Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.963
Peer-review started: May 3, 2022
First decision: May 11, 2022
Revised: May 22, 2022
Accepted: July 27, 2022
Article in press: July 27, 2022
Published online: September 27, 2022
Processing time: 142 Days and 1 Hours
Postoperative pancreatic fistula (PF) is a serious life-threatening complication after pancreaticoduodenectomy (PD). Our research aimed to develop a machine learning (ML)-aided model for PF risk stratification.
To develop an ML-aided model for PF risk stratification.
We retrospectively collected 618 patients who underwent PD from two tertiary medical centers between January 2012 and August 2021. We used an ML algorithm to build predictive models, and subject prediction index, that is, decision curve analysis, area under operating characteristic curve (AUC) and clinical impact curve to assess the predictive efficiency of each model.
A total of 29 variables were used to build the ML predictive model. Among them, the best predictive model was random forest classifier (RFC), the AUC was [0.897, 95% confidence interval (CI): 0.370–1.424], while the AUC of the artificial neural network, eXtreme gradient boosting, support vector machine, and decision tree were between 0.726 (95%CI: 0.191–1.261) and 0.882 (95%CI: 0.321–1.443).
Fluctuating serological inflammatory markers and prognostic nutritional index can be used to predict postoperative PF.
Core tip: Our research is based on machine learning (ML) algorithms and integrates the correlation between serum inflammatory factors and high risk of postoperative pancreatic fistula (PF), and constructs early warning models that can predict postoperative PF, and the predictive efficiency of these ML-based models may be at the population-based level. In the future, we expect these findings to expand external research to strengthen valuable supporting information and guide treatment decisions.
- Citation: Long ZD, Lu C, Xia XG, Chen B, Xing ZX, Bie L, Zhou P, Ma ZL, Wang R. Personal predictive model based on systemic inflammation markers for estimation of postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastrointest Surg 2022; 14(9): 963-975
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/963.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.963
Pancreaticoduodenectomy (PD), also known as a Whipple procedure, is one of the most difficult and complex surgeries that carries a high rate of major complications[1]. Post-operative pancreatic fistula (PF), as one of the most difficult complications after PD, can seriously endanger the lives of patients, so it has become a field of continuous concern for pancreatic surgeons[1,2]. Although the safety of PD has improved significantly in the past three decades[3,4]. Alarmingly, previous prospective studies have reported that postoperative PF occupied an incidence of > 10%[5-7].
In recent years, people have studied different styles of surgery and perioperative attempts to reduce the incidence of postoperative PF. However, regardless of the type of surgery, PF is still the most common fatal complication after pancreatectomy. Understanding the potential complications and early warning of these complications is important for the care of these severe patients.
Previous studies have utilized preoperative radiology and clinical variables combined with specific intraoperative factors to predict the risk of postoperative PF[8-11]. Despite advances in predictive platforms for postoperative PF, they have undergone a constantly changing approach. However, because of its unsatisfactory predictive performance, an improved delivery system is deemed necessary. Therefore, exploring an optimal risk score range model may contribute to eliminating potential life-threatening complications, and stratifying patients with postoperative PF risk, which can be better applied to clinical management.
Nowadays, a series of serum markers suggest that detecting systemic inflammation may be ass
Given this situation, we searched for the help of inflammatory factors and ML-based algorithms to optimize the predictive accuracy for postoperative PF. In this study, we tried to identify alternative predictors independently related to postoperative PF and develop an optimal risk stratification model that can accurately identify high-risk patients with postoperative PF.
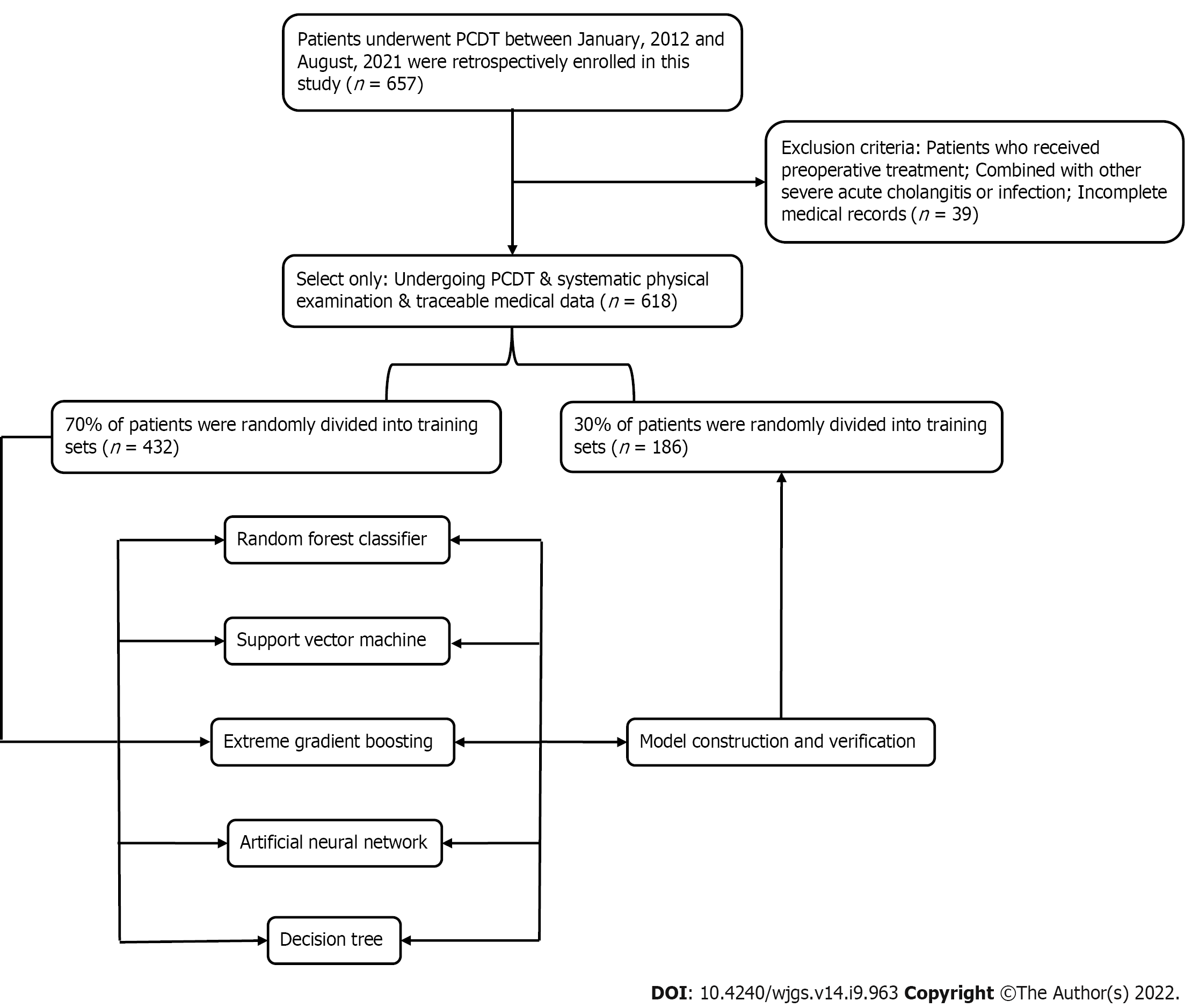
Patients who underwent PD to treat various periampullary tumors from two tertiary medical centers (Jingzhou Hospital and Lu’an Hospital of Anhui Medical University) between January 2012 and August 2021 were retrospectively reviewed. The inclusion criteria were: (1) Resected tumor specimens were confirmed to be malignant by pathological examination; (2) Blood routine examination and liver function examination results were found within 3 d before surgery; and (3) The patient had complete case data and relevant indicators of imaging, pathology and laboratory examination. The exclusion criteria were: (1) Patients receiving preoperative treatment, such as thermal ablation, neoadjuvant chemotherapy or radiotherapy; (2) Severe respiratory and circulatory diseases; (3) Severe acute cholangitis or infection in other parts of the body before surgery; (4) Metastasis from other parts of the primary tumor or direct invasion of adjacent organs from the primary tumor; and (5) Parathyroid diseases or other factors interfering with abnormal changes of procalcitonin (PCT). This study was a retrospective cohort study, which was approved by the Ethics Committee of Jingzhou Central Hospital (Reference: 2021-JH005) and conformed to the Declaration of Helsinki. Because this study adopted anonymous follow-up, the patients’ personal privacy information was strictly confidential. The detailed research flow chart is shown in Figure 1.
According to the standards defined by the International Study Group for Pancreatic Fistula (ISGPF) in 2016, that is, drainage flow > 30 mL for ≥ 72 h after an operation, the amylase content of the drainage fluid is measured. If it exceeds ≥ 3 times the upper limit of normal and had a clinical impact (such as abdominal pain or fever) and needed clinical treatment, it is judged that PF has occurred. The grade of PF updated by ISGPF in 2016 removes the diagnosis of grade A PF. The increase in amylase in asym
We chose to collect 3–5 mL blood samples from each patient on an empty stomach in the morning of 3 d before the operation, and included the latest blood routine and liver function tests in this study. Peripheral venous blood was taken in the morning of d 1, 3 and 5 after the operation, and the changes in C-reactive protein (CRP), serum PCT, and white blood cells were continuously observed.
We obtained population baseline data and clinical pathological data from the patients’ medical records. For instance, the pancreatic texture was evaluated by the surgeon during the operation (soft 1, hard 0), and the diameter of the main pancreatic was obtained by computed tomography or magnetic resonance imaging before the operation. We also collected routine laboratory measurement results, and when the missing value was ≥ 10% of the bias of the total variable, the variable was directly discarded and not included in the final model variable screening[20]. Finally, a total of 29 variables that met the inclusion criteria were used to build ML-based models.
At the beginning of building the model, we randomly divided the population data into two parts, namely, the training queue and the verification queue. The training queue was used to construct the predictive model, and the validation queue was used as the internal validation of the model to evaluate the robustness of the model. When screening candidate variables, we adopted the “two-step segmentation evaluation”, that is, the principle of random sorting to obtain the intersection[21]. In short, by sorting the intersection of variable sets, the optimal subset modeling was obtained. Finally, these models were evaluated through inspection, discrimination and calibration.
As for descriptive variables (i.e. continuous or classified variables), the median (interquartile range) or frequency (percentage) were used for statistical analysis. The χ2 test or Mann–Whitney test was used to calculate the variables between groups to evaluate whether there was a statistical difference. Stepwise regression based on the minimum value of the Akaike information standard was used to select the variables. All data analysis was completed with the help of R language software (version 4.0.4, http://www.r-project.org/). All P values were double tailed, and P < 0.05 was statistically significant.
In this study, all patients were randomly divided into a training set (n = 432, 70%) and validation set (n = 186, 30%) via the caret package. Seventy-eight (18.06%) and 20 (10.75%) patients developed postoperative PF in the training and validation group, respectively, as shown in Table 1. There were 76 (12.3%) grade B and 22 (3.6%) grace C. One patient died of multiple organ failure due to drug-resistant bacterial infection; five underwent reoperation because of continuous blood drainage via the drainage tube, which was confirmed to be abdominal bleeding caused by intraoperative PF; and two were transferred to intensive care.
| Variables | Training set | Testing set | ||||||
| Overall (n = 432) | Non-POPF (n = 354) | POPF (n = 78) | P value | Overall (n = 186) | Non-POPF (n = 166) | POPF (n = 20) | P value | |
| Age, median (IQR) | 55.0 (49.0–61.0) | 55.0 (49.0–61.0) | 53.0 (47.25–61.0) | 0.147 | 55.0 (50.0–60.0) | 55.0 (50.0–60.0) | 51.50 (45.75–59.50) | 0.182 |
| BMI, median (IQR) | 23.10 (21.80–24.60) | 22.80 (21.50–24.20) | 25.0 (23.33–26.92) | < 0.001 | 22.85 (21.72–24.30) | 22.70 (21.52–23.98) | 24.35 (22.88–26.13) | < 0.001 |
| Gender (%) | ||||||||
| Male | 283 (65.5) | 227 (64.1) | 56 (71.8) | 0.247 | 127 (68.3) | 110 (66.3) | 17 (85.0) | 0.148 |
| Female | 149 (34.5) | 127 (35.9) | 22 (28.2) | 59 (31.7) | 56 (33.7) | 3 (15.0) | ||
| Smoking (%) | ||||||||
| Yes | 198 (45.8) | 143 (40.4) | 55 (70.5) | < 0.001 | 89 (47.8) | 76 (45.8) | 13 (65.0) | 0.165 |
| No | 234 (54.2) | 211 (59.6) | 23 (29.5) | 97 (52.2) | 90 (54.2) | 7 (35.0) | ||
| Drinking history (%) | ||||||||
| Yes | 129 (29.9) | 78 (22.0) | 51 (65.4) | < 0.001 | 54 (29.0) | 40 (24.1) | 14 (70.0) | < 0.001 |
| No | 303 (70.1) | 276 (78.0) | 27 (34.6) | 132 (71.0) | 126 (75.9) | 6 (30.0) | ||
| Diabetes (%) | ||||||||
| Yes | 110 (25.5) | 49 (13.8) | 61 (78.2) | < 0.001 | 44 (23.7) | 30 (18.1) | 14 (70.0) | < 0.001 |
| No | 322 (74.5) | 305 (86.2) | 17 (21.8) | 142 (76.3) | 136 (81.9) | 6 (30.0) | ||
| Hypertension (%) | ||||||||
| Yes | 164 (38.0) | 129 (36.4) | 35 (44.9) | 0.208 | 59 (31.7) | 49 (29.5) | 10 (50.0) | 0.108 |
| No | 268 (62.0) | 225 (63.6) | 43 (55.1) | 127 (68.3) | 117 (70.5) | 10 (50.0) | ||
| Abdominal operation (%) | ||||||||
| Yes | 130 (30.1) | 103 (29.1) | 27 (34.6) | 0.409 | 53 (28.5) | 47 (28.3) | 6 (30.0) | 1 |
| No | 302 (69.9) | 251 (70.9) | 51 (65.4) | 133 (71.5) | 119 (71.7) | 14 (70.0) | ||
| Remnant texture (%) | ||||||||
| Soft | 121 (28.0) | 62 (17.5) | 59 (75.6) | < 0.001 | 44 (23.7) | 27 (16.3) | 17 (85.0) | < 0.001 |
| Hard | 311 (72.0) | 292 (82.5) | 19 (24.4) | 142 (76.3) | 139 (83.7) | 3 (15.0) | ||
| Blood transfusion (%) | ||||||||
| Yes | 232 (53.7) | 188 (53.1) | 44 (56.4) | 0.686 | 96 (51.6) | 84 (50.6) | 12 (60.0) | 0.577 |
| No | 200 (46.3) | 166 (46.9) | 34 (43.6) | 90 (48.4) | 82 (49.4) | 8 (40.0) | ||
| Anemia (%) | ||||||||
| Yes | 218 (50.5) | 179 (50.6) | 39 (50.0) | 1 | 84 (45.2) | 69 (41.6) | 15 (75.0) | 0.009 |
| No | 214 (49.5) | 175 (49.4) | 39 (50.0) | 102 (54.8) | 97 (58.4) | 5 (25.0) | ||
| Lesion size (%), cm | ||||||||
| > 3 | 182 (42.1) | 125 (35.3) | 57 (73.1) | < 0.001 | 67 (36.0) | 54 (32.5) | 13 (65.0) | 0.009 |
| ≤ 3 | 250 (57.9) | 229 (64.7) | 21 (26.9) | 119 (64.0) | 112 (67.5) | 7 (35.0) | ||
| Pancreatic duct diameter (%), mm | ||||||||
| < 3 | 154 (35.6) | 93 (26.3) | 61 (78.2) | < 0.001 | 63 (33.9) | 49 (29.5) | 14 (70.0) | 0.001 |
| ≥ 3 | 278 (64.4) | 261 (73.7) | 17 (21.8) | 123 (66.1) | 117 (70.5) | 6 (30.0) | ||
| ASA classification (%) | ||||||||
| I + II | 231 (53.5) | 188 (53.1) | 43 (55.1) | 0.843 | 85 (45.7) | 78 (47.0) | 7 (35.0) | 0.436 |
| III + IV | 201 (46.5) | 166 (46.9) | 35 (44.9) | 101 (54.3) | 88 (53.0) | 13 (65.0) | ||
| CRP, median (IQR), mg/L | 32.0 (22.0–44.0) | 29.0 (21.0–38.0) | 88.50 (56.0–120.0) | < 0.001 | 30.0 (22.0–40.0) | 29.0 (21.0–38.0) | 84.50 (42.25–109.25) | < 0.001 |
| WBC, median (IQR), 109 | 5.70 (5.30–6.30) | 5.70 (5.20–6.20) | 6.0 (5.60–6.60) | < 0.001 | 5.70 (5.20–6.30) | 5.60 (5.20–6.20) | 6.40 (5.52–6.82) | 0.002 |
| PCT, median (IQR), μg/L | 0.54 (0.37–0.68) | 0.49 (0.34–0.61) | 1.06 (0.78–1.21) | < 0.001 | 0.52 (0.37–0.67) | 0.49 (0.35–0.63) | 0.84 (0.68–1.09) | < 0.001 |
| AGR, median (IQR) | 1.50 (1.30–1.60) | 1.50 (1.40–1.60) | 1.35 (1.20–1.40) | < 0.001 | 1.50 (1.30–1.60) | 1.50 (1.40–1.60) | 1.35 (1.17–1.52) | 0.003 |
| PNI, median (IQR) | 49.60 (48.10–51.23) | 49.90 (48.32–51.60) | 48.60 (47.35–49.60) | < 0.001 | 50.10 (48.40–51.48) | 50.30 (48.42–51.60) | 49.30 (46.85–50.37) | 0.02 |
| Neutrophil count, median (IQR), 109 | 4.02 (3.49–4.59) | 4.18 (3.70–4.68) | 3.36 (3.03–3.74) | < 0.001 | 3.94 (3.51–4.54) | 4.03 (3.57–4.57) | 3.46 (3.11–3.76) | < 0.001 |
| Lymphocyte count, median (IQR), 109 | 1.64 (1.51–1.78) | 1.63 (1.50–1.76) | 1.79 (1.60–1.94) | < 0.001 | 1.64 (1.53–1.76) | 1.63 (1.52–1.73) | 1.83 (1.69–1.98) | < 0.001 |
| Platelet count, median (IQR), 109 | 230.0 (208.0–252.0) | 236.0 (213.0–255.0) | 206.0 (185.25–229.75) | < 0.001 | 229.0 (206.0–253.75) | 232.0 (208.25–257.75) | 200.0 (182.50–225.0) | < 0.001 |
| Monocyte count, median (IQR), 109 | 0.52 (0.45–0.60) | 0.55 (0.47–0.62) | 0.44 (0.39–0.49) | < 0.001 | 0.53 (0.46–0.61) | 0.54 (0.47–0.62) | 0.48 (0.42–0.52) | 0.003 |
| Hemoglobin, median (IQR), g/L | 132.0 (124.0–139.0) | 130.0 (121.25–138.0) | 138.0 (133.0–142.75) | < 0.001 | 132.0 (126.0–140.0) | 132.0 (126.0–139.75) | 134.50 (130.0–141.0) | 0.026 |
| NLR, median (IQR) | 2.0 (1.70–2.30) | 1.90 (1.70–2.20) | 2.70 (2.22–3.10) | < 0.001 | 2.0 (1.70–2.30) | 1.90 (1.60–2.20) | 2.80 (2.42–3.05) | < 0.001 |
| NAR, median (IQR) | 0.08 (0.07–0.09) | 0.08 (0.07–0.09) | 0.60 (0.30–0.88) | < 0.001 | 0.08 (0.07–0.09) | 0.08 (0.07–0.09) | 0.65 (0.38–0.80) | < 0.001 |
| PLR, median (IQR) | 136.20 (116.68–157.43) | 143.85 (123.23–161.70) | 113.15 (102.58–128.0) | < 0.001 | 136.45 (120.62–155.80) | 141.0 (121.22–159.78) | 120.15 (104.78–128.57) | < 0.001 |
| LMR, median (IQR) | 3.40 (2.90–3.80) | 3.30 (2.80–3.70) | 3.90 (3.52–4.70) | < 0.001 | 3.50 (3.0–3.80) | 3.40 (2.90–3.70) | 4.15 (3.75–4.48) | < 0.001 |
| HALP, median (IQR) | 53.95 (51.08–56.50) | 52.90 (50.50–55.20) | 72.75 (69.32–75.25) | < 0.001 | 52.45 (50.40–55.18) | 51.95 (50.10–54.30) | 70.10 (68.18–72.62] | < 0.001 |
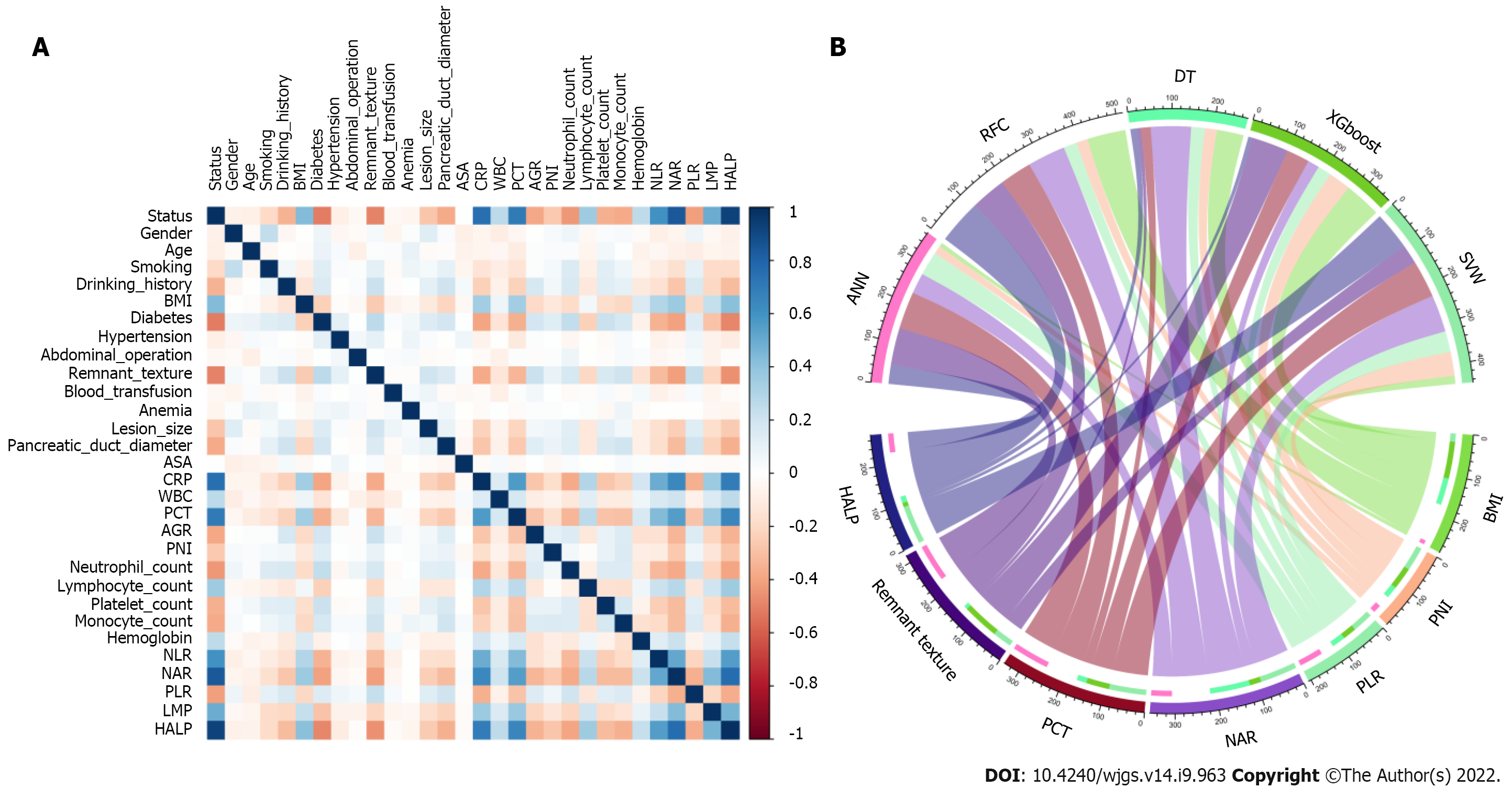
Feature selection is a universal problem in ML[22]. We performed an iterative analysis of 29 potential candidate variables, and the correlation matrix showed that there was a significant correlation between postoperative PF and inflammatory factors and some clinical variables (Figure 2A), including CRP, PCT, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and hemoglobin level × albumin level × lymphocyte count/platelet count ratio (HALP). As shown in Figure 2B, HALP, PCT, neutrophil-to-albumin ratio (NAR), PLR and PNI were the top important predictors. Meanwhile, the seven top-ranked predictors were HALP, remnant texture, PCT, NAR, PLR, PNI, and body mass index (BMI).
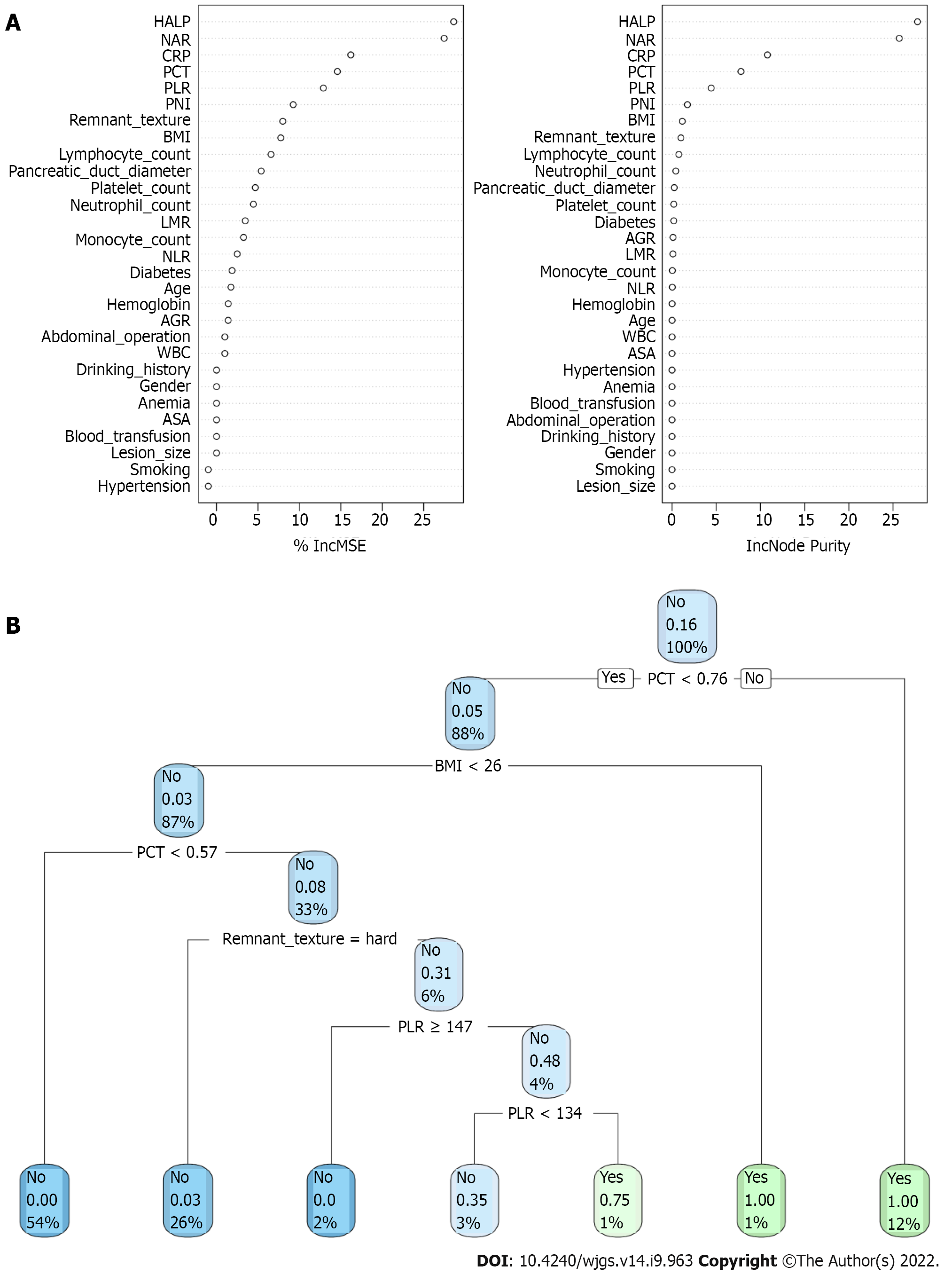
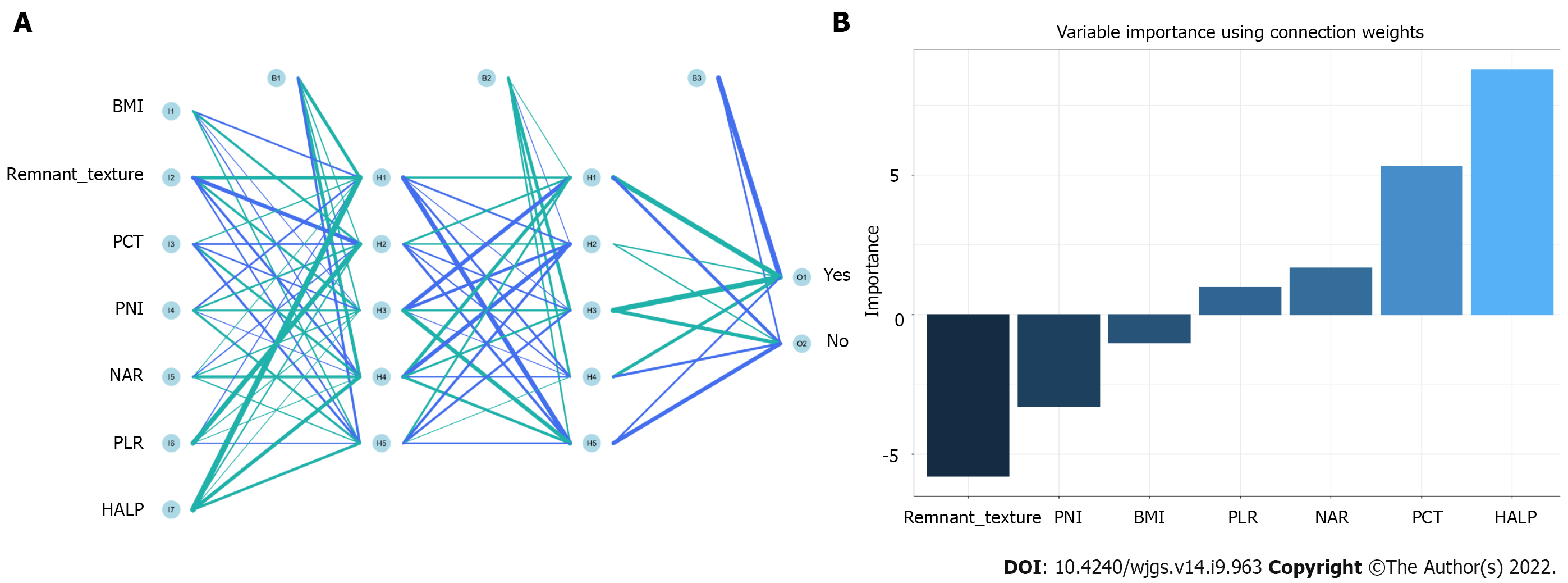
In the training queue, each patient could use positive or negative training and output the final judgment results. For example, a random forest classifier (RFC) algorithm could be used to effectively navigate the free parameter space to obtain a robust model (Figure 3A). The variable Gini index in the RFC model is shown in Supplementary Table 1. In addition, data mining through the decision tree (DT) model was useful, as shown in Figure 3B, among the candidate variables related to inflammatory factors, PCT and BMI also played an important role in DT as branch weight, which could be used as an important predictor of postoperative PF. The artificial neural network (ANN) model also showed relatively robust predictive performance, but slightly lower than that of RFC (Figure 4). We also constructed nomographs, which depended on the parameters obtained by LR, as shown in Supplementary Table 2. Compared with traditional predictive models, inflammatory factors also accounted for an important proportion.
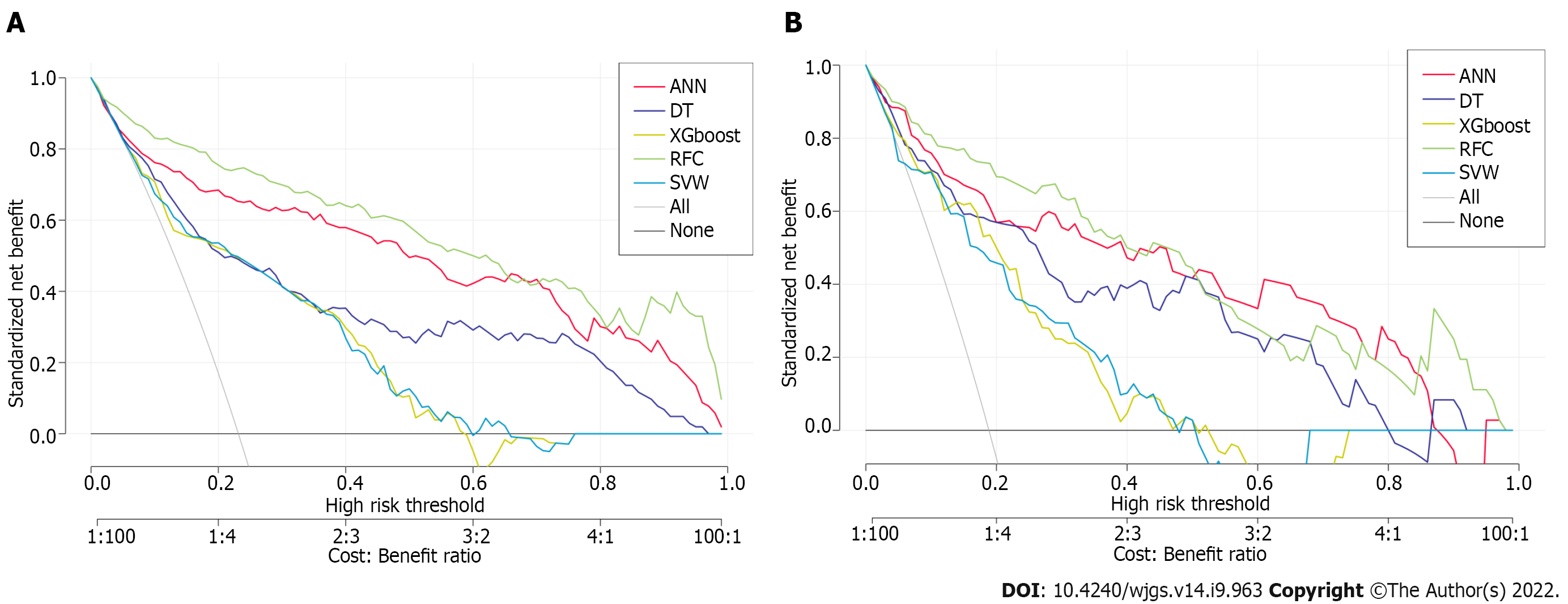
To explore the effectiveness of five supervised learning models for postoperative PF evaluation, we used decision curve analysis (DCA) for evaluation, which was consistent with the results of the included candidate variables. Even if different predictive models included the same variables, there were certain differences in their predictive effectiveness, as shown in Figure 5. In addition, as shown in Table 2, the predictive efficiency of RFC was the best [0.897, 95% confidence interval (CI): 0.370–1.424] compared with the other four predictive models, followed by ANN (0.882, 95%CI: 0.321–1.443), DT (0.807, 95%CI: 0.250–1.364), extreme gradient boosting (XGboost) (0.793, 95%CI: 0.270–1.316), and support vector machine (SVM) (0.726, 95%CI: 0.191–1.261). In conclusion, the iterative algorithm analysis using supervised learning, RFC and ANN, as well as DT (ML-aided decision support) models were properly used to guide postoperative PF prediction.
| Model | AUC | No. of candidate variables | |
| Mean | 95%CI | ||
| RFC | 0.897 | 0.370–1.424 | 7 |
| SVM | 0.726 | 0.191–1.261 | 8 |
| DT | 0.807 | 0.250–1.364 | 8 |
| ANN | 0.882 | 0.321–1.443 | 7 |
| XGboost | 0.793 | 0.270–1.316 | 9 |
We evaluated the clinical predictive efficiency of the optimal prediction model (RFC), as shown in Supplementary Figure 1. RFC can be used to achieve accurate stratification of patients’ postoperative PF via clinical impact curve (CIC). In general, RFC performed best in the construction of prediction models by fusing inflammatory markers.
Our study revealed two major findings. First, accurate risk stratification of postoperative PF in patients who received PD, which mainly depended on the added value of systemic inflammation markers. Second, the ML-based predictive model is better than the traditional predictive algorithm model, which is suitable for identifying whether patients have postoperative PF.
Several risk factors leading to such complications have been reported in the relevant literature, including pancreas texture, BMI, intraoperative blood loss, blood transfusion, and operating time[9,23,24]. We summarize updated literature on predicting postoperative PF, in combination with various candidate predictive markers in Supplementary Table 3. Guo et al[25] reported that the texture of pancreas, size of the main pancreatic duct, portal vein invasion and confirmed pathology are the risk factors of postoperative PF. Tajima et al[26] summarized that preoperative imaging evaluation of pancreatic pathologies would be also beneficial for stratifying. Not surprisingly, systemic inflammatory markers such as neutrophils, lymphocytes, platelets, CRP, albumin, and biomarkers may help predict postoperative PF. The systemic response to postoperative local inflammatory stimulation is tightly related to the complications after gastrointestinal surgery[27]. Gasteiger et al[15] reported that postoperative pancreatitis and inflammatory reaction are the main determinants of postoperative PF[15]. Intriguingly, our calculated risk factors for postoperative PF and inflammatory factors accounted for an irreplaceable weight in the predictive model.
In this study, an attempt was made to improve early postoperative risk stratification by combining local pancreatic residual inflammatory status and systemic response. We found that abnormal HALP, PCT, NAR, PLR and PNI showed reliable predictive value for postoperative PF. Previous studies have confirmed that neutrophils, as the source of vascular endothelial growth factor and tissue inhibitor protease, can promote tumor infiltration and distant metastasis[28-30]. Additionally, the number of lymphocytes in cancer patients changes frequently, which seriously affects the prognosis and survival rate[31,32]. As noted above, it appears that inflammatory factors were highly related to the presence of postoperative PF. Combined with these findings, our analysis showed that systemic inflammatory markers are of value in predicting postoperative PF.
Our ML-based model was based on clinical parameters and laboratory test results, which were consistent with previous research results. Clinical indicators including preoperative serum albumin, lipase level, and amount of intraoperative fluid infusion were independent risk factors of postoperative PF[23,24,33]. Therefore, we further analyzed the accuracy of the predictive model constructed between clinical parameters and systemic inflammatory markers based on an ML-based algorithm. Not surprisingly, we found that systemic inflammatory markers accounted for a high weight in each model. Among these predictive models, RFC allowed the calculation of risk level based on candidate variables, so the best predictive efficiency was obtained. It is not surprising that RFC adopted the resampling technique of bootstrapping to repeatedly focus on the “bagging” procedure[34]. To detect the discrimination of the ML-based model, the DCA and CIC methods were used to evaluate the predictive performance, and the results were consistent with the expected goal. Taken together, our model may apply to patients who intended to receive PD, especially to help surgeons decide whether to prevent postoperative PF after surgery.
Despite several strengths, there were some noteworthy limitations to this study. First, patients included were from two tertiary referral hospitals, which may have resulted in selection bias. Second, although we have established a perfect predictive model through an ML-based algorithm, our model still needs to be confirmed in other hospital settings. Although we adopted internal data cross-validation, we still need more external data to verify its feasibility in the future. Third, we only adopted simple data obtained from classification, missing clinical data were not considered throughout the study. Hence, incorporating specific new technologies such as immunodiagnostic biomarkers may help to improve the accuracy of predictive models.
Our results provide new insights into candidate predictive markers associated with high risk of PF. With the help of HALP, NAR, CRP, PCT and PLR, we developed ML-based predictive models, and the performance of these unsupervised integrated models was superior to that of traditional predictive models. We expect these findings to extend research to strengthen clinical decision-making and guide treatment.
We provide insights into the candidate predictive markers associated with a high risk of postoperative pancreatic fistula (PF) via serum inflammatory secretion. With the help of hemoglobin level × albumin level × lymphocyte count/platelet count ratio, neutrophil-to-albumin ratio, C-reactive protein, procalcitonin and platelet-to-lymphocyte ratio, we develop machine learning (ML)-based predictive models, and the predictive performance of these unsupervised integrated models was superior to that of traditional predictive models. We expect these findings to extend research to strengthen clinical decision-making and guide treatment.
Fluctuating serological inflammation markers and prognostic nutritional index can be detected in the early postoperative period, and clinically well established to predict postoperative PF; in particular, random forest classifier (RFC) performed best, which can guide optimal treatment, clinical management and prevent or mitigate adverse consequences.
A total of 29 variables were used to build the ML predictive model. Among them, the best predictive model was RFC, the area under the curve (AUC) was [0.897, 95% confidence interval (CI): 0.370–1.424], while the AUC of the artificial neural network, eXtreme gradient boosting, support vector machine, and decision tree were between 0.726 (95%CI: 0.191–1.261) and 0.882 (95%CI: 0.321–1.443).
As for descriptive variables (i.e., continuous or classified variables), the median (interquartile range) or frequency (percentage) were used for statistics in this study. The χ2 test or Mann–Whitney test was used to calculate the variables between groups to evaluate whether there was a statistical difference. Stepwise regression based on the minimum value of the Akaike information standard was used to select the variables. All data analysis was completed with the help of R language software (version 4.0.4, http://www.r-project.org/). All P values were double tailed, and P < 0.05 was statistically significant.
A total of 29 variables were used to build the ML predictive model. Among them, the best predictive model was RFC, the area under the curve (AUC) was [0.897, 95% confidence interval (CI): 0.370–1.424], while the AUC of the artificial neural network, eXtreme gradient boosting, support vector machine, and decision tree were between 0.726 (95%CI: 0.191–1.261) and 0.882 (95%CI: 0.321–1.443).
Fluctuating serological inflammatory markers and prognostic nutritional index (PNI) can be detected in the early postoperative period, which has been clinically proved to predict postoperative PF. In particular, RFC performed best, which can guide optimal treatment, clinical management, and prevent or mitigate adverse consequences.
PD, also known as a Whipple procedure, is one of the most difficult and complex surgeries that carries a high rate of major complications. Postoperative PF, as one of the most difficult complications after PD, can seriously endanger the lives of patients, so it has become an area of continuous concern for pancreatic surgeons. Although the safety of PD has improved significantly in the past three decades, previous prospective studies have reported that postoperative PF has an incidence of > 10%. Understanding the potential complications and early warning of these complications is important for the care of these patients.
The authors thank all medical workers and patients involved in this study, including those involved in data collection and compilation.
| 1. | Karim SAM, Abdulla KS, Abdulkarim QH, Rahim FH. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int J Surg. 2018;52:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Xiang Y, Wu J, Lin C, Yang Y, Zhang D, Xie Y, Yao X, Zhang X. Pancreatic reconstruction techniques after pancreaticoduodenectomy: a review of the literature. Expert Rev Gastroenterol Hepatol. 2019;13:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Narayanan S, Martin AN, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Mortality after pancreaticoduodenectomy: assessing early and late causes of patient death. J Surg Res. 2018;231:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Kawaida H, Kono H, Hosomura N, Amemiya H, Itakura J, Fujii H, Ichikawa D. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol. 2019;25:3722-3737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (4)] |
| 5. | Wellner UF, Brett S, Bruckner T, Limprecht R, Rossion I, Seiler C, Sick O, Wegener I, Hopt UT, Keck T; RECOPANC Trial Group. Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after partial PANCreatoduodenectomy (RECOPANC): study protocol of a randomized controlled trial UTN U1111-1117-9588. Trials. 2012;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Chen BP, Bennett S, Bertens KA, Balaa FK, Martel G. Use and acceptance of the International Study Group for Pancreatic Fistula (ISGPF) definition and criteria in the surgical literature. HPB (Oxford). 2018;20:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Fu SJ, Shen SL, Li SQ, Hu WJ, Hua YP, Kuang M, Liang LJ, Peng BG. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: an audit of 532 consecutive cases. BMC Surg. 2015;15:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Akgul O, Merath K, Mehta R, Hyer JM, Chakedis J, Wiemann B, Johnson M, Paredes A, Dillhoff M, Cloyd J, Pawlik TM. Postoperative Pancreatic Fistula Following Pancreaticoduodenectomy-Stratification of Patient Risk. J Gastrointest Surg. 2019;23:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 976] [Article Influence: 69.7] [Reference Citation Analysis (2)] |
| 10. | You Y, Han IW, Choi DW, Heo JS, Ryu Y, Park DJ, Choi SH, Han S. Nomogram for predicting postoperative pancreatic fistula. HPB (Oxford). 2019;21:1436-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, de Pastena M, Klompmaker S, Marchegiani G, Ecker BL, van Dieren S, Bonsing B, Busch OR, van Dam RM, Erdmann J, van Eijck CH, Gerhards MF, van Goor H, van der Harst E, de Hingh IH, de Jong KP, Kazemier G, Luyer M, Shamali A, Barbaro S, Armstrong T, Takhar A, Hamady Z, Klaase J, Lips DJ, Molenaar IQ, Nieuwenhuijs VB, Rupert C, van Santvoort HC, Scheepers JJ, van der Schelling GP, Bassi C, Vollmer CM, Steyerberg EW, Abu Hilal M, Groot Koerkamp B, Besselink MG; Dutch Pancreatic Cancer Group. Alternative Fistula Risk Score for Pancreatoduodenectomy (a-FRS): Design and International External Validation. Ann Surg. 2019;269:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 307] [Article Influence: 43.9] [Reference Citation Analysis (1)] |
| 12. | Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 934] [Article Influence: 155.7] [Reference Citation Analysis (0)] |
| 13. | Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 622] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 14. | Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1704] [Article Influence: 189.3] [Reference Citation Analysis (0)] |
| 15. | Gasteiger S, Primavesi F, Göbel G, Braunwarth E, Cardini B, Maglione M, Sopper S, Öfner D, Stättner S. Early Post-Operative Pancreatitis and Systemic Inflammatory Response Assessed by Serum Lipase and IL-6 Predict Pancreatic Fistula. World J Surg. 2020;44:4236-4244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | van Hilst J, Brinkman DJ, de Rooij T, van Dieren S, Gerhards MF, de Hingh IH, Luyer MD, Marsman HA, Karsten TM, Busch OR, Festen S, Heger M, Besselink MG; Dutch Pancreatic Cancer Group. The inflammatory response after laparoscopic and open pancreatoduodenectomy and the association with complications in a multicenter randomized controlled trial. HPB (Oxford). 2019;21:1453-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 21079] [Article Influence: 1916.3] [Reference Citation Analysis (2)] |
| 18. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3551] [Article Influence: 169.1] [Reference Citation Analysis (35)] |
| 19. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 3196] [Article Influence: 355.1] [Reference Citation Analysis (35)] |
| 20. | Miot HA. Anomalous values and missing data in clinical and experimental studies. J Vasc Bras. 2019;18:e20190004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Fan J, Lv J. A Selective Overview of Variable Selection in High Dimensional Feature Space. Stat Sin. 2010;20:101-148. [PubMed] |
| 22. | Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (7)] |
| 23. | Peng YP, Zhu XL, Yin LD, Zhu Y, Wei JS, Wu JL, Miao Y. Risk factors of postoperative pancreatic fistula in patients after distal pancreatectomy: a systematic review and meta-analysis. Sci Rep. 2017;7:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Han IW, Cho K, Ryu Y, Shin SH, Heo JS, Choi DW, Chung MJ, Kwon OC, Cho BH. Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence. World J Gastroenterol. 2020;26:4453-4464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Guo CX, Shen YN, Zhang Q, Zhang XZ, Wang JL, Gao SL, Lou JY, Que RS, Ma T, Liang TB, Bai XL. Prediction of postoperative pancreatic fistula using a nomogram based on the updated definition. Ann Surg Treat Res. 2020;98:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Tajima Y, Kawabata Y, Hirahara N. Preoperative imaging evaluation of pancreatic pathologies for the objective prediction of pancreatic fistula after pancreaticoduodenectomy. Surg Today. 2018;48:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Rettig TC, Verwijmeren L, Dijkstra IM, Boerma D, van de Garde EM, Noordzij PG. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann Surg. 2016;263:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Balestrieri ML, Balestrieri A, Mancini FP, Napoli C. Understanding the immunoangiostatic CXC chemokine network. Cardiovasc Res. 2008;78:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 334] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 31. | Ménétrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: an opportunity for combination with Cytokines? J Immunother Cancer. 2019;7:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 32. | Blankenstein T, Coulie PG, Gilboa E, Jaffee EM. The determinants of tumour immunogenicity. Nat Rev Cancer. 2012;12:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 33. | Ellis RJ, Brock Hewitt D, Liu JB, Cohen ME, Merkow RP, Bentrem DJ, Bilimoria KY, Yang AD. Preoperative risk evaluation for pancreatic fistula after pancreaticoduodenectomy. J Surg Oncol. 2019;119:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Rigatti SJ. Random Forest. J Insur Med. 2017;47:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 534] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Gaspar AF, Brazil S-Editor: Chen YL L-Editor: Kerr C P-Editor: Chen YL