Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2423
Peer-review started: August 7, 2023
First decision: August 24, 2023
Revised: September 18, 2023
Accepted: October 11, 2023
Article in press: October 11, 2023
Published online: November 27, 2023
Processing time: 112 Days and 1.6 Hours
Polycystic ovary syndrome (PCOS) is closely related to obesity, and weight loss can significantly improve the metabolic, endocrine and reproductive functions of obese individuals with PCOS. However, the efficacy of laparoscopic sleeve gastrectomy (LSG) for obesity with PCOS are unclear.
The purpose of the study was to investigate the effect of LSG on related variables in obese patients with PCOS.
A retrospective analysis was performed on 32 obese patients with PCOS who received LSG treatment at the Third Hospital of Shanxi Medical University from 2013 to 2020. The changes in anthropometric indices, insulin, testosterone, estradiol, follicle stimulating hormone (FSH), luteinizing hormone (LH), menstrual cycle and LH/FSH ratio before and 1 mo, 3 mo, 6 mo and 12 mo after the operation were statistically analyzed.
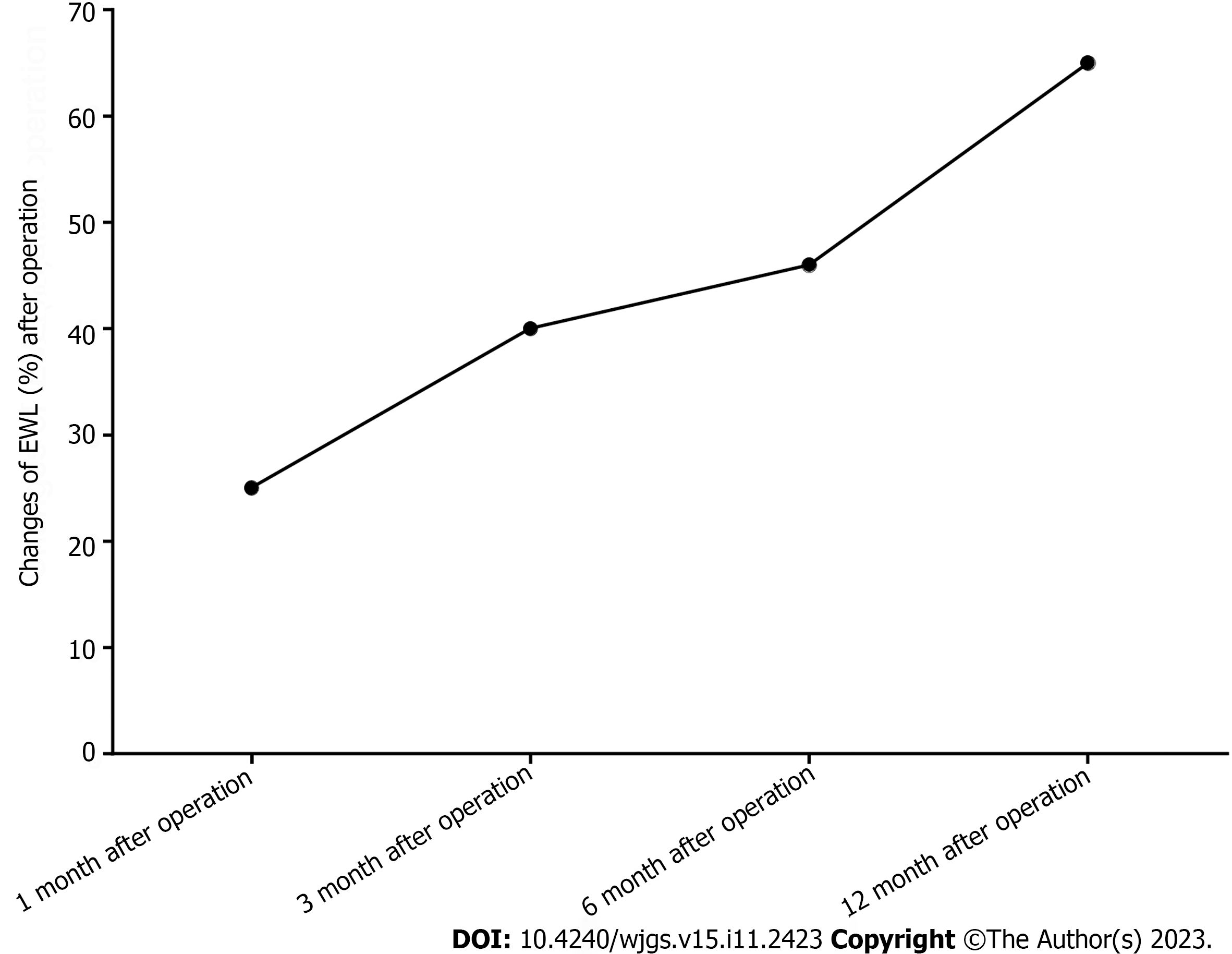
At 1 mo, 3 mo, 6 mo and 12 mo after surgery, the anthropometric indices, such as body weight and body mass index, of all patients were lower than those before the operation. The percentage excess weight loss (EWL%) at 1 mo, 3 mo, 6 mo and 1 year of follow-up were 25, 40, 46 and 65, respectively. The PCOS-related indices, such as insulin, testosterone, estradiol, follicle stimulating hormone (FSH), luteinizing hormone (LH) and menstrual cycle, were improved to varying degrees. During the 1-year follow-up, the average serum testosterone decreased from preoperative 0.72 ng/mL to 0.43 ng/mL (P < 0.05), average fasting insulin level (9.0 mIU/mL, preoperative 34.2 mil, LH level, 4.4 mIU/mL, preoperative 6.1 mIU/mL). The level of FSH (3.8 U/L, 4.8 U/p0.05) and the ratio of LH/FSH (0.7, 1.3/p0.05) were more relieved than those before surgery. During the postoperative follow-up, it was found that the menstrual cycle of 27 patients (nasty 27) returned to normal, and 6 patients (18%) who intended to become pregnant became pregnant within 1 year after surgery.
The weight loss effect of LSG is obvious and affirmative, and the endocrine index of obese patients with PCOS is also improved to some extent, although the mechanism is not clear. Laparoscopic sleeve gastrectomy is expected to become a backup choice for patients with polycystic ovaries in the future.
