Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.940
Peer-review started: April 14, 2022
First decision: June 10, 2022
Revised: July 2, 2022
Accepted: August 7, 2022
Article in press: August 7, 2022
Published online: September 27, 2022
Processing time: 161 Days and 0.3 Hours
There are many staging systems for gastrointestinal stromal tumors (GISTs), and the risk indicators selected are also different; thus, it is not possible to quantify the risk of recurrence among individual patients.
To develop and internally validate a model to identify the risk factors for GIST recurrence after surgery.
The least absolute shrinkage and selection operator (LASSO) regression model was performed to identify the optimum clinical features for the GIST recurrence risk model. Multivariable logistic regression analysis was used to develop a prediction model that incorporated the possible factors selected by the LASSO regression model. The index of concordance (C-index), calibration curve, receiver operating characteristic curve (ROC), and decision curve analysis were used to assess the discrimination, calibration, and clinical usefulness of the predictive model. Internal validation of the clinical predictive capability was also evaluated by bootstrapping validation.
The nomogram included tumor site, lesion size, mitotic rate/50 high power fields, Ki-67 index, intracranial necrosis, and age as predictors. The model presented perfect discrimination with a reliable C-index of 0.836 (95%CI: 0.712-0.960), and a high C-index value of 0.714 was also confirmed by interval validation. The area under the curve value of this prediction nomogram was 0.704, and the ROC result indicated good predictive value. Decision curve analysis showed that the predicting recurrence nomogram was clinically feasible when the recurrence rate exceeded 5% after surgery.
This recurrence nomogram combines tumor site, lesion size, mitotic rate, Ki-67 index, intracranial necrosis, and age and can easily predict patient prognosis.
Core Tip: This is a retrospective study to explore the risk factors for gastrointestinal stromal tumors recurrence after surgery. The nomogram included tumor site, lesion size, mitotic rate/50 high power fields, Ki-67 index, intracranial necrosis, and age as predictors. The model presented perfect discrimination with a reliable index of concordance (C-index) of 0.836 (95%CI: 0.712-0.960), and a high C-index value of 0.714 was also confirmed by interval validation. The area under the curve value of this prediction nomogram was 0.704, indicating good predictive value. Decision curve analysis showed that the predicting recurrence nomogram was clinically feasible.
- Citation: Guan SH, Wang Q, Ma XM, Qiao WJ, Li MZ, Lai MG, Wang C. Development of an innovative nomogram of risk factors to predict postoperative recurrence of gastrointestinal stromal tumors. World J Gastrointest Surg 2022; 14(9): 940-949
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/940.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.940
Gastrointestinal stromal tumors (GISTs) originate from gastrointestinal Cajal cells and are the most common mesenchymal tumors in the gastrointestinal tract, accounting for 1% to 3% of gastrointestinal malignancies[1]. GISTs can occur anywhere in the digestive tract, most commonly in the stomach (50%-60%) and the small intestine (30%-50%)[2]. Surgical resection is the main treatment for GIST. However, even with complete surgical resection, approximately 40% to 50% of patients with high-risk GISTs will have recurrence and metastasis[3]. Therefore, by accurately determining the risk factors for pos
Clinical characteristics including tumor site, tumor size, and mitotic rate are the most common indicators for analyzing the risk factors for recurrence after surgery for GIST. Some studies also suggest that the systemic inflammatory response plays an important role in the progression and metastasis of tumors[4]. The grade of risk classification after operation for GIST is mainly evaluated by the 2008 modified National Institutes of Health (NIH) risk grading standards[5], the 2020 edition of the World Health Organization soft tissue tumor classification[6], the National Comprehensive Cancer Network guidelines (6th edition, 2019)[7] and the Armed Forces Institute of Pathology criteria[8]. In addition, Joensuu et al[9] developed a new contour map to predict the prognosis of patients with GIST by monitoring the follow-up results of more than 2000 patients with GIST. However, the use of a single grading method to predict the probability of postoperative recurrence in patients with GIST has certain limitations, especially for some GIST patients who only evaluate the two key indicators of tumor size and mitotic rate. Therefore, there is currently no consensus on which risk grading system to use. Nomograms have been developed for most malignant tumors[10,11]. The use of nomograms has been compared to many traditional cancer staging systems, and it is proposed as an alternative or even a new standard.
Based on the above factors, a predictive nomogram may provide a more accurate prognostic assessment and basis for postoperative recurrence of GIST. To our knowledge, reports on the establishment of a nomogram for the postoperative recurrence of GIST are rare. Therefore, the aim of this study was to develop an effective and simple predictive tool for the risk assessment of postoperative recurrence after GIST and to evaluate the risk of postoperative recurrence using only postoperative pathological features and general clinical data.
The clinical and pathological data of 130 patients with GIST from January 2010 to January 2017 were retrospectively analyzed. The inclusion criteria were as follows: first, complete surgical resection and postoperative pathology and immunohistochemistry confirmed as GIST; second, complete medical records were available; third, patients presented with no other gastrointestinal malignancies; and fourth, patients reported no history of neoadjuvant targeted therapy. A total of 130 patients were included in the study according to the inclusion criteria. The classification criteria were as follows: the risk of recurrence of primary GIST was divided into 4 groups according to the 2008 NIH risk grading standards[5]: very low risk, low risk, middle risk, and high risk. Tumor size was based on the largest diameter of the lesion. The Ki-67 indicator was divided into two groups: < 5% and ≥ 5%. The mitotic rate/50 high power fields were divided into three groups: ≤ 5, > 5 and ≤ 10, and > 10. The tumors were divided into two groups according to whether there was bleeding or necrosis.
All cases were followed up mainly by telephone and outpatient and inpatient review after surgery. Recurrence was confirmed by imaging examination (abdominal B-ultrasound, computed tomography or magnetic resonance imaging) and pathological confirmation by biopsy. The last follow-up time was until June 2019, and the endpoint event was recurrence or metastasis of the patient. Recurrence-free survival was defined as the time from the date of surgery to the time of recurrence or metastasis or the last follow-up time.
Data processing was performed using R language (version 3.6.0) statistical software. The best predictive risk factors for recurrence were selected from the clinical pathological data of patients with GIST using the least absolute shrinkage and selection operator (LASSO) method suitable for reducing high-dimensional data[12,13]. The process was as follows: select the factor with a nonzero coefficient in the LASSO regression model[14], combine the factors selected in the LASSO regression model, and use multivariate logistic regression analysis to establish the prediction model and obtain the odds ratio value of the corresponding factor, 95%CI and P value. Statistical significance levels were relative, variables with a P value of < 0.05 were included in the model, and variables associated with disease and treatment factors were also included. All potential predictors have been used to develop predictive models for the risk of GIST recurrence.
