Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.72
Peer-review started: July 24, 2022
First decision: September 19, 2022
Revised: October 14, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: January 27, 2023
Processing time: 178 Days and 0.4 Hours
The impact of obesity on surgical outcomes in elderly patients candidate for liver surgery is still debated.
To evaluate the impact of high body mass index (BMI) on perioperative and oncological outcome in elderly patients (> 70 years old) treated with laparoscopic liver resection for hepatocellular carcinoma (HCC).
Retrospective multicenter study including 224 elderly patients (> 70 years old) operated by laparoscopy for HCC (196 with a BMI < 30 and 28 with BMI ≥ 30), observed from January 2009 to January 2019.
After propensity score matching, patients in two groups presented comparable results, in terms of operative time (median range: 200 min vs 205 min, P = 0.7 respectively in non-obese and obese patients), complications rate (22% vs 26%, P = 1.0), length of hospital stay (median range: 4.5 d vs 6.0 d, P = 0.1). There are no significant differences in terms of short- and long-term postoperative results.
The present study showed that BMI did not impact perioperative and oncologic outcomes in elderly patients treated by laparoscopic resection for HCC.
Core Tip: In order to evaluate the impact of a high body mass index (BMI) in elderly patients who underwent laparoscopic resection for hepatocellular carcinoma (HCC), we compared perioperative data and long-term outcomes from 10 European centers before and after propensity score matching. The present study showed that BMI did not impact perioperative and oncologic outcomes in elderly patients treated by laparoscopic resection for HCC.
- Citation: Conticchio M, Inchingolo R, Delvecchio A, Ratti F, Gelli M, Anelli MF, Laurent A, Vitali GC, Magistri P, Assirati G, Felli E, Wakabayashi T, Pessaux P, Piardi T, di Benedetto F, de’Angelis N, Briceño J, Rampoldi A, Adam R, Cherqui D, Aldrighetti LA, Memeo R. Impact of body mass index in elderly patients treated with laparoscopic liver resection for hepatocellular carcinoma. World J Gastrointest Surg 2023; 15(1): 72-81
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/72.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.72
Obesity is a significant contributing factor for the development of liver disease, starting from the stage of non-alcoholic steatohepatitis up to cirrhosis and hepatocellular carcinoma (HCC)[1-4]. Due to the constant increase of population aging, the treatment of HCC in elderly obese patient has become a global clinical issue[5]. Laparoscopic liver resection (LLR) provides the benefits of minimally invasive approach in terms of short-term outcomes[6,7], guaranteeing oncological results comparable to the open surgical approach[8,9]. However, data about the impact of obesity in patients undergoing LLR remain controversial, with some studies reporting higher body mass index (BMI) as a predictor of an adverse postoperative outcome[10] and other studies not reporting an increased risk of postoperative morbidity linked to obesity[11]. The aim of this study was to evaluate the impact of BMI in elderly patients undergoing LLR for HCC, by comparing short- and long-term outcomes.
This multicenter retrospective study included 224 patients treated between January 2009 and January 2019, at the following centers: Policlinico di Bari, Bari, Italy; Policlinico di Modena, Modena, Italy; Ospedale San Raffaele, Milan, Italy; Grande Ospedale Metropolitano Niguarda, Milan, Italy; Centre hépato-biliaire Paul Brousse, Villejuif, France; Hôpitaux Universitaires Henri Mondor, Créteil, France; Hospital Universitario Sofía, Córdoba, Spain; Hôpitaux Universitaires de Genève, Geneva, Switzerland; Nouvel Hôpital Civil, Strasbourg, France; Centre Hospitalier Universitaire, Reims, France.
This study investigated patients resected for HCC demonstrating the following inclusion criteria: Child-Pugh class A and B disease; age ≥ 70 years; no evidence of major vessel branch invasion and no distant metastases. Based on the World HealthOrganization (WHO) definition of obesity (BMI > 30 kg/m2)[12] patients were divided in two groups: BMI < 30 kg/m2 group and BMI > 30 kg/m2 group.
The diagnosis of HCC was done, according to the European Association for Study of Liver (EASL) consensus criteria[13], based on non-invasive findings or histopathology. The type of treatment was planned following multidisciplinary tumor board discussions.