Core Tip: The clinical data of 32 obese patients with polycystic ovary syndrome (PCOS) after laparoscopic sleeve gastrectomy were retrospectively analyzed, and the changes in PCOS after laparoscopic sleeve gastrectomy were analyzed.
- Citation: Wang XT, Hou YS, Zhao HL, Wang J, Guo CH, Guan J, Lv ZG, Ma P, Han JL. Effect of laparoscopic sleeve gastrectomy on related variables of obesity complicated with polycystic ovary syndrome. World J Gastrointest Surg 2023; 15(11): 2423-2429
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2423.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2423
The World Health Organization (WHO) confirms that obesity is a 21st century epidemic that can lead to hormonal imbalances, such as polycystic ovary syndrome (PCOS)[1-3]. PCOS is a common endocrine disease in young women, with a prevalence rate of 5%-18%[4-5]. The widely accepted mechanisms include insulin resistance leading to hyperinsulinemia, stimulating ovarian sheath cells to produce androgen, which is characterized by hypomenorrhea and hyperandrogenemia, and an increased risk of gynecological diseases such as hirsutism, infertility, and ovarian and endometrial cancer[6-8]. The incidence of PCOS in obese women is as high as 50%.
PCOS is closely related to obesity, and an increase in obesity will enhance the severity and expression of the PCOS phenotype. Current data suggest that weight loss is associated with metabolic, endocrine and reproductive improvements in obese women with PCOS. In addition to significant weight loss, bariatric surgery can also lead to recovery of the hypothalamic-pituitary axis, normal menstruation, improvement of hirsutism, reduction of cardiovascular risk, and improvement of fertility[9]. At present, the methods of weight loss are complex and diverse. The two most common types of bariatric surgery are laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux gastric bypass (LRYGB)[10]. LRYGB is found to be superior in long-term remission of dyslipidemia and hypertension while LSG is relatively low difficulty, small changes to the original structure of the human body and few postoperative complications[11]. Laparoscopic sleeve gastrectomy has been the most commonly performed bariatric procedure since 2014 and continues to steadily increase in number and percentage of all bariatric procedures year after year[12]. In this study, the clinical data of 32 obese patients with PCOS after laparoscopic sleeve gastrectomy were retrospectively analyzed, and the changes in PCOS after laparoscopic sleeve gastrectomy were analyzed.
The clinical data of 32 patients who were diagnosed with obesity complicated with polycystic ovary syndrome and underwent laparoscopic sleeve gastrectomy in the Department of Weight Loss and Metabolic Surgery of the Third Hospital of Shanxi Medical University from January 2013 to December 2020 were reviewed. Body weight, fasting insulin, testosterone, estrogen, FSH, LH and other endocrine and metabolic indices were statistically analyzed before the operation and 1 mo, 3 mo, 6 mo and 12 mo after the operation. The inclusion criteria were as follows: (1) The diagnosis of obesity was clear, and there were surgical indications according to the guidelines; (2) according to the guidelines, the diagnosis of polycystic ovary syndrome was clear; (3) the patient signed informed consent for the operation and successfully completed laparoscopic sleeve gastrectomy; and (4) there were no postoperative complications or secondary operations. Exclusion criteria: (1) Polycystic ovary received surgical treatment or nearly 2 mo of treatment; (2) choose other methods of weight loss; and (3) morbid obesity.
After successful anesthesia, the supine split leg position was taken, the skin 1.0 cm was cut along the navel, the visible Trocar was punctured into the abdomen once, and 1.2 cm Trocar was placed on the right side of the umbilical 10 cm; the left clavicle line of 10 cm was disposed into 5 mm Trocar, and a Kirschner needle was placed under the xiphoid process to block the left lobe of the liver, and the omentum tissue was cut off along the great curvature of the stomach, close to the gastric wall, up to 1.5 cm on the left side of the His angle of the cardia, and down to the pyloric 4 cm; a gastric tube approximately 1.2 cm in diameter was placed through the mouth. Close to the pylorus 5 cm, close to the gastric tube, the gastric tissue on the large curved side was resected so that the residual gastric cavity was tubular at approximately 100 mL. Inverted thorns can be absorbed by purse sutures to strengthen the seromuscular layer. The specimen was removed, and it was confirmed that there was no blood oozing or bleeding. The abdomen was closed, and the operation was ended.
Body weight-related parameters [body weight, body mass index (BMI), percentage excess weight loss (EWL%)] and polycystic ovary syndrome-related parameters [fasting insulin, testosterone, estrogen, FSH, LH, menstrual cycle and LH/FSH ratio] were measured before and 1, 3, 6 and 12 mo after the operation.
SPSS 23.0 statistical software was used. The measurement data in accordance with the normal distribution are expressed as the mean ± standard deviation (mean ± SD), and the counting data are expressed as n (%). The related indices before and after the operation were compared by paired sample t tests, and P < 0.05 was considered statistically significant.
Between January 2013 and December 2020, 32 obese women with PCOS participated in the study and underwent surgery without intraoperative or postoperative complications. The baseline information of 32 patients were shown in Table 1. The average age of the sample was 33.78 ± 7.31 years old. The body weight and BMI decreased significantly at 1, 3, 6 and 12 mo after the operation (mean preoperative = 41.22 kg/m2; postoperative mean = 28.98 kg/m2). The patient's body mass and BMI were basically stable at 1 year after the operation. See Table 2 and Figure 1.
| Characteristic | n = 32 |
| Age, yr | 33.78 ± 7.31 |
| Marital status | |
| Unmarried | 6 (18.8) |
| Married | 24 (75.0) |
| Divorced | 2 (6.2) |
| Smoking | 4 (12.5) |
| Alcohol | 3 (9.4) |
During the postoperative period, the average level of estradiol increased (mean preoperatively = 81.1 PG/dL; postoperative mean = 89.2 pg/dL), and the difference was not statistically significant. The average serum testosterone decreased from 0.72 ng/mL to 0.43 ng/mL (P = 0.05), and the insulin level decreased significantly after the operation (preoperative value = 34.2 mIU/mL; postoperative insulin level = 9.0 mIU/mL). After the operation, the level of FSH decreased (mean before the operation = 4.8 U/L, average after the operation = 3.8 U/L), and the difference was not statistically significant. At the same time, the level of LH decreased significantly after the operation (P < 0.05) (mean preoperative = 6.1 U/L; postoperative mean = 4.4 U/L). The average LH/FSH ratio decreased after the operation (1.3 before and after the operation). The preoperative average value was greater than 1, indicating that the level of LH was higher than that of FSH. On the other hand, after surgical treatment, it was found that the average ratio was less than 1, indicating that the average FSH level after bariatric surgery was higher than that of LH. The decrease in the LH/FSH ratio was statistically significant (P = 0.05). The menstrual cycle of 27 patients (nasty 27, 84%) returned to normal, and 6 patients (18%) who intended to become pregnant became pregnant within 1 year after the operation. See Table 3.
| Time | Estradiol (pg/dL) | Testosterone (ng/mL) | Insulin (mIU/mL) | LH (U/L) | FSH (U/L) | LH/FSH |
| Preoperative | 81.1 ± 25.6 | 0.72 ± 0.32 | 34.2 ± 16.0 | 6.1 ± 2.0 | 4.8 ± 1.6 | 1.3 ± 0.6 |
| 1 mo after operation | 83.4 ± 21.2 | 0.68 ± 0.29a | 30.3 ± 12.8a | 6.3 ± 1.8a | 5.3 ± 2.1 | 1.1 ± 0.5a |
| 3 mo after operation | 78.7 ± 18.8 | 0.57 ± 0.24a | 24.8 ± 9.2a | 5.8 ± 2.4a | 5.5 ± 1.8 | 1.1 ± 0.5a |
| 6 mo after operation | 82.6 ± 16.4 | 0.51 ± 0.25a | 15.8 ± 6.1a | 5.5 ± 2.2a | 5.0 ± 2.5 | 0.9 ± 0.4a |
| 12 mo after operation | 89.2 ± 29.3 | 0.43 ± 0.19a | 9.0 ± 2.5a | 4.4 ± 1.9a | 3.8 ± 2.3 | 0.7 ± 0.4a |
According to the clinical research and theoretical basis of PCOS for many years, China's Diagnostic Criteria of Polycystic Ovary Syndrome[13] points out that oligomenorrhea or amenorrhea is a necessary diagnostic condition. In addition, hyperandrogenaemia and/or clinical manifestations and ultrasound polycystic ovaries are nonessential conditions, and one of them can diagnose polycystic ovary syndrome[14]. Clinical hyperandrogenism can be detected by the serum testosterone index. Normal serum testosterone in females is generally below 0.68 ng/L. If it exceeds 0.7 ng/L, it can be diagnosed as hyperandrogenemia. The symptoms can be characterized by exuberant hair growth, male characteristics, oligomenorrhea or amenorrhea. High serum androgen levels directly inhibit follicle stimulating hormone secretion and eventually lead to infertility. The indices of FSH and LH in patients with polycystic ovary syndrome are higher than normal to varying degrees. The ratio of LH/FSH can also be used as an important index for the diagnosis of polycystic ovary syndrome. The pathogenesis of PCOS is not clear, and genetic, environmental and other factors interact with each other. The widely accepted theory is that hyperandrogenemia and hyperinsulinemia caused by insulin resistance are its characteristics. In this study, insulin was also used as an important indicator to evaluate the remission of PCOS[15].
According to the diagnostic criteria of polycystic ovary syndrome, the testosterone index was used as the indication of hyperandrogenism, the insulin index was used as the manifestation of insulin resistance, and oligoovulation showed abnormal gonadotropin secretion, which could be observed by FSH and LH. The changes in related indices in patients with PCOS before and after the operation were analyzed. Anovulation in patients with PCOS is characterized by abnormal secretion of gonadotropin, which is usually characterized by increased serum LH concentration and normal or low FSH, resulting in an increase in the ratio of LH/FSH. Clinically, the ratio is used as an index to predict ovarian reserve function, and an increase in the ratio indicates a decrease in female ovarian reserve function[16]. In this study, there were important changes in gonadotropin secretion after LSG; that is, FSH synthesis increased, and LH synthesis decreased, resulting in the reversal of the LH/FSH ratio. In this way, in addition to the reduction in plasma insulin, it helps to reduce hyperandrogenemia, promote the complete development of antral follicles and is conducive to ovulation. During the return visit, we found that some patients became pregnant within six months after the operation. This study did not evaluate the long-term effects of reproductive parameters such as infertility, but a systematic review published by Dağ et al[17] showed that bariatric surgery can significantly improve menstrual irregularity and hirsutism, and fertility may be improved after bariatric surgery. The ovulation process leads to the formation of the corpus luteum and the continuous release of progesterone from the luteal structure, which reduces the risk of endometrial hyperplasia and cancer[13]. Gonadotropin levels show hormonal oscillations during the menstrual cycle, which makes the study of gonadotropin more meaningful by taking the LH/FSH ratio as a variable rather than just focusing on a gonadotropin index. In this study, LH showed a downward trend compared with that before the operation, the change in FSH showed periodic changes, and LH/FSH was significantly improved compared with that before the operation. During the follow-up, it was also found that the menstrual cycle of 27 patients returned to normal, and 6 patients with pregnancy intention (18%) became pregnant within 1 year after the operation. According to the guidelines, women of childbearing age should avoid pregnancy for at least one year after weight loss, mainly because of the patient's own nutritional status and physical recovery. In this study, some patients became pregnant within 1 year after the operation due to age, family and other factors; that is, laparoscopic sleeve gastrectomy has a definite effect on obesity complicated with polycystic ovary syndrome.
The fasting insulin level reflects compensatory hyperinsulinemia. The diagnostic criterion of insulin resistance is that fasting insulin is greater than or equal to 85 pmol/L, especially in patients with hypertension and obesity. Fat accumulation often weakens the biological activity of insulin and makes the body resistant to insulin. Although the effects of obesity are different, it interferes with the pathophysiology of PCOS and affects insulin resistance and hyperinsulinemia[15]. Hyperinsulinemia stimulates androgen production and maintains abnormal gonadotropin secretion in the pituitary gland. In contrast, weight loss can improve insulin resistance, reduce circulating LH concentration, and increase reproductive potential[18]. In this study, it was observed that the level of serum insulin decreased from 34.2 mIU/mL to 9 mIU/mL, which reflected the improvement of insulin resistance and had a positive effect on the attenuation of androgen, resulting in the secretion of gonadotropin tending to normal. The above results can be explained by the increase in postoperative gastric emptying, which further leads to a decrease in GLP-1-mediated insulin secretion and related auxin-releasing peptide and leptin, thus increasing serum insulin and reducing insulin resistance[19].
In this study, EWL% finally reached 65% and stabilized 12 mo after the operation, and BMI gradually decreased to 28.98 at 12 mo after the operation (P < 0.05), which confirmed the significant weight loss effect of LSG. During the postoperative follow-up, it was found that 15 patients with acanthosis nigricans improved to varying degrees. At present, the etiology of acanthosis nigricans is not clear, and it is often associated with abnormal metabolism of hormones and insulin and is often associated with polycystic ovary syndrome. It is undeniable that the improvement of acanthosis nigricans is related to polycystic ovary syndrome. However, the specific mechanism and causality still need to be further studied. Hyperandrogenemia can also show hirsutism, especially in female patients. Although there was no specific score in this study, it was found that the endocrine and metabolic indices gradually stabilized and hirsutism significantly improved during the follow-up.
In summary, LSG can improve hyperandrogenaemia and irregular menstruation in obese patients with PCOS, significantly reduce weight loss and improve a series of complications related to PCOS. such as acanthosis nigricans and hirsutism. Laparoscopic sleeve gastrectomy can improve patients' physical, hormonal and reproductive indicators and can be considered part of the treatment of infertility in obese patients. Further research is needed to draw more reliable conclusions.
Polycystic ovary syndrome (PCOS) is a common endocrine disease in young women, with a prevalence rate of 5%-18%. PCOS is closely related to obesity, and an increase in obesity will enhance the severity and expression of the PCOS phenotype.
The efficacy of laparoscopic sleeve gastrectomy (LSG) for obesity with PCOS are still unclear.
The purpose of the study was to investigate the effect of LSG on related variables in obese patients with PCOS.
The clinical data of 32 patients who were diagnosed with obesity complicated with polycystic ovary syndrome and underwent laparoscopic sleeve gastrectomy from January 2013 to December 2020 were reviewed. The changes in anthropometric indices, insulin, testosterone, estradiol, follicle stimulating hormone (FSH), luteinizing hormone (LH), menstrual cycle and LH/FSH ratio before and 1 mo, 3 mo, 6 mo and 12 mo after the operation were statistically analyzed.
The patient's body mass and BMI were basically stable at 1 year after the operation. During the postoperative period, the average level of estradiol increased; the average serum testosterone and the insulin level decreased significantly after the operation. On the other hand, after surgical treatment, it was found that the average ratio was less than 1, indicating that the average FSH level after bariatric surgery was higher than that of LH.
Laparoscopic sleeve gastrectomy can improve hyperandrogenaemia and irregular menstruation in obese patients with polycystic ovary syndrome, significantly reduce weight loss and improve a series of complications related to polycystic ovary syndrome.
The impact of laparoscopic sleeve gastrectomy on obese patients with polycystic ovary syndrome's physical, hormonal and reproductive indicators.
| 1. | Waxman A; World Health Assembly. WHO global strategy on diet, physical activity and health. Food Nutr Bull. 2004;25:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Hoeger KM, Dokras A, Piltonen T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J Clin Endocrinol Metab. 2021;106:e1071-e1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 3. | Khan MJ, Ullah A, Basit S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl Clin Genet. 2019;12:249-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 4. | Messinis IE, Messini CI, Anifandis G, Dafopoulos K. Polycystic ovaries and obesity. Best Pract Res Clin Obstet Gynaecol. 2015;29:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Naz MSG, Tehrani FR, Majd HA, Ahmadi F, Ozgoli G, Fakari FR, Ghasemi V. The prevalence of polycystic ovary syndrome in adolescents: A systematic review and meta-analysis. Int J Reprod Biomed. 2019;17:533-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Ye W, Xie T, Song Y, Zhou L. The role of androgen and its related signals in PCOS. J Cell Mol Med. 2021;25:1825-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 7. | Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. 2021;44:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 8. | Rodriguez Paris V, Bertoldo MJ. The Mechanism of Androgen Actions in PCOS Etiology. Med Sci (Basel). 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Jamal M, Gunay Y, Capper A, Eid A, Heitshusen D, Samuel I. Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: a 9-year analysis. Surg Obes Relat Dis. 2012;8:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Grönroos S, Helmiö M, Juuti A, Tiusanen R, Hurme S, Löyttyniemi E, Ovaska J, Leivonen M, Peromaa-Haavisto P, Mäklin S, Sintonen H, Sammalkorpi H, Nuutila P, Salminen P. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss and Quality of Life at 7 Years in Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg. 2021;156:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Han Y, Jia Y, Wang H, Cao L, Zhao Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int J Surg. 2020;76:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 12. | Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, Buchwald H, Scopinaro N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg. 2018;28:3783-3794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 755] [Article Influence: 94.4] [Reference Citation Analysis (1)] |
| 13. | Diagnosis of polycystic ovary syndrome -- Health industry standard of the People's Republic of China. Zhonghua Fuchanke Zazhi. 2012;47:74-75. [DOI] [Full Text] |
| 14. | Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol. 2018;132:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 15. | Marshall JC, Dunaif A. Should all women with PCOS be treated for insulin resistance? Fertil Steril. 2012;97:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Jiang NX, Li XL. The Disorders of Endometrial Receptivity in PCOS and Its Mechanisms. Reprod Sci. 2022;29:2465-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Dağ ZÖ, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 1164] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 19. | Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, Calvo M, Bermúdez V. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med. 2014;2014:719050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahn S, South Korea; Bouras E, Greece S-Editor: Liu JH L-Editor: A P-Editor: Cai YX