Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.997
Peer-review started: December 18, 2021
First decision: April 19, 2022
Revised: May 4, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: September 27, 2022
Processing time: 277 Days and 22.1 Hours
The prognosis for oesophageal carcinoma is poor, but once distant metastases emerge the prognosis is considered hopeless. There is no consistent protocol for the early identification and aggressive management of metastases.
To examine the outcome of a policy of active postoperative surveillance with aggressive treatment of confirmed metastases.
A prospectively maintained database of 205 patients diagnosed with oesophageal carcinoma between 1998 and 2019 and treated with curative intent was inter
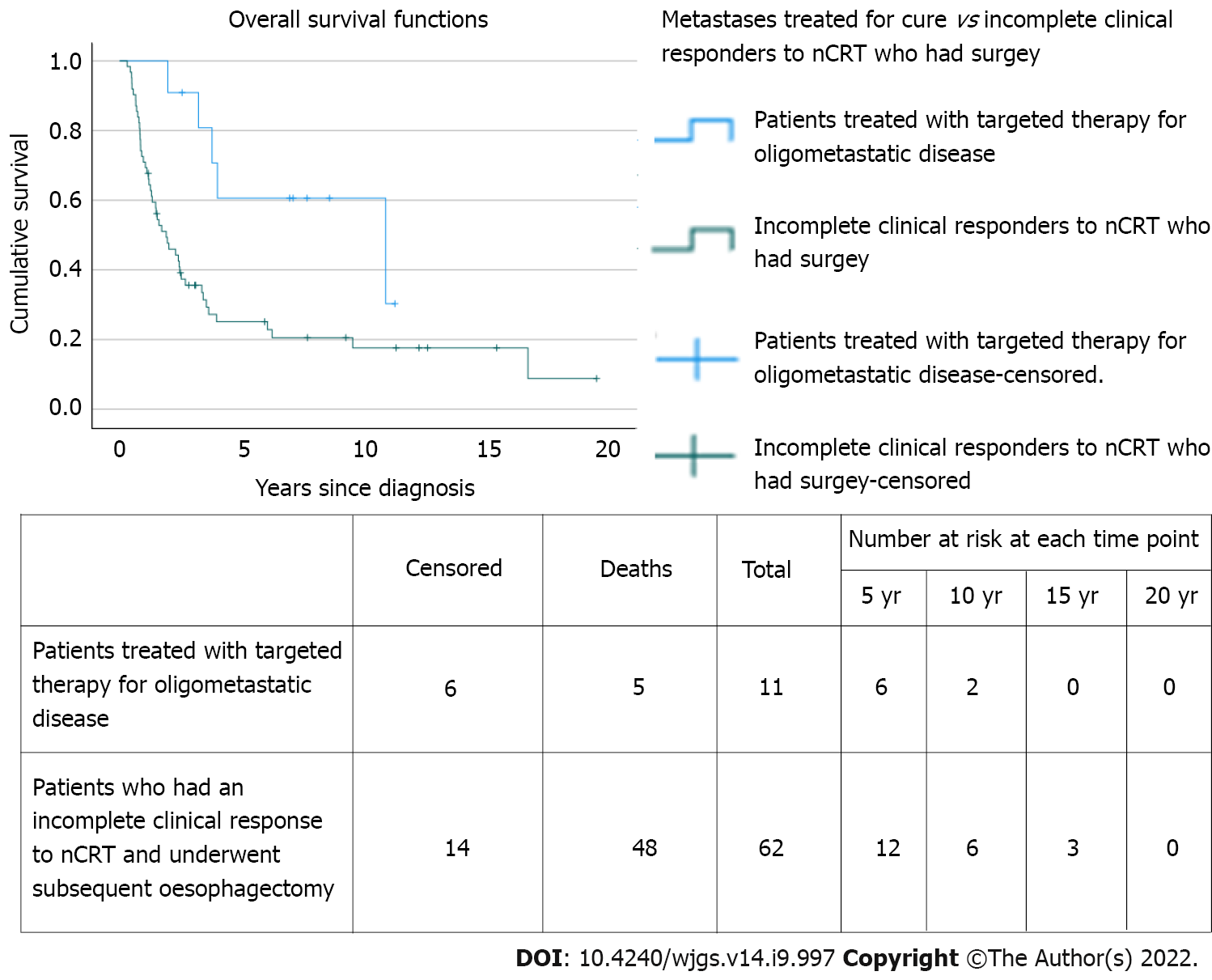
Of 205 patients, 11 (5.4%) had metastases treated for cure (82% male; median age 60 years; 9 adenocarcinoma and 2 squamous cell carcinomas). All had undergone neoadjuvant chemotherapy or chemoradiotherapy, followed by surgery in all but 1 case. Of the 11 patients, 4 had metastatic disease at diagnosis, of whom 3 were successfully downstaged with nCRT before definitive surgery; 2 of these 4 also developed oligometastatic recurrence and were treated with curative intent. Following definitive treatment, 7 had treatment for metachronous oligometastatic disease; 5 of whom underwent metastasectomy (adrenal × 2; lung × 2; liver × 1). The median overall survival was 10.9 years [95% confidence interval (CI): 0.7-21.0 years], which was statistically significantly longer than incomplete clinical responders undergoing surgery on the primary tumour without metastatic intervention [n = 62; median overall survival = 1.9 (95%CI: 1.1-2.7; P = 0.012]. The cumulative proportion surviving 1, 3, and 5 years was 100%, 91%, and 61%, respectively compared to 71%, 36%, and 25% for incomplete clinical responders undergoing surgery on the primary tumour who did not undergo treatment for metastatic disease.