The surgical procedure was planned based on tumor features and liver function. Minor and major liver resections were performed according Brisbane classification[14]. The choice of position and the size of trocars depended by tumor location. Intraoperative ultrasonography represented a standardized initial step of surgical procedure. Liver parenchymal transection was performed with laparoscopic instruments using various energy devices such as the cavitation ultrasonic surgical aspirator ultrasonic, monopolar and bipolar forceps. The extent of resection depended on the size and anatomical location of the tumor and they were defined as “minor” for the resection of two or fewer Couinaud’s liver segments, and ‘major’ for the resection ≥ 3 liver segments. The hepatic hilum was prepared for the Pringle’s maneuver. The specimen was placed in an endocatch bag and removed from one of the trocars’ incision sites.
Short-term outcomes after liver resection included the evaluation of the parameters in the perioperative period, including intraoperative variables such as operative time, and blood transfusion rate, and postoperative variables as complications rate (based on the Clavien-Dindo classification[15]), and length of hospitalization. Long-term outcomes included oncological results in terms of overall survival and disease-free survival (DFS). Liver blood tests were assessed on first, third and fifth postoperative day. Follow-up was performed once every 3 mo during the first year and every 4 mo thereafter with CT-scan and blood tests (including liver function and oncologic markers). Recurrence after treatment included repeat resection, locoregional treatment, till liver transplantation, or supportive care based on the patient’s general status and liver disease according to the EASL-EORTC clinical practice guidelines[13].
Statistical analyses were carried out using the IBM SPSS Statistics 20 software. The t-test and Mann-Whitney U test were used to compare continuous variables. The chi-square test and Kruskal-Wallis test respectively was performed to compare categorical variables. The Kaplan-Meier method was used to assess recurrence-free survival (RFS) and overall survival (OS) curves. The Cox proportional hazard model was performed to analyse independent prognostic factors of longterm survival. A propensity score matching (PSM) analysis was performed to reduce selection bias obtaining two more homogeneous matched groups of patients in the resection and ablation groups. Variables included in our propensity model included age, comorbidities ≥ 2, American Society of Anesthesiologists (ASA) score, Child-Pugh and model for end-stage liver disease (MELD) scores, extent of resection, tumor number and size. A one-to-one PSM was performed with a caliper width of < 0.2 of the pooled standard deviation of estimated propensity scores, applying these variables to a logistic regression model and calculated C-statistics. A total of 27 out of the 196 patients in the BMI < 30 group and a total of 27 out of the 28 patients in the BMI > 30 group were matched for further analyses.
We included 224 patients treated with LLR for an HCC and aged ≥ 70 years. One hundred and ninty-six patients presented a BMI < 30 kg/m2 and 28 patients presented a BMI > 30 kg/m2. Demographic data were similar between two groups, except for a higher rate of male in BMI ≥ 30 kg/m2 group than in BMI < 30 kg/m2 group (69% vs 93%, P = 0.001). Associated comorbidities were not increased in obese patients, as ASA and MELD score (Table 1).
| LLR before PSM (224) | After PSM (54) | |||||
| BMI < 30 (196) | BMI ≥ 30 (28) | P | BMI < 30 (27) | BMI ≥ 30 (27) | P | |
| MALE | 135 (69) | 26 (93) | 0.00 | 17 | 25 | 0.02 |
| Age (yr), median (range) | 75.2 (69.5-90.0) | 75.3 (69.7-86.6) | 0.70 | 76.3 (70.6-81.2) | 73.1 (70-82.3) | 0.70 |
| BMI (kg/cm²), median (range) | 26.7 (19.0-29.0) | 32.5 (30.0-52.0) | 0.00 | 26.7 (25.0-267.0) | 33 (30-37) | |
| Co-morbidities > 2, n (%) | 77 (80) | 9 (32) | 0.50 | 14 | 9 | 0.27 |
| Cause of Cirrhosis, n (%) | ||||||
| Hepatitis C virus | 102 (52) | 12 (43) | 0.40 | 16 | 11 | 0.27 |
| Hepatitis B virus | 39 (20) | 4 (14) | 0.60 | 7 | 4 | 0.50 |
| Alcohol | 23 (12) | 7 (25) | 0.07 | 1 | 7 | 0.05 |
| Others | 32 (16) | 5 (18) | 0.80 | 3 | 5 | 0.70 |
| ASA score, n (%) | 0.80 | 1.00 | ||||
| I-II | 84 (43) | 11 (39) | 10 | 10 | ||
| III-IV | 112 (57) | 17 (61) | 17 | 17 | ||
| Blood tests median (range) | ||||||
| Bilirubin (mg/dL) | 0.9 (0.2-4.2) | 0.9 (0.2-2.1) | 0.8 | 0.9 (0.3-1.1) | 0.5 (0.2-1.1) | 0.70 |
| Creatinine (mg/dL) | 0.9 (0.2-2.5) | 0.9 (0.4-1.9) | 1.00 | 0.9 (0.8-1.5) | 0.9 (0.7-2) | 0.80 |
| Platelet count, × 109/L | 176 (45-421) | 187 (72-468) | 0.3 | 144 (47-337) | 168 (117-396) | 0.80 |
| INR | 1.1 (0.6-2.0) | 1.08 (0.7-1.67) | 0.3 | 1.1 (1-1.5) | 1 (1-1.3) | 0.50 |
| CHILD-PUGH, n (%) | 0.2 | 1.00 | ||||
| A | 177 (90) | 23 (82) | 23 | 22 | ||
| B | 19 (68) | 5 (18) | 4 | 5 | ||
| MELD median (range) | 6 (6-16) | 6 (6-13) | 0.6 | 8 (6-12.5) | 8 (6-13) | 0.40 |
| Tumors number n (%) | 0.06 | 1.00 | ||||
| Single nodule | 191 (97) | 25 (89) | 24 | 24 | ||
| Multi nodules | 5 (3) | 3 (11) | 3 | 3 | ||
| Tumors size (mm), median (range) | 30 (9-50) | 30 (18-50) | 0.6 | 30 (9-50) | 27 (24-35) | 0.30 |
| Bilobar tumor, n (%) | 1 (0.5) | 1 (3) | 0.2 | 1 | 1 | 1.00 |
| Tumor in PS segment, n (%) | 41 (21) | 6 (21) | 1.00 | 5 | 5 | 1.00 |
| Histologically proven n (%) | 31 (16) | 8 (29) | 0.11 | |||
| Previous treatment, n (%) | 12 (6) | 3 (10) | 0.4 | |||
| Major hepatectomy, n (%) | 22 (11) | 2 (7) | 0.7 | 1 | 2 | 1.00 |
| Operative time > 240 min | 73 (37) | 12 (43) | 0.7 | 150 (80-210) | 150 (65-155) | 0.70 |
Perioperative and postoperative data are described in Table 2. There were no significant differences in surgical time (median range: 200 min vs 220 min, P = 0.70, in the BMI < 30 and BMI > 30 respectively), rate of blood transfusion (16% vs 3%, P = 0.09), length of hospitalization (median range: 6.0 d vs 5.5 d, P = 0.20)
| LLR before PSM (224) | After PSM (54) | |||||
| BMI < 30 (196) | BMI ≥ 30 (28) | P | BMI < 30 (27) | BMI ≥ 30 (27) | P | |
| Operative time (min), median (range) | 200 (70-600) | 220 (65-337) | 0.70 | 200 (80-320) | 205 (65-337) | 0.7 |
| Blood transfusion, n (%) | 32 (16) | 1 (3) | 0.09 | 5 (18) | 1 (3) | 0.2 |
| Dindo-Clavien classification, n (%) | 0.23 | 0.6 | ||||
| I-II | 18 (92) | 27 (97) | 23 (93) | 26 (96) | ||
| III-IV | 16 (8) | 1 (3) | 2 (7) | 1 (4) | ||
| Postoperative complications, n (%) | 0.02 | 1.0 | ||||
| Yes | 93 (47) | 7 (25) | 6 (22) | 7 (26) | ||
| No | 103 (53) | 21 (75) | 21 (78) | 20 (74) | ||
| Type of complication, n (%) | ||||||
| Liver failure | 15 (8) | 1 (3) | 0.70 | 2 (7) | 1 (4) | 1.0 |
| Ascites | 24 (12) | 2 (7) | 0.70 | 3 (11) | 2 (7) | 1.0 |
| Biliary leakage | 2 (1) | 1 (3) | 0.30 | 0 | 1 (4) | 1.0 |
| Hemorrhage | 8 (4) | 0 | 0.60 | 2 (7) | 0 | 0.5 |
| Systemic infection | 14 (7) | 1 (3) | 0.70 | 2 (7) | 1 (4) | 1.0 |
| Intra-abdominal abscess | 7 (3) | 0 | 0.60 | 2 (7) | 1 (4) | 1.0 |
| Wound infection | 4 (2) | 3 (11) | 0.04 | 0 | 3 (11) | 0.2 |
| Portal thrombosis | 2 (1) | 0 | 1.00 | 1 (4) | 0 | 1.0 |
| Pulmonary | 15 (7) | 0 | 0.20 | 2 (7) | 0 | 0.5 |
| Cardiac | 11 (5) | 1 (3) | 1.00 | 2 (7) | 1 (4) | 1.0 |
| Renal | 8 (4) | 0 | 0.60 | 2 (7) | 0 | 0.5 |
| Reoperation, n (%) | 6 (6) | 1 (3) | 1.00 | 0 | 1 (4) | 1.0 |
| Postoperative death, n (%) | 7 (3) | 0 | 0.60 | 2 (7) | 0 | 0.5 |
| Postoperative treatment, n (%) | 6 (3) | 1 (3) | 1.00 | 0 | 1 (4) | 1.0 |
| Length of hospital stay, median (range) | 6 (2-40) | 5.5 (3-21) | 0.20 | 4.5 (2-40) | 6 (3-21) | 0.1 |
| Mortality at 90 d, n (%) | 9 (5) | 0 | 0.60 | 2 (7) | 0 | 0.5 |
The global rate of postoperative complication was higher in the non-obese group (47% vs 25%, P = 0.02) compared to the obese group. Only the rate of wound infection was higher in the obese group (11% vs 2%, P = 0.04).
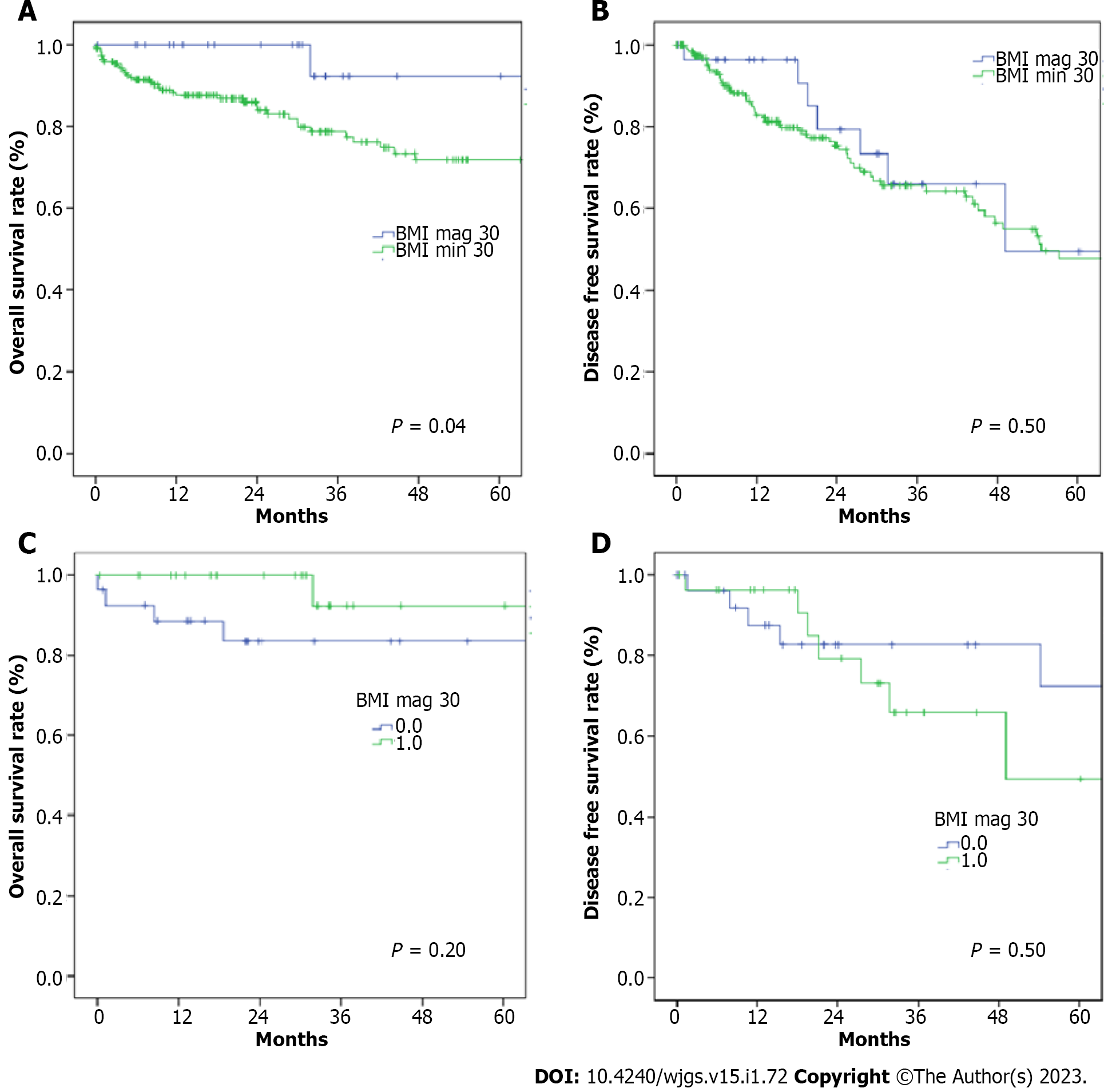
The 90-d mortality rate didn’t present significative difference between the two groups (5% in BMI < 30 group and 0% in BMI > 30 group, P = 0.60). The estimated 1- and 3-year OS rates were 100% and 92.3% in BMI > 30 group, and 96% and 91.4% in BMI < 30 group (P = 0.004; Figure 1A) respectively. The estimated 1- and 3-year DFS rates were 96% and 67% in BMI > 30 group, and 82% and 36% in BMI < 30 group (P = 0.50; Figure 1B) respectively.
