[14] | 2016 | 26.4 | 73 | 56.85 (10.7) | 56/17 | NA | NA | NA | NA |
| Poon et al[29] | 2002 | 56 | 368 | 54.1 (12.2) | 295/73 | 5.4 (2.6) | 203 (55) | NA | NA |
| Shah et al[30] | 2007 | 34 | 165 | 62.0 (14.0) | NA | 4.7 (2.2) | NA | 145 (88) | 14 (8) |
| Tanaka et al[31] | 2015 | 39 | 291 | 67 (61-73) | 220/71 | 4 (2.3 – 5) | 134 (46) | 270 (93) | 21 (7) |
| Taniai et al[32] | 2008 | 22.5 | 291 | 64.1 (8.7) | 225/66 | 3.71 (1.91) | 156 (54) | 209 (72) | 82 (28) |
| Thng et al[33] | 2015 | 22 | 63 | 59 (27-81) | 50/13 | NA | NA | 60 (95) | 3 (5) |
| Wakayama et al[34] | 2017 | 57 | 521 | 62.8 (10.1) | 427/94 | 4 (2.1) | NA | 511 (98) | 8 (2) |
| Yamashita et al[35] | 2011 | NA | 412 | 64.0 (3.0) | 328/84 | 3.8 (2.2) | NA | 246 (60) | 166 (40) |
| Yang et al[37] | 2013 | NA | 293 | 47.0 (13.0) | 263/57 | 6.7 (3.8) | 201 (69) | 231 (79) | 62 (21) |
| Yang et al[36] | 2014 | NA | 781 | NA | 635/146 | NA | NA | 768 (98) | 51 (7) |
| Yeh et al[38] | 2003 | 16.4 | 985 | 55.7 (13.11) | 776/209 | 4.5 (2.4) | NA | NA | NA |
| Zhong et al[39] | 2017 | NA | 707 | NA | 612/95 | NA | 520 (74) | 672 (95) | 35 (5) |
| Zhu et al[40] | 2015 | 29.4 | 495 | 50.3 (11.2) | 436/59 | 4.8 (2.3) | 129 (26) | 431 (87) | 64 (13) |
| Study | Year | Follow-up, mo | No. | Age, yr | Sex (M/F) | Tumour size, cm | Cirrhosis, n (%) | Child-Pugh class, n (%) | |
| A | B + C | ||||||||
| Allemann et al[15] | 2013 | 25 | 22 | 72 (36-88) | NA | 13.5 (10-21) | 9 (41) | 22 (100) | 0 (0) |
| Chang et al[21] | 2016 | 72.5 | 912 | NA | 740/162 | NA | 166 (18) | NA | NA |
| Choi et al[22] | 2009 | 36 | 50 | 50.8 (12.5) | 34/16 | NA | 13 (26) | 48 (96) | 2 (4) |
| Fang et al[16] | 2019 | 20 | 84 | NA | 76/8 | NA | 72 (86) | 77 (92) | 7 (8) |
| Giuliante et al[23] | 2013 | NA | 37 | 62.2 (11) | 28/9 | 12 (11-15) | NA | 36 (97) | 1 (3) |
| Huang et al[24] | 2016 | 26 | 127 | NA | 114/13 | NA | 90 (71) | NA | NA |
| Jo et al[25] | 2011 | 30 | 11 | 52.4 (8.4) | 6/5 | 14.5 (4.11) | NA | 11 (100) | 0 (0) |
| Lee et al[17] | 2021 | NA | 426 | 55.7 (14.3) | 345/81 | 13.14 (4.95) | NA | NA | NA |
| Lewis et al[26] | 2019 | 22 | 16 | NA | NA | NA | NA | NA | NA |
| Liau et al[27] | 2005 | 27 | 82 | 62.0 (14.0) | 48/34 | 14.7 (4.1) | 8 (10) | 73 (89) | 5 (6) |
| Nagano et al[28] | 2005 | NA | 26 | 56.2 (12.2) | 19/7 | 14.8 (10-30) | 5 (19) | 22 (85) | NA |
| Noh et al[14] | 2016 | 26.4 | 41 | 55.1 (10.8) | 33/8 | NA | NA | NA | NA |
| Poon et al[29] | 2002 | 56 | 120 | 50.9 (12.8) | 99/21 | 13.8 (3) | 32 (27) | NA | NA |
| Shah et al[30] | 2007 | 34 | 24 | 57.0 (15.0) | NA | 13.1 (2.9) | NA | 24 (100) | 0 (0) |
| Tanaka et al[31] | 2015 | 39 | 24 | 64.5 (54-71) | 20/4 | 13 (11.2-14.1) | 7 (29) | 20 (83) | 1 (4) |
| Taniai et al[32] | 2008 | 22.5 | 29 | 62.0 (9.4) | 26/3 | 13.45 (2.77) | 12 (41) | 23 (79) | 6 (21) |
| Thng et al[33] | 2015 | 22 | 23 | 63 (34-84) | 20/3 | NA | NA | 20 (87) | 3 (13) |
| Wakayama et al[34] | 2017 | 57 | 54 | 63.9 (12.7) | 43/10 | 12.4 (3.7) | NA | 49 (92) | 4 (8) |
| Yamashita et al[35] | 2011 | NA | 53 | 60.0 (2.0) | 48/5 | 13.2 (0.4) | NA | 38 (72) | 15 (28) |
| Yang et al[37] | 2013 | NA | 258 | 45.0 (12) | 212/46 | 13.2 (4.1) | 171 (66) | 217 (84) | 41 (16) |
| Yang et al[36] | 2014 | NA | 304 | NA | 242/62 | NA | NA | 250 (83) | 16 (5) |
| Yeh et al[38] | 2003 | 16.4 | 211 | 47.8 (13.4) | 164/74 | 13.9 (3.4) | NA | NA | NA |
| Zhong et al[39] | 2017 | NA | 150 | 47.3 (10.9) | 123/27 | 12.4 (2.5) | 88 (59) | 142 (95) | 8 (5) |
| Zhu et al[40] | 2015 | 29.4 | 244 | 46.8 (11.3) | 209/35 | 12 (2.3) | 67 (27) | 210 (86) | 34 (14) |
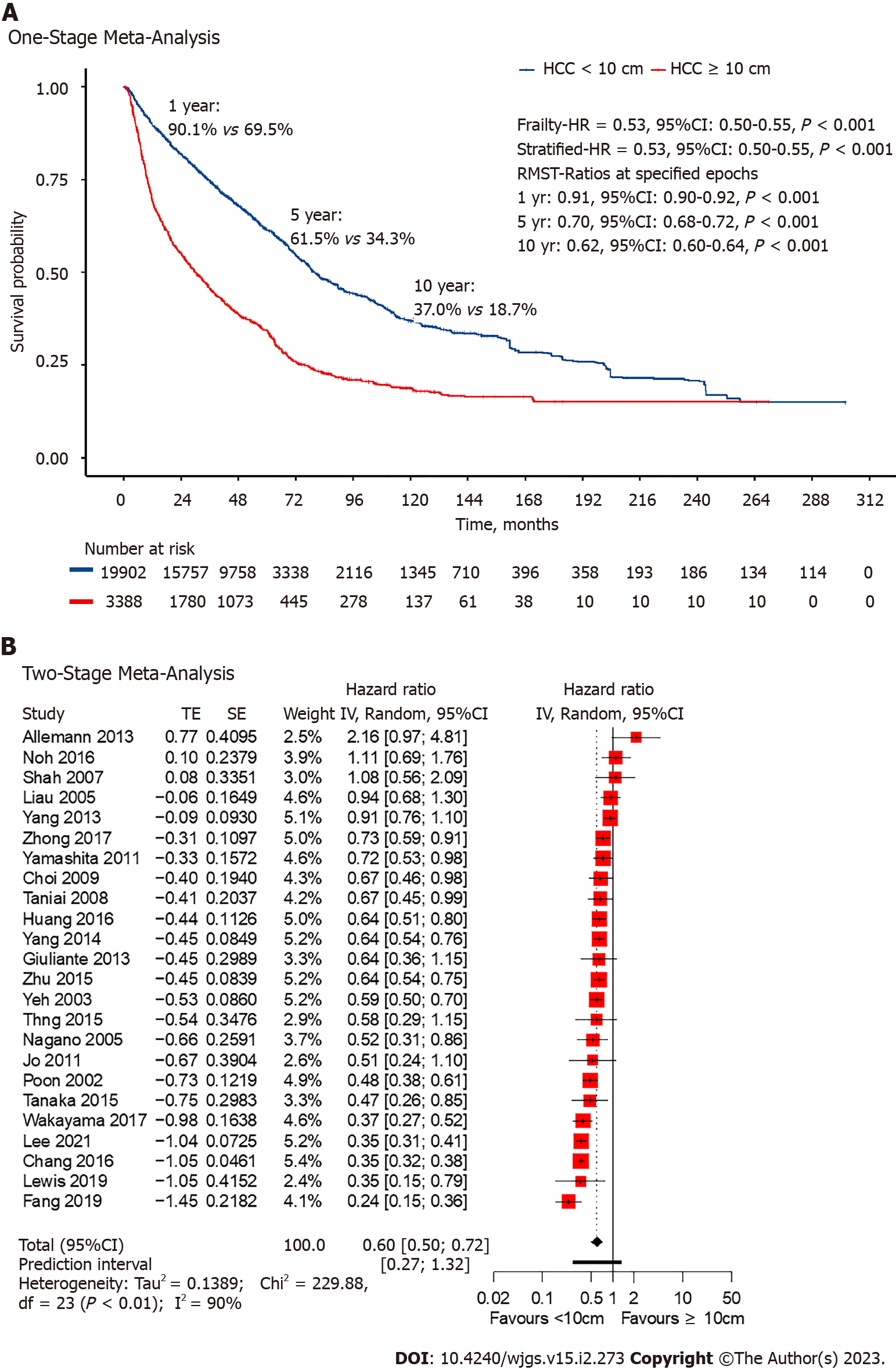
Among the included studies, all 24 had extractable data for OS. Non-giant HCC had a lower HR at 0.53 (95%CI: 0.50-0.55, P < 0.001; Figure 2) with the one-stage frailty model, and a similarly significant trend was seen with the stratified HR at 0.53 (95%CI: 0.50-0.55, P < 0.001; Figure 2). RMST at 1-, 5- and 10-years showed significantly increased hazards for giant HCC. The estimated 1-year OS from the reconstructed IPD was 90.1% for non-giant HCC and 69.5% for giant HCC (RMST 0.91, 95%CI: 0.90-0.92, P < 0.001; Figure 2). Two-stage meta-analysis showed that non-giant HCC has a HR of 0.60 (95%CI: 0.50-0.72, P < 0.01; Figure 2).
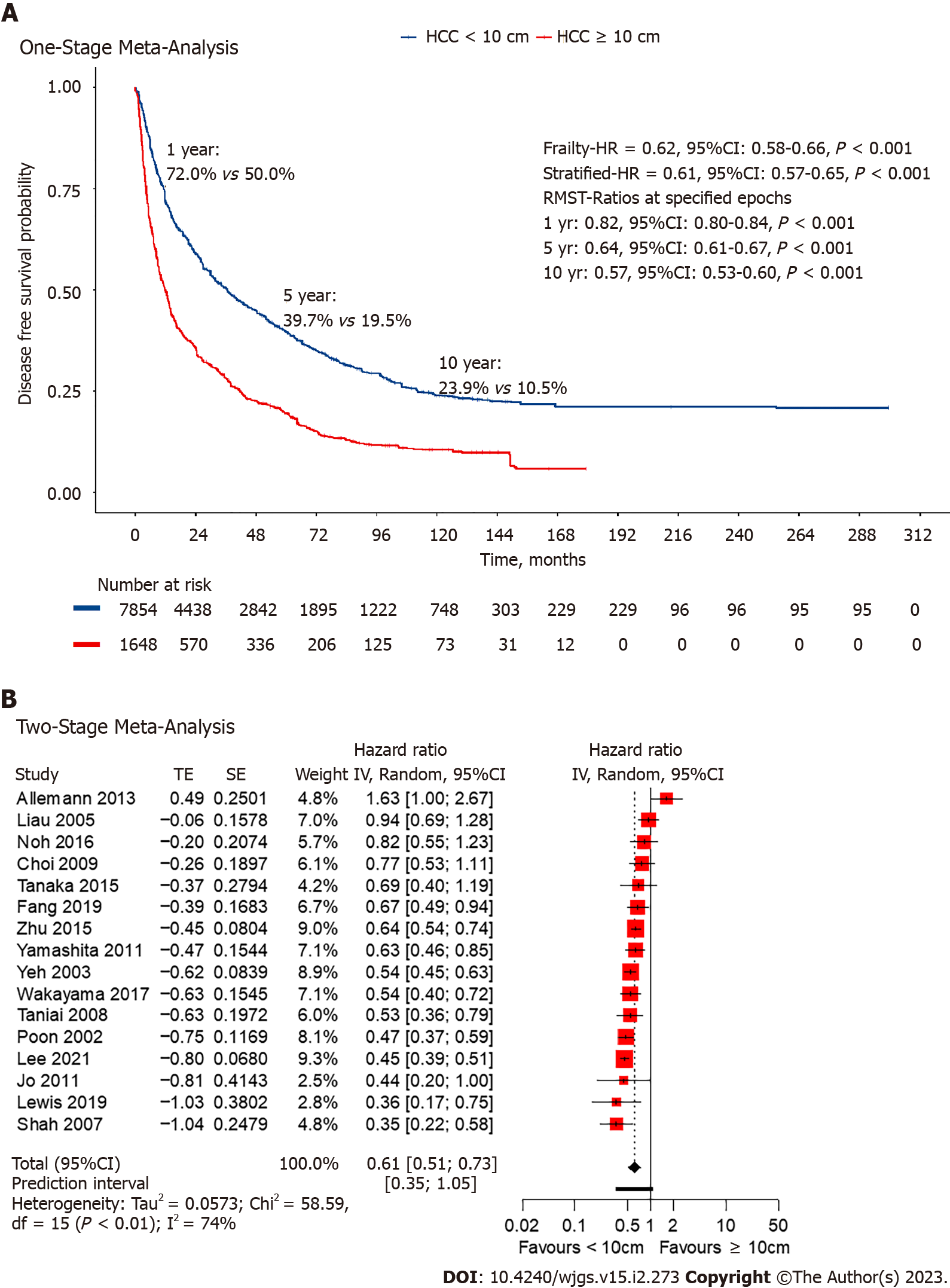
Among the included studies, 17 studies[14-17,22,25-27,29-32,34,35,37,40] had extractable data for DFS. Non-giant HCC had a lower HR at 0.62 (95%CI: 0.58-0.84, P < 0.001; Figure 3) in the one-stage frailty model, and a similarly significant trend was seen with the stratified HR at 0.61 (95%CI: 0.57-0.65, P < 0.001; Figure 3). RMST at 1-, 5- and 10-years all shown significantly increased hazards for giant HCC. The estimated 1-year DFS from the reconstructed IPD was 58.9% for non-giant HCC and 35.7% for giant HCC (RMST 0.82, 95%CI: 0.80-0.84, P < 0.001; Figure 3). Two-stage meta-analysis showed that non-giant HCC has a HR of 0.63 (95%CI: 0.52-0.76, P < 0.01; Figure 3).
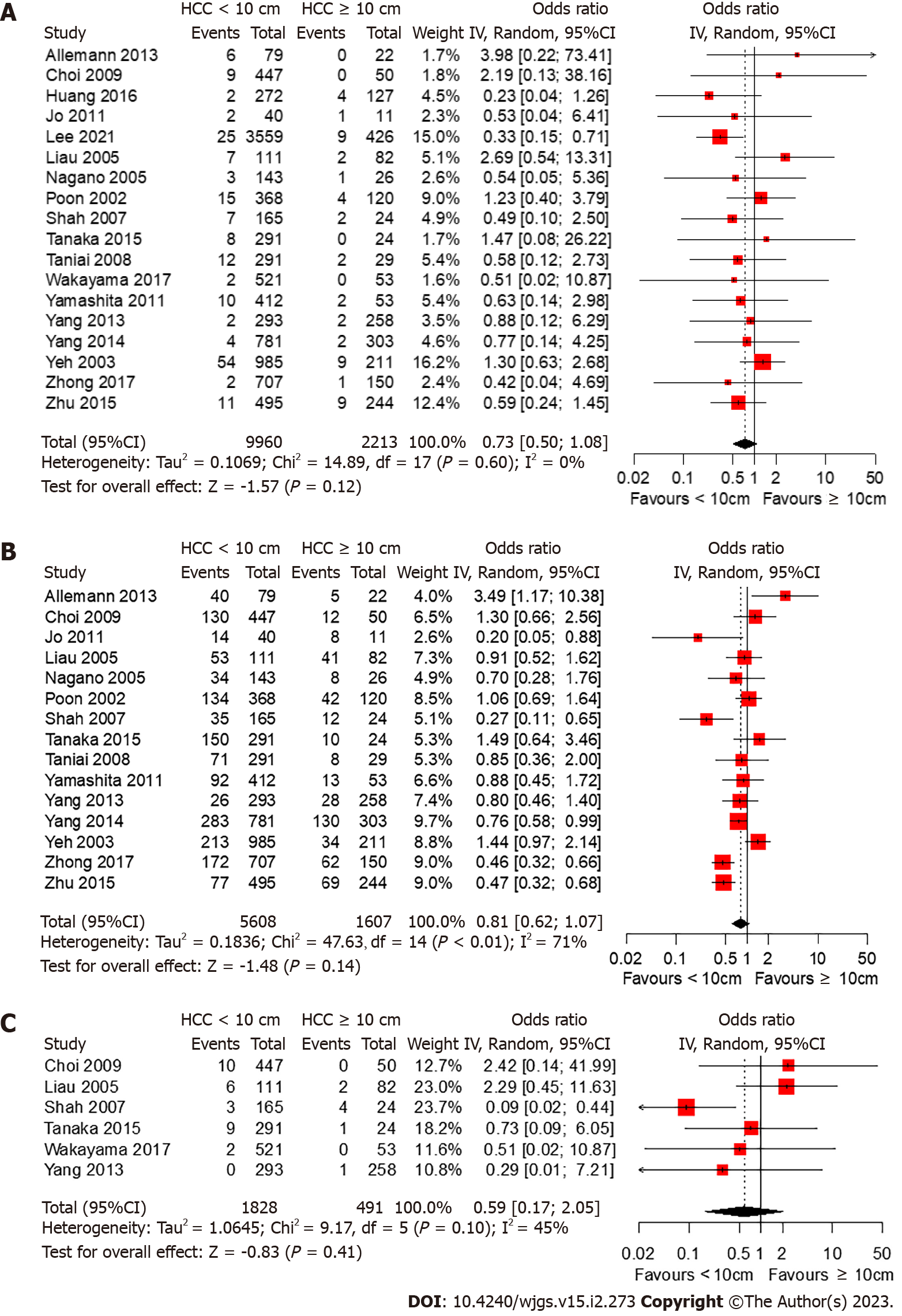
Among the included studies, 18 studies[15,17,22,24,25,27-32,34-40] reported 30-d mortality rates whereas only two studies[36,39] reported 90-d mortality rates (Figure 4). While resection of non-giant HCC had lower odds of death within the first 30 d after surgery, the difference was not statistically significant (OR 0.73, 95%CI: 0.50-1.08, P = 0.116). No significant heterogeneity was observed (I2 = 0%, P = 0.60). In the two studies that reported the 90-d mortality rate, the 90-d mortality rate was higher than the 30-d mortality rate; however, no significant difference was found between the different tumor size groups.
Among the studies included, 15 studies[15,22,25,27-32,35-40] reported major postoperative complications (Figure 4). While resection of non-giant HCC had lower odds of major postoperative complications, the difference was not statistically significant (OR 0.81, 95%CI: 0.62-1.06, P = 0.140). Substantial heterogeneity was observed among the included studies (I2 = 71%, P < 0.01).
Among the included studies, six studies[22,27,30,31,34,37] reported post-hepatectomy liver failure (PHLF) (Figure 4). While resection of non-giant HCC had lower odds of PHLF, the difference was not statistically significant (OR 0.59, 95%CI: 0.17-2.05, P = 0.41). No significant heterogeneity was observed (I2 = 45%, P = 0.10).
Among the included studies, 20 studies[14-16,21-25,27-34,36-38,40] reported on vascular invasion, 13 studies[15,16,21,22,24,27-29,31,32,37,39,40] on cirrhosis, 16 studies[15,16,22,23,25,27,28,30-37,39,40] on Child Pugh’s score and 9 studies[21,22,24,27,29,32,34,37,40] on tumor number (Table 3). While non-giant HCC was found to have significantly lower odds of vascular invasion (OR 0.367, 95%CI: 0.236-0.572, P < 0.0001) and multinodular tumors (OR 0.592, 95%CI: 0.376-0.939, P < 0.0259), it was found to have significantly higher odds of cirrhosis (OR 1.955, 95%CI: 1.317-2.903, P = 0.0009). No significant di
| Factor | OR | 95%CI | P value |
| Vascular invasion | 0.367 | 0.236-0.572 | < 0.0001 |
| Multinodular | 0.592 | 0.374-0.939 | 0.0259 |
| Child-Pugh score | 1.008 | 0.745-1.364 | 0.9592 |
| Cirrhosis | 1.955 | 1.317-2.903 | 0.0009 |
In this meta-analysis of 23747 patients, surgical resection of non-giant HCC was associated with approximately half the rate of death from any cause and a lower rate of disease recurrence than surgical resection of giant HCC. These pooled associations showed a significant disparity in long-term outcomes between the two groups despite the use of the same treatment modality. Furthermore, giant HCC is shown to be associated with higher odds of vascular invasion and multinodular tumors, factors that have been shown to be associated with poorer outcomes[41,42]. In contrast, the short-term perioperative outcomes and safety profiles, measured by 30-d mortality and postoperative complications, respectively, did not differ significantly between the two groups. Hence, while HCC size may not affect the safety and efficacy of surgical resection in the short term, this study illustrates not only a possible correlation between a larger tumor size and poorer outcomes, but also demonstrates that giant HCC have different tumor characteristics from non-giant HCC. Therefore, giant HCC should be staged differently because they are associated with poorer outcomes and prognostically poorer tumor characteristics.
Despite being a major risk factor for the development of HCC[43], cirrhosis and cirrhotic severity were not found to be associated with larger tumor size. In this study, non-giant HCC were found to have a higher risk of developing cirrhosis. A possible explanation for this is that cirrhotic patients are more likely receiving 6 moly ultrasound scan surveillance[44]. Therefore, tumors are likely to be detected before they reach larger sizes. Similarly, no association was found between the presence of Child-Pugh B cirrhosis and higher and larger tumor sizes. This shows that larger tumor size may not be correlated with greater odds of cirrhosis or more severe cirrhosis.
The myriad of HCC staging systems testifies that no single system is ‘ideal’. The BCLC staging system is widely accepted in clinical practice and classifies patients into stages based on their performance status (PS) and Child-Pugh score[11]. The BCLC staging system does not place sufficient importance on tumor size when stratifying patients. Tumor size only plays a role in sorting patients with a single tumor, PS 0, and Child-Pugh A into very early stage (0) and early-stage (A), for which < 2 cm is the cut-off set for being classified as stage 0. However, this classification into stages 0 and A seems inconsequential for patients with single tumors, since the final determinant of management options in this group of patients is portal pressure and bilirubin levels, with no consideration given to size. This is evident because surgical resection is the first option for patients with normal total bilirubin levels and no evidence of clinically significant portal hypertension. Given the findings of this study, BCLC stage A patients with single tumors should be further classified, based on tumor size, into giant and non-giant subgroups since survival after surgical resection differs significantly between these two groups. As a cut-off size of 10 cm was used, this study was unable to determine the exact size beyond which the oncological prognosis was inferior.
Similarly, in other staging systems, other prognostic factors have taken precedence over tumor size. In the latest AJCC 8th edition staging system[12], solitary tumors ≤ 2 cm are now staged as T1a regardless of microvascular invasion, which differs from the 7th edition, where microvascular invasion determines whether the tumor is T1 or T2. However, for tumors > 2 cm in diameter, vascular invasion and multifocality play a larger role in staging; the absence of these factors would place the tumor in T1b, regardless of tumor size. In both the Cancer of the Liver Italian Program score[45,46] and Okuda staging system[47], the criteria for tumor size are ambiguous, using relative tumor size compared to the liver (tumor burden) as the cut-off. In contrast, the HKLC classification was constructed solely based on PS, Child-Pugh score, liver tumor status, and the presence of extrahepatic vascular invasion or metastasis, without considering size[13]. Hence, many of the current staging systems ignore tumor size, and even in those that include size, size plays a limited role in staging the tumors. However, as giant HCC has been shown to be associated with vascular invasion and multinodular tumors, these factors should not be treated as mutually exclusive. From a technical perspective, the surgical resection of giant HCC is challenging. A large tumor size limits the surgical working space, increases the risk of tumor seeding from surgical manipulation, and distorts liver anatomy, thus potentially increasing operative difficulty. Further, it is likely that resection of large tumor entails dissection zone in proximity to hilum or major vessels, thus increasing the likelihood of bleeding or bile leak. In addition, surgical resection of giant HCC is in general entails major hepatectomy with small future liver remnant and associated risk of PHLF.
Although both groups had similar 30-d postoperative mortality and major complication rates, these may not accurately reflect the safety profile of surgical resection in each group. As the 90-d postoperative mortality rate has rarely been reported, only the 30-d mortality rate could be used as an indicator of postoperative mortality. However, a review by Egger et al[48] found that most studies reported an approximate doubling of mortality rates between 30 and 90 d following surgery. As the findings of this study were based on 30-d mortality rates, they may not accurately reflect the safety profile of surgical resection. Additionally, many studies did not specify which postoperative complications the patients experienced, and only 6 of the 24 studies[22,27,30,31,34,37] specified if the patients developed PHLF. Since PHLF has been found to be an independent predictor of mortality[2], the development of PHLF after HCC resection may be more indicative of the safety profile than complication rates alone. Thus, to improve the safety profile assessment of surgical resection, more precise reporting of major postoperative complications, particularly PHLF, and reporting of the 90-d mortality rate are required.
Although long-term outcomes for giant HCCs are significantly worse than those for non-giant HCCs, surgery continues to be the preferred treatment option. There is consensus that non-surgical treatment options for single giant HCC are associated with poorer outcomes than surgical resection, although many studies supporting surgical resection in the management of giant HCC have used transarterial chemoembolization (TACE) as a comparison[49-51]. In a recent meta-analysis of 1892 patients, Gui et al[52] found that TACE + radiofrequency ablation offers oncological outcomes comparable to surgical resection with lower morbidity. Although the meta-analysis was not specific to the treatment of giant HCC, it opens up the possibility of exploring the multimodal and combination approaches in patients with giant HCC. While surgical resection remains the current preferred treatment option for patients with giant HCC, future prospective studies should investigate different modalities of intervention for single or multiple giant HCC to determine whether these treatments can provide better quality of life outcomes with low therapy-associated morbidity. In addition, with scientific progress and innovation, radiation therapies including external beam radiation and selective internal radiation therapy, have a complementary role in the multidisciplinary care of patients with HCC[53].
This study has several limitations that should be considered. First, all included studies were retrospective studies with a risk of selection bias. As such, the favorable safety profile of giant HCC resection and the similar liver function in both giant and non-giant HCC may in part be due to the selection of younger and fitter patients with well-preserved liver function, or a publication bias. Second, there was a high degree of heterogeneity among studies. Hence, caution should be exercised when interpreting the results. Third, survival data, such as OS and DFS, were manually extracted from the survival curves. Hence, the possibility of errors during the data extraction cannot be eliminated. Fourth, although the algorithm used allows for a close approximation of the original IPD, it does not provide further details, such as patient-level covariates, which may provide greater insight. Lastly, this study was not able to assess whether total tumor volume (calculated by the equation (4π × r1 × r2 × r3)/3; where r1, r2, and r3 are half of the largest, intermediate, and shortest tumor dimensions respectively) could be a prognosticator of oncological outcomes.
In summary, the results of this study show that surgical resection of giant HCC is associated with poorer long-term survival outcomes and should therefore be treated as a separate disease entity. While it was found that surgical resection of both giant and non-giant HCC had similar safety profiles, this may be confounded by poor reporting of the 90-d mortality rate. HCC staging systems should account for these size differences.
There is currently no consensus on the inclusion of tumor size in hepatocellular carcinoma (HCC) staging systems. Furthermore, the size cut-off may vary in systems that incorporate tumor size, and a consensus is warranted for inclusion of size into the staging criteria with cut-off to be determined by multi-center collaborative clinical studies.
Research on long-term survival after resection of giant (≥ 10 cm) and non-giant HCC (< 10 cm) has produced conflicting results.
This study aimed to investigate whether oncological outcomes and safety profiles of resection differ between giant and non-giant HCC.
PubMed, MEDLINE, EMBASE, and Cochrane databases were searched. Studies designed to investigate the outcomes of giant vs non-giant HCC were included. The primary endpoints were overall survival (OS) and disease-free survival (DFS). The secondary endpoints were postoperative complications and mortality rates. All studies were assessed for bias using the Newcastle–Ottawa Scale.
24 retrospective cohort studies involving 23747 patients (giant = 3326; non-giant = 20421) who underwent HCC resection were included. OS was reported in 24 studies, DFS in 17 studies, 30-d mortality rate in 18 studies, postoperative complications in 15 studies, and post-hepatectomy liver failure (PHLF) in six studies. The HR was significantly lower for non-giant HCC in both OS (HR 0.53, 95%CI: 0.50-0.55, P < 0.001) and DFS (HR 0.62, 95%CI: 0.58-0.84, P < 0.001). No significant difference was found for 30-d mortality rate (OR 0.73, 95%CI: 0.50-1.08, P = 0.116), postoperative complications (OR 0.81, 95%CI: 0.62-1.06, P = 0.140), and PHLF (OR 0.81, 95%CI: 0.62-1.06, P = 0.140).
Resection of giant HCC is associated with poorer long-term outcomes. The safety profile of resection was similar in both groups; however, this may have been confounded by reporting bias. HCC staging systems should account for the size differences.
Future prospective studies should investigate different modalities of intervention for giant HCC to determine whether these treatments can provide better quality of life outcomes with low therapy-associated morbidity.
We thank Dr. Chan Yiong Huak (National University Health System) for reviewing and providing statistical guidance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li JX, China; Yu YB, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52:1898-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 2. | Sheriff S, Madhavan S, Lei GY, Chan YH, Junnarkar SP, Huey CW, Low JK, Shelat VG. Predictors of mortality within the first year post-hepatectomy for hepatocellular carcinoma. J Egypt Natl Canc Inst. 2022;34:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1477] [Article Influence: 295.4] [Reference Citation Analysis (1)] |
| 4. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1487] [Cited by in RCA: 1456] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 5. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 3091] [Article Influence: 441.6] [Reference Citation Analysis (17)] |
| 6. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1156] [Article Influence: 128.4] [Reference Citation Analysis (0)] |
| 7. | Halabi S, Owzar K. The importance of identifying and validating prognostic factors in oncology. Semin Oncol. 2010;37:e9-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, Lee FY, Lin HC, Huo TI. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 2016;64:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 9. | Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: a population-based study. Cancer Manag Res. 2018;10:4401-4410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Yang A, Xiao W, Chen D, Wei X, Huang S, Lin Y, Zhang C, Lin J, Deng F, Wu C, He X. The power of tumor sizes in predicting the survival of solitary hepatocellular carcinoma patients. Cancer Med. 2018;7:6040-6050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3060] [Article Influence: 765.0] [Reference Citation Analysis (61)] |
| 12. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4658] [Article Influence: 517.6] [Reference Citation Analysis (4)] |
| 13. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 547] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 14. | Noh JH, Kim TS, Ahn KS, Kim YH, Kang KJ. Prognostic factors after hepatic resection for the single hepatocellular carcinoma larger than 5 cm. Ann Surg Treat Res. 2016;91:104-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Allemann P, Demartines N, Bouzourene H, Tempia A, Halkic N. Long-term outcome after liver resection for hepatocellular carcinoma larger than 10 cm. World J Surg. 2013;37:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Fang Q, Xie QS, Chen JM, Shan SL, Xie K, Geng XP, Liu FB. Long-term outcomes after hepatectomy of huge hepatocellular carcinoma: A single-center experience in China. Hepatobiliary Pancreat Dis Int. 2019;18:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Lee CW, Yu MC, Wang CC, Lee WC, Tsai HI, Kuan FC, Chen CW, Hsieh YC, Chen HY. Liver resection for hepatocellular carcinoma larger than 10 cm: A multi-institution long-term observational study. World J Gastrointest Surg. 2021;13:476-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Jung YK, Jung CH, Seo YS, Kim JH, Kim TH, Yoo YJ, Kang SH, Yim SY, Suh SJ, An H, Yim HJ, Yeon JE, Byun KS, Um SH. BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A. J Gastroenterol Hepatol. 2016;31:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wan L, Dong DH, Wu XN, Ding HF, Lu Q, Tian Y, Zhang XF, Li W. Single Large Nodule (>5 cm) Prognosis in Hepatocellular Carcinoma: Kinship with Barcelona Clinic Liver Cancer (BCLC) Stage A or B? Med Sci Monit. 2020;26:e926797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 1896] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 21. | Chang YJ, Chung KP, Chang YJ, Chen LJ. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg. 2016;103:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 22. | Choi GH, Han DH, Kim DH, Choi SB, Kang CM, Kim KS, Choi JS, Park YN, Park JY, Kim DY, Han KH, Chon CY, Lee WJ. Outcome after curative resection for a huge (>or=10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg. 2009;198:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Giuliante F, De Rose AM, Guerra V, Ardito F, Nuzzo G, Carr BI. Clinical characteristics and survival of European patients with resectable large hepatocellular carcinomas. J Gastrointest Cancer. 2013;44:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Huang P, Liu C, Li B, Zheng Y, Zou R, Huang J, Hu Z, Yuan Y. Preoperative mean corpuscular hemoglobin affecting long-term outcomes of hepatectomized patients with hepatocellular carcinoma. Mol Clin Oncol. 2016;4:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Jo S. Outcome of Hepatectomy for Huge Hepatocellular Carcinoma. Korean J Hepatobiliary Pancreat Surg. 2011;15:90-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Lewis RH, Glazer ES, Bittenbinder DM, O'Brien T, Deneve JL, Shibata D, Behrman SW, Vanatta JM, Satapathy SK, Dickson PV. Outcomes Following Resection of Hepatocellular Carcinoma in the Absence of Cirrhosis. J Gastrointest Cancer. 2019;50:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, Fong Y, D'Angelica MI, Blumgart LH, DeMatteo RP. Outcome of partial hepatectomy for large (> 10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Nagano Y, Tanaka K, Togo S, Matsuo K, Kunisaki C, Sugita M, Morioka D, Miura Y, Kubota T, Endo I, Sekido H, Shimada H. Efficacy of hepatic resection for hepatocellular carcinomas larger than 10 cm. World J Surg. 2005;29:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 29. | Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Shah SA, Wei AC, Cleary SP, Yang I, McGilvray ID, Gallinger S, Grant DR, Greig PD. Prognosis and results after resection of very large (>or=10 cm) hepatocellular carcinoma. J Gastrointest Surg. 2007;11:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Tanaka S, Iimuro Y, Hirano T, Hai S, Suzumura K, Fujimoto J. Outcomes of hepatic resection for large hepatocellular carcinoma: special reference to postoperative recurrence. Am Surg. 2015;81:64-73. [PubMed] |
| 32. | Taniai N, Yoshida H, Tajiri T. Adaptation of hepatectomy for huge hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 33. | Thng Y, Tan JK, Shridhar IG, Chang SK, Madhavan K, Kow AW. Outcomes of resection of giant hepatocellular carcinoma in a tertiary institution: does size matter? HPB (Oxford). 2015;17:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Wakayama K, Kamiyama T, Yokoo H, Orimo T, Shimada S, Einama T, Kamachi H, Taketomi A. Huge hepatocellular carcinoma greater than 10 cm in diameter worsens prognosis by causing distant recurrence after curative resection. J Surg Oncol. 2017;115:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Yamashita Y, Taketomi A, Shirabe K, Aishima S, Tsuijita E, Morita K, Kayashima H, Maehara Y. Outcomes of hepatic resection for huge hepatocellular carcinoma (≥ 10 cm in diameter). J Surg Oncol. 2011;104:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 36. | Yang J, Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Is hepatectomy for huge hepatocellular carcinoma (≥ 10 cm in diameter) safe and effective? Asian Pac J Cancer Prev. 2014;15:7069-7077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Yang L, Xu J, Ou D, Wu W, Zeng Z. Hepatectomy for huge hepatocellular carcinoma: single institute's experience. World J Surg. 2013;37:2189-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Yeh CN, Lee WC, Chen MF. Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann Surg Oncol. 2003;10:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Zhong JH, Pan LH, Wang YY, Cucchetti A, Yang T, You XM, Ma L, Gong WF, Xiang BD, Peng NF, Wu FX, Li LQ. Optimizing stage of single large hepatocellular carcinoma: A study with subgroup analysis by tumor diameter. Medicine (Baltimore). 2017;96:e6608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Zhu SL, Chen J, Li H, Li LQ, Zhong JH. Efficacy of hepatic resection for huge (≥ 10 cm) hepatocellular carcinoma: good prognosis associated with the uninodular subtype. Int J Clin Exp Med. 2015;8:20581-20588. [PubMed] |
| 41. | Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular Carcinoma with Macrovascular Invasion: Defining the Optimal Treatment Strategy. Liver Cancer. 2017;6:360-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Selçuk H. Prognostic Factors and Staging Systems in Hepatocellular Carcinoma. Exp Clin Transplant. 2017;15:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 43. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4380] [Article Influence: 876.0] [Reference Citation Analysis (4)] |
| 44. | Frenette CT, Isaacson AJ, Bargellini I, Saab S, Singal AG. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin Proc Innov Qual Outcomes. 2019;3:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 45. | . A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 966] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 46. | . Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 378] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of primary hepatocellular carcinoma. Hepatology. 1984;4:3S-6S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Egger ME, Ohlendorf JM, Scoggins CR, McMasters KM, Martin RC 2nd. Assessment of the reporting of quality and outcome measures in hepatic resections: a call for 90-day reporting in all hepatectomy series. HPB (Oxford). 2015;17:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Wei CY, Chen PC, Chau GY, Lee RC, Chen PH, Huo TI, Huang YH, Su YH, Hou MC, Wu JC, Su CW. Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma. Ann Transl Med. 2020;8:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Zhu SL, Zhong JH, Ke Y, Ma L, You XM, Li LQ. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol. 2015;21:9630-9637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Min YW, Lee JH, Gwak GY, Paik YH, Rhee PL, Koh KC, Paik SW, Yoo BC, Choi MS. Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol. 2014;29:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Gui CH, Baey S, D'cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020;46:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 53. | Tong VJW, Shelat VG, Chao YK. Clinical application of advances and innovation in radiation treatment of hepatocellular carcinoma. J Clin Transl Res. 2021;7:811-833. [PubMed] |