Metastatic oesophageal cancer represents a unique challenge, but aggressive treatment can be rewarded with impressive survival data. In view of recent advances in targeted therapies, intensive follow-up may yield a greater number of patients with curative potential and thus improved long-term survival.
Core Tip: Modern imaging technologies can detect oligometastatic oesophageal cancer earlier than ever before, and targeted multimodal therapies, combined with innovative surgery, increases the potential for cure. Unfortunately, current guidelines do not reflect these advances and all too often consign patients to palliation. This approach is incongruous with other oligometastatic cancers such as colorectal cancer. Based on the survival outcomes of patients with oligometastatic disease treated for cure at our institution we advocate for more intensive surveillance strategies for earlier identification of patients with curative potential to improve overall long-term survival.
- Citation: Pickett L, Dunne M, Monaghan O, Grogan L, Breathnach O, Walsh TN. Oesophageal cancer metastases: An observational study of a more aggressive approach. World J Gastrointest Surg 2022; 14(9): 997-1007
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/997.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.997
Oesophageal cancer is an aggressive disease that presents insidiously, disseminates early, and spreads rapidly in most patients. It remains a leading cause of death from cancer worldwide and fewer than 5%-12% will survive 5 years[1,2]. At least 40% of patients present with distant metastasis at initial diagnosis[3], and only 5% of these patients will be alive at 5 years[4]. Even when presenting with early disease, 29%-54% of patients undergoing surgical resection with curative intent will develop locoregional or distant recurrence[5-7]. Of patients with a ypT0N0M0 tumour at resection following neoadjuvant chemoradiotherapy (nCRT), up to 17% will succumb to distant metastases[8-10]. Because of these poor survival outcomes, the role of intensive surveillance post-oesophagectomy and treatment of metastatic disease remains controversial.
The management of oesophageal cancer has undergone major advances over the past 30 years. Specifically, both neoadjuvant chemotherapy and nCRT have been shown to increase survival over surgery alone[11-13]. While neoadjuvant chemotherapy has achieved this increase by targeting occult micrometastases[14], combined CRT has increased survival by both targeting micrometastases and sterilizing locoregional disease, thus up to 50% of patients with squamous cell carcinoma (SCC) and up to 25% of patients with adenocarcinoma (AC) undergoing CRT have a complete pathological response in the resected specimen, depending on the regimen and the disease stage[11,12].
Nevertheless, metastatic oesophageal cancer remains a challenge. Oligometastases are defined as a state of limited metastatic disease characterized by fewer than five metastases[5,15]. Synchronous oligometastases may be detected at the time of diagnosis of the primary cancer, while metachronous oligometastases are those detected during follow-up[5,16]. Metastasectomy is well-established in the treatment of certain oligometastatic cancers, such as colorectal cancer, where partial hepatectomy and pulmonary resection are well established[5]. Both the United Kingdom’s National Institute for Health and Clinical Excellence and the United States’ National Comprehensive Cancer Network recommend surveillance strategies to identify recurrence as well as liver and pulmonary metastasectomy where possible[17,18]. In contrast, the National Institute for Health and Clinical Excellence recommends neither routine clinical follow-up nor radiological follow-up be offered to patients who have no sym
Over the past decades, efforts have focused on the molecular and biological alterations that lead to oesophageal cancer, specifically the influence of angiogenesis on micrometastatic tumour growth[21,22]. This has resulted in the development of novel molecularly targeted agents that target a variety of relevant pathways, such as vascular endothelial growth factor, cyclooxygenase-2, epidermal growth factor receptor, and mammalian target of rapamycin[23] as well as targeted radiotherapy in the form of stereotactic radiotherapy[24]. Leading the way are HER-2 inhibitors for the treatment of HER-2 expressing metastatic ACs[23]. It is intuitive that aggressive treatment of oligometastatic disease would improve disease control and provide a survival benefit for patients with recurrent cancer detected at its earliest stage. The purpose of this study was to examine survival outcomes in patients who underwent active surveillance and targeted therapy at our institution for their oligometastatic disease.