Calibration curves were drawn to evaluate the accuracy of the recurrence nomogram. The recognition performance of the recurrence nomogram was quantified by measuring Harrell’s index of concordance (C-index). Bootstrap verification (1000 bootstrap resampling) was performed on the recurrence nomogram to determine the relative corrected C-index[15]. Decision curve analysis was performed to quantify the clinical values of the recurrence nomogram by quantifying the net benefit at different threshold probabilities in the GIST cohort[16]. The proportion of all false-positive patients was subtracted from the proportion of true positive patients, and the net benefit was calculated by weighing the relative harm of the intervention with the negative consequences of unnecessary interventions[17].
In this study, 130 patients with GIST radical surgery were included, including 101 gastric stromal tumors, 24 small intestinal stromal tumors, and 5 Large intestinal stromal tumors. All patients were divided into a recurrence group (13 cases) and a nonrecurrence group (117 cases) according to the presence or absence of recurrence. The ratio of males to females was close to 1:1. The patients were aged 25-82 years old, and the mean age was 57.0 ± 11.8 years old. All data and proportions of the two groups of patients, including general information and clinicopathological features are shown in Table 1.
| Demographic characteristics | n (%) | ||
| Recurrence (n = 13) | Nonrecurrence (n = 117) | Total (n = 130) | |
| Age (yr) | |||
| < 60 | 8 (61.5) | 62 (54.0) | 70 (53.8) |
| ≥ 60 | 5 (38.5) | 55 (47.0) | 60 (46.2) |
| Sex | |||
| Male | 6 (46.2) | 61 (52.1) | 67 (51.5) |
| Female | 7 (53.8) | 56 (47.9) | 63 (48.5) |
| Tumor site | |||
| Stomach | 9 (69.2) | 92 (78.6) | 101 (77.7) |
| Small intestine | 1 (7.7) | 23 (19.7) | 24 (18.5) |
| Large intestine | 3 (23.1) | 2 (1.7) | 5 (3.8) |
| Tumor size | |||
| < 2 cm | 2 (15.4) | 25 (21.4) | 27 (20.8) |
| ≥ 2 and ≤ 5 cm | 6 (46.1) | 56 (47.9) | 62 (47.7) |
| > 5 and ≤ 10 cm | 1 (7.7) | 30 (25.6) | 31 (23.8) |
| > 10 cm | 4 (30.8) | 6 (5.1) | 10 (7.7) |
| NIH risk category | |||
| Very low | 3 (23.1) | 31 (26.5) | 34 (26.2) |
| Low | 2 (15.4) | 31 (26.5) | 33 (25.4) |
| Middle | 1 (7.7) | 27 (23.1) | 28 (21.5) |
| High | 7 (53.8) | 28 (23.9) | 35 (26.9) |
| Mitotic rate | |||
| ≤ 5 cm | 7 (53.8) | 87 (74.4) | 94 (72.3) |
| > 5 cm and ≤ 10 cm | 2 (15.4) | 22 (18.8) | 24 (18.5) |
| > 10 cm | 4 (30.8) | 8 (6.8) | 12 (9.2) |
| Ki-67 | |||
| < 5% | 4 (30.8) | 70 (59.8) | 74 (56.9) |
| ≥ 5% | 9 (69.2) | 47 (40.2) | 56 (43.1) |
| Intratumoral hemorrhage | |||
| Yes | 10 (76.9) | 100 (85.5) | 110 (84.6) |
| No | 3 (23.1) | 17 (14.5) | 20 (15.4) |
| Intratumoral necrosis | |||
| Yes | 8 (61.5) | 99 (84.6) | 107 (82.3) |
| No | 5 (38.5) | 18 (15.4) | 23 (17.7) |
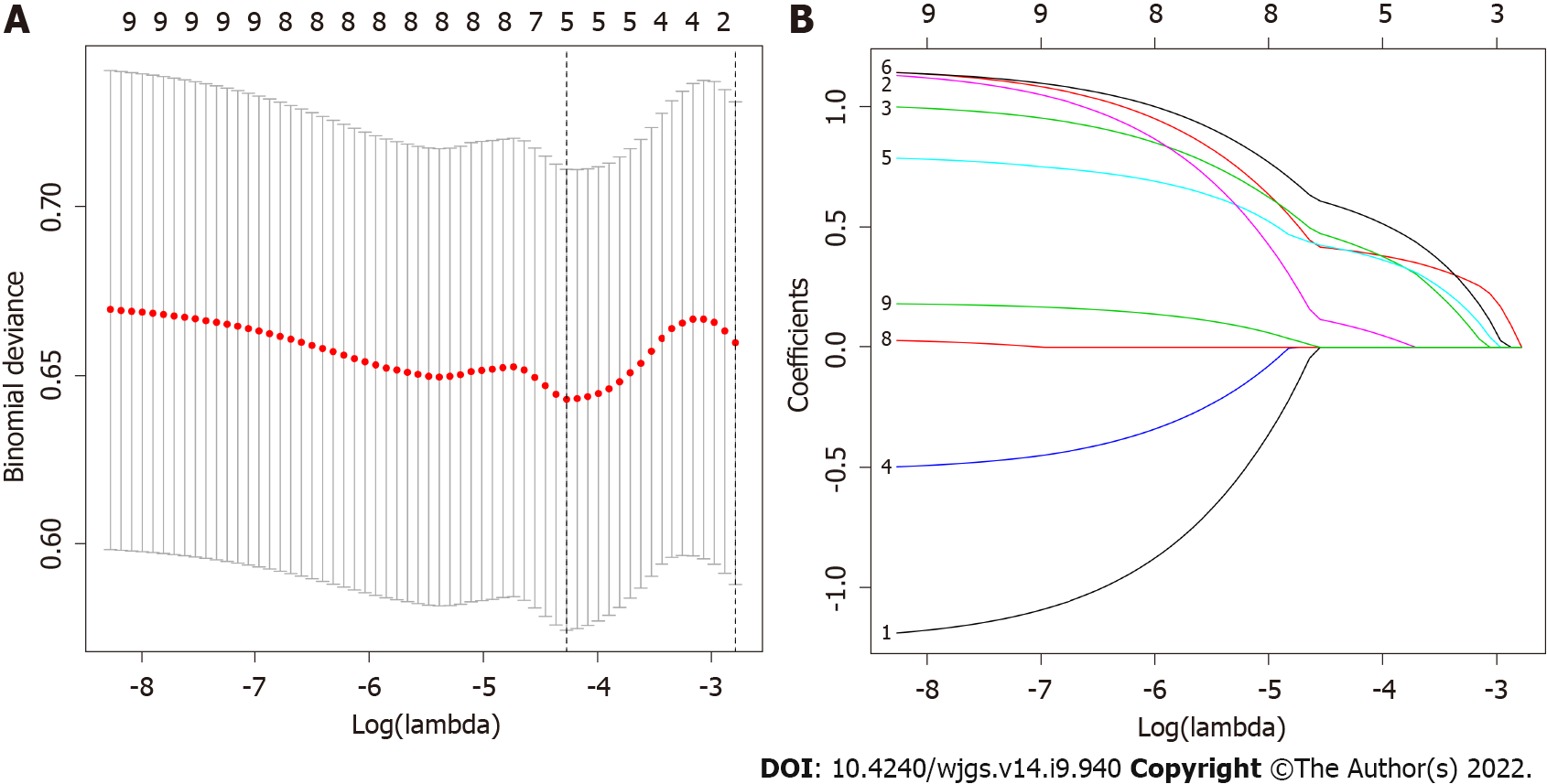
Of the 130 patients’ general information and clinical pathological features, 9 factors were calculated using the LASSO regression model, and 5 factors with nonzero coefficients were considered potential predictors. These factors included the mitotic rate, Ki-67, intratumoral necrosis, tumor size and tumor site (Figure 1A and B).
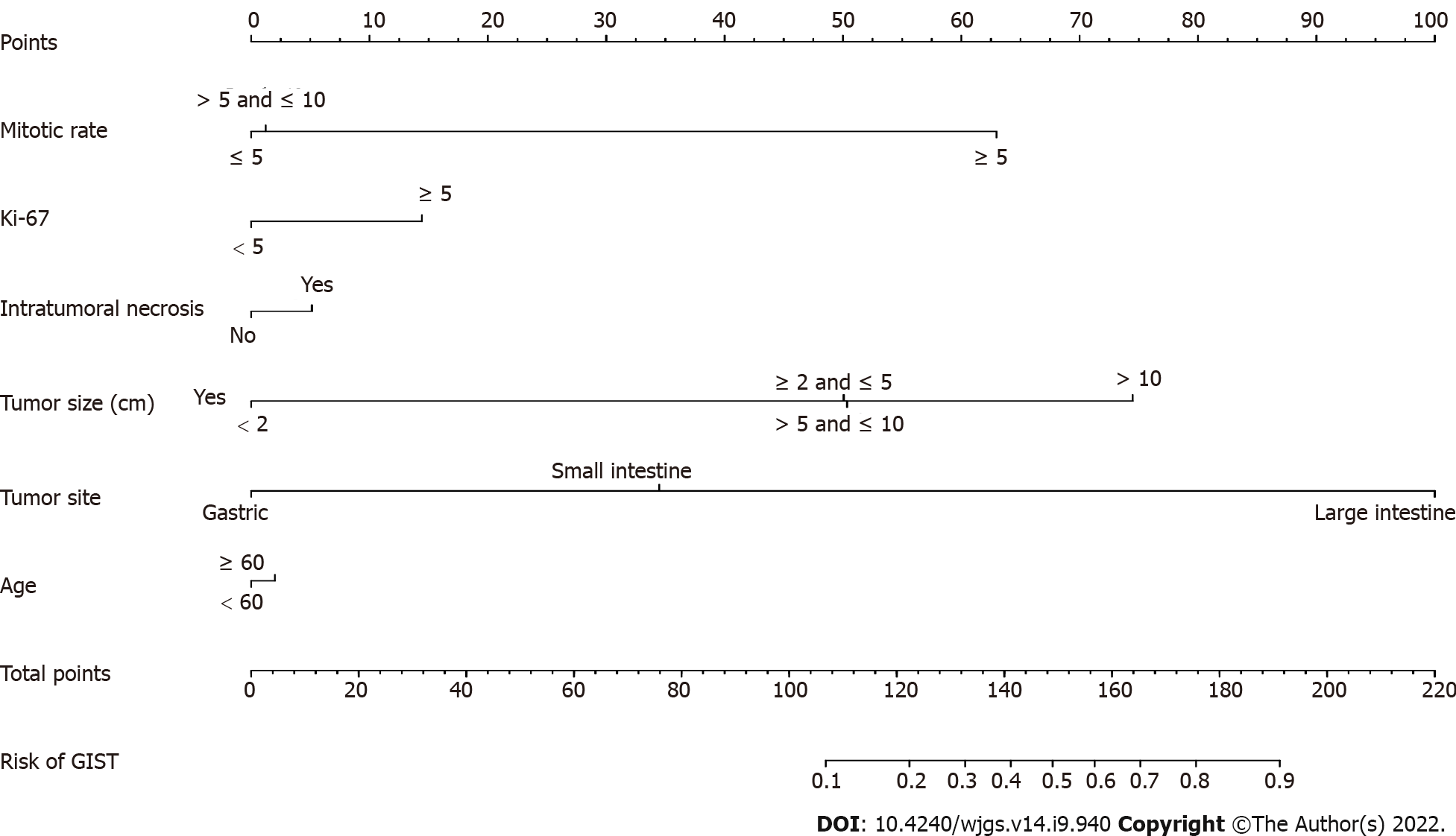
Multivariate logistic regression analysis was performed on factors with nonzero coefficients in the LASSO regression model. In addition, considering the importance of age in oncology, an additional age factor was added to this analysis is shown in Table 2. Therefore, a total of 6 potential predictors were mitotic rate, Ki 67, intratumoral necrosis, tumor size, tumor site and age. The potential predictive factors are integrated together, and scaled line segments are drawn on the same plane to a certain scale to express the relationship between variables in the predictive model, represented by a nomogram (Figure 2).
| Intercept and variable | Prediction model | ||
| β | Odds ratio (95%CI) | P value | |
| Intercept | -3.0092 | 0.049 (0.006-0.245) | 0.001 |
| Mitotic rate | 3.2152 | 24.907 (2.215-707.556) | 0.020 |
| Ki-67 | 0.7514 | 2.120 (0.340-15.083) | 0.425 |
| Intratumoral necrosis | -0.2675 | 0.765 (0.081-5.421) | 0.799 |
| Tumor size | -0.0147 | 0.985 (0.115-10.405) | 0.989 |
| Tumor site | 3.4115 | 30.313 (3.265-405.088) | 0.003 |
| Age | 0.1048 | 1.110 (0.228-5.611) | 0.895 |
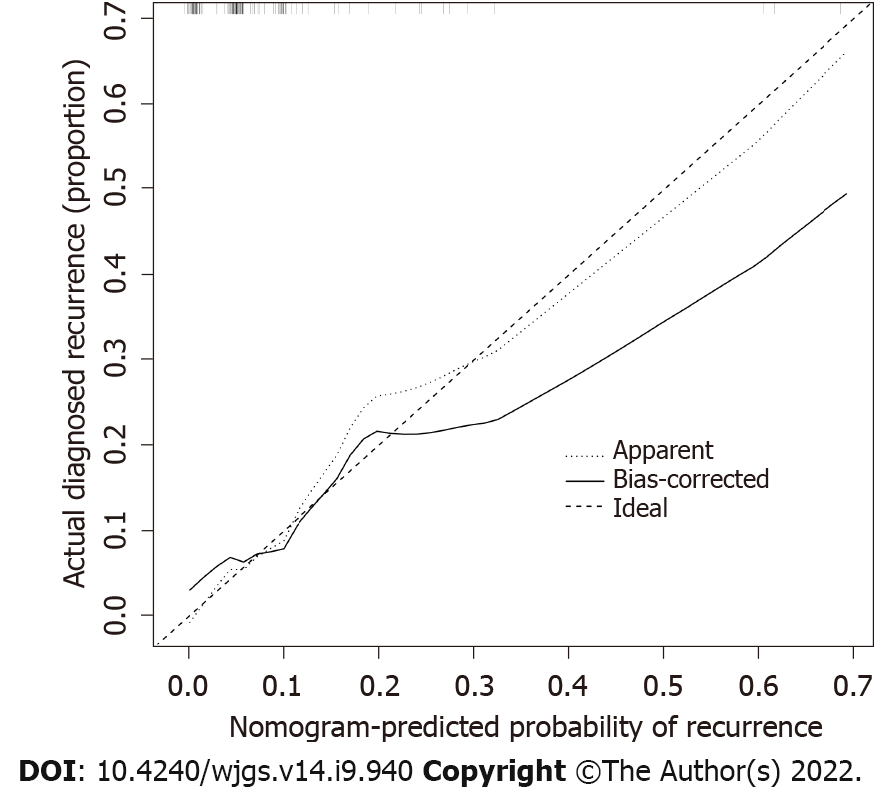
The calibration curve of the recurrence risk nomogram used to predict recurrence risk in patients with GIST showed good consistency (Figure 3). The C-index of the predictive nomogram of this cohort was 0.836 (95%CI: 0.712-0.960), and it was confirmed as 0.714 by bootstrapping validation, which indicated that this model had great differentiation. In the recurrence risk nomogram, the apparent performance possessed a good prediction capability.
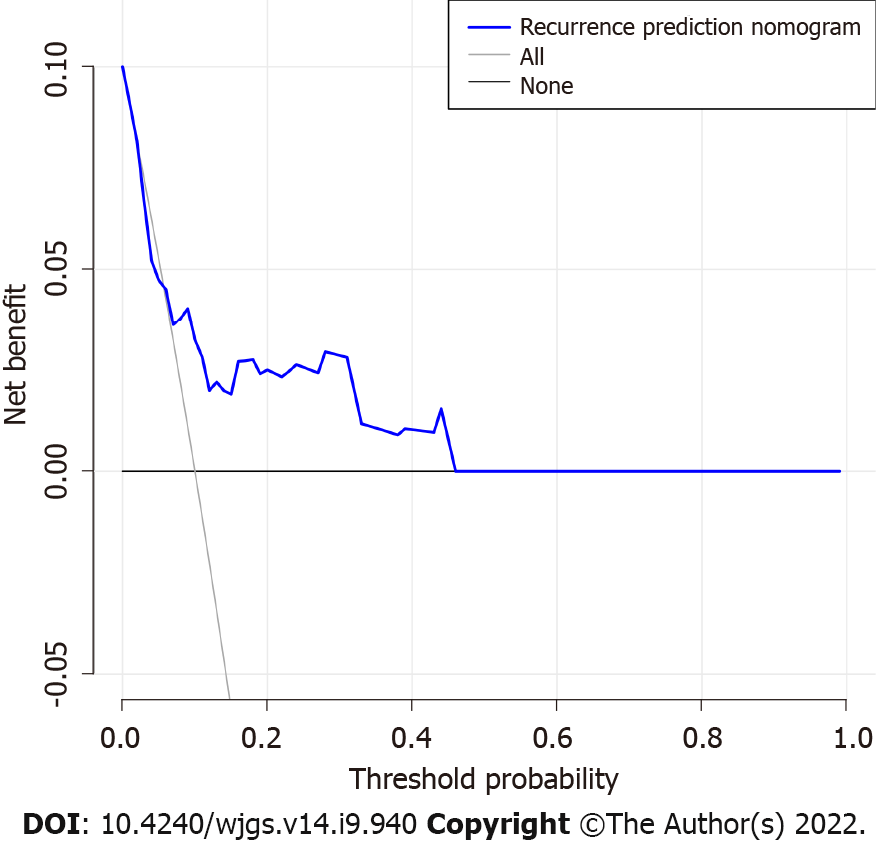
The decision curve analysis for the GIST recurrence risk nomogram showed that if the threshold probability of a patient and a doctor is > 5 and < 100%, respectively, using this recurrence nomogram to predict recurrence risk adds more benefit than the scheme (Figure 4). As the threshold probability increases, the predictive power will not increase. In this range, according to the risk of recurrence nomogram, the net benefit is comparable to several overlaps.
The global incidence of GIST is approximately 11.0-14.5/1 million[18]. Although it is rare compared with other tumors in the digestive tract, China has a large population base, so a considerable number of patients are diagnosed with GISTs every year. In clinical work, an increasing number of patients with GIST have been diagnosed and treated, and the number should not be underestimated. Although the use of small molecule targeted drugs such as imatinib has significantly improved the prognosis of patients with moderate and high-risk GISTs, there is still tumor recurrence or metastasis during or after adjuvant therapy[19]. Therefore, accurate assessment of the factors affecting the recurrence of GIST in patients is essential for guiding the individualized treatment of patients.
Four staging systems are commonly used for GIST. At present, the classification of different staging systems is mainly based on the following three influencing factors: the size of the tumor, the mitotic rate, and the location of the tumor. However, none of these systems were specifically developed for postoperative prognosis predictions. Similarly, it is not possible to quantify the risk of recurrence among individual patients. Currently, nomograms are widely used in prognostic studies in oncology and medicine. To predict the prognosis of certain cancers, some researchers have developed more accurate scales than conventional staging systems[20,21]. Therefore, the aim of the study was to establish a recurrence risk nomogram for patients with GIST to achieve higher accuracy and predictions that are easier to understand to help better clinical decision-making and maximize patient benefit.
We developed and validated a new predictive tool that uses six easily available variables to predict recurrence risk after radical surgery in patients with GIST. Incorporating general information and risk factors for clinicopathological features into an easy-to-use nomogram can help individualize the prediction of the recurrence of GIST. Nomograms are based on statistical models that use a combination of prognostic variables to determine the likelihood of a particular event and perform well in predicting postoperative recurrence. The predictions are supported by a C-index of 0.836 (95%CI: 0.712-0.960) and a calibration curve. The C-index, an internal verification method, in this study cohort was 0.714, showing good discrimination and calibration ability. Our high C-index in all cohort verifications indicates that this nomogram can be widely and accurately used due to its large sample size. This study provides a relatively accurate predictive tool for postoperative recurrence in patients with GIST. Each postoperative patient was scored according to the nomogram. The higher the score, the higher the probability of postoperative recurrence and the higher the follow-up frequency.
GISTs can occur in any part of the digestive tract or in the omentum, mesentery, peritoneum, and abdominal pelvic cavity, but the stomach (approximately 60%) is the most common, followed by the small intestine (25% to 30%), while a few cases occur in the colorectal (approximately 5%), esophagus and other areas[22]. The results of this group of cases show that the stomach and small intestine are the most common sites of GISTs, similar to previous research reports. Tumors in different parts have large differences in their malignancy and prognosis. For GISTs, the location of tumor growth is also an extremely important prognostic factor. A retrospective study of 332 patients with GIST showed that the tumors with good prognosis were the esophagus, stomach, duodenum, small intestine, parenteral and colorectal[23]. We screened tumor sites for potential predictors of postoperative recurrence using LASSO regression analysis, and further differences in tumor location were found in the multivariate logistic regression analysis (P < 0.003). In this study, nomograms showed that tumors in the colorectal region had the highest risk of postoperative recurrence, followed by the small intestine, and finally the stomach region. Studies have shown that the prognosis of gastric stromal tumors is significantly better than that of small intestinal stromal tumors, which is mainly due to the invasive growth of small intestinal stromal tumors, often with early peritoneal metastasis, and the ease with which they rupture; therefore, duodenal stromal tumors should be actively treated as soon as possible[23]. With larger tumors, preoperative treatment should first be considered, and the rate of pancreaticoduodenectomy should be minimized. The degree of malignancy of colorectal stromal tumors is higher than that of small intestine and gastric stromal tumors[24], and the risk of recurrence is the highest. GISTs generally occur most frequently in middle-aged and elderly people, and the most common onset is between 50 and 70 years old[25]. In this study, the mean age was 57.0 ± 11.8 years, and 71.5% of patients were aged 50 years or older. There was no difference based on sex, which was consistent with the above study reports.
At present, the influence of mitotic rate and tumor size on the prognosis of GIST has been generally recognized, and multiple staging systems have been applied to the risk assessment of recurrence after GIST. It has been reported in a study that univariate survival analysis showed that the factors that had a significant impact on prognosis were the primary site of the tumor, tumor diameter and the mitotic rate (P < 0.05)[26]. Multivariate survival analysis showed that the mitotic rate is an independent prognostic factor for patients with GIST metastasis or recurrence. Catena et al[27] showed that tumor size, mitotic rate, and microscopic resection margins predicted disease-free survival in GIST patients. In general, the larger the tumor size is, the higher the malignant biological behavior, and the relatively poor the prognosis. The prognosis of patients with GIST is closely related to the mitotic rate, and those with a high mitotic rate often show a worse prognosis[28]. The high mitotic rate and larger lesion range in this study significantly increased the risk of recurrence after GIST, consistent with most studies.
In recent years, with the development of immunohistochemistry technology, we often use tumor immunohistochemical markers for tumor prognosis analysis. Ki-67 is a nuclear antigen expressed in proliferating cells, and its antibody marks proliferating cells in the non-G0 phase of the whole cell cycle, so it can be used as a marker of cell proliferation. In breast cancer, Ki-67 positivity has been shown to be negatively correlated with disease-free survival and overall survival[28]. It has been reported[29] that the expression level of Ki-67 is important for judging the malignant degree of GIST. By analyzing the correlation between immunohistochemical markers and prognosis in GIST samples, Kadado et al[30] showed that there was a statistically significant difference in the Ki-67 proliferation index between localized GIST and patients with recurrence and metastasis (P < 0.001). The nomograms in this study showed that Ki-67 ≥ 5 increased the risk of recurrence after GIST, consistent with the results of the above studies. It is suggested that Ki-67 can be used as an important factor to evaluate the recurrence or metastasis of GIST. In addition, for patients treated with imatinib before surgery, due to tumor liquefaction necrosis, the capsule is prone to spontaneous rupture, resulting in tumor cell dissemination, postoperative recurrence or distant metastasis. The 5-year recurrence-free survival rate of tumor necrosis was significantly lower than that of nonnecrotic rupture (P < 0.016), and the risk of death in the former was 2.79-3.03 times that of the latter[28]. Clinically, some patients with GISTs often have necrosis of the lesion at the beginning of diagnosis, which may be associated with metastasis of the abdomen and liver. Distant metastasis is one of the important factors affecting the prognosis of GIST. Patients with distant metastasis or local infiltration metastasis are more aggressive, although the prognosis is still poor after combined resection of the metastatic lesions. This is consistent with the fact that nomogram tumor intratumoral necrosis in this study can increase the risk of recurrence after GIST. Therefore, tumor necrosis may also be an important factor in predicting prognosis.
The occurrence, development and prognosis of tumors are the result of a multifactor interaction. It is generally believed that the biological behavior of GIST is the most important factor in determining its prognosis. At present, among the influencing factors of GIST prognosis, it is most common to consider the tumor location, size, and mitotic rate. The prediction model developed in this study also includes Ki-67, tumor intratumoral necrosis and age-related indicators. Comprehensive assessment of patient outcomes will assist in guiding individualized treatment.
There are many staging systems for gastrointestinal stromal tumors (GISTs), and the risk indicators selected are also different; thus, it is not possible to quantify the risk of recurrence among individual patients.
To develop a nomogram of postoperative recurrence risk factors in GIST patients to further guide individualized treatment.
To investigate the risk factors for postoperative recurrence in GIST patients.
We retrospectively analyzed the clinical and pathological data of 130 patients with GIST. The least absolute shrinkage and selection operator regression model and multivariable logistic regression analysis were used to develop a prediction model. The index of concordance (C-index), calibration curve, receiver operating characteristic curve, and decision curve analysis were used to assess the discrimination, calibration, and clinical usefulness of the predictive model.
The nomogram included tumor site, lesion size, mitotic rate/50 high power fields, Ki-67 index, intracranial necrosis, and age as predictors. The model presented a perfect discrimination with a reliable C-index. The receiver operating characteristic curve indicated a good predictive value. Decision curve analysis showed that the predicting recurrence nomogram was clinically feasible.
This recurrence nomogram combines tumor site, lesion size, mitotic rate, Ki-67 index, intracranial necrosis, and age and can easily predict patient prognosis.
We look forward to conducting a multicenter large-sample prospective controlled study in the future to further explore risk factors after GIST surgery, to better guide individualized treatment.
| 1. | Wang MX, Devine C, Segaran N, Ganeshan D. Current update on molecular cytogenetics, diagnosis and management of gastrointestinal stromal tumors. World J Gastroenterol. 2021;27:7125-7133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Tan AD, Willemsma K, MacNeill A, DeVries K, Srikanthan A, McGahan C, Hamilton T, Li H, Blanke CD, Simmons CE. Tyrosine kinase inhibitors significantly improved survival outcomes in patients with metastatic gastrointestinal stromal tumour: a multi-institutional cohort study. Curr Oncol. 2020;27:e276-e282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 4. | Bae JH, Lee CS, Han SR, Park SM, Lee YS, Lee IK. Differences in the prognostic impact of post-operative systemic inflammation and infection in colorectal cancer patients: Using white blood cell counts and procalcitonin levels. Surg Oncol. 2020;35:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 5. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 897] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 6. | Kallen ME, Hornick JL. The 2020 WHO Classification: What's New in Soft Tissue Tumor Pathology? Am J Surg Pathol. 2021;45:e1-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 7. | Zhao WY, Zhao G, Wang M. [Updates and interpretations of the NCCN Clinical Practice Guidelines (2019 6th version) on gastrointestinal stromal tumor]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Khoo CY, Chai X, Quek R, Teo MCC, Goh BKP. Systematic review of current prognostication systems for primary gastrointestinal stromal tumors. Eur J Surg Oncol. 2018;44:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 688] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Jiang L, Zhu X. A Novel Nomogram for Prediction of Early Postoperative Complications of Total Gastrectomy for Gastric Cancer. Cancer Manag Res. 2021;13:7579-7591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Wang Z, Li H, Liu T, Sun Z, Yang F, Jiang G. Development and External Validation of a Nomogram for Predicting Cancer-Specific Survival of Non-Small Cell Lung Cancer Patients With Ipsilateral Pleural Dissemination. Front Oncol. 2021;11:645486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33:1-22. [PubMed] |
| 13. | Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512-5528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 859] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 14. | Kidd AC, McGettrick M, Tsim S, Halligan DL, Bylesjo M, Blyth KG. Survival prediction in mesothelioma using a scalable Lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. 2018;5:e000240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1192] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 16. | Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 1041] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 17. | Li M, Zhang J, Dan Y, Yao Y, Dai W, Cai G, Yang G, Tong T. A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J Transl Med. 2020;18:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Mantese G. Gastrointestinal stromal tumor: epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol. 2019;35:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 19. | Yin XN, Tang SM, Yin Y, Shen CY, Zhang B, Chen ZX. [Associations of Preoperative Platelet-to-lymphocyte Ratio and Derived Neutrophil-to-lymphocyte Ratio with thePrognosis of Gastrointestinal Stromal Tumor]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:239-243. [PubMed] |
| 20. | Lou S, Meng F, Yin X, Zhang Y, Han B, Xue Y. Comprehensive Characterization of RNA Processing Factors in Gastric Cancer Identifies a Prognostic Signature for Predicting Clinical Outcomes and Therapeutic Responses. Front Immunol. 2021;12:719628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Cao R, Yuan L, Ma B, Wang G, Qiu W, Tian Y. An EMT-related gene signature for the prognosis of human bladder cancer. J Cell Mol Med. 2020;24:605-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 22. | Sugiyama Y, Sasaki M, Kouyama M, Tazaki T, Takahashi S, Nakamitsu A. Current treatment strategies and future perspectives for gastrointestinal stromal tumors. World J Gastrointest Pathophysiol. 2022;13:15-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Qi Y, Zhao W, Wang Z, Li T, Meng X. Tumor sites and microscopic indicators are independent prognosis predictors of gastrointestinal stromal tumors. Tohoku J Exp Med. 2014;233:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Roşulescu A, Pechianu N, Hortopan M, Mihai M, Dima S, Stroescu C, Zamfir R, Braşoveanu V, Leonard D, Vasilescu C, Popescu I, Herlea V. Gastrointestinal stromal tumors of the colon and rectum. Pol J Pathol. 2020;71:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Serrano C, George S. Gastrointestinal Stromal Tumor: Challenges and Opportunities for a New Decade. Clin Cancer Res. 2020;26:5078-5085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 26. | Zemła P, Stelmach A, Jabłońska B, Gołka D, Mrowiec S. A Retrospective Study of Postoperative Outcomes in 98 Patients Diagnosed with Gastrointestinal Stromal Tumor (GIST) of the Upper, Middle, and Lower Gastrointestinal Tract Between 2009 and 2019 at a Single Center in Poland. Med Sci Monit. 2021;27:e932809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Catena F, Di Battista M, Ansaloni L, Pantaleo M, Fusaroli P, Di Scioscio V, Santini D, Nannini M, Saponara M, Ponti G, Persiani R, Delrio P, Coccolini F, Di Saverio S, Biasco G, Lazzareschi D, Pinna A; GISTologist Study Group. Microscopic margins of resection influence primary gastrointestinal stromal tumor survival. Onkologie. 2012;35:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Cavnar MJ, Seier K, Curtin C, Balachandran VP, Coit DG, Yoon SS, Crago AM, Strong VE, Tap WD, Gönen M, Antonescu CR, Brennan MF, Singer S, DeMatteo RP. Outcome of 1000 Patients With Gastrointestinal Stromal Tumor (GIST) Treated by Surgery in the Pre- and Post-imatinib Eras. Ann Surg. 2021;273:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Zhu X, Chen L, Huang B, Wang Y, Ji L, Wu J, Di G, Liu G, Yu K, Shao Z, Wang Z. The prognostic and predictive potential of Ki-67 in triple-negative breast cancer. Sci Rep. 2020;10:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 30. | Kadado KJ, Abernathy OL, Salyers WJ, Kallail KJ. Gastrointestinal Stromal Tumor and Ki-67 as a Prognostic Indicator. Cureus. 2022;14:e20868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chetty R, United Kingdom; Fusaroli P, Italy S-Editor: Zhang H L-Editor: A P-Editor: Zhang H