Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2619
Peer-review started: June 13, 2023
First decision: July 6, 2023
Revised: August 3, 2023
Accepted: August 28, 2023
Article in press: August 28, 2023
Published online: November 27, 2023
Processing time: 166 Days and 17 Hours
Rectal sparing is an option for some rectal cancers with complete or good response after chemoradiotherapy (CRT); however, it has never been evaluated in patients with metastases. We assessed long-term outcomes of a rectal-sparing approach in a liver-first strategy for patients with rectal cancer with resectable liver metastases.
We examined patients who underwent an organ-sparing approach for rectal cancer with synchronous liver metastases using a liver-first strategy during 2010-2015 (n = 8). Patients received primary chemotherapy and pelvic CRT. Liver surgery was performed during the interval between CRT completion and rectal tumor re-evaluation. Clinical and oncological characteristics and long-term outcomes were assessed.
All patients underwent liver metastatic resection with curative intent. The R0 rate was 100%. Six and two patients underwent local excision and a watch-and-wait (WW) approach, respectively. All patients had T3N1 tumors at diagnosis and had good clinical response after CRT. The median survival time was 60 (range, 14-127) mo. Three patients were disease free for 5, 8, and 10 years after the procedure. Five patients developed metastatic recurrence in the liver (n = 5) and/or lungs (n = 2). Only one patient developed local recurrence concurrent with metastatic recurrence 24 mo after the WW approach. Two patients died during follow-up.
The results suggest good local control in patients undergoing organ-sparing strategies for rectal cancer with synchronous liver metastasis. Prospective trials are required to validate these data and identify good candidates for these strategies.
Core Tip: Our liver first strategy allows long course randomized controlled trial achievement without compromising systemic treatment. In case of good response after chemoradiotherapy, rectal sparing has never been evaluated in patients with metastases. Rectal sparing strategy results in low morbidity and improved patient’s long-term quality of life. With a follow-up more than 5 years, we described a good local control in 8 patients with metastases. Prospective trials are required to validate these data and identify good candidates for these strategies.
- Citation: Meillat H, Garnier J, Palen A, Ewald J, de Chaisemartin C, Tyran M, Mitry E, Lelong B. Organ sparing to cure stage IV rectal cancer: A case report and review of literature. World J Gastrointest Surg 2023; 15(11): 2619-2626
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2619.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2619
Rectal cancer affects nearly 10000 new patients every year in France, among whom 20%-25% present with synchronous liver metastases. Despite oncological advances, the only potentially curative therapy remains surgical resection or destruction of lesions at both sites[1]. Rectal and liver resections can achieve 5-year survival rates of > 50%[1,2] compared with only approximately 5% for patients treated with palliative intent[3].
Because the prognosis of these patients is directly related to the presence of liver metastases and because complications of rectal surgery are common after chemoradiotherapy (CRT) and may therefore delay the start of appropriate metastatic treatments, the liver-first approach has been proposed for patients with locally advanced rectal cancer and synchronous liver metastases[4-7]. Thus, patients receive computed tomography (CT) first, followed by liver surgery, before and/or after CRT depending on the team. Triplet CT and newer targeted therapies such as cetuximab and bevacizumab have led to improved response rates at both sites[8] and conversion rates to hepatic resectability[9,10].
Rectal pathological complete response has been observed in 15%-20% of patients after standard CRT[11] and in up to one-third of cases after adding triplet CT, following the same pattern as that for patients with metastases[12].
In these conditions, the question of whether to maintain the indication for radical surgery or total mesorectal excision (TME) has been raised by several therapeutic trials evaluating rectal-sparing strategies in patients without metastasis[13,14]. In France, the most widely evaluated strategy is local excision (LE) via the transanal approach. This strategy is reserved for patients with an initially favorable lesion (T2 or low T3 of less than 40 mm). The rationale of this strategy compared to radical surgery is based on the preservation of quality of life (QoL) and digestive and urogenital functions with identical oncological efficacy owing to rectal preservation and the absence of surgical nerve damage[15,16]. Recent studies have shown that LE is a safe alternative for TME for patients who are good responders after CRT for T2T3N0-1 mid-to-low rectal cancer[13,17] with a 5-year local recurrence rate of 7%. Although this strategy has not been evaluated in patients with metastases, the rationale remains similar, i.e., to improve the QoL of patients whose prognosis is related to a higher risk of hepatic recurrence than the risk of local recurrence. Thus, this study aimed to assess long-term outcomes of a rectal-sparing approach in a liver-first strategy for selected patients with rectal cancer with resectable liver metastases.
Between 2010 and 2015, 65 patients were treated for rectal cancer (≤ 8 cm from the anal verge) with synchronous resectable liver metastases at the Institut Paoli-Calmettes, Marseille (France). Eight (12.3%) underwent a rectal-sparing strategy.
Data were prospectively collected from a clinical database labeled by the National Institute for Data Protection (NCT 02869503). The study was approved by institutional review board and consent was waived owing to the retrospective nature of the study.
Seven patients were men, and the mean age of the patients was 65 years. Patient characteristics are summarized in Table 1. All patients had poor long-term prognoses with elevated carcinoembryonic antigen (CEA) levels (n = 2) and more than two lesions (n = 5).
| Patient No. | Age (yr) | Sex | BMI | Comorbid conditions | ASA score | CEA level | Rectal tumor | Liver metastasis | ||||
| Size (mm) | Distance to anal verge (mm) | staging | Nb of lesions | bilobar | Size of the largest metastasis | |||||||
| 1 | 65 | M | 31 | HTN, DM, Smocking | 2 | 14000 | 40 | 30 | T3N1 | 15 | 1 | 73 |
| 2 | 78 | M | 29 | HTN | 2 | 55 | 25 | 40 | T3N1 | 1 | 0 | 46 |
| 3 | 58 | M | 23 | DM, Smocking | 3 | 18 | 18 | 25 | T3N1 | 3 | 1 | 25 |
| 4 | 77 | M | 24 | HTN | 2 | 134 | 30 | 50 | T3N1 | 2 | 0 | 42 |
| 5 | 68 | M | 26 | Myasthenia | 2 | 7 | 30 | 38 | T3N0 | 5 | 1 | 70 |
| 6 | 58 | M | 25 | Smocking | 2 | 1 | 40 | 25 | T3N1 | 13 | 1 | 20 |
| 7 | 59 | M | 27 | COPD | 2 | 27 | 40 | 20 | T3N1 | 6 | 1 | 26 |
| 8 | 64 | F | 23 | HTN | 2 | 281 | 25 | 35 | T3N1 | 2 | 0 | 52 |
Tumors were classified using the 8th Union for International Cancer Control/tumour-node-metastasis staging system[18]. R0 resection included a surgical margin of at least 1 mm for both LE and TME specimens. Tumor regression grade (TRG) was scored according to the Dworak classification[19].
Based on histopathological findings, LE was considered adequate, and patients were observed without further surgery when the following favorable features were present: YpT0, ypT1, in-depth and lateral R0 resection, and on a case-by-case basis, ypT2 with favorable TRG 1 or 2. LE was considered inadequate and TME was recommended in other cases (ypT3 or higher, positive margins, TRG of at least 3, or lymphovascular invasion). An R0 Liver resection was defined as microscopically tumor-free resection margin.
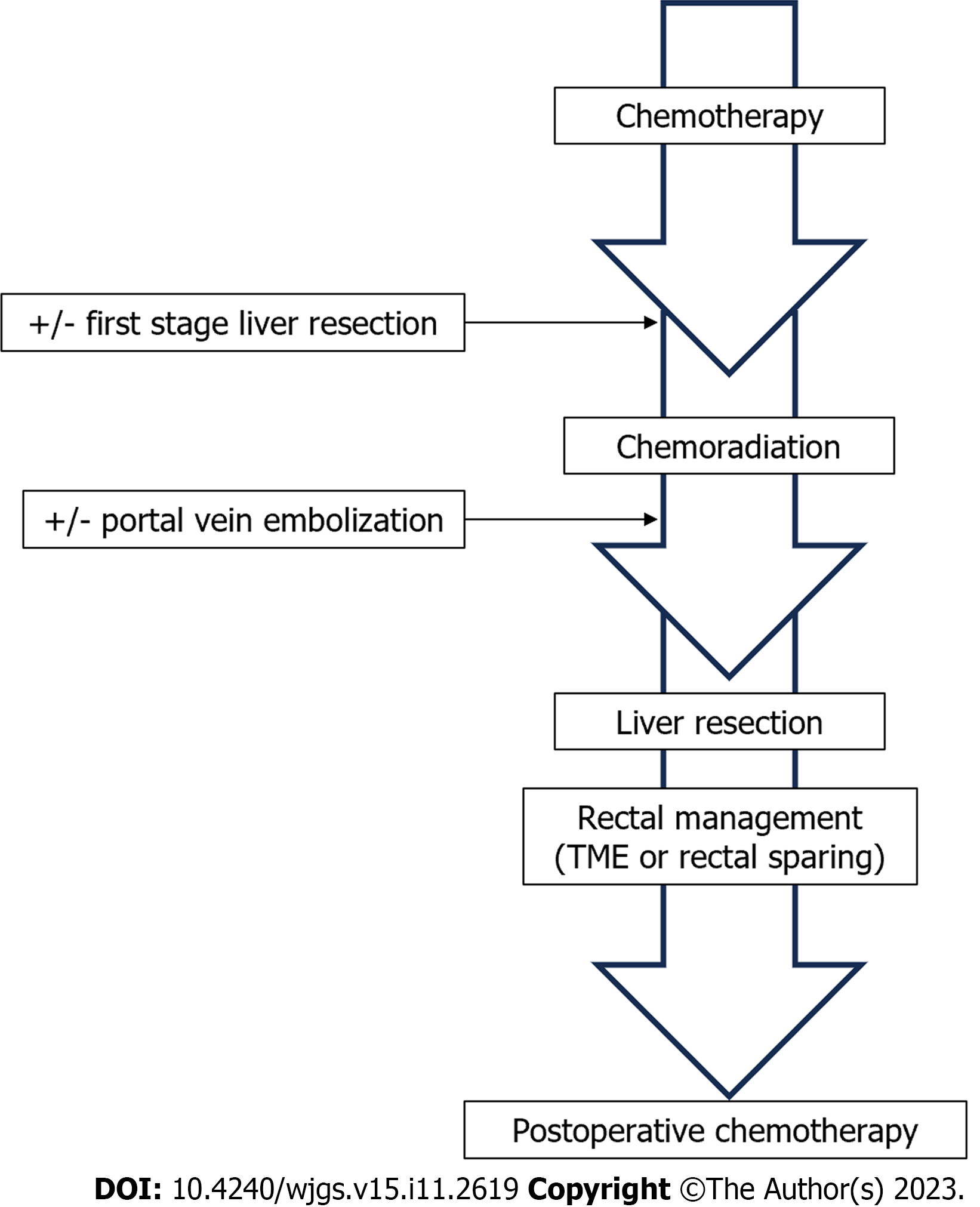
Initial evaluation included thoracoabdominopelvic CT, rectal and liver magnetic resonance imaging (MRI), endorectal ultrasound (EUS) and CEA test before and after 4-6 cycles of CT. All patients suitable for neoadjuvant treatment and surgery (performance status < 3) first received CT. Complete reassessment was systematically performed after 4-6 cycles of CT according to the same modalities. In patients with stable liver disease or those with expected clinical response after margin negative resection (R0), pelvic CRT was performed followed by liver surgery in the interval between pelvic CRT completion and planned rectal surgery, as an optimized liver-first strategy (Figure 1).
The oncological strategy was chosen as a function of the overall condition of the patient and the resectability of the liver metastasis and rectal tumor in our multidisciplinary meetings (including liver surgeons, rectal surgeons, oncologists, radiotherapists, radiologists, and pathologists).
Rectal sparing within a liver-first strategy for rectal cancer with resectable liver metastases.
All patients received neoadjuvant CT in line with current recommendations[2,9] and concomitant normofractionated chemoradiation (45-50 Gy in 25 fractions combined with capecitabine).
Liver surgery was scheduled according to response to CT. When the expected future liver remnant was < 30% of the initial volume, portal venous embolization was performed to prevent postoperative liver failure. Liver surgery was performed in one or two stages and consisted of anatomical or non-anatomical resections, and/or thermoablations.
Rectal surgery was performed 8-12 wk after CRT completion. A rectal-sparing strategy was proposed for patients with initially favorable lesions (low T3 or < 40 mm with extramural vascular invasion < 3) and a good or complete clinical response after CT and CRT. A good clinical response was defined by the absence of a mass on digital rectal examination and a residual scar of 2 cm or less with no vegetative component, significant hollow, or deep infiltration into the muscular layer[13].
A watch-and-wait (WW) strategy was proposed in the absence of residual lesions. In other cases, an LE was performed with conventional full-thickness excision of the tumor or scar and the rectal wall via direct or transanal endoscopic microsurgery, including 1-cm lateral tissue margins. The deep margin corresponding to mesorectal fat was inked by the surgeon before being sent for histopathological analysis.
Follow-up in all patients consisted of physical examination and thoracoabdominal CT 1 mo after the last surgery and then every 3 mo. In addition, EUS and pelvic MRI were performed every 3 mo. Local recurrence was defined as a radiologically and biopsy-proven pelvic tumor. Distant recurrence was defined as radiological evidence of a tumor in any distant organ. Disease recurrence was defined as a suspicious lesion on imaging in the setting of an elevated CEA level and pathological confirmation. Overall survival and disease-free survival were determined based on the diagnosis. Patients considered disease free were censored at the time of the latest follow-up clinical assessment.
All patients had unfavorable long-term prognoses with multiple (n = 6), often bilobar (n = 5), or bulky (n = 4) lesions (Table 2). An increased CEA level was observed in seven patients. Liver surgery was performed in one (n = 6) or two stages (n = 2). Portal vein embolization was necessary in three patients. The postoperative mortality rate was nil. Only one patient had severe complications and required radiological drainage of the bilioma. The R0 resection rate was 100%.
| Patient No. | Preoperative treatment | Liver surgery | Rectal strategy | Postoperative treatment | |||||
| Type | PVE | Two stage | Restaging | Type | Delay1 | Histologic analysis | |||
| 1 | Folfox × 12 then CRT | Major hepatectomy | 1 | 0 | T0N0 | LE | 9 | T0R0 | 0 |
| 2 | Folfiri × 6 then CRT | Segmentectomy | 0 | 0 | T0N0 | LE | 11 | T0R0 | Patient’s refusal |
| 3 | Folfox × 10 then CRT | Major hepatectomy | 0 | 0 | T1N0 | LE | 9 | T0R0 | 0 |
| 4 | Folfox × 4 then CRT | Major hepatectomy | 0 | 0 | T0N0 | LE | 12 | T2R0 (TRG1) | Folfiri-cetux × 8 |
| 5 | Folfirinox × 8 then CRT | Major hepatectomy + Tum + RF | 1 | 1 | T2N0 | LE | 11 | T0R0 | |
| 6 | Folfirinox × 6 then CRT | Major hepatectomy + Tum + RF | 1 | 1 | T1N0 | LE | 10 | T2R0 (TRG1) | Folfox × 6 |
| 7 | Folfirinox × 4 then CRT | Tum + RF | 0 | 0 | T0N0 | WW | - | - | Folfox × 8 |
| 8 | Folfiri cetux × 6 then CRT | Tum + segmentectomy | 0 | 0 | T0N0 | WW | - | - | Folfox × 6 |
All patients had locally advanced rectal tumors at diagnosis and were good (n = 6) or complete (n = 2) clinical responders to CRT (Table 2). The median interval between CRT completion and rectal examination was 10 (range, 9-12) mo. In the absence of a visible scar, the WW strategy was performed in two patients. In other cases, patients underwent LE and histopathological analysis confirmed a good tumor response in all patients. No TME completion was necessary. Four patients had tumors defined as ypT0 and two patients had tumors defined as ypT2 with a favorable TRG score; the R0 resection rate was 100%. Postoperative mortality and severe morbidity rates were nil.
The median follow-up duration was 82 mo (range, 48-142). Two patients developed metastatic recurrence of the disease in the liver at 8 and 11 mo and underwent curative treatment for the recurrence. Currently, the patients are in remission. Local rectal recurrence concomitant with liver recurrence occurred in one patient after the WW strategy at 24 mo after rectal examination. The patient underwent second-line CT followed by curative surgery for liver recurrence but refused TME. Only one patient died owing to laryngeal cancer, which was diagnosed 3 years after completing treatment for rectal cancer.
Currently, the treatment of colorectal liver metastases (CRLM) remains a major clinical challenge without a consensus[20]. The case-by-case treatment strategy is determined according to: (1) Tumor and disease-related characteristics, patient-related factors, and treatment-related factors such as toxicity and main oncological problems; (2) presence or absence of predictive factors for rectal and liver resection morbidity; and (3) response to initial CT. New regional and systemic chemotherapies associated with biological agents combined with technical advances in liver surgery have made it possible to broaden indications for CRLM resection by offering personalized treatment.
For rectal tumors, TME remains the only available treatment option with curative intent in patients with metastatic rectal cancer, regardless of the response to neoadjuvant therapy. However, a complete clinical response or a very good response is observed in 15%-20% of patients after standard CRT and in up to one-third of cases after addicting CT, as suggested by a recent randomized controlled trial (RCT) in patients without metastasis[12,21].
Rare cases of rectal-sparing strategies in patients with metastases have been described: WW[22,23] and LE[4] in the liver-first strategy. A WW strategy was used in nine cases as a result of primary tumor disappearance after RCT[22-24]. Unfortunately, no study has specified the characteristics of rectal lesions or oncological outcomes of these patients. Mentha et al[4] and Buchs et al[25] reported two cases of LE with complete clinical response after RCT. One case in 2006[4] did not have any long-term data. Another case in 2015[25] had a confirmed pathological response after RCT but had recurrence 11 mo later and underwent abdominoperineal resection with a final staging of pT3Nx.
In a Dutch study[7], a rectal-sparing strategy could have been proposed in ten patients who had a complete response of their primary tumor after complete treatment according to a liver-first strategy, as introduced by Mentha et al[4]. This strategy involves systematic preoperative CT and resection of CRLM, followed by pelvic RCT and rectal resection. In our optimised liver-first strategy, liver surgery is performed at the interval between radiotherapy completion and rectal surgery. This strategy allows rectal re-evaluation without increasing the time without CT. Prolonging the interval between CRT completion and rectal staging increases the complete clinical response rate[26]. Thus, it allows for a better selection of patients who can benefit from a rectal-sparing strategy without increasing surgical morbidity[26,27].
Short-course radiotherapy followed by CT and delayed rectal surgery[21] is an option in the neoadjuvant setting of resectable rectal cancer that could potentially be adapted for patients with metastases[24]. This would make it possible to limit the time without CT while maintaining a delayed rectal reassessment and possibly proposing a rectal-sparing strategy in cases of good clinical response. Nevertheless, the oncological safety of this strategy has not been evaluated in specific studies.
It is important to note that we have a highly selected population after applying the two-stage selection criteria in the organ preservation for rectal cancer (GRECCAR 2) trial; we considered the initial rectal tumor characteristics and the clinical response to CRT. Seven of the eight patients studied had an initial N + tumor according to routine EUS and MRI. The initial lymph node involvement, especially the lymph node response after CT and RCT, is difficult to specify formally[28].
In addition to oncological multidisciplinary meetings, weekly meetings are organized with specialized radiologists and colorectal surgeons to review all examinations, including surveillance MRI, to improve our patient selection. Our results are consistent with those of GRECCAR 2 study[13], as we observed no lymph node recurrence among patients undergoing LE. Four patients had no residual tumor (ypT0), but two patients had residual ypT2 tumors equivalent to a risk of residual lymph node involvement evaluated at 8%. This risk is probably lower given the low TRG (TRG 1: few residual cells). Given the discordant results and the absence of validated criteria, the WW strategy seems to be reserved only for patients without residual scarring and is subject to very strict surveillance.
In patients without metastasis, the GRECCAR 2 trial’s 5-year results provide no evidence of differences in long-term survival (84% vs. 82%; P = 0.85) or cancer-specific mortality (7% vs. 10%; P = 0.53) between LE and TME[17].
In all cases, a favorable pathological response is associated with good prognosis and survival benefit[29]. Under these conditions, whether to maintain the indication for radical surgery in good responders or even in complete clinical responders is an issue that has never been raised in patients with metastases.
The oncological safety of rectal-sparing strategy has never been evaluated in patients with metastases but needs to be balanced with morbidity or functional benefits. Minimizing operative morbidity is a major issue for strategy treatment choice as it is an independent factor for overall survival and disease-free survival after CRLM resection[30]. The rectal-sparing strategy induces a more favorable global health status and bowel function than TME after CRT[16,31]. The effect of rectal cancer treatment on functional outcomes and patients’ QoL must now be considered in the decision-making process whenever possible.
To the best of our knowledge, this is the first study to provide detailed characteristics and long-term results of patients undergoing a rectal-sparing strategy for rectal cancer with synchronous liver metastasis. Our results are encouraging compared to the prognoses of patients with metastases in the literature because only one patient had a local rectal recurrence with concurrent hepatic recurrence using the WW strategy 3 years after liver surgery.
The present study has some limitations and caution must be exercised in interpreting its results given the small sample size. The rectal-sparing strategy requires coordinated action by a multidisciplinary team and depends on many criteria, including treatment times and tumor response to therapy. Moreover, patients are not always referred to our center at the time of diagnosis and have already started CRT, which does not allow for a first liver strategy and limits potential inclusions.
Second, this was a retrospective single-center study. In the absence of clear recommendations, practices vary widely from one center to another in the surgical and oncological management of CRLM, which hinders the realization of a multicenter study. Imposing the same protocol on several teams and institutions, with selection criteria often different from their usual practice, is an obstacle to its large-scale implementation.
In conclusion, although our findings should be interpreted with caution given the small sample size and high patient selection, we suggest that rectal-sparing strategies must become an option in expert centers to improve the QoL of patients with CRLM.
| 1. | Petrowsky H, Fritsch R, Guckenberger M, De Oliveira ML, Dutkowski P, Clavien PA. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17:755-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 974] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 3. | Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann Surg. 2012;255:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | D'Hondt M, Lucidi V, Vermeiren K, Van Den Bossche B, Donckier V, Sergeant G. The interval approach: an adaptation of the liver-first approach to treat synchronous liver metastases from rectal cancer. World J Surg Oncol. 2017;15:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Andres A, Toso C, Adam R, Barroso E, Hubert C, Capussotti L, Gerstel E, Roth A, Majno PE, Mentha G. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases: a LiverMetSurvey-based study. Ann Surg. 2012;256:772-8; discussion 778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Nierop PMH, Verseveld M, Galjart B, Rothbarth J, Nuyttens JJME, van Meerten E, Burger JWA, Grünhagen DJ, Verhoef C. The liver-first approach for locally advanced rectal cancer and synchronous liver metastases. Eur J Surg Oncol. 2019;45:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2545] [Article Influence: 254.5] [Reference Citation Analysis (41)] |
| 9. | Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1046] [Article Influence: 61.5] [Reference Citation Analysis (1)] |
| 10. | Beppu T, Miyamoto Y, Sakamoto Y, Imai K, Nitta H, Hayashi H, Chikamoto A, Watanabe M, Ishiko T, Baba H. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol. 2014;21 Suppl 3:S405-S413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F, Hallböök O, Syk I, Glimelius B, Martling A. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 447] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 12. | Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 884] [Article Influence: 176.8] [Reference Citation Analysis (0)] |
| 13. | Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, Faucheron JL, Jafari M, Portier G, Meunier B, Sileznieff I, Prudhomme M, Marchal F, Pocard M, Pezet D, Rullier A, Vendrely V, Denost Q, Asselineau J, Doussau A. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (1)] |
| 14. | Huisman JF, Schoenaker IJH, Brohet RM, Reerink O, van der Sluis H, Moll FCP, de Boer E, de Graaf JC, de Vos Tot Nederveen Cappel WH, Beets GL, van Westreenen HL. Avoiding Unnecessary Major Rectal Cancer Surgery by Implementing Structural Restaging and a Watch-and-Wait Strategy After Neoadjuvant Radiochemotherapy. Ann Surg Oncol. 2021;28:2811-2818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | D'Alimonte L, Bao QR, Spolverato G, Capelli G, Del Bianco P, Albertoni L, De Paoli A, Guerrieri M, Mantello G, Gambacorta MA, Canzonieri V, Valentini V, Coco C, Pucciarelli S. Long-Term Outcomes of Local Excision Following Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Ann Surg Oncol. 2021;28:2801-2808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Brachet S, Meillat H, Chanez B, Ratone JP, Brunelle S, Tyran M, Poizat F, de Chaisemartin C, Lelong B. Case-Matched Comparison of Functional and Quality of Life Outcomes of Local Excision and Total Mesorectal Excision Following Chemoradiotherapy for Rectal Cancer. Dis Colon Rectum. 2022;65:1464-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Rullier E, Vendrely V, Asselineau J, Rouanet P, Tuech JJ, Valverde A, de Chaisemartin C, Rivoire M, Trilling B, Jafari M, Portier G, Meunier B, Sieleznieff I, Bertrand M, Marchal F, Dubois A, Pocard M, Rullier A, Smith D, Frulio N, Frison E, Denost Q. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol. 2020;5:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 18. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4711] [Article Influence: 523.4] [Reference Citation Analysis (4)] |
| 19. | Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1076] [Article Influence: 37.1] [Reference Citation Analysis (1)] |
| 20. | Sabbagh C, Cosse C, Ravololoniaina T, Chauffert B, Joly JP, Mauvais F, Regimbeau JM. Oncological strategies for middle and low rectal cancer with synchronous liver metastases. Int J Surg. 2015;23:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 1123] [Article Influence: 224.6] [Reference Citation Analysis (1)] |
| 22. | Conrad C, Vauthey JN, Masayuki O, Sheth RA, Yamashita S, Passot G, Bailey CE, Zorzi D, Kopetz S, Aloia TA, You YN. Individualized Treatment Sequencing Selection Contributes to Optimized Survival in Patients with Rectal Cancer and Synchronous Liver Metastases. Ann Surg Oncol. 2017;24:3857-3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | de Jong MC, van Dam RM, Maas M, Bemelmans MH, Olde Damink SW, Beets GL, Dejong CH. The liver-first approach for synchronous colorectal liver metastasis: a 5-year single-centre experience. HPB (Oxford). 2011;13:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Kok END, Havenga K, Tanis PJ, de Wilt JHW, Hagendoorn J, Peters FP, Buijsen J, Rutten HJT, Kuhlmann KFD; Dutch Stage IV Rectal Cancer Group. Multicentre study of short-course radiotherapy, systemic therapy and resection/ablation for stage IV rectal cancer. Br J Surg. 2020;107:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Buchs NC, Ris F, Majno PE, Andres A, Cacheux W, Gervaz P, Roth AD, Terraz S, Rubbia-Brandt L, Morel P, Mentha G, Toso C. Rectal outcomes after a liver-first treatment of patients with stage IV rectal cancer. Ann Surg Oncol. 2015;22:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Marchegiani F, Palatucci V, Capelli G, Guerrieri M, Belluco C, Rega D, Morpurgo E, Coco C, Restivo A, De Franciscis S, Aschele C, Perin A, Bonomo M, Muratore A, Spinelli A, Ramuscello S, Bergamo F, Montesi G, Spolverato G, Del Bianco P, Gambacorta MA, Delrio P, Pucciarelli S. Rectal Sparing Approach After Neoadjuvant Therapy in Patients with Rectal Cancer: The Preliminary Results of the ReSARCh Trial. Ann Surg Oncol. 2022;29:1880-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Zhuang Z, Zhang Y, Wei M, Yang X, Wang Z. Magnetic Resonance Imaging Evaluation of the Accuracy of Various Lymph Node Staging Criteria in Rectal Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:709070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 29. | Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S, Mattia FO, Friso ML, Genovesi D, Vidali C, Gambacorta MA, Buffoli A, Lupattelli M, Favretto MS, La Torre G. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 30. | Gamboa AC, Lee RM, Turgeon MK, Varlamos C, Regenbogen SE, Hrebinko KA, Holder-Murray J, Wiseman JT, Ejaz A, Feng MP, Hawkins AT, Bauer P, Silviera M, Maithel SK, Balch GC. Impact of Postoperative Complications on Oncologic Outcomes After Rectal Cancer Surgery: An Analysis of the US Rectal Cancer Consortium. Ann Surg Oncol. 2021;28:1712-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Hupkens BJP, Martens MH, Stoot JH, Berbee M, Melenhorst J, Beets-Tan RG, Beets GL, Breukink SO. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection - A Matched-Controlled Study. Dis Colon Rectum. 2017;60:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maher H, China; Sahin TT, Turkey S-Editor: Qu XL L-Editor: A P-Editor: Cai YX