After matching, we obtained a more homogeneous population for both groups (Table 1). The variables included in the PSM were age, comorbidities, ASA and MELD score, Child Pugh score, tumor size, tumor number and extent of resection. Peri-operative and post-operative results are analytically described in Table 2. The post-operative follow up didn’t reveal any difference in the complication’s rate between BMI > 30 and BMI < 30 group (26% vs 22%, P = 1.0), nor in grade of severity (Clavien-Dindo grades III-IV) (4% vs 7% P = 0.6). Moreover, operative time (median range: 205 min vs 200 min, P = 0.7) and rate of blood transfusion (3% vs 18%, P = 0.2) were similar. The estimated 1- and 3-year overall survival rates were 100% and 92.3% in the BMI > 30 group, and 88.4% and 83.5% in the BMI < 30 group (P = 0.2; Figure 1C).
The estimated 1- and 3-year DFS rates were 96.2% and 65.8% in the BMI > 30 group, and 87.5% and 86.2% in the BMI < 30 group (P = 0.5; Figure 1D) respectively.
The impact of obesity on surgical results in elderly patients who underwent liver resections remains a subject of vivid debate. An increased surgical risk has been expected because of comorbidities, associated to obesity and old age, underlying liver disease and technical difficulties[16-20]. Our multicenter study did not confirm this hypothesis and showed that LLR can be safely performed in treatment of HCC also in elderly patients with a BMI ≥ 30 kg/m2. The evaluation of the influence of BMI in elderly population is important because of the increasing prevalence of this condition associated to an higher average life expectancy[21,22].
The increasing BMI has been reported as a predisposition to develop various diseases, including diabetes mellitus, hypertension, respiratory disease and certain type of cancers[23-25]. Our data did not show differences in term of rate of comorbidities or tumor characteristics, even after PMS analysis, according to various preoperative parameters (age, comorbidities, ASA and MELD score, Child Pugh score, tumor size, and tumor number and extent of resection), which resulted in a more homogeneous and therefore comparable population.
Even though the initial hypothesis that obesity negatively affected the outcomes of minimally invasive approach was not verified[26], data regarding LLR in obese patients were controversial. After the evaluation of surgical procedures ended with Second International Consensus Conference on LLR[27], a scoring system was built to stratify LLR into groups with increasing degree of difficulty[28]. This IWATE score aimed to preoperatively predict, the technical difficulty of various LLR, but without including body habitus. So, the question whether anthropometric variables really have an impact on perioperative outcomes, remains.
Using operative time, rate of blood transfusion and rate of conversion as surrogates of surgical difficulty, Ome et al[29] reported significantly longer median operation time in obese compared to non-obese patients, while for Uchida et al[30] BMI was an independent predictor of longer operative time > 200 min. Lee et al[31] reported a significant difference in operative time and incidence of blood transfusion in overweight compared to normal weight patients, but no difference for obese patients. In accordance to the abovementioned data, the results of this study also suggest similar rate of blood transfusion and operative time in patients with BMI < 30 kg/m2 and those with BMI ≥ 30 kg/m2.
The advantages of a minimally invasive approach in liver surgery, including lower abdominal wall morbidity and early postoperative rehabilitation[32,33], may be more beneficial for the subgroup of obese patients. A recent systematic review[34] reported similar rates of postoperative complications between obese and non-obese patients, although several issues including discrepancy in the obesity definition, limit the validity of these results. Nomi et al[35] reported that the postoperative course of obese patients was not negatively affected by a higher incidence of infectious complications nor liver specific complications. Yu et al[36] reported a higher rate of bile leak in obese compared to non-obese patients. The herein presented data demonstrate a similar postoperative outcome, with no significant differences in major complications (Clavien Dindo III-IV) nor liver related complications in obese compared to non-obese patients.
View magnification of, optimal exposure with liver mobilization and the increase of dedicated tools allow a clearer visualization of deep structures, small vessels and biliary ducts[7,36,37]. The authors speculate this “power” of laparoscopy can justify a lower rate of postoperative complications not only in terms of preservation of abdominal wall integrity, linked with prevention on respiratory diseases and reduction of postoperative pain, but also with a greater accuracy in resection technique, especially in the hands of experienced surgeons.
Oncological outcomes following PSM were also similar, as no differences were noted in disease-free and overall survival in obese vs non-obese patients, undergoing LLR for HCC. This is also in accordance with in the majority of published data[34]. These results suggest that also elderly obese patients can benefit from surgical treatment in terms of long-term outcomes, mainly driven by the excellent short-term outcome of laparoscopy.
In conclusion, according to the present study, BMI does not impact surgical outcomes of LLR in elderly patients treated for HCC. Thorough patient selection, based on liver volume and function evaluation, as well as patient habitus and comorbidities, could result in safe and feasible LLR in elderly obese patients.
A high body mass index (BMI) could represent a factor which impacts perioperative outcomes in elderly patients who underwent laparoscopic liver resection (LLR).
To evaluate of postoperative outcomes between elderly (age > 70 years) patients with a BMI ≥ 30 and BMI < 30 who underwent a LLR for hepatocellular carcinoma (HCC).
The analysis of short (perioperative) and long-term (oncological results) outcomes.
The analysis of data was performed before and after propensity score matching.
After propensity score matching, patients in two groups presented comparable results, in terms of operative time complications rate length of hospital stay. There are no significant differences in terms of short- and long-term postoperative results.
The present study showed that BMI did not impact perioperative and oncologic outcomes in elderly patients treated by laparoscopic resection for HCC.
Randomized controlled studies are needed to better explore these results.
| 1. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8578] [Article Influence: 408.5] [Reference Citation Analysis (9)] |
| 2. | Pascale A, Pais R, Ratziu V. An overview of nonalcoholic steatohepatitis: past, present and future directions. J Gastrointestin Liver Dis. 2010;19:415-423. [PubMed] |
| 3. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 981] [Article Influence: 61.3] [Reference Citation Analysis (1)] |
| 4. | Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Zhao LY, Huo RR, Xiang X, Torzilli G, Zheng MH, Yang T, Liang XM, Huang X, Tang PL, Xiang BD, Li LQ, You XM, Zhong JH. Hepatic resection for elderly patients with hepatocellular carcinoma: a systematic review of more than 17,000 patients. Expert Rev Gastroenterol Hepatol. 2018;12:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 7. | Martin RC, Scoggins CR, McMasters KM. Laparoscopic hepatic lobectomy: advantages of a minimally invasive approach. J Am Coll Surg. 2010;210:627-634, 634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D'Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 525] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 9. | Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS; World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1174] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 10. | Mathur AK, Ghaferi AA, Osborne NH, Pawlik TM, Campbell DA, Englesbe MJ, Welling TH. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010;14:1285-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Utsunomiya T, Okamoto M, Kameyama T, Matsuyama A, Yamamoto M, Fujiwara M, Mori M, Aimitsu S, Ishida T. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14:1553-1558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1. [PubMed] |
| 13. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6425] [Article Influence: 803.1] [Reference Citation Analysis (9)] |
| 14. | Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford). 2002;4:99; author reply 99-99; author reply100. [PubMed] |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26171] [Article Influence: 1189.6] [Reference Citation Analysis (2)] |
| 16. | Berkalp B, Cesur V, Corapcioglu D, Erol C, Baskal N. Obesity and left ventricular diastolic dysfunction. Int J Cardiol. 1995;52:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 785] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 20. | Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D'Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 318] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5072] [Cited by in RCA: 4754] [Article Influence: 528.2] [Reference Citation Analysis (2)] |
| 22. | Woolf AD. Number 17 World Health Organization World Report on Ageing and Health. J Can Rheumatol Assoc. 2018;. |
| 23. | Henry ZH, Caldwell SH. Obesity and Hepatocellular Carcinoma: A Complex Relationship. Gastroenterology. 2015;149:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 635] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 25. | Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3786] [Cited by in RCA: 3703] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 26. | Loffer FD, Pent D. Laparoscopy in the obese patient. Am J Obstet Gynecol. 1976;125:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schön MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 416] [Reference Citation Analysis (0)] |
| 28. | Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 431] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 29. | Ome Y, Hashida K, Yokota M, Nagahisa Y, Okabe M, Kawamoto K. The safety and efficacy of laparoscopic hepatectomy in obese patients. Asian J Surg. 2019;42:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Uchida H, Iwashita Y, Saga K, Takayama H, Watanabe K, Endo Y, Yada K, Ohta M, Inomata M. Benefit of laparoscopic liver resection in high body mass index patients. World J Gastroenterol. 2016;22:3015-3022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Lee SJ, Hauch A, Kane E, DuCoin C, Darden M, Parker G, Kandil E, Buell JF. Effect of Obesity on Perioperative Outcomes after Laparoscopic Hepatectomy. Hepatoma Res. 2016;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Nomi T, Fuks D, Kawaguchi Y, Mal F, Nakajima Y, Gayet B. Laparoscopic major hepatectomy for colorectal liver metastases in elderly patients: a single-center, case-matched study. Surg Endosc. 2015;29:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Delvecchio A, Conticchio M, Ratti F, Gelli M, Anelli FM, Laurent A, Vitali GC, Magistri P, Assirati G, Felli E, Wakabayashi T, Pessaux P, Piardi T, Di Benedetto F, de'Angelis N, Briceño-Delgado J, Adam R, Cherqui D, Aldrighetti L, Memeo R. Laparoscopic major hepatectomy for hepatocellular carcinoma in elderly patients: a multicentric propensity scorebased analysis. Surg Endosc. 2021;35:3642-3652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Kwan B, Waters PS, Keogh C, Cavallucci DJ, O'Rourke N, Bryant RD. Body mass index and surgical outcomes in laparoscopic liver resections: a systematic review. ANZ J Surg. 2021;91:2296-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Nomi T, Fuks D, Ferraz JM, Kawaguchi Y, Nakajima Y, Gayet B. Influence of body mass index on postoperative outcomes after laparoscopic liver resection. Surg Endosc. 2015;29:3647-3654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Yu HB, Dong YD, Wang LC, Tian GJ, Mu SM, Cao Y, Peng YN, Lou CY, Liu P, Li DY. Laparoscopic Liver Resection can be an Effective Way in Obese Patients: A Single Center of 2-Year Experience. Surg Laparosc Endosc Percutan Tech. 2016;26:e69-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang XQ, China; Zhang CZ, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL