Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2553
Peer-review started: August 1, 2023
First decision: September 1, 2023
Revised: September 3, 2023
Accepted: September 26, 2023
Article in press: September 26, 2023
Published online: November 27, 2023
Processing time: 118 Days and 4.8 Hours
Although the location of proximal cancer of the remnant stomach is the same as that of primary proximal cancer of the stomach, its clinical characteristics and prognosis are still controversial.
To evaluate the clinicopathological features and prognosis factors of gastric stump cancer (GSC) and primary proximal gastric cancer (PGC).
From January, 2005 to December, 2016, 178 patients with GSC and 957 cases with PGC who received surgical treatment were enrolled. Patients in both groups underwent 1:1 propensity score matching analysis, and both clinical and pathological data were systematically collected for statistical purposes. Quality of life was evaluated by the C30 and STO22 scale between GSC-malignant (GSC following gastric cancer) and GSC-benign (GSC following benign lesions of the stomach).
One hundred and fifty-two pairs were successfully matched after propensity score matching analysis. Of the 15 demographic and pathological variables collected, the analysis further revealed that the number of lymph nodes and positive lymph nodes were different prognostic and clinicopathological factors between PGC and GSC. Univariate and multivariate analyses showed that gender, differentiation degree and tumor-node-metastasis stage were independent risk factors for patients with GSC. Gender, vascular invasion, differentiation degree, depth of infiltration, positive lymph nodes, and tumor-node-metastasis stage were independent risk factors for patients with PGC. The 5-year overall survival and cancer-specific survival of patients with GSC were significantly lower than those in the PGC group, the scores for overall quality of life in the GSC-malignant group were lower than the GSC-benign, and the differences were statistically significant.
The differences in clinicopathological characteristics between GSC and PGC were clarified, and PGC had a better prognosis than GSC.
Core Tip: Although the location of gastric stump cancer (GSC) is the same as that of primary proximal gastric cancer (PGC) , its clinical characteristics and prognosis are still controversial. In our research, 152 pairs of patients were successfully matched after propensity score matching analysis. The differences in clinicopathological characteristics between GSC and PGC were clarified, and PGC had a better prognosis than GSC. The scores for overall quality of life in the GSC-malignant group were lower than the GSC-benign group, and the differences were statistically significant.
- Citation: Wang SH, Zhang JC, Zhu L, Li H, Hu KW. Does gastric stump cancer really differ from primary proximal gastric cancer? A multicentre, propensity score matching-used, retrospective cohort study. World J Gastrointest Surg 2023; 15(11): 2553-2563
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2553.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2553
Gastric cancer is one of the most common malignant tumours of the digestive tract worldwide. According to the latest statistics, there were approximately 1.034 million new cases of gastric cancer worldwide in 2018, resulting in a total of approximately 783000 deaths[1-3]. The 5-year survival rate of early gastric cancer patients exceeds 90%. However, the diagnostic rate of early gastric cancer is < 10%[4], and the 5-year survival rate of advanced gastric cancer is still < 50%[5,6]. In recent years, gastric stump cancer (GSC), which accounts for only approximately 1%-7% of gastric cancers, has attracted more attention from scholars[7-10].
The concept of GSC was first proposed as the occurrence of residual cancer after surgery for benign lesions in 1922 by Balfour[11]. The current definition of GSC is, regardless of the method of first surgical resection or type of reconstruction, cancer found in the stump stomach 5 years after primary surgery for benign diseases or 10 years after primary surgery for malignant diseases. Although the detection rate of early gastric cancer continues to increase, due to the lack of typical symptoms and longer postoperative time leading to a decrease in patients’ willingness to undergo gastroscopy, GSC is often still in the late stage when detected, which seriously reduces the survival time of patients. Although radical surgery is still the only treatment method for GSC, this complex surgery still has a high incidence of postoperative complications and mortality. Anatomical changes, intra-abdominal adhesions, and frequent combined resection of other organs make the surgery of GSC difficult. Currently, most studies on this surgical treatment have only registered a few patients and provided a brief descriptive analysis of their complications.
It is worth noting that although GSC originates from the same region after distal gastrectomy for gastric cancer and proximal gastric cancer (PGC), the lymphatic drainage direction of GSC patients and PGC patients is different due to the influence of first-time surgical lymph node dissection. Moreover, intra-abdominal adhesions in GSC patients may affect the quality of lymph node dissection. Although the clinical and pathological characteristics of GSC and PGC have been compared in the past, clinical studies on GSC are very rare, especially high-quality, large-scale randomised controlled studies. In recent years, there has been continuous literature exploring the prognosis of GSC and PGC, there is still controversy in this regard, partly due to the limited number of GSC patients. In addition, the scope of lymph node dissection and how these patients should be staged are still unresolved issues. It is necessary to understand the characteristics of GSC to determine its prognosis and appropriate treatment strategies.
This study aims to evaluate the differences in clinical pathological characteristics and prognosis between PGC and GSC. Moreover, for patients with GSC caused by benign or malignant lesions, we evaluated their postoperative quality of life (QoL) to explore the impact of disease duration and psychological factors.
This article is in line with the STROCSS criteria[12].
One hundred and seventy-eight patients with GSC and 957 patients with PGC were enrolled as the control group from January, 2005 to December, 2016. None of the patients received neoadjuvant therapy. The clinical and pathological data of the patients were collected, including age, gender, tumor-node-metastasis (TNM) stage (T and N stages were classified according to the criteria described in the American Joint Committee on Cancer Staging Manual, 8th edition), number of lymph nodes obtained, nerve invasion, vascular invasion, surgical methods, blood transfusion, length of hospital stay, American Society of Anaesthesiologists (ASA) grade, and bypass type. Variables that were initially recorded as continuous variables were also included in the current analysis.
In this study, the survival time ranged from the day of surgery to the day of death via telephone and outpatient visits, which included enhanced computed tomography every 6 mo, routine blood tests, and biochemical and tumour indicators, and terminated when the patients died. In the first year after surgery, all GSC patients who were still alive during the follow-up period were followed up to assess QoL, and the scoring scale was used to record the patient’s general living conditions.
QoL was evaluated using the Chinese version of the EORTC QLQ-C30 and QLQ-STO22[13,14]. After the patients were introduced, they completed the questionnaire. Based on the EORTC QLQ-C30 and QLQ-STO22 scoring manuals, the original data of each scale were converted into 0-100. Statistical processing was performed using the EORTC QLQ-C30 questionnaire survey. For the QLQ-STO22 questionnaire survey, the higher the score, the worse the QoL. The t-test was used to compare the QoL.
In this propensity score matching (PSM) analysis, the following variables were considered potential confounders between the groups and were adjusted: Gender (female vs male), age (> 55 vs ≤ 55 years), and ASA score (ASA I/II vs III/IV). Propensity scores were calculated by bivariate logistic regression, using a 1:1 case-control match with a caliper value of 0.1 (one-to-one nearest-neighbor matching). The standardized difference (10% or 0.1) was used to compare the distribution of all paired.
The Cox proportional hazards regression model with backward variable selection was used to determine the factors independently related to survival time. It has also been reported that the 95% confidence interval (CI) of the hazard ratio (HR) has a significant effect. In this study, a P value of < 0.05 was used to define statistical significance, and all analyses were performed using SPSS 19.0.
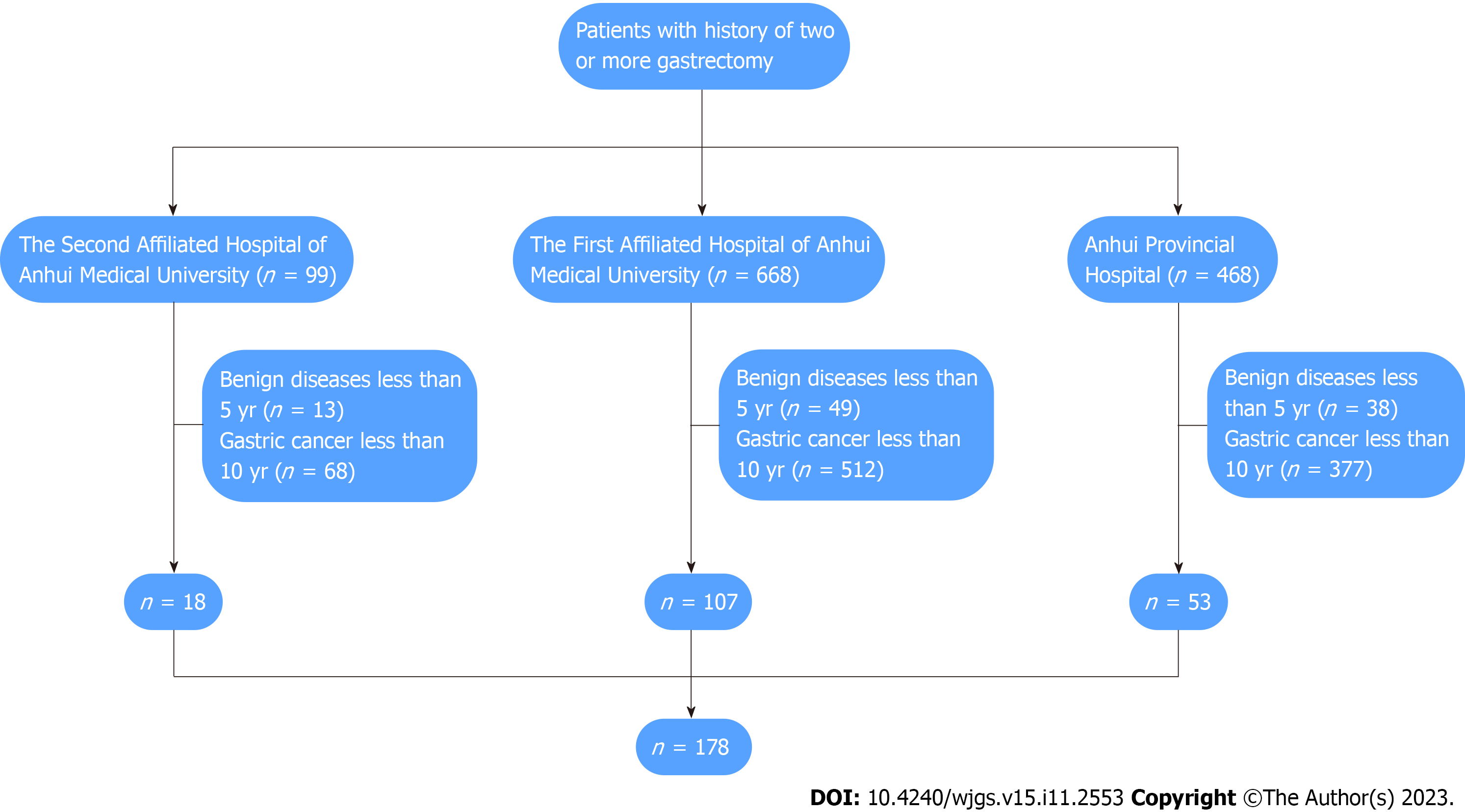
In this cohort, a total of 178 patients with GSC underwent surgical treatment in the general surgery department of the three hospitals (Figure 1). The mean age was 63 years. According to the American Joint Committee on Cancer Staging Manual, there were 15, 43 and 94 cases of stage I, II and III GSC, respectively. Nine hundred and fifty-seven patients with PGC underwent surgical treatment in the three hospitals. There were 736 male patients and 221 female patients. The mean age was 67 years. According to the American Joint Committee on Cancer Staging Manual, there were 132, 168, and 657 cases of stage I, II and III PGC, respectively.
Before PSM, there were significant differences in the number of lymph nodes, blood transfusion, TNM stage and differentiation degree between the PGC and GSC group. After PSM, there were 152 cases in these two groups, the statistical results showed that there were significant differences in the number of lymph nodes, positive lymph nodes, and differentiation degree between two groups (Table 1).
| Variables | Before PSM | After PSM | ||||||
| GSC (n = 178) | PGC (n = 957) | χ2/Z | P value | GSC (n = 152) | PGC (n = 152) | χ2/Z | P value | |
| Gender | 0.222 | 0.638 | 0.017 | 0.895 | ||||
| Female | 44 | 221 | 38 | 39 | ||||
| Male | 134 | 736 | 114 | 113 | ||||
| Age (yr) | 0.452 | 0.502 | 0.020 | 0.0889 | ||||
| > 55 | 127 | 706 | 120 | 119 | ||||
| ≤ 55 | 51 | 251 | 32 | 33 | ||||
| Tumor size | 2.300 | 0.129 | 2.608 | 0.106 | ||||
| > 3.5 cm | 103 | 611 | 91 | 77 | ||||
| ≤ 3.5 cm | 75 | 346 | 61 | 75 | ||||
| ASA grade | 2.590 | 0.108 | 0.058 | 0.809 | ||||
| I/II | 112 | 540 | 99 | 101 | ||||
| III/IV | 66 | 417 | 53 | 51 | ||||
| Hospital stay after surgery (d) | 12.65 ± 5.13 | 12.77 ± 4.42 | 1.023 | 0.133 | 11.13 ± 4.71 | 11.45 ± 5.90 | 1.156 | 0.232 |
| Blood transfusion | 10.705 | 0.001 | 2.114 | 0.156 | ||||
| Yes | 42 | 127 | 34 | 57 | ||||
| No | 126 | 780 | 108 | 85 | ||||
| Vascular invasion | 0.405 | 0.525 | 0.920 | 0.337 | ||||
| Positive | 36 | 339 | 32 | 70 | ||||
| Negative | 51 | 415 | 49 | 82 | ||||
| Missing | 91 | 203 | 71 | - | ||||
| Nerve invasion | 0.475 | 0.491 | 0.280 | 0.596 | ||||
| Positive | 49 | 389 | 40 | 63 | ||||
| Negative | 55 | 378 | 49 | 89 | ||||
| Missing | 74 | 190 | 63 | - | ||||
| Differentiation degree | 18.537 | 0.000 | 1.452 | 0.028 | ||||
| High/median | 38 | 100 | 27 | 21 | ||||
| Low | 128 | 824 | 115 | 131 | ||||
| Missing | 12 | 33 | 10 | - | ||||
| Depth of infiltration | 0.310 | 0.578 | 0.838 | 0.360 | ||||
| T1/T2 | 40 | 198 | 36 | 43 | ||||
| T3/T4 | 138 | 762 | 116 | 109 | ||||
| Number of lymph nodes | 3.859 | 0.049 | 6.752 | 0.009 | ||||
| ≥ 7 | 101 | 617 | 94 | 115 | ||||
| < 7 | 77 | 340 | 58 | 37 | ||||
| Positive lymph nodes | 0.570 | 0.450 | 19.667 | 0.000 | ||||
| ≥ 3 | 86 | 433 | 71 | 109 | ||||
| < 3 | 92 | 524 | 81 | 43 | ||||
| TNM stage | 10.367 | 0.006 | 0.062 | 0.969 | ||||
| I | 15 | 132 | 17 | 18 | ||||
| II | 43 | 168 | 38 | 39 | ||||
| III | 94 | 657 | 97 | 95 | ||||
Table 2 shows that gender, degree of differentiation, and TNM stage were found to be risk factors for GSC. The prognostic factors in PGC determined by the univariate analysis were as follows: Gender, vascular invasion, degree of differentiation, depth of infiltration, number of positive lymph nodes, and TNM stage were found to be risk factors for PGC. Multivariate analyses were conducted to identify the independent prognostic factors, and the results are shown in Table 3. The degree of differentiation, and TNM stage were independent prognostic factors for patients with GSC and differentiation degree, depth of infiltration, positive lymph nodes and TNM stage were independent prognostic factors for PGC patients.
| Variables | GSC | PGC | ||||||
| n = 152 | HR | 95%CI | P value | n = 152 | HR | 95%CI | P value | |
| Gender | 1.991 | 0.937-3.422 | 0.038a | 1.991 | 0.937-3.422 | 0.038a | ||
| Female | 38 | 38 | ||||||
| Male | 114 | 114 | ||||||
| Age (yr) | 1.117 | 0.681-1.833 | 0.900 | 1.111 | 0.690-1.804 | 0.893 | ||
| > 55 | 120 | 119 | ||||||
| ≤ 55 | 32 | 33 | ||||||
| Tumor size | 1.012 | 0.622-1.646 | 0.961 | 1.405 | 0.598-1.837 | 0.902 | ||
| > 3.5 cm | 91 | 77 | ||||||
| ≤ 3.5 cm | 61 | 75 | ||||||
| ASA grade | 1.338 | 0.792-2.260 | 0.276 | 1.257 | 0.777-2.900 | 0.331 | ||
| I/II | 99 | 101 | ||||||
| III/IV | 53 | 51 | ||||||
| Hospital stay after surgery (d) | 11.13 ± 4.71 | 0.635 | 0.308-1.307 | 0.218 | 12.45 ± 5.90 | 0.873 | 0.299-1.780 | 0.412 |
| Blood transfusion | 1.114 | 0.655-1.896 | 0.690 | 1.296 | 0.588-2.001 | 0.255 | ||
| Yes | 44 | 67 | ||||||
| No | 108 | 85 | ||||||
| Vascular invasion | 1.662 | 0.210-2.138 | 0.630 | 1.603 | 1.000-9.568 | 0.049a | ||
| Positive | 32 | 70 | ||||||
| Negative | 49 | 82 | ||||||
| Missing | 71 | - | ||||||
| Nerve invasion | 1.710 | 0.971-3.012 | 0.063 | 4.660 | 0.981-22.134 | 0.053 | ||
| Positive | 40 | 63 | ||||||
| Negative | 49 | 89 | ||||||
| Missing | 63 | - | ||||||
| Differentiation degree | 2.714 | 1.603-4.596 | 0.000a | 3.503 | 1.734-11.385 | 0.000a | ||
| High/median | 27 | 21 | ||||||
| Low | 115 | 131 | ||||||
| Missing | 10 | - | ||||||
| Depth of infiltration | 3.614 | 2.290-4.289 | 0.080 | 2.332 | 0.074-4.498 | 0.041a | ||
| T1/T2 | 36 | 43 | ||||||
| T3/T4 | 116 | 109 | ||||||
| Number of lymph nodes | 0.792 | 0.336-1.869 | 0.595 | 3.432 | 0.874-12.441 | 0.077 | ||
| ≥ 7 | 94 | 115 | ||||||
| < 7 | 58 | 37 | ||||||
| Positive lymph nodes | 0.223 | 0.110-0.881 | 0.124 | 0.485 | 0.260-0.906 | 0.023a | ||
| ≥ 3 | 71 | 109 | ||||||
| < 3 | 81 | 43 | ||||||
| TNM stage | 5.727 | 2.579- 12.715 | 0.000a | 5.446 | 2.555-11.992 | 0.000a | ||
| I | 17 | 18 | ||||||
| II | 38 | 39 | ||||||
| III | 97 | 95 | ||||||
| GSC | PGC | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | 1.552 | 0.129-3.428 | 0.058 | 1.847 | 0.135-2.990 | 0.043 |
| Differentiation degree | 1.430 | 1.055-1.938 | 0.021a | 1.999 | 0.636-3.004 | 0.027a |
| Depth of infiltration | 2.929 | 1.383-4.691 | 0.000a | |||
| Positive lymph nodes | 2.452 | 1.085-3.942 | 0.012a | |||
| TNM stage | 1.426 | 1.040-1.955 | 0.027a | 2.771 | 1.448-4.662 | 0.000a |
| Vascular invasion | 1.269 | 0.680-3.998 | 0.070 | |||
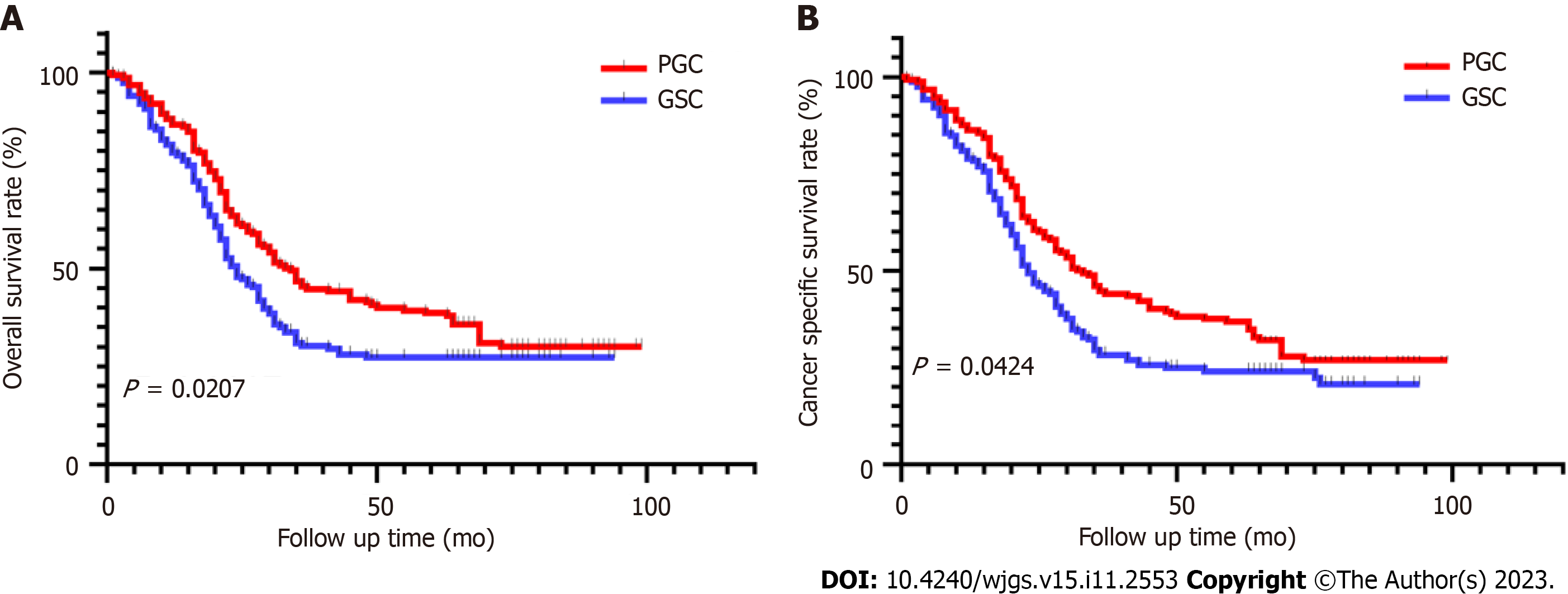
The median follow-up time in the PGC group was 83 mo. At the last follow-up in June 2022, 72.2% of patients had died. The median follow-up time in the GSC group was 80 mo, and 82.1% of patients had died. The overall median survival in the PGC group was 34 mo and was 24 mo in the GSC group. The risk of death after GSC radical surgery was not constant. Most patients with GSC experienced overall-cause death or cancer-specific death in the first 3 years after surgery. After a period of evaluation, the probability of all-cause death and cancer-specific death peaked at 12 mo after surgery and then gradually decreased. We also evaluated the probability of survival for patients with GSC over a period and showed that the probability of cancer-specific survival increased with prolongation of postoperative survival. Correspondingly, with the prolongation of survival time, the recurrence rate in patients with GSC decreased. In the GSC control group, the overall survival during the follow-up period was significantly lower than that in the PGC group (HR = 0.7290, 95%CI: 0.5578-0.9529, P = 0.0207, Figure 2A), the cancer specific survival in the PGC group was also significantly higher than that in the GSC group (HR = 0.7504; 95%CI: 0.5686-0.9902, P = 0.0424, Figure 2B).
According to the QLQ-C30 questionnaire, the overall health status scores of patients with GSC-benign (GSC-B) and those with GSC-malignant (GSC-M) were 67.15 ± 20.1 and 56.2 ± 18.5, respectively. There was a significant difference between the two groups by statistical analysis, which showed that the overall health status of the GSC-M group was worse than that of the GSC-B group. In terms of function scale, the scores for physical, emotional and cognitive function in patients on the symptom scale, and the scores for fatigue, pain, diarrhea, economic difficulties, and reflux in the two groups were not different.
There has been no large-scale high-quality study in the field of GSC. Previous studies on GSC are few, especially clinical trials with more than 100 cases. A study by Japanese scholars included 156 GSC patients and 755 PGC patients and the authors believed that the prognosis of GSC patients was worse than that of PGC patients, moreover, GSC secondary to malignant lesions occurred earlier than that of benign lesions after surgery[15]. Wang et al[16] focused on cardiac cancer, and included 48 GSC patients and 96 primary cardiac cancer patients. The results confirmed that the survival rate of patients with residual gastric cardia cancer after radical resection was lower than that of primary cardiac cancer patients, but the survival rate of patients without serous infiltration or lymph node metastasis was similar to that of primary cardiac cancer patients. Ramos et al[17] also obtained similar results, indicating that there is still a lot of controversy regarding the prognosis of GSC and PGC patients, and further clarification is needed in large-scale clinical trials, especially high-quality randomised controlled trials.
At present, the definition of GSC is still controversial. These disputes easily make researchers focus on the time interval and the nature of the primary disease, and often ignore the nature of GSC, its cause. The incidence of GSC has been increasing in recent years, and the reason for this is unclear. However, some scholars believe that damage to the epithelial cells of the gastric mucosa and weakening of the gastric mucosal barrier by alkaline reflux after the previous surgery are important factors in the occurrence of GSC. Healed anastomoses or suture ulcers are important factors in stress stimulation; the occurrence and development of some GSCs may be related to Epstein-Barr virus infection; the occurrence of GSCs is also related to the previous surgical method[18,19]. After partial resection, Billroth II (B-II) surgery is associated with a higher incidence of GSC due to its higher reflux rate. In this study, more than half of patients in the GSC group underwent B-II anastomosis during their first surgery, while the proportion of Roux-en-Y (R-Y) anastomosis was less than 11%. It can be seen that the proportion of GSC in patients with B-II anastomosis was higher. Undeniably, R-Y anastomosis performs better in resisting digestive reflux.
R-Y anastomosis can reduce reflux, the occurrence of residual gastritis, and the incidence of GSC[20-22]. Cutting the vagus nerve during distal gastrectomy also causes cancer. After cutting, the gastric defence factors are reduced, and the blood circulation, secretion, and regeneration of the gastric mucosa are affected, resulting in cell DNA mutations during the proliferation process. This is carcinogenic[23], and its occurrence is related to factors such as age, heredity, and sex. Research shows that in patients diagnosed with GSC, the median age is between 67 and 71 years and male patients are at greater (4-9 times) risk of developing GSC than female patients[24]. In this study, the number of male patients with GSC was more than three times that of female patients, with a mean age of 63 (range, 39-76) years.
It is worth noting that in this study, only 36.2% of patients who underwent surgical treatment for benign diseases developed GSC, while the proportion of patients with GSC-M was 63.8%. Due to the fact that the biological behavior of tumor cells, especially their metastatic ability, may vary depending on the location of the tumor, in order to avoid this bias, we only selected one-third of primary PGC patients as the control group. Overall, the GSC group exhibited similar characteristics to PGC patients. In addition, survival data processed by statistical methods showed a difference in survival time between the GSC group and the PGC group, which is contrary to the previous research results of Ramos et al[17]. As expected, among the patients we included, the number of lymph nodes after GSC surgery was significantly lower than that in the PGC group. Some studies have shown that the characteristics of lymph node metastasis in GSC are different due to the interruption of lymphatic pathways during the first operation, which may lead to more involvement of the splenic artery, splenic hilum, lower mediastinum and jejunum mesentery lymph nodes[25-27]. However, the standard extension for lymph node resection has not yet been determined. It is well known that an enlarged lymph node resection in this area can seriously affect the QoL after surgery. Therefore, the scope of mesentery lymph node resection should be determined according to the extent of lymph node involvement, taking into account the risks and benefits[28].
In recent years, the application of neoadjuvant therapy in the perioperative period of gastric cancer has become a consensus. However, the application of this conclusion in GSC still needs more evidence. Patients with neoadjuvant therapy were not included in this study as the number of patients with GSC receiving neoadjuvant therapy was small, and the inclusion of too many patients with neoadjuvant therapy in the PGC group may have a significant impact on the results. There is no denying that neoadjuvant therapy has several potential advantages, including improving R0 removal rates, testing tumour response to a specific treatment regimen, and not only that, it provides a time window to evaluate tumour biology. Despite local control, an important risk of neoadjuvant therapy is that it may introduce a greater probability of distant metastasis if treatment fails to control tumour progression. The best approach, however, is unclear. In conclusion, selective addition of neoadjuvant chemotherapy and/or radiotherapy is beneficial in specific anatomical and histopathological subtypes.
The clinical symptoms of GSC lack specificity, the resection rate is low after diagnosis, and the prognosis is poor. It causes damage to the patients’ physical, psychological, and social functions and affects their health-related QoL (HRQOL). However, few studies have evaluated the postoperative QoL in patients with GSC. In this study, the HRQOL in two groups of GSC patients caused by benign (GSC-B) and malignant (GSC-M) lesions was comprehensively evaluated using the QLQ-C30 and gastric cancer-specific scale QLQ-STO22. The results of this study show that the scores for overall QoL in the GSC-B group were higher than those in the GSC-M group and there was no significant statistical difference in other aspects. We speculate that this may be related to the postoperative chemotherapy received by patients in the GSC-M group, as the proportion of postoperative chemotherapy in the GSC-M group was significantly higher. On the other hand, we found that the differentiation level of patients in the GSC-M group was worse than that in the GSC-B group, and the proportion of poorly differentiated patients was higher, which may also be a reason for the decline in their QoL. Early clinical diagnosis, appropriate treatment, timely control of disease progression, and reduction of physical symptoms are conducive to improving patients’ HRQOL. While improving their physiological function, patients should recognise the positive role of psychological and spiritual factors in the course of cancer, carry out necessary psychological treatment and intervention, alleviate psychological obstacles, and eliminate the negative impact of bad emotions on HRQOL as far as possible.
The differences in clinicopathological characteristics between GSC and PGC were clarified, and PGC had a better prognosis than GSC.
The clinicopathological characteristics of gastric stump cancer (GSC) and proximal gastric cancer (PGC) have not yet been confirmed. There has always been controversy regarding the differences in treatment and prognosis prediction.
Evaluation of the differences between GSC and primary PGC using a larger sample size.
The object of this study was to evaluate the clinicopathological features, and prognostic factors of GSC and primary PGC.
After detailed data statistics and data collection, 178 GSC patients and 957 PGC patients underwent surgical treatment at multiple centers. A 1:1 propensity score matching analysis was conducted on the two groups of patients, with 152 patients in each group entering the final analysis. Single factor and multivariate analysis were used to study the risk factors in gastric cancer patients. The survival curve was plotted to compare the differences in survival time between the two groups. The quality of life (QoL) of GSC-malignant (GSC-M) (post cancer GSC) and GSC-benign (GSC-B) (post benign gastric lesion GSC) patients was evaluated using the C30 and STO22 scales.
The number of lymph nodes and positive lymph nodes were different prognostic and clinicopathological factors between PGC and GSC. The 5-year overall survival and cancer-specific survival of patients with GSC were significantly lower than the PGC group, the scores for overall QoL in the GSC-M group were lower than the GSC-B group, and the differences were statistically significant.
The differences in clinicopathological characteristics between GSC and PGC were significant, and compared to GSC patients, PGC patients had a better prognosis, and the overall health status of the GSC-M group was worse than that of the GSC-B group.
More large-scale randomised controlled trial studies are needed to provide higher-level evidence regarding the comparison between PGC and GSC.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56637] [Article Influence: 7079.6] [Reference Citation Analysis (134)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13321] [Article Influence: 1332.1] [Reference Citation Analysis (4)] |
| 3. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20715] [Article Influence: 1883.2] [Reference Citation Analysis (23)] |
| 4. | Hussain I, Ang TL. Evidence based review of the impact of image enhanced endoscopy in the diagnosis of gastric disorders. World J Gastrointest Endosc. 2016;8:741-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 5. | Picado O, Dygert L, Azab B, Franceschi D, Sleeman D, Livingstone AS, Merchant N, Yakoub D. Surgical Management of Metastatic Gastric Cancer: A National Cancer Database Analysis. Gastroenterology. 2017;152:S1247. [DOI] [Full Text] |
| 6. | Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, Meershoek-Klein Kranenbarg E, Boot H, Trip AK, Swellengrebel HAM, van Laarhoven HWM, Putter H, van Sandick JW, van Berge Henegouwen MI, Hartgrink HH, van Tinteren H, van de Velde CJH, Verheij M; CRITICS investigators. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 396] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 7. | Kaneko K, Kondo H, Saito D, Shirao K, Yamaguchi H, Yokota T, Yamao G, Sano T, Sasako M, Yoshida S. Early gastric stump cancer following distal gastrectomy. Gut. 1998;43:342-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ohashi M, Katai H, Fukagawa T, Gotoda T, Sano T, Sasako M. Cancer of the gastric stump following distal gastrectomy for cancer. Br J Surg. 2007;94:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Shimada H, Fukagawa T, Haga Y, Oba K. Does remnant gastric cancer really differ from primary gastric cancer? A systematic review of the literature by the Task Force of Japanese Gastric Cancer Association. Gastric Cancer. 2016;19:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Sinning C, Schaefer N, Standop J, Hirner A, Wolff M. Gastric stump carcinoma - epidemiology and current concepts in pathogenesis and treatment. Eur J Surg Oncol. 2007;33:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 11. | Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg. 1922;76:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G; STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1230] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 13. | Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S, Kvikstad A. The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer. 1995;31A:2260-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 300] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Huang CC, Lien HH, Sung YC, Liu HT, Chie WC. Quality of life of patients with gastric cancer in Taiwan: validation and clinical application of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-STO22. Psychooncology. 2007;16:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Tokunaga M, Sano T, Ohyama S, Hiki N, Fukunaga T, Yamada K, Yamaguchi T. Clinicopathological characteristics and survival difference between gastric stump carcinoma and primary upper third gastric cancer. J Gastrointest Surg. 2013;17:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Huang CM, Wang JB, Zheng CH, Li P, Xie JW, Lin JX, Lu J. Survival and surgical outcomes of cardiac cancer of the remnant stomach in comparison with primary cardiac cancer. World J Surg Oncol. 2014;12:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Ramos MFKP, Pereira MA, Dias AR, Dantas ACB, Szor DJ, Ribeiro U Jr, Zilberstein B, Cecconello I. Remnant gastric cancer: An ordinary primary adenocarcinoma or a tumor with its own pattern? World J Gastrointest Surg. 2021;13:366-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Zhang DW, Dong B, Li Z, Dai DQ. Clinicopathologic features of remnant gastric cancer over time following distal gastrectomy. World J Gastroenterol. 2015;21:5972-5978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Morgagni P, Gardini A, Marrelli D, Vittimberga G, Marchet A, de Manzoni G, Di Cosmo MA, Rossi GM, Garcea D, Roviello F; Italian Research Group for Gastric Cancer. Gastric stump carcinoma after distal subtotal gastrectomy for early gastric cancer: experience of 541 patients with long-term follow-up. Am J Surg. 2015;209:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Hirao M, Takiguchi S, Imamura H, Yamamoto K, Kurokawa Y, Fujita J, Kobayashi K, Kimura Y, Mori M, Doki Y; Osaka University Clinical Research Group for Gastroenterological Study. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Xiong JJ, Altaf K, Javed MA, Nunes QM, Huang W, Mai G, Tan CL, Mukherjee R, Sutton R, Hu WM, Liu XB. Roux-en-Y versus Billroth I reconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19:1124-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Tornese S, Aiolfi A, Bonitta G, Rausa E, Guerrazzi G, Bruni PG, Micheletto G, Bona D. Remnant Gastric Cancer After Roux-en-Y Gastric Bypass: Narrative Review of the Literature. Obes Surg. 2019;29:2609-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Ohira M, Toyokawa T, Sakurai K, Kubo N, Tanaka H, Muguruma K, Yashiro M, Onoda N, Hirakawa K. Current status in remnant gastric cancer after distal gastrectomy. World J Gastroenterol. 2016;22:2424-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Costa-Pinho A, Pinto-de-Sousa J, Barbosa J, Costa-Maia J. Gastric stump cancer: more than just another proximal gastric cancer and demanding a more suitable TNM staging system. Biomed Res Int. 2013;2013:781896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Deng J, Liang H, Wang D, Sun D, Ding X, Pan Y, Liu X. Enhancement the prediction of postoperative survival in gastric cancer by combining the negative lymph node count with ratio between positive and examined lymph nodes. Ann Surg Oncol. 2010;17:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Son SY, Kong SH, Ahn HS, Park YS, Ahn SH, Suh YS, Park DJ, Lee HJ, Kim HH, Yang HK. The value of N staging with the positive lymph node ratio, and splenectomy, for remnant gastric cancer: A multicenter retrospective study. J Surg Oncol. 2017;116:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Nakagawa M, Choi YY, An JY, Hong JH, Kim JW, Kim HI, Cheong JH, Hyung WJ, Choi SH, Noh SH. Staging for Remnant Gastric Cancer: The Metastatic Lymph Node Ratio vs. the UICC 7th Edition System. Ann Surg Oncol. 2016;23:4322-4331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Chowdappa R, Tiwari AR, Ranganath N, Kumar RV. Is there difference between anastomotic site and remnant stump carcinoma in gastric stump cancers?-a single institute analysis of 90 patients. J Gastrointest Oncol. 2019;10:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Neri V, Italy; Uhlmann D, Germany S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