We conducted a retrospective review of a prospectively maintained database of all patients diagnosed with oesophageal carcinoma and treated with curative intent between 1998 and 2019 at Connolly Hospital Blanchardstown, Dublin, Ireland. Patients were treated with either CRT alone, or CRT followed by surgery, or surgery alone.
Over a 21-year period, 205 patients with oesophageal carcinoma underwent curative management. Following discharge, patients were followed up in the clinic every 3 mo for the first 3 years with esophagogastroduodenoscopy performed every 3 mo and computed tomography (CT) performed every 6 mo. Between 3 years and 5 years they were followed up in the clinic every 6 mo with esophagogastroduodenoscopy every 6 mo and CT scanning performed annually. After 5 years patients were followed up annually with endoscopy and a clinic visit (which was on the same day for patients who had to travel from a distance). In addition, patients had access to their oncology coordinator and were encouraged to call at any time with any concern. On receipt of a call, the coordinator would offer them a clinic visit or an esophagogastroduodenoscopy (or other imaging) depending on their symptoms or concerns.
A patient database was maintained over the study period, both by nursing and clinical staff. This was scrutinized for patients with synchronous and metachronous oligometastases. Only patients who underwent curative treatment of oligometastatic disease were included in this study. A second group of patients (with non-metastatic disease) who had an incomplete clinical response to nCRT and subsequently underwent surgery on the primary tumour were identified for comparison of survival outcomes.
Of 205 patients treated with curative intent, 62 had an incomplete response to nCRT for non-metastatic oesophageal cancer and subsequently underwent surgery, and 11 had oligometastases treated for cure. The medical and electronic records of the oligometastatic cohort treated for cure were reviewed for demographic, clinical, and histopathologic variables. Notably, staging of the primary oesophageal cancer was prospectively assigned according to the TNM classification of the American Joint Committee for Cancer Staging, initially the 6th edition and then the 7th following its publication. Each case was assessed with respect to the use of neoadjuvant therapy, history of oesophagectomy, and timing of metastasis. Further details regarding the site and treatment of metastasis were included. Survival data was included for analysis and comparison.
As this was a retrospective audit ethical approval was not required, but audit approval was sought and granted by the Connolly Hospital Ethics Committee.
The statistical analysis of this study was performed by biostatistician Mary Dunne from St Luke’s Radiation Oncology Network, Dublin D06 HH36, Ireland. Overall survival was estimated using the Kaplan-Meier method and was defined as the duration from the date of diagnosis until death from any cause or last follow-up at study endpoint on February 26, 2020. The log-rank test was used to compare survival differences between groups (assessed for significance at the 0.05 level). Statistical analysis was performed using IBM SPSS Statistics 25 (Chicago, IL, United States).
Of the 205 patients, 11 (5.4%) patients diagnosed with oesophageal carcinoma [146 (71.0%) male; 135 (65.9%) AC; 68 (33.2%) SCC; 2 adenosquamous)] between 1998 and 2019 and treated with curative intent had metastases treated for cure. Of these, 4 had synchronous oligometastatic oesophageal cancer, 2 of which also had treatment for cure for oligometastatic recurrence. A further 7 had metachronous oligometastatic oesophageal cancer only. The median age of patients with synchronous metastasis was 65 years (range: 53-71 years; AC 75%) and in patients with metachronous carcinoma was 57 years (range: 36-72 years; AC 86%) (Table 1). The majority of both cohorts were male (75% and 86%, respectively).
| Patient | Age in yr | Sex | Primary tumour location | Histologic type of tumour | Differentiation | Clinical stage of primary tumour | Neoadjuvant therapy | Oesophagectomy | ypTNM |
| Synchronous and Metachronous Oligometastatic Disease | |||||||||
| 11 | 62 | Female | Upper third | SCC | Moderate | T4N1M1 | No | NA | |
| 22 | 53 | Male | OGJ | AC | Poor | T3N2M1 | Walsh Regimen + CROSS | Yes | T2bN1Mx |
| Synchronous Oligometastatic Disease Only | |||||||||
| 3 | 71 | Male | Lower third/OGJ | AC | Poor | T3N1M1 | Carbo5FU; 60Gy | Yes | T3N0M0 |
| 4 | 68 | Male | OGJ | AC | Moderate-poor | T3N2M0 | Walsh Regimen | Yes | T2N0Mx |
| Metachronous Oligometastatic Disease Only | |||||||||
| 5 | 56 | Male | Middle/lower third | SCC | Moderate | T3N2M0 | Walsh Regimen | Yes | T2N1Mx |
| 6 | 36 | Male | Lower | AC | Moderate | T3N1M0 | CROSS | Yes | T3N0Mx |
| 73 | 72 | Female | OGJ | AC | Moderate | T3N0M0 | CROSS | Yes | T2N0 |
| 8 | 70 | Male | OGJ | AC | Poor | Nodal disease/Stage IIIA | MAGIC | Yes | T2N1Mx |
| 9 | 48 | Male | Lower third | AC | Poor | Stage IIB | Walsh Regimen | Yes | T1N0Mx |
| 104 | 57 | Male | Lower third | AC | Poor | T3N0M0 | CROSS | Yes | T2N0M0 |
| 11 | 60 | Male | OGJ | AC | Poor | T3N0M0 | CROSS | Yes | T0N0Mx |
The 4 patients that had metastatic disease at presentation were treated with nCRT, 3 of whom underwent subsequent oesophagectomy and achieved a margin free R0 resection and 1 of whom declined surgery following a clinical complete response to nCRT (Table 1). Two of these patients subsequently presented with metachronous metastases, which were also treated for cure (Table 2).
| Patient | Synchronous metastases | Type | Treatment | Metachronous metastases | Type | Time to recurrence in mo | Treatment | Survival post recurrence in mo | Alive at study endpoint | Overall survival in mo |
| 1 | Yes | Locally advanced1 | Walsh regimen | Yes | Lung | 11.5 | Stereotactic radiotherapy | 36.4 | No | 47.9 |
| 2 | Yes | Coeliac axis | Walsh regimen + CROSS + radial gastrectomy | Yes | Left para-aortic nodes | 17.9 | Chemotherapy (Epirubicin, Oxaliplatin + Capecitabine) | 65.4 | Yes | 83.3 |
| 3 | Yes | Liver | Carbo5FU; 60 Gy + oesophagectomy | No | NA | NA | NA | NA | No | 23.6 |
| 4 | Yes | Locally advanced2 | Walsh regimen + oesophagectomy | No | NA | NA | NA | NA | Yes | 102.8 |
| 5 | No | NA | NA | Yes | Lung | 32.9 | Left upper lobectomy (VATS) | 97.4 | No | 130.3 |
| 6 | No | NA | NA | Yes | Lung | 16.7 | Chemotherapy (carbo/taxol + FOLFOX) | 21.9 | No | 38.6 |
| 7 | No | NA | NA | Yes | Lung | 19.2 | Wedge resection (VATS) | 26.1 | No | 45.3 |
| 8 | No | NA | NA | Yes | Adrenal | 29.7 | Adrenalectomy | 62.1 | Yes | 91.8 |
| 9 | No | NA | NA | Yes | Adrenal | 15.9 | Adrenalectomy + chemotherapy (irinotecan) | 118.9 | Yes | 134.8 |
| 10 | No | NA | NA | Yes | Liver | 33.0 | Resection + chemotherapy | 51.9 | Yes | 84.9 |
| 11 | No | NA | NA | Yes | Paraaortic + Retroperitoneal | 15.7 | Chemotherapy (FOLFOX) | 14.9 | Yes | 30.6 |
Patient 1 had locally advanced SCC at diagnosis (T4N1M1). Despite a complete clinical response to definitive CRT, routine surveillance positron emission tomography–CT (PET-CT) almost 12 mo later (11.5 mo) revealed fluorodeoxyglucose (FDG) avid lung lesions bilaterally. These were subsequently treated with stereotactic radiotherapy. The patient survived for 3 years post metastatic recurrence (36.4 mo). Patient 2 had a 12 mm short-axis FDG-positive lymph node lying immediately to the right of the coeliac axis on staging PET-CT (AC, T3N2M1). The patient was treated with nCRT and radical oesophagogastrectomy for a poorly differentiated junctional/cardia AC (ypT2bN1Mx). Almost 18 mo later (17.9 mo) a radiological work-up for a pulmonary embolus revealed a 1.9 cm left para-aortic node with FDG uptake on PET-CT, which was subsequently treated with chemotherapy (Table 2). Follow-up CT showed a reduction in tumour size and subsequent surveillance with endoscopy and CT revealed stable disease with no evidence of recurrence. The patient was alive and well at the conclusion of this study, 83.3 mo after his initial diagnosis (65.4 mo post-recurrence).
Two further patients (Patient 3 and Patient 4) had treatment for cure of synchronous oligometastatic disease only (Tables 1 and 2). Patient 3 had liver metastasis on staging PET-CT (AC, T3N1M1). Restaging CT post nCRT was negative for liver metastasis, and the patient subsequently underwent oesophagectomy (ypT3N0M0). Patient 4 had a 1 cm FDG avid right supraclavicular node on staging PET-CT (AC, T3N2M0) and underwent nCRT and subsequent oesophagectomy for a moderate to poorly differentiated AC at the oesophagogastric junction (ypT2N0Mx). The patient was alive and well at the conclusion of this study, 8.5 years after his initial diagnosis (102.8 mo).
The remaining 7 patients did not have clinical evidence of metastatic oesophageal cancer at diagnosis. These patients had mostly T3 disease with or without nodal involvement (Table 1; Patient 5-11). All underwent nCRT or neoadjuvant chemotherapy followed by surgery for their primary cancer. Of this cohort (n = 7), 3 developed pulmonary recurrence, 2 adrenal, 1 liver, and 1 patient had biopsy proven retroperitoneal nodal recurrence. All 7 patients underwent targeted treatment for metastatic recurrence with intent to cure, the details of which are summarized in Table 2. The median time from diagnosis to recurrence was 19.2 mo (range: 15.7-33.0 mo), and the median survival post recurrence was 97.4 mo [95% confidence interval (CI): 0-204 mo). The median overall survival (MOS) was 130 mo (95%CI: 3-258 mo), or the MOS was 10.9 years (95%CI: 0.2-21.5 years).
The MOS of the 11 patients who underwent curative treatment for synchronous or metachronous metastatic disease or both was 10.9 years (95%CI: 0.7-21) which was statistically significantly longer than patients with an incomplete clinical response following nCRT undergoing surgery [n = 62; MOS = 1.9 years (95%CI: 1.1-2.7); P = 0.012] (Figure 1). Of note, the latter did not undergo curative treatment for any future proven or probable metastatic recurrence. The cumulative proportion of patients with metastatic disease treated for cure surviving 1, 3, and 5 years was 100%, 91%, and 61%, respectively, with 6 patients still alive at the end of the study period, compared to 71%, 36%, and 25% for incomplete clinical responders without metastatic disease undergoing surgery on the primary tumour.
Patients that underwent surgical resection for their recurrence (n = 5) had a MOS of 10.9 years (95%CI: 0.6-21.2) from date of diagnosis, 8.1 years (95%CI: 0-16.8 years) post recurrence, and a 5-year survival of 80% from the date of diagnosis.
Patients with metastatic oesophageal cancer present a unique challenge. Although solitary metastases of oesophageal cancer are uncommon[25], the evolution of imaging will ensure ever-earlier detection, which challenges oncologists and surgeons to detect and deal with them. Treatment of oligometastatic oesophageal cancer is controversial, and to date formal guidelines are lacking. There are no large randomized multicenter trials, and thus case series, such as ours, remain an important source of infor
Those patients treated surgically for recurrence in our study had a MOS of 10.9 years, or 130.3 mo and a 5-year survival of 80%. Depypere et al[26] conducted a large retrospective study comparing different treatment options for different subtypes of recurrence following curative resection, including single solid organ metastasis and single metastasis at another location. Of 1754 patients that had curative resection, 43.7% had recurrence, 14.4% of whom had clinical solitary solid organ recurrence (liver, lung, brain, or adrenal)[26]. Only 20 patients (1.14%) had their recurrence resected with or without systemic therapy and had a significantly better median and 5-year survival than 63 non-surgically treated patients [54.8 mo (5-year survival 43.9%) vs 11.6 mo (5-year survival 4.6%)][25,26]. Arguably, those suitable for resection self-select, but the survival statistics for metastatic resection in a disease as aggressive as oesophageal cancer are impressive.
The patients in our study who underwent adrenalectomy were alive at 62.1 and 118.9 mo post recurrence. The oesophagus is the third most frequent site of origin of adrenal metastasis[27], and there are only a few reports of adrenalectomy for recurrence with survival ranging from 28 mo to over 5 years[27-30]. These findings confirm that adrenalectomy for isolated adrenal metastases from oesophageal carcinoma is worthwhile. A disease-free interval of over 6 mo and an AC subtype are reported as predictors of improved survival and should be considered in patient selection[31,32]. As adrenal metastases are clinically silent, intensive surveillance imaging is indicated if they are to be identified early enough for curative resection.
The remaining patients who underwent metastasectomy in our case series had either lung or liver metastases. All had metachronous oligometastases, had received nCRT, and had undergone resection of their primary tumour. Those who underwent pulmonary metastasectomy lived for 26.1 and 97.4 mo post recurrence, while the patient who underwent liver metastasectomy was alive and disease-free at 51.9 mo post recurrence. While hepatectomy and pulmonary resection are universally recommended for colorectal cancer metastases[17,18], they are not recommended for oesophageal cancer[19,20]. A nationwide study by Seesing et al[33] of the Dutch national registry for histopathology and cyto
Oesophageal cancer patients very frequently present with metastases, which almost inevitably consigns them to palliative management. Until recently primary cancer resection in these circumstances was rarely considered. Of the 4 patients who presented with metastatic oesophageal cancer in our case series, 3 underwent surgery to the primary cancer. All 3 had nCRT and all achieved an R0 resection, with a cumulative proportion surviving 2 years of 67%. Zhang et al[35] analysed a large population-based cohort of 4367 metastatic oesophageal cancer patients (M1b-stage) from the SEER database[35] and found a significant survival benefit for surgery for the primary tumour with a median survival for the surgery group of 14 mo compared with 9 mo for the no surgery AC group, and a similar significant survival advantage for surgery (11 mo) compared with the no surgery SCC group (7 mo)[35]. Of note, patients who had not received neoadjuvant chemotherapy failed to benefit from resection for either tumour subtype[35]. Thus, when combined with neoadjuvant chemotherapy, surgery for the primary tumour is associated with improved survival in a select group of patients with metastatic oesophageal cancer[35].
Three of the patients in our series received chemotherapy alone for recurrent oligometastatic oesophageal cancer (patients 2, 6, and 11). Although chemotherapy is commonly considered as merely palliative in recurrent metastatic cancer, it also has the potential to cure[36]. Taxanes as single agents have a slightly higher response rate in patients with AC (34%) than in patients with SCC (28%), resulting in an overall survival rate of 13.2 mo[37]. Parry et al[38] reported complete tumour regression in 2 patients after chemotherapy alone, with both patients alive at last follow-up (35 and 112 mo)[38]. Developments in proton beam therapy and stereotactic ablative radiation increases its conformality and reduces radiation toxicity[39]. Sachdeva et al[40] recently reported on the use of external beam radiotherapy for the treatment of oligometastatic sacral metastases in a 46-year-old male with a rare case of primary oesophageal lymphoma[40]. Moreover, 1-year and 2-year progression-free survival and overall survival rates have been reported at 62% and 48% and 90% and 72%, respectively, following stereotactic ablative therapy for pulmonary metastases[41].
With few predictive factors for survival of metastatic oesophageal cancer in the literature[42], it is unclear which patients or which tumour characteristics predict the best survival outcomes. The current approach to metastatic disease all too often consigns the patient to palliative care and a dismal outcome. We have previously reported that bone marrow positivity for micrometastases at the time of oesophagectomy is a predictor of increased risk of cancer-related death and can identify patients requiring intensive surveillance for early detection of metastases with intent to treat[43]. Our current findings suggest that a more optimistic approach can be rewarded with impressive survival data. It is intuitive that aggressive treatment can improve survival, but it implies a need for more intensive surveillance strategies, especially in the first 3 years post-resection, to identify salvageable patients and consider curative intent. In an era of molecularly targeted agents, the identification of such patients is more important than ever as identified by the CheckMate 557 trial where the addition of nivolumab for patients with residual disease following CRT provided a median disease-free survival of 22.4 mo vs 11.0 mo in the placebo arm, which was significant[44].
The obvious limitation of our study is the small sample size of patients with metastatic oesophageal cancer treated for cure. Moreover, the survival data reported in our study reflects a policy of aggressive treatment of confirmed limited metastases only. Such patients self-select, and our survival data cannot be applied to all patients with metastatic oesophageal cancer.
In conclusion, as advances in imaging facilitate earlier metastatic disease detection and advances in multimodal and targeted treatments improve survival outcomes, surveillance strategies must be intensified to diagnose metastatic disease earlier in the recurrence process to institute medical or surgical measures with a greater possibility of success. Future studies are needed to prospectively identify the rate of oligometastatic recurrence in oesophageal carcinoma in the context of today’s imaging technologies to update surveillance and treatment guidelines in line with those for cancers of the lower gastrointestinal tract.
The prognosis of metastatic oesophageal cancer is poor. The rate of oligometastatic oesophageal cancer is not well established nor is the survival benefit of intervention. As a result, current guidelines advocate against a proactive approach, which is incongruent with other oligometastatic cancers such as colorectal cancer. Based on a policy of active postoperative surveillance and survival outcomes of patients with oligometastatic disease treated with curative intent at our institution, we advocate for more intensive surveillance strategies to identify patients with curative potential early and thus improve long-term survival.
To evaluate the impact of a policy of active surveillance and aggressive management of confirmed metastases on long-term survival.
To examine survival outcomes in patients who underwent active surveillance and targeted therapy of their oligometastatic disease, either at diagnosis or on follow-up surveillance, at our institution. When compared to incomplete clinical responders to neoadjuvant chemoradiotherapy (nCRT) for non-metastatic oesophageal cancer who underwent surgery on their primary tumour, the median overall survival of the oligometastatic cohort was statistically significantly longer. These findings suggest that aggressive treatment of confirmed metastases can be rewarded with impressive survival data and that a more proactive approach to oesophageal oligometastases should be considered.
A prospectively maintained database of patients diagnosed with oesophageal carcinoma and treated with curative intent in a single institution was interrogated for patients with metastases, either at diagnosis or on follow-up surveillance, and treated for cure. This cohort was compared with incomplete clinical responders to nCRT who subsequently underwent surgery on their primary tumour. Overall survival was estimated using the Kaplan-Meier method, and the log-rank test was used to compare survival differences between groups.
The overall survival of patients with oligometastatic disease who were treated for cure at our institution is impressive and statistically significantly longer than incomplete clinical responders without metastatic disease who subsequently underwent surgery on their primary tumour. These results suggest that intensive follow-up and aggressive management of confirmed metastases may improve long-term survival. Further studies are needed to prospectively identify the rate of oligometastatic recurrence in oesophageal carcinoma and evaluate the cost-benefit ratio of a policy of active surveillance and aggressive management of confirmed oligometastatic disease.
In view of recent diagnostic and therapeutic advances, intensive follow-up and aggressive treatment of confirmed metastases may improve long-term survival in patients with oligometastatic oesophageal carcinoma.
Further research should prospectively establish the rate of oligometastatic recurrence in oesophageal carcinoma to evaluate the cost-benefit ratio of active surveillance and aggressive management and inform future clinical guidelines.
| 1. | Verhoef C, van de Weyer R, Schaapveld M, Bastiaannet E, Plukker JT. Better survival in patients with esophageal cancer after surgical treatment in university hospitals: a plea for performance by surgical oncologists. Ann Surg Oncol. 2007;14:1678-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Anderson LA, Tavilla A, Brenner H, Luttmann S, Navarro C, Gavin AT, Holleczek B, Johnston BT, Cook MB, Bannon F, Sant M; EUROCARE-5 Working Group:. Survival for oesophageal, stomach and small intestine cancers in Europe 1999-2007: Results from EUROCARE-5. Eur J Cancer. 2015;51:2144-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1033] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 4. | American Cancer Society [Internet]. Cancer Facts and Statistics. [cited 22 June 2020]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html. |
| 5. | Jamel S, Tukanova K, Markar S. Detection and management of oligometastatic disease in oesophageal cancer and identification of prognostic factors: A systematic review. World J Gastrointest Oncol. 2019;11:741-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Sugiyama M, Morita M, Yoshida R, Ando K, Egashira A, Takefumi O, Saeki H, Oki E, Kakeji Y, Sakaguchi Y, Maehara Y. Patterns and time of recurrence after complete resection of esophageal cancer. Surg Today. 2012;42:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Knight WRC, Zylstra J, Van Hemelrijck M, Griffin N, Jacques AET, Maisey N, Baker CR, Gossage JA, Largergren J, Davies AR. Patterns of recurrence in oesophageal cancer following oesophagectomy in the era of neoadjuvant chemotherapy. BJS Open. 2017;1:182-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, van der Sangen MJ, Beukema JC, Rütten H, Spruit PH, Reinders JG, Richel DJ, van Berge Henegouwen MI, Hulshof MC. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 383] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 10. | Meguid RA, Hooker CM, Taylor JT, Kleinberg LR, Cattaneo SM 2nd, Sussman MS, Yang SC, Heitmiller RF, Forastiere AA, Brock MV. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. 2009;138:1309-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4248] [Article Influence: 303.4] [Reference Citation Analysis (3)] |
| 12. | Bass GA, Furlong H, O'Sullivan KE, Hennessy TP, Walsh TN. Chemoradiotherapy, with adjuvant surgery for local control, confers a durable survival advantage in adenocarcinoma and squamous cell carcinoma of the oesophagus. Eur J Cancer. 2014;50:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1925] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 14. | Eguchi T, Kodera Y, Nakanishi H, Yokoyama H, Ohashi N, Ito Y, Nakayama G, Koike M, Fujiwara M, Nakao A. The effect of chemotherapy against micrometastases and isolated tumor cells in lymph nodes: an in vivo study. In Vivo. 2008;22:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 738] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 16. | Niibe Y, Chang JY. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm Med. 2012;2012:261096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | National Institute for Health and Care Excellence [Internet]. Colorectal Cancer. NICE Guideline [NG151]. Jan 2020. Updated Dec 2021. [cited 29 May 2022]. Available from: https://www.nice.org.uk/guidance/ng151. |
| 18. | National Comprehensive Cancer Network [Internet]. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. Feb 2022. [cited 29 May 2022]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| 19. | National Institute for Health and Care Excellence [Internet]. Oesophago-gastric cancer: assessment and management in adults. NICE Guideline [NG83]. Jan 2018. [cited 29 May 2022]. Available from: https://www.nice.org.uk/guidance/ng83/chapter/Recommendations#follow-up. |
| 20. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 720] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 21. | O'sullivan GC, Sheehan D, Clarke A, Stuart R, Kelly J, Kiely MD, Walsh T, Collins JK, Shanahan F. Micrometastases in esophagogastric cancer: high detection rate in resected rib segments. Gastroenterology. 1999;116:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | McDonnell CO, Hill AD, McNamara DA, Walsh TN, Bouchier-Hayes DJ. Tumour micrometastases: the influence of angiogenesis. Eur J Surg Oncol. 2000;26:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020;5:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 348] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 24. | Milano MT, Katz AW, Schell MC, Philip A, Okunieff P. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1516-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Schizas D, Lazaridis II, Moris D, Mastoraki A, Lazaridis LD, Tsilimigras DI, Charalampakis N, Liakakos T. The role of surgical treatment in isolated organ recurrence of esophageal cancer-a systematic review of the literature. World J Surg Oncol. 2018;16:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Depypere L, Lerut T, Moons J, Coosemans W, Decker G, Van Veer H, De Leyn P, Nafteux P. Isolated local recurrence or solitary solid organ metastasis after esophagectomy for cancer is not the end of the road. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Fumagalli U, de Carli S, de Pascale S, Rimassa L, Bignardi M, Rosati R. Adrenal metastases from adenocarcinoma of the esophagogastric junction: adrenalectomy and long-term survival. Updates Surg. 2010;62:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Saito H, Shuto K, Ota T, Toma T, Ohira G, Natsume T, Uesato M, Akutsu Y, Kono T, Matsubara H. [A case of long-term survival after resection for postoperative solitary adrenal metastasis from esophageal adenocarcinoma]. Gan To Kagaku Ryoho. 2010;37:2406-2408. [PubMed] |
| 29. | O'Sullivan KE, Moriarty AR, Larkin JO, Reynolds JV. Curative surgical management of isolated adrenal recurrence of oesophageal adenocarcinoma. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Kanaya N, Noma K, Okada T, Maeda N, Tanabe S, Sakurama K, Shirakawa Y, Fujiwara T. A case of long-term survival after surgical resection for solitary adrenal recurrence of esophageal squamous carcinoma. Surg Case Rep. 2017;3:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 617] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 32. | Howell GM, Carty SE, Armstrong MJ, Stang MT, McCoy KL, Bartlett DL, Yip L. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol. 2013;20:3491-3496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Seesing MFJ, van der Veen A, Brenkman HJF, Stockmann HBAC, Nieuwenhuijzen GAP, Rosman C, van den Wildenberg FJH, van Berge Henegouwen MI, van Duijvendijk P, Wijnhoven BPL, Stoot JHMB, Lacle M, Ruurda JP, van Hillegersberg R; Gastroesophageal Metastasectomy Group. Resection of hepatic and pulmonary metastasis from metastatic esophageal and gastric cancer: a nationwide study. Dis Esophagus. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Liu J, Wei Z, Wang Y, Xia Z, Zhao G. Hepatic resection for post-operative solitary liver metastasis from oesophageal squamous cell carcinoma. ANZ J Surg. 2018;88:E252-E256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Zhang R, Zou J, Li P, Li Q, Qiao Y, Han J, Huang K, Ruan P, Lin H, Song Q, Fu Z. Surgery to the primary tumor is associated with improved survival of patients with metastatic esophageal cancer: propensity score-matched analyses of a large retrospective cohort. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Mohamed A, El-Rayes B, Khuri FR, Saba NF. Targeted therapies in metastatic esophageal cancer: advances over the past decade. Crit Rev Oncol Hematol. 2014;91:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Ajani JA, Ilson DH, Daugherty K, Pazdur R, Lynch PM, Kelsen DP. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst. 1994;86:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Parry K, Visser E, van Rossum PS, Mohammad NH, Ruurda JP, van Hillegersberg R. Prognosis and Treatment After Diagnosis of Recurrent Esophageal Carcinoma Following Esophagectomy with Curative Intent. Ann Surg Oncol. 2015;22 Suppl 3:S1292-S1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Welsh J, Amini A, Likhacheva A, Erasmus J J, Gomez D, Davila M, Mehran RJ, Komaki R, Liao Z, Hofstetter WL, Lee H J, Bhutani MS, Ajani JA. Update: modern approaches to the treatment of localized esophageal cancer. Curr Oncol Rep. 2011;13:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Sachdeva S, Jain A, Dalal A, Puri AS. Primary Esophageal Lymphoma: A Rare Entity. GE Port J Gastroenterol. 2021;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Jang BS, Kim HJ, Kim BH, Kim DW, Kim YT, Kim YW, Jang MJ, Wu HG. Clinical outcomes of stereotactic ablative radiotherapy in patients with pulmonary metastasis. Jpn J Clin Oncol. 2017;47:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Li B, Wang R, Zhang T, Sun X, Jiang C, Li W, Zou B, Xie P, Meng X, Wang L, Yu J. Development and validation of a nomogram prognostic model for esophageal cancer patients with oligometastases. Sci Rep. 2020;10:11259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Ryan P, Furlong H, Murphy CG, O'Sullivan F, Walsh TN, Shanahan F, O'Sullivan GC. Prognostic significance of prospectively detected bone marrow micrometastases in esophagogastric cancer: 10-year follow-up confirms prognostic significance. Cancer Med. 2015;4:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M; CheckMate 577 Investigators. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384:1191-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 1228] [Article Influence: 245.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sachdeva S, India; Serban ED, Romania S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR