Published online Feb 27, 2023. doi: 10.4240/wjgs.v15.i2.234
Peer-review started: October 7, 2022
First decision: January 3, 2023
Revised: January 5, 2023
Accepted: February 3, 2023
Article in press: February 3, 2023
Published online: February 27, 2023
Processing time: 143 Days and 11.3 Hours
Hepatobiliary manifestations occur in ulcerative colitis (UC) patients. The effect of laparoscopic restorative proctocolectomy (LRP) with ileal pouch anal anastomosis (IPAA) on hepatobiliary manifestations is debated.
To evaluate hepatobiliary changes after two-stages elective laparoscopic restorative proctocolectomy for patients with UC.
Between June 2013 and June 2018, 167 patients with hepatobiliary symptoms underwent two-stage elective LRP for UC in a prospective observational study. Patients with UC and having at least one hepatobiliary manifestation who underwent LRP with IPAA were included in the study. The patients were followed up for four years to assess the outcomes of hepatobiliary manifestations.
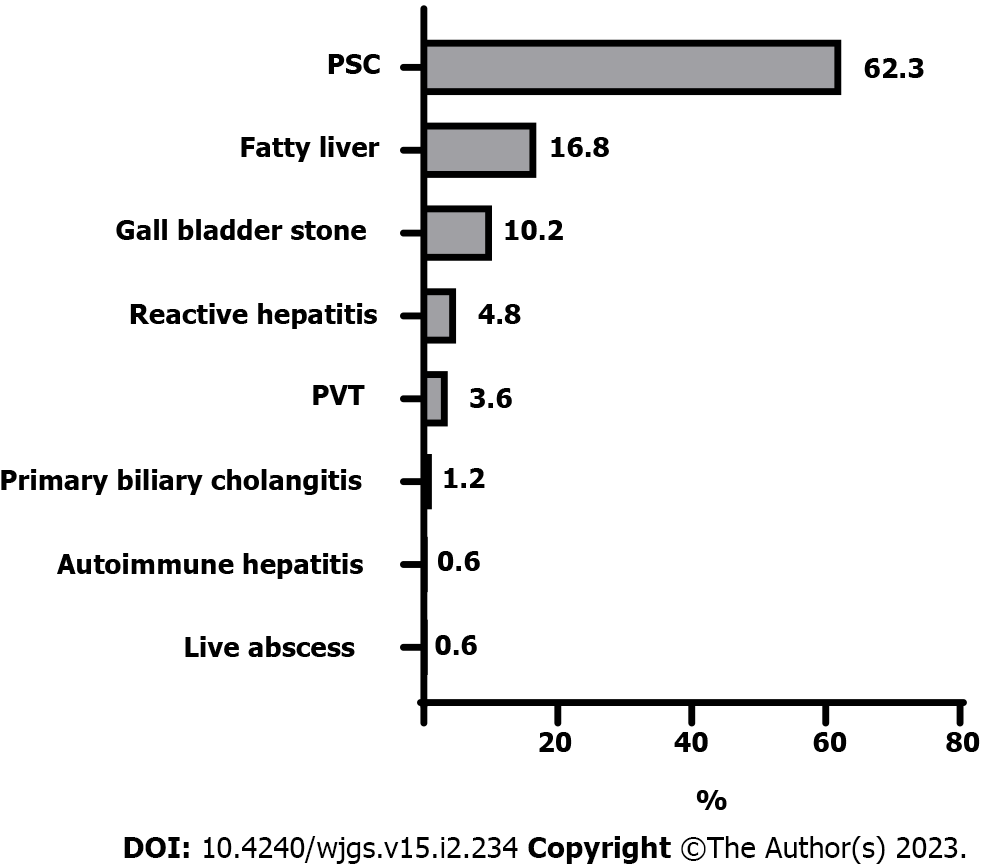
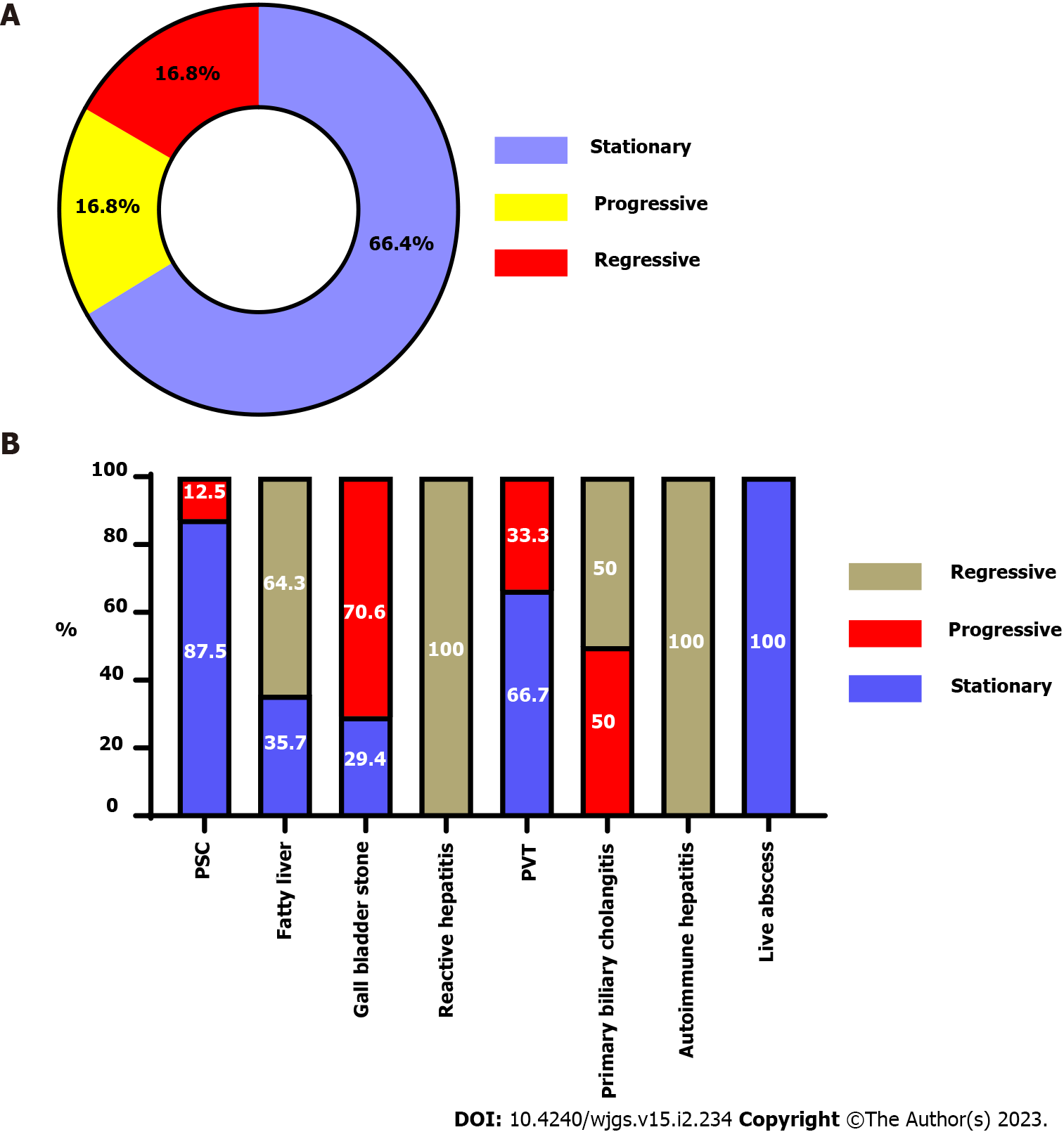
The patients' mean age was 36 ± 8 years, and males predominated (67.1%). The most common hepatobiliary diagnostic method was liver biopsy (85.6%), followed by Magnetic resonance cholangiopancreatography (63.5%), Antineutrophil cytoplasmic antibodies (62.5%), abdominal ultrasonography (35.9%), and Endoscopic retrograde cholangiopancreatography (6%). The most common hepatobiliary symptom was Primary sclerosing cholangitis (PSC) (62.3%), followed by fatty liver (16.8%) and gallbladder stone (10.2%). 66.4% of patients showed a stable course after surgery. Progressive or regressive courses occurred in 16.8% of each. Mortality was 6%, and recurrence or progression of symptoms required surgery for 15%. Most PSC patients (87.5%) had a stable course, and only 12.5% became worse. Two-thirds (64.3%) of fatty liver patients showed a regressive course, while one-third (35.7%) showed a stable course. Survival rates were 98.8%, 97%, 95.8%, and 94% at 12 mo, 24 mo, 36 mo, and at the end of the follow-up.
In patients with UC who had LRP, there is a positive impact on hepatobiliary disease. It caused an improvement in PSC and fatty liver disease. The most prevalent unchanged course was PSC, while the most common improvement was fatty liver disease.
Core Tip: There has been little research on the efficacy of proctocolectomy in ulcerative colitis patients with hepatobiliary manifestations. The course of hepatobiliary symptoms after proctocolectomy is being evaluated prospectively in our study. The main finding of this study was that two-thirds of patients had an unchanged course following surgery, whereas 16.8% had a progressive or regressive course. The mortality rate was 6%, and 15% of patients required surgery due to recurrence or worsening symptoms. Most primary sclerosing cholangitis patients (87.5%) had an unchanged course, with only 12.5% progressing. Two-thirds (64.3%) of fatty liver patients progressed, whereas one-third (35.7%) remained stationary. At 12, 24, 36, and 48 mo, the survival rates were 98.8%, 97%, 95.8%, and 94%, respectively.
- Citation: Habeeb TAAM, Hussain A, Podda M, Cianci P, Ramshaw B, Safwat K, Amr WM, Wasefy T, Fiad AA, Mansour MI, Moursi AM, Osman G, Qasem A, Fawzy M, Alsaad MIA, Kalmoush AE, Nassar MS, Mustafa FM, Badawy MHM, Hamdy A, Elbelkasi H, Mousa B, Metwalli AEM, Mawla WA, Elaidy MM, Baghdadi MA, Raafat A. Hepatobiliary manifestations following two-stages elective laparoscopic restorative proctocolectomy for patients with ulcerative colitis: A prospective observational study. World J Gastrointest Surg 2023; 15(2): 234-248
- URL: https://www.wjgnet.com/1948-9366/full/v15/i2/234.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i2.234
Inflammatory bowel disease (IBD) is expected to affect 1% of the population over the next decade[1]. Although the primary clinical manifestations of IBD are centred on the gastrointestinal tract, 25%–40% of IBD patients develop at least one extraintestinal manifestation (EIM)[2].
Hepatobiliary manifestations constitute one of the most common EIMs in IBD[3]. Primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH), fatty liver, cholelithiasis, primary biliary cholangitis, portal vein thrombosis, and hepatic abscess are the most prevalent hepatobiliary manifestations of ulcerative colitis (UC)[4,5].
Most UC patients can be managed with medications, but minorities require proctocolectomy[6]. Two-stage laparoscopic proctocolectomy (LRP) with ileal pouch-anal anastomosis (IPAA) is a cure for UC, but its effect on hepatobiliary diseases is controversial[6-10]. Therefore, we conducted a prospective observational study to examine the effects of LRP with IPAA on hepatobiliary symptoms to evaluate the role of surgery in preventing or ameliorating liver damage from the disease progression.
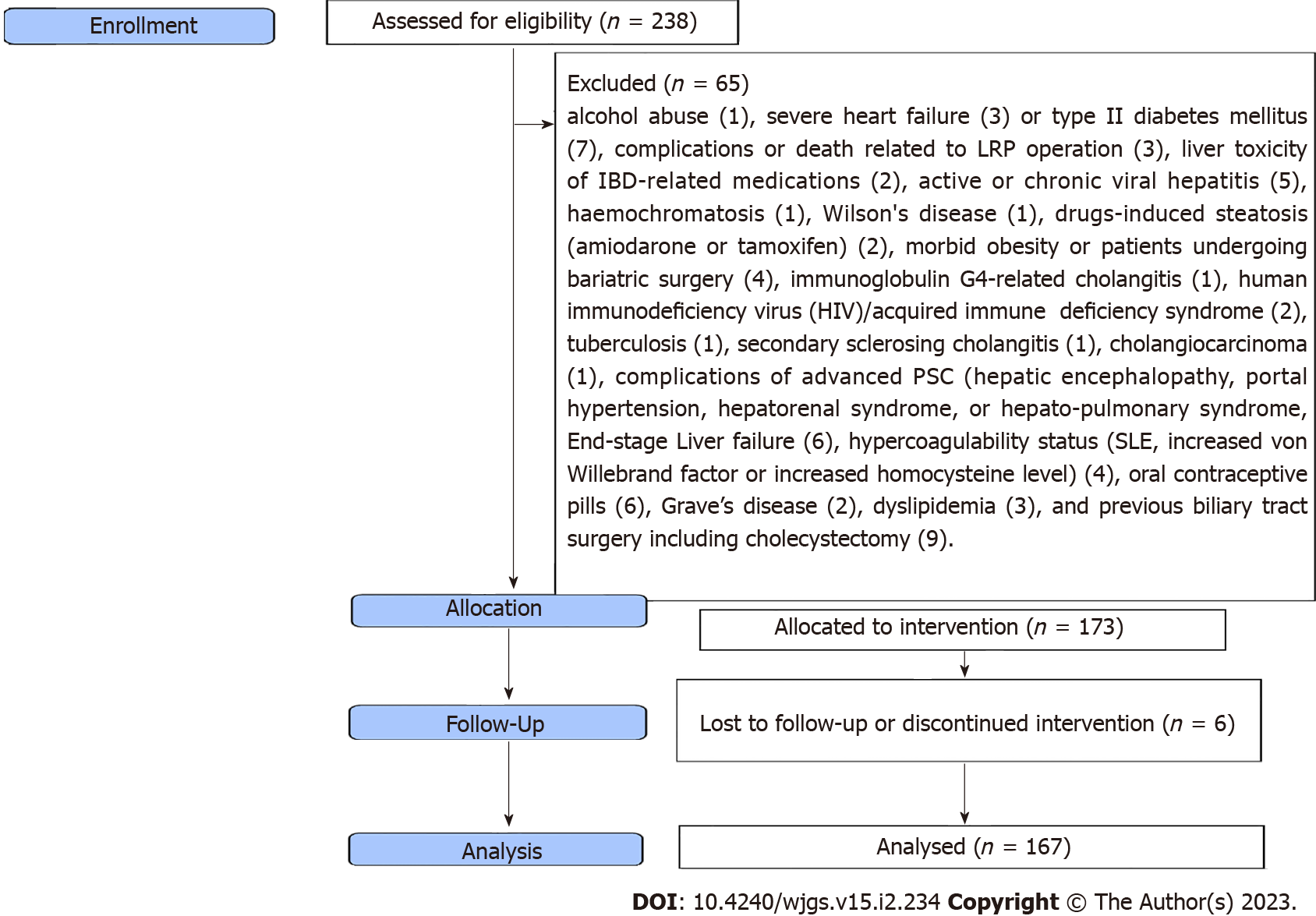
This is a prospective observational study on 167 patients with hepatobiliary manifestations who underwent two-stage elective LRP with IPAA for UC from June 2013 to June 2018 at our universities’ hospitals. Inclusion criteria were all patients between 18 and 69 years; men and women with at least one hepatobiliary manifestation. In patients with UC, surgery was decided according to The European Crohn’s and Colitis Organisation guidelines on therapeutics in UC[11]. Exclusion criteria included: Alcohol abuse, severe heart failure or type II diabetes mellitus, complications or death related to LRP operation, liver toxicity of IBD-related medications, active or chronic viral hepatitis, hemochromatosis, Wilson's disease, drugs-induced steatosis (amiodarone or tamoxifen), morbid obesity or patients undergoing bariatric surgery, immunoglobulin G4-related cholangitis; human immunodeficiency virus/acquired immune deficiency syndrome; tuberculosis; secondary sclerosing cholangitis; cholangiocarcinoma; complications of advanced PSC (hepatic encephalopathy, portal hypertension, hepatorenal syndrome, or hepato-pulmonary syndrome; end-stage liver failure), hypercoagulability status (systemic lupus erythematosus, increased von Willebrand factor or increased homocysteine level), oral contraceptive pills, Grave's disease, dyslipidemia, and previous biliary tract surgery including cholecystectomy.
The Institutional Review Board approved the study (Approval No. ZU IRB#9841). Each patient signed a written consent form, and the study followed the rules of the 1975 Declaration of Helsinki principles. In addition, the study was registered on ClinicalTrials.gov (NCT05495178) and done according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
PSC is progressive biliary fibrosis affecting intra and/or extrahepatic bile ducts[12] and diagnosed by laboratory tests [(cholestasis, Antineutrophil cytoplasmic antibodies (ANCA)], radiology [abdominal ultrasonography (US), abdominal computed tomography (CT), endoscopic retrograde cholangiopancreatography (ERCP), or magnetic resonance cholangiopancreatography (MRCP)], and liver biopsy. Primary biliary cholangitis (PBC) is characterized by the loss of small and medium-sized bile ducts on liver biopsy, elevated anti-mitochondrial antibodies, and altered gamma-glutamyl transferase and alkaline phosphatase (ALP) levels[13]. Non-alcoholic fatty liver disease (NAFLD) is characterized by fat storage in ≥ 5% of hepatic steatosis in the absence of concomitant liver disease (chronic viral hepatitis), use of steatosis-inducing medications (amiodarone or tamoxifen), autoimmune hepatitis, hemochromatosis, Wilson's disease, or excessive alcohol consumption[14]. Diagnosis of NAFLD was made by liver biopsies or US[15], and the severity score was previously stated[16]. Autoimmune hepatitis diagnosis based on the International Autoimmune Hepatitis Group criteria with a score of > 15 points consisting of demographic, histologic, and laboratory markers, including antinuclear antibodies with a titer of at least 1:40 and liver histology[17]. An aseptic liver abscess is diagnosed based on IBD history, US, and CT[18]. Ultrasound, colour Doppler, and/or CT scans were used to detect portal vein thrombosis.
For patients with UC who require surgery, two stages of LRP with IPAA and a diverting loop ileostomy are the gold standard[19]. The diverting loop ileostomy was reversed 2-3 mo following surgery. Because of the increased risk of thromboembolic events, prophylactic anticoagulation medication was scheduled and continued for six months after surgery. The follow-up period was four years, and cases lost during the follow-up period were excluded from the study.
Follow-up with the clinical progression of patients' conditions and laboratory evaluations was performed at six months, one year, two years, three years, and four years, or at any time of patients’ complaint. These data were compared to data obtained immediately before surgery (at the time of surgery). Follow-ups were performed at outpatient clinics, via phone, or by email. Laboratory (Liver function tests, antibodies, Cancer antigen 19-9) and radiology (abdominal ultrasonography, colour Doppler, CT, MRCP) tests were performed as part of the follow-up assessments. An endoscopic examination of the ileal pouch on an annual basis was arranged. A liver biopsy was planned at the end of the study.
Version 28 of SPSS was used for data management and statistical analysis (IBM, Armonk, New York, United States). The normality of quantitative data was evaluated using the Kolmogorov–Smirnov test, the Shapiro-Wilk test, and direct data visualization methods. Means and standard deviations, or medians and ranges based on normality tests, were used to summarize the quantitative data. As a summary of categorical data, numbers and percentages were used. The McNemar test compared laboratory and clinical findings before and after surgery. We used a Kaplan-Meier analysis to estimate overall survival and recurrence-free survival. The independent t-test or the Mann-Whitney U test for normally and non-normally distributed quantitative variables was used to compare the regression rates of hepatobiliary manifestations in the two groups. We compared categorical data using the Chi-square test. Multivariate logistic regression analysis was done to predict no regression of hepatobiliary manifestations. Each significant variable on the univariate levels was included in a multivariate regression model and adjusted for age, gender, smoking, family history of UC, and UC duration. The odds ratios and confidence intervals at 95% were calculated. All statistical tests were two-sided. P values < 0.05 were considered statistically significant.
Figure 1 shows the flow diagram of the inclusion and exclusion criteria of the study cohort. As shown in Table 1, the mean age was 36 ± 8 years, with male predominance (67.1%). The most frequent diagnostic method for hepatobiliary manifestations was liver biopsy (85.6%), followed by MRCP (63.5%), ANCA (62.5%), US (35.9%), and ERCP (6%). Figure 2 shows the frequency of different hepatobiliary manifestations.
| General characteristics | |
| Age (yr) (mean ± SD) | 36 ± 8 |
| Sex | |
| Male | 112 (67.1) |
| Female | 55 (32.9) |
| Smoking | 50 (29.9) |
| Family history of ulcerative colitis | 35 (21.0) |
| Ulcerative colitis disease duration before surgery (mo) | 39 (4 - 90) |
| Treatment of ulcerative colitis | |
| Mesalazine | 137 (82.0) |
| Sulphasalazine | 30 (18.0) |
| Anti-TNF | 21 (12.6) |
| Corticosteroids | 65 (38.9) |
| Type of PSC1 | |
| Large duct PSC | 83 (79.8) |
| Small duct PSC | 21 (20.2) |
| Family history of PSC | 14 (8.4) |
| Diagnosis and treatment of hepatobiliary manifestations | |
| Diagnostic methods | |
| ANCA | 104 (62.3) |
| MRCP | 106 (63.5) |
| ERCP | 10 (6.0) |
| Liver biopsy | 143 (85.6) |
| Ultrasound | 60 (35.9) |
| Treatment | |
| UDCA | 106 (63.5) |
| LMWH | 6 (3.6) |
| Steroid | 2 (1.2) |
| Obeticholic acid | 2 (1.2) |
| Sonar guided drainage | 1 (0.6) |
| Fibrates | 2 (1.2) |
| Azathioprine | 1 (0.6) |
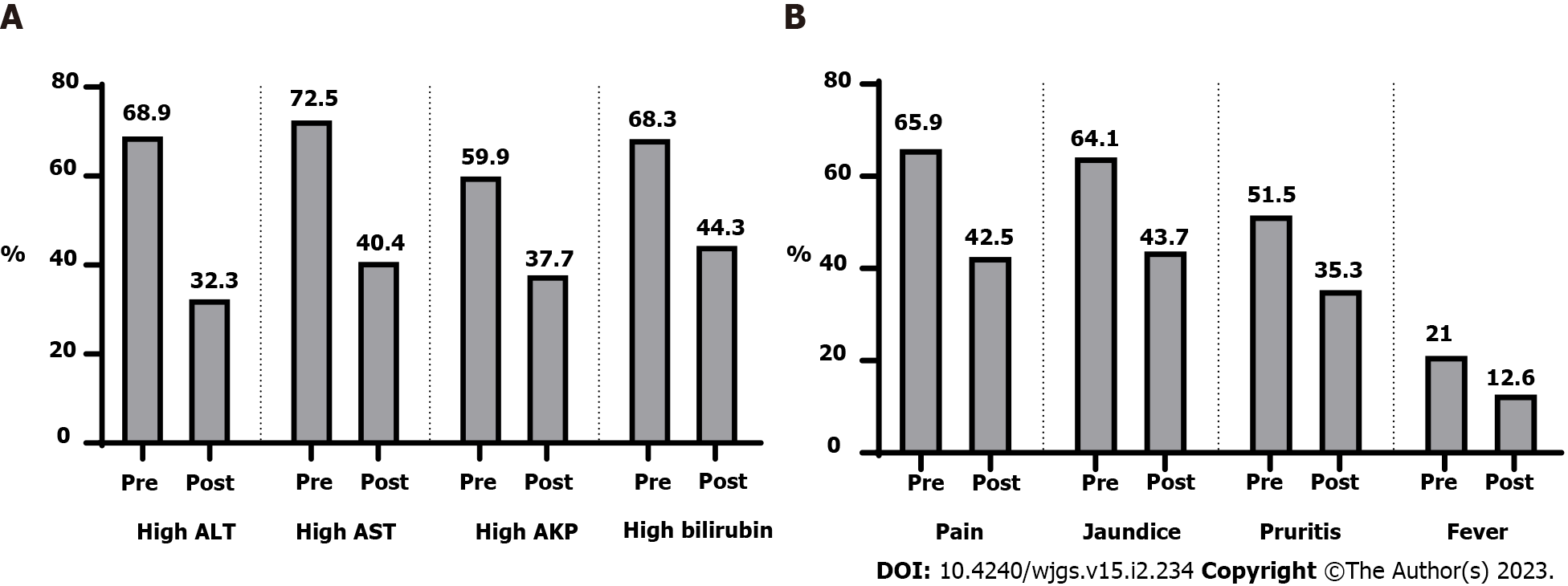
After surgery, there were clinical and laboratory improvements (Table 2 and Figure 3). Figure 4 showed that 66.4% of patients had a stationary course. In comparison, 16.8% of patients showed a progressive or regressive course, and there are variations in the courses of different types of hepatobiliary manifestations.
| P value | ||
| High ALT | ||
| Before surgery | 115 (68.9) | < 0.0011 |
| After surgery | 54 (32.3) | |
| High AST | ||
| Before surgery | 121 (72.5) | < 0.0011 |
| After surgery | 68 (40.7) | |
| High alkaline phosphatase | ||
| Before surgery | 100 (59.9) | < 0.0011 |
| After surgery | 63 (37.7) | |
| High bilirubin | ||
| Before surgery | 114 (68.3) | < 0.0011 |
| After surgery | 74 (44.3) | |
| Pain | ||
| Before surgery | 110 (65.9) | < 0.0011 |
| After surgery | 71 (42.5) | |
| Jaundice | ||
| Before surgery | 107 (64.1) | < 0.0011 |
| After surgery | 73 (43.7) | |
| Pruritus | ||
| Before surgery | 86 (51.5) | < 0.0011 |
| After surgery | 59 (35.3) | |
| Fever | ||
| Before surgery | 35 (21.0) | < 0.0011 |
| After surgery | 21 (12.6) | |
| Fatty liver score | ||
| Before surgery | 2 (1- 3) | < 0.0011 |
| After surgery | 1 (0-3) |
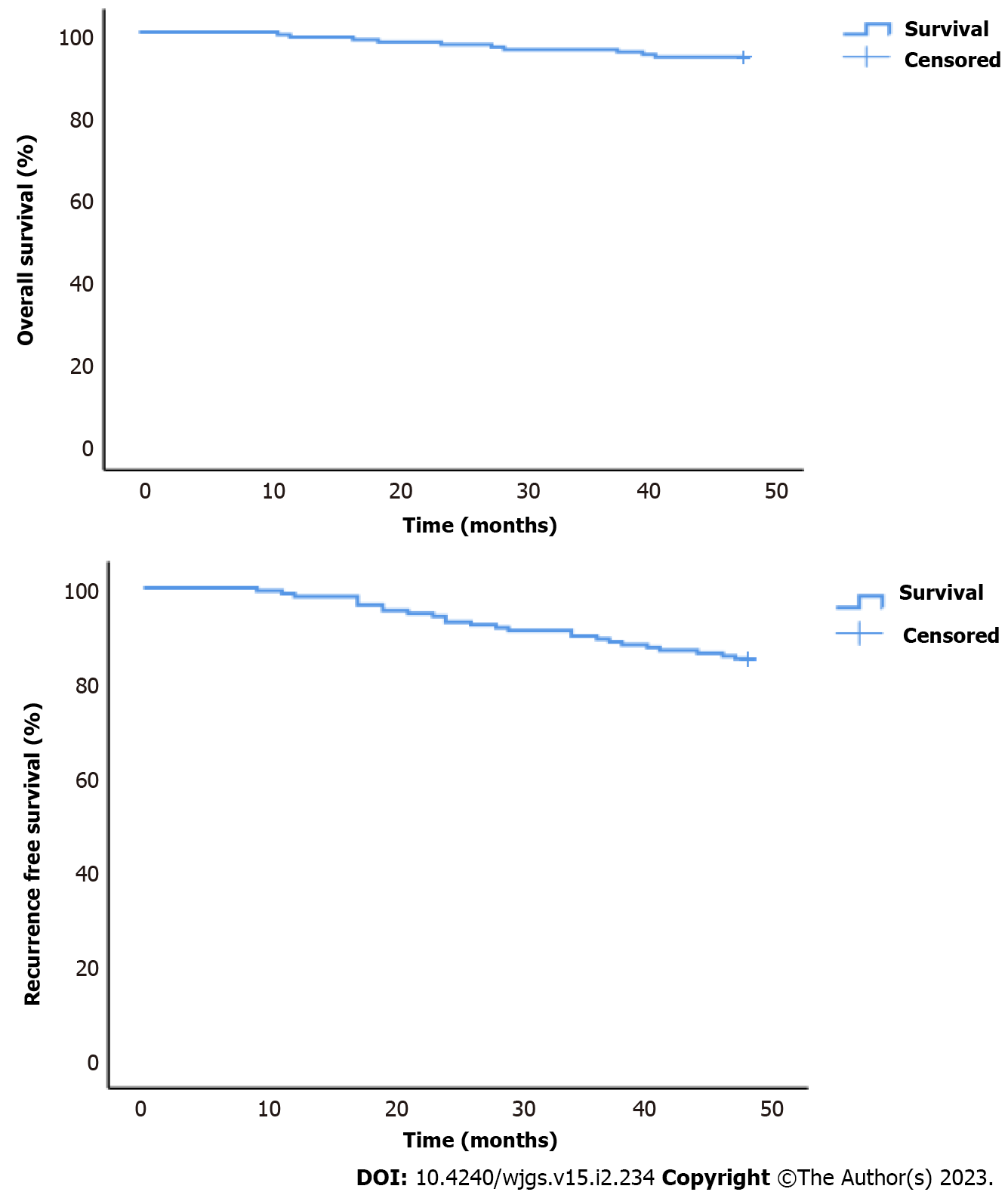
The survival rate was 98.8%, 97%, 95.8%, and 94% at 12 mo, 24 mo, 36 mo, and at the end of the follow-up. Regarding the recurrence or progression of symptoms requiring surgery, the recurrence-free rate at 12 mo was 98.2%. At 24 mo, it was 92.8%. At 36 mo, it was 89.2%. At the end of the follow-up, it reached 85% (Figure 5).
Patients with no regression (stationary and progressive course) demonstrated higher use of anti-Tumor necrosis factor (15.1% vs 0%, P = 0.028), corticosteroids (43.2% vs 17.9%, P = 0.012), and hepatobiliary treatment (80.6% vs 7.1%, P < 0.001). In addition, they demonstrated higher percentages of high alanine transaminase (ALT) (74.8 vs 39.3%, P < 0.001), high aspartate aminotransferase (AST) (75.5% vs 57.1%, P = 0.047), high alkaline phosphatase (71.2% vs 3.6%, P < 0.001), jaundice (67.6% vs 46.4%, P = 0.033), pruritus (59% vs 14.3%, P < 0.001), and fever (24.5% vs 3.6%, P = 0.013). Age (P = 0.578), gender (P = 0.327), smoking (P = 0.780), family history (P = 0.278), duration of UC (P = 0.877), treatment for UC (P = 0.601), family history of PSC (P = 0.079), pain (P = 0.496), and fatty liver score (P = 0.121) were not found to be significantly different (Table 3).
| Manifestations regression | P value | ||
| Yes (n = 28) | No (n = 139) | ||
| Age (yr) | 37 ± 7 | 36 ± 8 | 0.578 |
| Sex | |||
| Males | 21 (75) | 91 (65.5) | 0.327 |
| Females | 7 (25) | 48 (34.5) | |
| Smoking | 9 (32.1) | 41 (29.5) | 0.780 |
| Family history | 8 (28.6) | 27 (19.4) | 0.278 |
| UC duration (mo) | 37 (7-81) | 40 (4-90) | 0.877 |
| UC treatment | |||
| Mesalazine | 22 (78.6) | 115 (82.7) | 0.601 |
| Sulphasalazine | 6 (21.4) | 24 (17.3) | |
| Anti-TNF | 0 (0) | 21 (15.1) | 0.0281 |
| Corticosteroids | 5 (17.9) | 60 (43.2) | 0.0121 |
| Hepatobiliary manifestations | |||
| Autoimmune hepatitis | 1 (3.6) | 0 (0) | NA |
| Fatty liver | 18 (64.3) | 10 (7.2) | |
| Gall bladder stone | 0 (0) | 17 (12.2) | |
| Liver abscess | 0 (0) | 1 (0.7) | |
| Primary biliary cholangitis | 1 (3.6) | 1 (0.7) | |
| PSC | 0 (0) | 104 (74.8) | |
| Portal vein thrombosis | 0 (0) | 6 (4.3) | |
| Reactive hepatitis | 8 (28.6) | 0 (0) | |
| Type of PSC | |||
| Large duct PSC | 0 (0) | 83 (79.8) | NA |
| Small duct PSC | 0 (0) | 21 (20.2) | |
| Family history of PSC | 0 (0) | 14 (10.1) | 0.079 |
| Hepatobiliary treatment | 2 (7.1) | 112 (80.6) | < 0.0011 |
| High ALT | 11 (39.3) | 104 (74.8) | < 0.0011 |
| High AST | 16 (57.1) | 105 (75.5) | 0.0471 |
| High Alkaline phosphatase | 1 (3.6) | 99 (71.2) | < 0.0011 |
| High bilirubin | 14 (50) | 100 (71.9) | 0.0231 |
| Pain | 20 (71.4) | 90 (64.7) | 0.496 |
| Jaundice | 13 (46.4) | 94 (67.6) | 0.0331 |
| Pruritus | 4 (14.3) | 82 (59) | < 0.0011 |
| Fever | 1 (3.6) | 34 (24.5) | 0.0131 |
| Fatty liver score | 2 (1-3) | 2 (2-3) | 0.121 |
The predictors of no regression were steroid treatment (OR = 3.68, 95%CI = 1.29 – 10.45, P = 0.015), high ALT (OR = 5.39, 95%CI = 2.19 – 13.28, P < 0.001), high AST (OR = 2.59, 95%CI = 1.08 – 6.2, P = 0.032), high ALP (OR = 73.59, 95%CI = 9.52 – 568.93, P < 0.001), high bilirubin (OR = 2.72, 95%CI = 1.16 – 6.4, P = 0.022), jaundice (OR = 2.49, 95%CI = 1.07 – 5.8, P = 0.034), pruritus (OR = 9.75, 95%CI = 3.12 – 30.5, P < 0.001), and fever (OR = 9.7, 95%CI = 1.25 – 75.03, P = 0.029). The predictors with their odds ratios and 95% confidence intervals are illustrated in Table 4.
To the best of our knowledge, this is the first current study to investigate the course of hepatobiliary symptoms in patients with UC after elective two-stage LRP with IPAA. Colectomy was correlated to a considerably low rate of progressive course in this study of 167 patients: 66.4% for an unchanged course, 16.8% for a regressive course, and 16.8% for a progressive course. There were not many changes for PSC (91/104, 87.5%), while the most progressive cases were gallbladder disorders (12/17, 70.5%), and the most regressive cases were fatty liver (18/28, 64.3%).
Many theories have been proposed to explain the course of PSC after LRP with IPAA, including autoimmune phenomena[20], liver-gut crosstalk[21], the influence of saturated fat on changes in the bile acid pool, with an increase in the taurocholic acid[22], and bacterial translocation or absorption of bacterial endotoxins into the portal circulation via a chronically inflamed bowel with Kupffer cell activation[23-26]. The effects of colectomy on PSC have been documented in conflicting ways. Colectomy was beneficial according to a study by Lepistö et al[6] that stated that PSC severity increased in four (13%) patients, regressed in 15 (50%), and stayed stationary in 11 (37%).Regarding the incidence of progression, our findings are identical to those of this study, but not in the incidence of stationary and regressive courses. Our stationary course of PSC is higher (87.5% vs 37%), and none of the cases in our study demonstrated a regression course. We performed liver biopsies and MRCP on all PSC patients prior to surgery and during the follow-up period, whereas the previous study relied on liver function for diagnosis and did not perform liver biopsies on all patients. Furthermore, our study had a large number of patients, and cases lost to follow-up were excluded from our study (in comparison to earlier study). Our study's higher stable course of PSC may be attributable to Ursodeoxycholic acid (UDCA) (15 mg/kg/day) in all patients after LRP, whereas only 9/30 patients had UDCA following colectomy in the prior study. Treatment with UDCA has a beneficial effect on the course of PSC; studies have demonstrated efficacy[27-30]. A good proportion of PSC cases not progressing to a more severe form is associated with the absence of pouchitis in all of our patients' ileoanal pouch anastomosis. The study corroborated our conclusion that pouchitis may aggravate PSC[31].
In contrast, another study by Cangemi et al[7] stated that proctocolectomy exerted no beneficial effect on PSC, the stage of which has remained unchanged or has progressed with no statistically significant improvements in liver function test values[7]. On the contrary, our results showed a statistically significant improvement in liver function test values after surgery. Variations in the results could be attributed to methodological differences, diagnostic procedures (liver biopsy in 71% of cases only), and the number of cases. Perhaps, the effect of LRP on PSC is beneficial, as evidenced by the higher percentage of stable disease and smaller progressive cases. Another study by Treeprasertsuk et al[32] discovered that proctocolectomy had no benefit and a lower survival rate than expected. They experienced only progressive courses with higher mortality rates; LCF, acute cholangitis, right hepatic vein thrombosis with liver infarcts, and many cases that needed liver transplantation. The poor prognosis could be attributed to the study's small sample size, open approach, surgical difficulties, and heterogeneity in selection criteria, particularly the inclusion of cirrhotic patients with low platelet counts and albumin levels.
In patients with LRP, resection of a short segment of the ileum and the entire colon inhibits bile acid absorption, resulting in supersaturation of biliary cholesterol[8], pouch metaplasia with decreased primary bile acid absorption[33], and delayed gall bladder emptying[34]. We found a high incidence of recurrent biliary colic requiring surgery (12/17, 70.5%), while the remaining five cases had a stationary course without symptoms. Concomitant cholecystectomy with LRP may prolong the operative time (nearly 40 min) and add more risk of complications. However, it saves the patient from going through more difficult cholecystectomy operation /gallstone complications in the future[35]. There were no cases of gallbladder cancer in our study. This is because most gallstone cases develop symptoms following LRP, necessitating cholecystectomy. Another problem is the short duration of follow-up (4 years).
Multiple studies showed that proctocolectomy could help with fatty liver regression[7,36,37]. We agree with prior evidence that proctocolectomy is favourable for NAFLD. LRP had a positive effect on fatty liver, with 18 cases (18/28, 64%) showing complete regression to normal liver and the remaining ten patients (36%) showing a stationary course. This improvement is due to improvements in malnutrition, anaemia, and a reduction in corticosteroid dosage during the surgical follow-up period, as supported by a study[38]. NAFLD progression was not observed in our cases due to the absence of pouchitis. As a result, we concluded that proctocolectomy plays a definitive role in the management of NAFLD-complicating UC, as evidenced by radiology and liver biopsy (improvement of fatty liver score from a median of 2 (range 1-3) in preoperative biopsies to a median of 1 (range 0-3) in postoperative biopsies).
High incidence of portal vein thrombosis (PVT) in IBD may be due to increased factors V and VIII levels, platelet counts, fibrinogen levels, or decreased antithrombin III levels[39,40]. In our study, we identified six patients with PVT: Four cases before surgery, no recurrence after surgery, and two patients who developed PVT after surgery. One of the two postoperative PVT cases exhibited partial portal vein obstruction, which was treated with anticoagulants. In contrast, the second case exhibited complete obstruction of PVT with intestinal gangrene, necessitating resection of the majority of the small intestine with short bowel syndrome, and died two months later. The low incidence of postoperative PVT can be due to the small number of cases, the routine use of low molecular heparin in the postoperative period for six months in all cases according to the current society guidelines and expert opinion[40], absence of pouchitis which increases the incidence of PVT[41], and the possible occurrence of PVT not associated with specific symptoms or asymptomatic[42]. The use of abdominal ultrasound and colored Doppler at regular intervals unquestionably aided in detecting asymptomatic cases. We concluded that proctocolectomy decreases the incidence but not the severity of PVT with an unfavorable outcome, despite the small number of cases, with a mortality rate of 50% among those who developed PVT is similar to the findings of other studies[43].
Two isolated PBC accidentally discovered cases were included; one regressed to normal liver condition, while the other progressed to liver cell failure and required liver transplantation without mortality. We thought that the excellent prognosis was due to obeticholic acid that improved the course of the disease[44], removal of the colon, the site of antibody production, which helped make the prognosis better after surgery, and absence of pouchitis[9].
One patient with UC developed a liver abscess before surgery, whereas no such case was reported after LRP. We concur with the pathogenesis that liver abscess may be caused by antibodies produced by patients with UC attacking the liver, resulting in necrosis and abscess formation that was negative for bacteria[45]. Proctocolectomy permanently eliminates the site of antibody production. Furthermore, postoperative corticosteroids help to prevent recurrence[46]. Proctocolectomy prevents liver abscesses, according to our findings.
One case of AIH was diagnosed before surgery and regressed to normal following LRP with a favourable prognosis, demonstrating the positive effects of proctocolectomy[47]. The favorable prognosis of our patient was likely due to the removal of the inflamed colon and steroid-based immunosuppressive therapy[10].
An earlier study confirmed the efficacy of proctocolectomy for nonspecific reactive hepatitis[7]. In accordance with the previous study's findings, we diagnosed 8 patients with nonspecific reactive hepatitis, and complete regression in every case was confirmed.
This is a large prospective multicenter study of different hepatobiliary manifestations assessment after LRP with a relatively long duration of patient follow-up. We also included comprehensive clinical points evaluating different courses of hepatobiliary manifestations. A prospective study prevents selection bias with accurate results that could be generalized.
However, our study has some limitations. One is the lack a control group of patients that were not operated on. Therefore, this study did not handle the severity of preoperative colitis and its effect on the course of hepatobiliary manifestations in the postoperative period. Another limitation is that it did not evaluate the disease course after liver transplantation. Another limitation is that we did not evaluate the treatment of both UC and hepatobiliary manifestations during the postoperative course. Finally, we did not evaluate the causes of the high incidence of symptomatic gallbladder stones.
This is a large prospective multicenter study of different hepatobiliary manifestations assessment after LRP with a relatively long duration of patient follow-up. We also included comprehensive clinical points evaluating different courses of hepatobiliary manifestations. A prospective study prevents selection bias with accurate results that could be generalized.
However, our study has some limitations. One is the lack a control group of patients that were not operated on. Therefore, this study did not handle the severity of preoperative colitis and its effect on the course of hepatobiliary manifestations in the postoperative period. Another limitation is that it did not evaluate the disease course after liver transplantation. Another limitation is that we did not evaluate the treatment of both UC and hepatobiliary manifestations during the postoperative course. Finally, we did not evaluate the causes of the high incidence of symptomatic gallbladder stones.
Inflammatory bowel disease (IBD) is expected to affect 1% of the population over the next decade. Hepatobiliary manifestations constitute one of the most common extraintestinal manifestations in IBD. Primary sclerosing cholangitis (PSC), autoimmune hepatitis, fatty liver, cholelithiasis, primary biliary cholangitis, portal vein thrombosis, and hepatic abscess are the most prevalent hepatobiliary manifestations of ulcerative colitis (UC). Most UC patients can be managed with medications, but minorities require proctocolectomy.
Two-stage laparoscopic proctocolectomy (LRP) with ileal pouch-anal anastomosis (IPAA) is a cure for UC, but its effect on hepatobiliary diseases is controversial.
Therefore, we conducted a prospective observational study to examine the effects of LRP with IPAA on hepatobiliary symptoms to evaluate the role of surgery in preventing or ameliorating liver damage from the disease progression.
This is a prospective observational study on 167 patients with hepatobiliary manifestations who underwent two-stage elective LRP with IPAA for UC We examined the effects of LRP with IPAA on hepatobiliary symptoms to evaluate the role of surgery in preventing or ameliorating liver damage from the disease progression.
The course of hepatobiliary manifestations after surgery is improved in most forms. Most PSC patients had a stable course, Two-thirds of fatty liver patients showed a regressive course with an improved survival rate at the end of the study.
Our study emphasized the positive and improving effects of surgery on hepatobiliary manifestations in patients with UC.
Further studies are required in a larger sample size to evaluate the effect of surgery on different forms of hepatobiliary manifestations in patients with UC. further studies are required to compare the effect of surgery and effects of medical treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hakimi T, Afghanistan; Piltcher-da-Silva R, Brazil S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 901] [Article Influence: 180.2] [Reference Citation Analysis (0)] |
| 2. | Guillo L, D'Amico F, Serrero M, Angioi K, Loeuille D, Costanzo A, Danese S, Peyrin-Biroulet L. Assessment of extraintestinal manifestations in inflammatory bowel diseases: A systematic review and a proposed guide for clinical trials. United European Gastroenterol J. 2020;8:1013-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Venkatesh PG, Navaneethan U, Shen B. Hepatobiliary disorders and complications of inflammatory bowel disease. J Dig Dis. 2011;12:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Yarur AJ, Czul F, Levy C. Hepatobiliary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Navaneethan U. Hepatobiliary manifestations of ulcerative colitis: an example of gut-liver crosstalk. Gastroenterol Rep (Oxf). 2014;2:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 6. | Lepistö A, Kivistö S, Kivisaari L, Arola J, Järvinen HJ. Primary sclerosing cholangitis: outcome of patients undergoing restorative proctocolecetomy for ulcerative colitis. Int J Colorectal Dis. 2009;24:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Cangemi JR, Wiesner RH, Beaver SJ, Ludwig J, MacCarty RL, Dozois RR, Zinsmeister AR, LaRusso NF. Effect of proctocolectomy for chronic ulcerative colitis on the natural history of primary sclerosing cholangitis. Gastroenterology. 1989;96:790-794. [PubMed] |
| 8. | Gotthardt DN, Sauer P, Schaible A, Stern J, Stiehl A, Beuers U. Kinetics of primary bile acids in patients after proctocolectomy and ileal pouch-anal anastomosis. Digestion. 2014;90:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Sandborn WJ, Landers CJ, Tremaine WJ, Targan SR. Association of antineutrophil cytoplasmic antibodies with resistance to treatment of left-sided ulcerative colitis: results of a pilot study. Mayo Clin Proc. 1996;71:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Macsween R N M. Liver pathology associated with diseases of other organs. Pathology of the liver. Churchil Livingstone Inc.1987. Available from: https://cir.nii.ac.jp/crid/1571135650655409792. |
| 11. | Spinelli A, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Doherty G, Raine T, Panis Y. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J Crohns Colitis. 2022;16:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (1)] |
| 12. | Fousekis FS, Theopistos VI, Mitselos IV, Skamnelos A, Kavvadias A, Katsanos KH, Christodoulou DK. Specific Features of Patients With Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. J Clin Med Res. 2019;11:81-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 999] [Article Influence: 111.0] [Reference Citation Analysis (1)] |
| 14. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2645] [Article Influence: 188.9] [Reference Citation Analysis (1)] |
| 15. | Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 1164] [Article Influence: 77.6] [Reference Citation Analysis (2)] |
| 16. | Tiniakos DG. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: histological diagnostic criteria and scoring systems. Eur J Gastroenterol Hepatol. 2010;22:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, Lohse AW, Montano-Loza AJ. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 18. | Bollegala N, Khan R, Scaffidi MA, Al-Mazroui A, Tessolini J, Showler A, Colak E, Grover SC. Aseptic Abscesses and Inflammatory Bowel Disease: Two Cases and Review of Literature. Can J Gastroenterol Hepatol. 2017;2017:5124354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Stanciulea O, Eftimie MA, Mosteanu I, Ciortan R, Popescu I. Laparoscopic Restorative Proctocolectomy for Ulcerative Colitis - How I Do It? Chirurgia (Bucur). 2022;117:328-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Shah A, Macdonald GA, Morrison M, Holtmann G. Targeting the Gut Microbiome as a Treatment for Primary Sclerosing Cholangitis: A Conceptional Framework. Am J Gastroenterol. 2020;115:814-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Worthington J, Cullen S, Chapman R. Immunopathogenesis of primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2005;28:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1470] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 23. | Aoki CA, Bowlus CL, Gershwin ME. The immunobiology of primary sclerosing cholangitis. Autoimmun Rev. 2005;4:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hübscher SG, Briskin M, Salmon M, Adams DH. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 248] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Liaskou E, Karikoski M, Reynolds GM, Lalor PF, Weston CJ, Pullen N, Salmi M, Jalkanen S, Adams DH. Regulation of mucosal addressin cell adhesion molecule 1 expression in human and mice by vascular adhesion protein 1 amine oxidase activity. Hepatology. 2011;53:661-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, Bell H, Gangsøy-Kristiansen M, Matre J, Rydning A, Wikman O, Danielsson A, Sandberg-Gertzén H, Ung KA, Eriksson A, Lööf L, Prytz H, Marschall HU, Broomé U. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Cullen SN, Rust C, Fleming K, Edwards C, Beuers U, Chapman RW. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Shen B, Bennett AE, Navaneethan U, Lian L, Shao Z, Kiran RP, Fazio VW, Remzi FH. Primary sclerosing cholangitis is associated with endoscopic and histologic inflammation of the distal afferent limb in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2011;17:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Treeprasertsuk S, Björnsson E, Sinakos E, Weeding E, Lindor KD. Outcome of patients with primary sclerosing cholangitis and ulcerative colitis undergoing colectomy. World J Gastrointest Pharmacol Ther. 2013;4:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Kuisma J, Nuutinen H, Luukkonen P, Järvinen H, Kahri A, Färkkilä M. Long term metabolic consequences of ileal pouch-anal anastomosis for ulcerative colitis. Am J Gastroenterol. 2001;96:3110-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Damião AO, Sipahi AM, Vezozzo DP, Gonçalves AL, Habr-Gama A, Teixeira MG, Fukushima JT, Laudanna AA. Effects of colectomy on gallbladder motility in patients with ulcerative colitis. Dig Dis Sci. 1997;42:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Habeeb TAAM, Kermansaravi M, Giménez ME, Manangi MN, Elghadban H, Abdelsalam SA, Metwalli AM, Baghdadi MA, Sarhan AA, Moursi AM, El-Taher AK. Sleeve Gastrectomy and Cholecystectomy are Safe in Obese Patients with Asymptomatic Cholelithiasis. A Multicenter Randomized Trial. World J Surg. 2022;46:1721-1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Eade MN, Cooke WT, Brooke BN. Liver disease in ulcerative colitis. II. The long-term effect of colectomy. Ann Intern Med. 1970;72:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Broomé U, Glaumann H, Hultcrantz R. Liver histology and follow up of 68 patients with ulcerative colitis and normal liver function tests. Gut. 1990;31:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Matsumoto T, Yamasaki S, Arakawa A, Abe K, Abe H, Kon K, Kobayashi S, Takasaki Y. Exposure to a high total dosage of glucocorticoids produces non-alcoholic steatohepatits. Pathol Int. 2007;57:388-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Rojas-Feria M, Castro M, Suárez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013;19:7327-7340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Naymagon L, Tremblay D, Zubizarreta N, Moshier E, Naymagon S, Mascarenhas J, Schiano T. The Natural History, Treatments, and Outcomes of Portal Vein Thrombosis in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2021;27:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Ball CG, MacLean AR, Buie WD, Smith DF, Raber EL. Portal vein thrombi after ileal pouch-anal anastomosis: its incidence and association with pouchitis. Surg Today. 2007;37:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Maconi G, Bolzacchini E, Dell'Era A, Russo U, Ardizzone S, de Franchis R. Portal vein thrombosis in inflammatory bowel diseases: a single-center case series. J Crohns Colitis. 2012;6:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Talbot RW, Heppell J, Dozois RR, Beart RW Jr. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 412] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, Patanwala I, Pereira SP, Thain C, Thorburn D, Tiniakos D, Walmsley M, Webster G, Jones DEJ. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 45. | Hasan B, Khalid R, Charles R, Shen B. Abdominal Pain in a Patient With Diverted Bowel and Inflammatory Bowel Disease. ACG Case Rep J. 2020;7:e00437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Floreani A, Rizzotto ER, Ferrara F, Carderi I, Caroli D, Blasone L, Baldo V. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 47. | Epstein MP, Kaplan MM. A pilot study of etanercept in the treatment of primary sclerosing cholangitis. Dig Dis Sci. 2004;49:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |