Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.904
Peer-review started: March 3, 2022
First decision: April 19, 2022
Revised: April 28, 2022
Accepted: August 26, 2022
Article in press: August 26, 2022
Published online: September 27, 2022
Processing time: 203 Days and 4.5 Hours
Neoadjuvant chemotherapy (NC) improves the survival outcomes of selected patients with colorectal liver metastasis (CRLM). The benefits of irinotecan-based regimens in these patients are still under debate.
To compare the benefits of irinotecan- and oxaliplatin-based regimens in patients with resectable CRLM.
From September 2003 to August 2020, 554 patients received NC and underwent hepatectomy for CRLM. Based on a 1:1 propensity score matching (PSM) model, 175 patients who received irinotecan were matched to 175 patients who received oxaliplatin to obtain two balanced groups regarding demographic, therapeutic, and prognostic characteristics.
Chemotherapy was based on oxaliplatin in 353 (63.7%) patients and irinotecan in 201 (36.3%). After PSM, the 5-year progression-free survival (PFS) and overall survival (OS) rates with irinotecan were 18.0% and 49.7%, respectively, while the 5-year PFS and OS rates with oxaliplatin were 26.0% and 46.8%, respectively. Intraoperative blood loss, operating time, and postoperative complications dif
In NC in patients with CRLM, irinotecan is similar to oxaliplatin in survival outcomes, but irinotecan is superior regarding operating time, intraoperative blood loss, and postoperative complications.
Core Tip: This was the first retrospective cohort study to investigate irinotecan-based regimens for neoadjuvant chemotherapy in patients with colorectal liver metastasis (CRLM) in China. It highlighted the benefits of irinotecan and might contribute to modifying the treatment guidelines for CRLM. Che
- Citation: Liu W, Chen FL, Wang K, Bao Q, Wang HW, Jin KM, Xing BC. Irinotecan- vs oxaliplatin-based regimens for neoadjuvant chemotherapy in colorectal liver metastasis patients: A retrospective study. World J Gastrointest Surg 2022; 14(9): 904-917
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/904.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.904
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related mortality[1]. The liver is the most common site of metastatic involvement, and 25%-30% of CRC patients present with metastatic diseases initially. The long-term survival outcome has been sig
Currently, the administration of neoadjuvant chemotherapy (NC) in resectable colorectal liver metastasis (CRLM) patients is increasing as it can increase the radical resection rate and treat occult metastases[5]. 5-Fluorouracil (5-Fu) was previously one of the most common anticancer drugs for CRLM. FOLFIRI (irinotecan, 5-Fu, and leucovorin) and FOLFOX (oxaliplatin, 5-Fu, and leucovorin) regimens have been proven more effective. By combining with antibodies targeting epidermal growth factor receptor and vascular endothelial growth factor, a response rate of about 20% observed in the new era of modern chemotherapy has been greatly increased. Nevertheless, it has been shown that systemic chemotherapy for CRLM might cause injury to the nontumoral liver parenchyma. Sinusoidal obstruction syndrome (SOS) has been identified as being a complication to oxaliplatin-based che
For resectable CRLM, oxaliplatin-based regimens have been preferred to irinotecan-based regimens as the first-line treatment because of less alopecia and gastrointestinal toxicity[8]. Irinotecan has been administered to patients with resectable CRLM, but supporting evidence is absent, and whether survival outcomes are improved remains under debated. The present study investigated whether irinotecan might improve progression-free survival (PFS) or OS in patients with resectable CRLM.
This study collected the data from CRLM patients who received NC and underwent hepatic resection between September 2003 and August 2020 at the Hepatopancreatobiliary Surgery Department of Peking University Cancer Hospital. The demographic and clinical data were retrospectively obtained from a prospective patient database. The inclusion criteria were: (1) Evaluated to be resectable by a multidisciplinary team (MDT) that consisted of surgical oncologists, radiologists, and medical oncologists; (2) Received NC and underwent hepatic resection; (3) No other simultaneous malignancies; (4) 19-80 years of age; and (5) Eastern Cooperative Oncology Group performance status < 2. Patients who underwent only ablation or palliative hepatic resection (R2) were excluded. This study was approved by the Ethics Committee of Beijing Cancer Hospital (No. 2021YJZ06-GZ01), and the requirement for informed consent was waived.
All patients were evaluated by physical examination, routine hematology, biochemistry analyses, and measurement of levels of tumor markers including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (Ca19-9) before treatment. According to standard clinical protocols, computed tomography or magnetic resonance imaging of the abdomen and chest was performed for preoperative staging and evaluation of liver metastasis. In addition, positron emission tomography was performed to rule out any extrahepatic metastasis.
The NC regimens consisted mainly of 5-Fu, leucovorin, and oxaliplatin, or 5-Fu, leucovorin, and irinotecan, with or without bevacizumab or cetuximab. There were 353 patients who received a regimen based on oxaliplatin and 201 patients who were treated with a regimen based on irinotecan. Based on World Health Organization criteria, the response to NC was classified according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). MDT discussion assessed the treatment response and the possibility of surgery. If the patient presented with disease progression, a new second-line chemotherapy regimen was recommended.
In surgical treatment, the technical criteria for resectability related to the liver remnant after resection were: (1) Preserving two contiguous segments; (2) Preserving adequate vascular inflow, outflow, and biliary drainage; and (3) Preserving adequate future liver remnant volume (30% in normal liver and 40% in patients with preoperative chemotherapy)[9]. Major hepatic resection was defined to be any resection of three or more segments. All the patients underwent hepatic resection and primary tumor resection. All the specimens were examined for pathological diagnosis after surgery.
The continuous variables are expressed using median and range, and the categorical variables are expressed as number (n) and frequency (%). The c2 or Fisher’s exact test was used to compare categorical variables between groups, while the Mann-Whitney U test was afforded to compare the continuous variables between groups. Propensity score matching (PSM) was applied to compensate for the biases between the irinotecan and the oxaliplatin groups in the unmatched cohort with a matching ratio of 1:1 by the nearest neighbor method. The caliper value was set at 0.05. The imbalance before and after PSM was assessed by the standardized mean difference. The following variables were included in the PSM model: Age, sex, primary N stage, number of liver metastases, preoperative CEA/Ca19-9, preoperative clinical risk score (CRS) as proposed by Fong et al[10], RAS mutation status, cycles of NC, major hepatic resection, intraoperative radiofrequency ablation combined with hepatic resection, adjuvant chemotherapy, and response to NC. Short-term results were compared between the irinotecan and oxaliplatin groups before and after PSM, such as intraoperative blood loss, intraoperative red blood cell (RBC) transfusion, operating time, and Clavien-Dindo grade of general or surgical complications. PFS was defined as the time from treatment to recurrence, disease progression, or death, whichever occurred first[11]. OS was defined as the interval between hepatic resection and the date of death or last follow-up. Kaplan-Meier survival analysis was performed to compare the PFS and OS before and after PSM using the log-rank test. Uni- and multivariable analyses were conducted with Cox proportional hazards model to identify the independent prognostic factors for PFS after PSM. Significance level was set at 0.05, and SPSS version 23 was used for statistical analyses (IBM, Armonk, NY, United States).
We enrolled a total of 554 CRLM patients, with 201 in the irinotecan group and 353 in the oxaliplatin group. Primary N stage, timing of liver metastases, biological agent, staged resection, and operating time were significantly different between the two groups (P < 0.05) (Table 1).
| Patient demographic | All patients (n = 554) | Irinotecan group (n = 201) | Oxaliplatin group (n = 353) | P value |
| Age (yr) | 57.1 ± 9.5 | 56.1 ± 9.6 | 57.7 ± 9.4 | 0.056 |
| Sex ration (male:female) | 193:361 | 62:139 | 131:222 | 0.137 |
| Primary T stage | 0.736 | |||
| T1-2 | 64 | 22 | 42 | |
| T3-4 | 490 | 179 | 311 | |
| Primary N stage | 0.036 | |||
| N0 | 191 | 58 | 133 | |
| N1-2 | 363 | 143 | 220 | |
| Primary tumor location | 0.613 | |||
| Colon | 322 | 114 | 208 | |
| Rectum | 232 | 87 | 145 | |
| Primary tumor side | 0.839 | |||
| Right | 75 | 28 | 47 | |
| Left | 479 | 173 | 306 | |
| Timing of liver metastasis | < 0.001 | |||
| Synchronous | 482 | 157 | 325 | |
| Metachronous | 72 | 44 | 28 | |
| Tumor number (median) | 3 (1-10) | 3 (1-9) | 3 (1-10) | 0.706 |
| Tumor size (mm, mean ± SD) | 27.6 ± 18.2 | 26.78 ± 17.2 | 29.0 ± 17.8 | 0.160 |
| Localization of liver metastases | 0.250 | |||
| Unilobar | 226 | 90 | 176 | |
| Bilobar | 288 | 111 | 177 | |
| CEA level (ng/mL) | 31.44 ± 85.3 | 24.93 ± 54.1 | 35.17 ± 98.65 | 0.175 |
| CA 19-9 level (IU/mL) | 215.4 ± 877.9 | 194.8 ± 232.8 | 227.4 ± 185.4 | 0.847 |
| Extrahepatic metastasis | 0.572 | |||
| No | 462 | 170 | 292 | |
| Yes | 92 | 31 | 61 | |
| RAS mutation | 0.174 | |||
| Wildtype | 332 | 128 | 204 | |
| Mutation | 222 | 73 | 149 | |
| Biological agent | < 0.001 | |||
| Cetuximab | 118 | 57 | 61 | |
| Bevacizumab | 187 | 97 | 90 | |
| No | 249 | 47 | 202 | |
| Response | 0.209 | |||
| Complete response | 5 | 0 | 5 | |
| Partial response | 217 | 81 | 136 | |
| Stable disease | 301 | 112 | 189 | |
| Progressive disease | 31 | 8 | 23 | |
| Cycles | 4 (1-16) | 4 (1-12) | 4 (1-16) | 0.430 |
| Concomitant ablation therapy | 91 | 39 | 52 | 0.154 |
| CRS | ||||
| 0-2 | 274 | 95 | 179 | |
| 3-5 | 280 | 106 | 174 | |
| Resection | 0.002 | |||
| Simultaneous resection | 145 | 41 | 104 | |
| Staged resection | 409 | 160 | 249 | |
| Intraoperative blood loss (mL) | 213 ± 198 | 204 ± 172 | 218 ± 212 | 0.437 |
| Intraoperative RBC transfusion | 24 | 10 | 14 | 0.289 |
| Intraoperative RBC transfusion (U) | 2 (1-12) | 2 (1-6) | 4 (2-12) | 0.026 |
| Operating time (min) | 199 ± 74 | 190 ± 72 | 204 ± 76 | 0.039 |
| Hepatic resection | 0.357 | |||
| Major resection | 123 | 49 | 74 | |
| Minor resection | 431 | 152 | 279 | |
| Margin status | 0.308 | |||
| Positive | 72 | 30 | 42 | |
| Negative | 482 | 171 | 311 | |
| Clavien-Dindo classification | 0.057 | |||
| I-II | 164 | 53 | 111 | |
| II-V | 32 | 7 | 25 | |
| Adjuvant chemotherapy | 0.153 | |||
| No | 132 | 41 | 91 | |
| Yes | 422 | 160 | 262 |
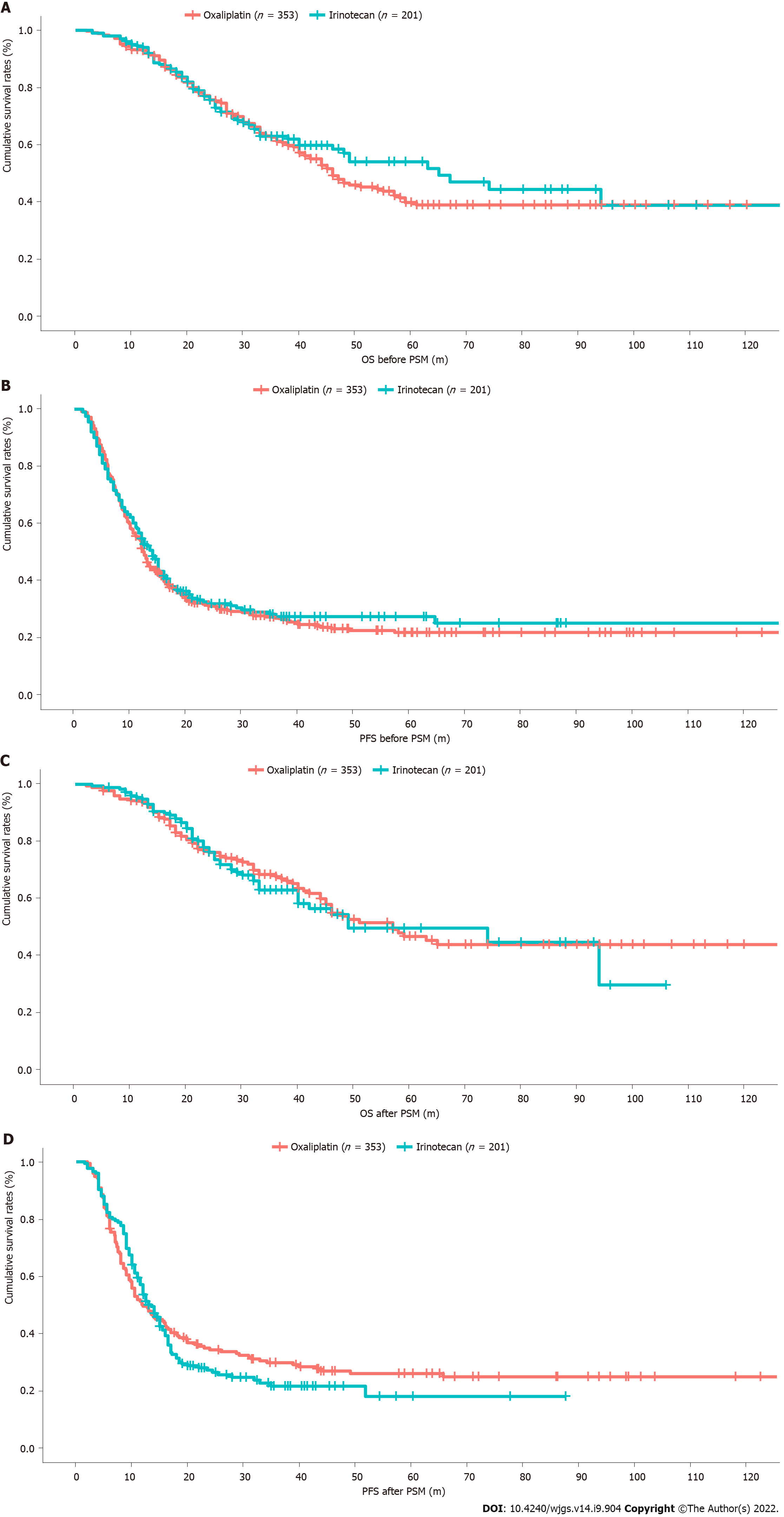
The median follow-up was 41 mo. The intrahepatic and extrahepatic recurrence rates were not significantly different between the irinotecan and oxaliplatin groups. There were no significant differences in 1-, 3-, or 5-year PFS and OS rates (P > 0.05; Figures 1A and 1B). In the irinotecan group, the median PFS was 14.0 mo and the 5-year PFS was 25.2%. The median OS was 65 mo and 5-year OS rates was 54.0%. In the oxaliplatin group, the median PFS was 12.5 mo and 5-year PFS was 22.0%. The median OS was 46 mo and 5-year OS was 39.8%.
After PSM for the significantly different preoperative and prognostic factors between the two groups, 175 patients from the irinotecan group and 175 from the oxaliplatin group were considered for the matched analyses. When the biases associated with the differences in primary N stage, timing of liver metastases, biological agent, staged resection, intraoperative RBC transfusion, and operating time were removed by PSM, differences in intraoperative blood loss, operating time, and postoperative complications were observed (Table 2).
| Patient demographic | All patients (n = 350) | Irinotecan group (n = 175) | Oxaliplatin group (n = 175) | P value |
| Age (yr) | 56.0 ± 4.2 | 56.2 ± 9.6 | 55.7 ± 10.1 | 0.632 |
| Sex ration (male:female) | 230:120 | 121:54 | 109:66 | 0.177 |
| Primary T stage | 0.433 | |||
| T1-2 | 47 | 21 | 26 | |
| T3-4 | 303 | 154 | 149 | |
| Primary N stage | 0.526 | |||
| N0 | 104 | 51 | 53 | |
| N1-2 | 246 | 125 | 121 | |
| Primary tumor location | 0.756 | |||
| Colon | 205 | 101 | 104 | |
| Rectum | 145 | 74 | 71 | |
| Primary tumor side | 0.745 | |||
| Right | 48 | 25 | 23 | |
| Left | 302 | 150 | 152 | |
| Timing of liver metastasis | 0.077 | |||
| Synchronous | 283 | 135 | 148 | |
| Metachronous | 67 | 40 | 27 | |
| Tumor number (median) | 2 (1-25) | 2 (1-25) | 2 (1-22) | 0.422 |
| Tumor size (mm, mean ± SD) | 28.8 ± 18.9 | 29.2 ± 20.3 | 28.4 ± 17.5 | 0.681 |
| Localization of liver metastases | 0.493 | |||
| Unilobar | 190 | 98 | 92 | |
| Bilobar | 160 | 77 | 83 | |
| CEA level (ng/mL) | 27.81 ± 64.87 | 24.26 ± 55.81 | 31.36 ± 72.81 | 0.307 |
| CA 19-9 level (IU/mL) | 228.71 ± 203.76 | 212.92 ± 145.70 | 244.51 ± 266.39 | 0.894 |
| Extrahepatic metastasis | 0.311 | |||
| No | 293 | 150 | 143 | |
| Yes | 57 | 25 | 32 | |
| RAS mutation | 0.912 | |||
| Wild type | 221 | 111 | 110 | |
| Mutation | 129 | 64 | 65 | |
| Biological agent | 0.169 | |||
| Cetuximab | 100 | 53 | 47 | |
| Bevacizumab | 167 | 88 | 79 | |
| No | 83 | 34 | 49 | |
| Response | 0.176 | |||
| Complete response | 1 | 0 | 1 | |
| Partial response | 144 | 70 | 74 | |
| Stable disease | 183 | 98 | 85 | |
| Progressive disease | 22 | 7 | 15 | |
| Cycles | 4 (0-10) | 4 (0-10) | 4 (0-10) | 0.948 |
| Concomitant ablation therapy | 66 | 36 | 30 | 0.464 |
| CRS | 0.669 | |||
| 0-2 | 166 | 81 | 85 | |
| 3-5 | 184 | 94 | 90 | |
| Simultaneous resection | 88 | 39 | 49 | 0.443 |
| Staged resection | 262 | 136 | 126 | |
| Intraoperative blood loss (mL) | 222 ± 211 | 201 ± 181 | 264 ± 235 | 0.024 |
| Intraoperative RBC transfusion | 15 | 8 | 7 | 0.117 |
| Intraoperative RBC transfusion (U) | 2 (1-12) | 2 (1-6) | 2 (2-6) | 0.281 |
| Operation time (min) | 198 ± 73 | 188 ± 73 | 208 ± 72 | 0.012 |
| Hepatic resection | 0.886 | |||
| Major resection | 90 | 42 | 45 | |
| Minor resection | 260 | 133 | 130 | |
| Margin status | 0.367 | |||
| Positive | 32 | 17 | 15 | |
| Negative | 318 | 158 | 160 | |
| Clavien-Dindo classification | 0.019 | |||
| I-II | 102 | 43 | 59 | |
| III-V | 22 | 7 | 15 | |
| Adjuvant chemotherapy | 0.352 | |||
| No | 132 | 41 | 91 | |
| Yes | 422 | 160 | 262 |
The median follow-up was 42 mo. The 1-, 3-, and 5-year OS rates were higher in the irinotecan group than in the oxaliplatin group, while the reverse trend was observed for PFS, but the differences were not significant (P > 0.05; Figures 1C and 1D). In the irinotecan group, the 5-year PFS and OS rates were 18.0% and 49.7%, respectively, and the median PFS and OS were 13.5 and 49 mo, respectively. In the oxaliplatin group, the 5-year PFS and OS rates were 26.0% and 46.8%, respectively, and the median PFS and OS were 12.0 and 57 mo, respectively.
Multivariable Cox regression analysis was performed for the PSM cohort. In the univariate analysis, primary tumor location, synchronous liver metastases, tumor size > 5 cm, tumor number > 1, CRS 3-5, concomitant ablation, bilobar distribution, CA 19-9 > 100 U/mL, RAS mutation, and response rate were associated with PFS (P < 0.05) (Table 3). In the multivariate analysis, tumor size > 5 cm, tumor number > 1, RAS mutation, CA 19-9 > 100 U/mL, and response rate to NC were independently associated with PFS (P < 0.05).
| Variable | Univariable analysis | Multivariable analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| > 60 | Ref | |||||
| ≤ 60 | 0.878 | 0.682-1.131 | 0.314 | |||
| Gender | ||||||
| Male | Ref | |||||
| Female | 0.949 | 0.733-1.230 | 0.694 | |||
| Primary T stage | ||||||
| 1-2 | Ref | |||||
| 3-4 | 1.183 | 0.820-1.706 | 0.369 | |||
| Primary N stage | ||||||
| N0 | Ref | |||||
| N1-2 | 1.090 | 0.952-1.248 | 0.212 | |||
| Location tumor | ||||||
| Colon | Ref | |||||
| Rectum | 0.869 | 0.676-1.116 | 0.270 | |||
| Primary tumor location | ||||||
| Left | Ref | Ref | ||||
| Right | 1.508 | 1.072-2.121 | 0.018 | 1.413 | 0.991-2.015 | 0.056 |
| Disease-free interval | ||||||
| > 12 mo | Ref | Ref | ||||
| ≤ 12 mo | 1.487 | 1.068-2.071 | 0.019 | 1.156 | 0.788-1.696 | 0.459 |
| CEA | ||||||
| ≤ 200 | Ref | |||||
| > 200 | 1.340 | 0.689-2.607 | 0.388 | |||
| CA 19-9 | ||||||
| ≤ 100 | Ref | Ref | ||||
| > 100 | 1.528 | 1.077-2.167 | 0.017 | 1.521 | 1.032-2.241 | 0.034 |
| Tumor size | ||||||
| ≤ 5 cm | Ref | Ref | ||||
| > 5 cm | 1.149 | 1.019-1.554 | 0.028 | 1.479 | 1.062-2.060 | 0.021 |
| Tumor no. | ||||||
| ≤ 1 | Ref | Ref | ||||
| > 1 | 1.702 | 1.284-2.255 | 0.000 | 1.446 | 1.077-2.146 | 0.014 |
| CRS | ||||||
| 0-2 | Ref | Ref | ||||
| 3-5 | 1.665 | 1.298-2.135 | 0.000 | 1.256 | 0.894-1.765 | 0.189 |
| RAS status | ||||||
| Wild | Ref | Ref | ||||
| Mutation | 1.641 | 1.276-2.110 | 0.000 | 1.468 | 1.127-1.913 | 0.004 |
| Extrahepatic metastases | ||||||
| No | Ref | |||||
| Yes | 1.081 | 0.781-1.496 | 0.638 | |||
| Biological agent | ||||||
| Cetuximab | ||||||
| Bevacizumab | Ref | |||||
| No | 1.057 | 0.910-1.228 | 0.469 | |||
| Response | ||||||
| Complete response | ||||||
| Partial response | ||||||
| Stable disease | Ref | Ref | ||||
| Progressive disease | 1.564 | 1.067-2.292 | 0.022 | 1.830 | 1.211-2.764 | 0.004 |
| Hepatic resection | ||||||
| Minor | Ref | |||||
| Major | 0.997 | 0.753-1.320 | 0.984 | |||
| Concomitant ablation | ||||||
| No | Ref | Ref | ||||
| Yes | 1.634 | 1.195-2.236 | 0.002 | 1.002 | 0.641-1.568 | 0.992 |
| Stage resection | ||||||
| No | Ref | |||||
| Yes | 0.839 | 0.682-1.033 | 0.098 | |||
| Margin status | ||||||
| R0 | Ref | |||||
| R1 | 0.878 | 0.581-1.327 | 0.537 | |||
| Distribution | ||||||
| Unilobar | Ref | Ref | ||||
| Bilobar | 1.277 | 1.067-1.528 | 0.008 | 1.112 | 0.875-1.413 | 0.385 |
| Extrahepatic metastases | ||||||
| Yes | Ref | |||||
| No | 1.081 | 0.781-1.496 | 0.638 | |||
| Adjuvant chemotherapy | ||||||
| No | Ref | |||||
| Yes | 0.885 | 0.654-1.198 | 0.430 | |||
| Clavien-Dino classification | ||||||
| I-II | Ref | |||||
| III-V | 1.018 | 0.833-1.244 | 0.859 | |||
| RBC transfusion | ||||||
| Yes | Ref | |||||
| No | 0.857 | 0.456-1.614 | 0.634 | |||
Compared with 5-Fu alone, irinotecan-based preoperative chemotherapy increased the response rates up to 39%[12], and oxaliplatin improved the response rate from 22% to 51%[13]. With newly developed biological agents, further significant benefits were achieved. Almost 60% of populations were evaluated to have tumor response by combining oxaliplatin-based or irinotecan-based chemotherapy with such targeted agents[14]. In the present study, the 5-year PFS and OS rates were 25.2% and 54.0% for the irinotecan group, respectively. In the oxaliplatin group, the 5-year PFS and OS rates were 22.0% and 39.8%, respectively. Our study was the first retrospective cohort analysis to compare the survival outcomes of irinotecan and oxaliplatin in patients with CRLM.
During the past few years, perioperative chemotherapy for CRLM has been developed remarkably. NC is recommended for resectable CRLM patients to increase the possibility of radical resections. It also might crush the occult metastasis in the liver remnant. Moreover, NC could test whether cancer cells are chemosensitive in situ. According to the responses mentioned above, physicians might determine the individualized adjuvant chemotherapy regimen and identify patients who would not benefit from immediate hepatic resection because of tumor progression. Nevertheless, it is still controversial whether NC should be applied for all patients with resectable CRLM. It was reported that a significant improvement in PFS was observed for resectable CRLM patients after NC with FOLFOX4 in the EORTC Intergroup Trial 40983. In contrast, 64% of CRLM patients achieved an objective radiological response after NC, and disease-free survival also improved significantly according to a systematic review of 23 studies comprising 3278 patients. In the present study, tumor size > 5 cm, tumor number > 1, RAS mutation, CA 19-9 > 100 U/mL, and response to NC were independent factors for PFS. This was consistent with previous studies. Hepatic resection is considered a standard treatment for CRLM patients, including special populations, such as those treated with hyperthermic intraperitoneal chemotherapy (HIPEC) and pregnant women[15,16]. HIPEC can be administered before or after surgery, and future studies should examine which HIPEC strategy, and combined with which chemotherapy regimen, would achieve better outcomes.
Oxaliplatin- and/or irinotecan-based NC might cause histological damage, vascular lesions, or steatohepatitis although there are conflicting results in the literature[6,7]. Chemotherapy-induced liver injury could reduce the function of the future remnant liver with an increase in postoperative complications[17]. Non-parenchymal-sparing strategies have been advocated for radical resection of CRLM and the outcomes associated with these strategies have been reported. Nakano et al[17] have reported that major hepatic resection for patients with CRLM with SOS might increase the risk of postoperative complications. Sinusoidal lesions have been associated with an increased blood requirement and higher postoperative liver failure[18,19].
Many studies have attempted to identify predictive factors for chemotherapy-induced liver damage[20]. It is reported that the following could induce SOS: High γ-glutaryl transferase levels, low platelet counts, high aspartate aminotransferase to platelet ratios, and enlarged spleen[21,22]. However, prospective studies are required to confirm the relevance of these factors, and a combination of parameters may provide evidence to establish a diagnosis of SOS preoperatively. Bevacizumab offers an opportunity to prevent SOS and reduces the incidence from 46% to 5% when added to preoperative chemotherapy[23]. It was hypothesized that endothelial cells might secret matrix metalloprotease-9 (MMP-9) and induce SOS in murine models. Bevacizumab might improve SOS by inhibiting vascular endothelial growth factor-dependent induction of MMP-9 and subsequent matrix degradation[24].
The present study had some limitations. First, it was a retrospective cohort study without randomizing for enrolled patients. Second, the included patients were limited after PSM. The sample size should be enlarged in a randomized controlled trial. Third, a validation group would strengthen the present conclusions.
In NC for CRLM, irinotecan is similar to oxaliplatin in improving the survival outcomes, but irinotecan is superior in reducing operating time, intraoperative blood loss, and postoperative complications.
Colorectal cancer (CRC) represents an important disease burden worldwide, being the third most common malignancy and the second leading cause of cancer mortality. Many patients are de novo metastatic at presentation, and liver metastasis is common in CRC. In selected patients with colorectal liver metastases (CRLM) (i.e., the liver as the only metastatic site), surgery can be performed directly, but some patients with resectable CRLM will require neoadjuvant chemotherapy (NC) to increase the radical resection rate and treat occult metastases. On the other hand, chemotherapy can cause liver injury that will lead to impaired remnant liver function.
For resectable CRLM, oxaliplatin-based regimens have been preferred to irinotecan-based regimens as the first-line treatment because of lower occurrences of alopecia and gastrointestinal toxicity. Irinotecan has been suggested for patients with resectable CRLM, but data for such patients are limited and whether outcomes are improved remains debatable. Therefore, even though NC improves the survival outcomes for selected patients with CRLM, the benefits of irinotecan-based regimens are still under debate.
This study investigated the benefits of irinotecan- vs oxaliplatin-based NC regimens in patients with resectable CRLM.
At a single hospital in China, 554 patients received NC and underwent hepatectomy for CRLM from September 2003 to August 2020. In order to manage confounding factors, a 1:1 propensity score matching (PSM) was performed. Overall survival (OS), progression-free survival (PFS), intraoperative blood loss, operation time, and postoperative complications were compared between the two groups.
In the present study, NC regimens were based on oxaliplatin in 353 (63.7%) patients and on irinotecan in 201 (36.3%). Finally, 175 patients who received irinotecan-based NC were matched to 175 who received oxaliplatin-based NC. Hence, the two groups were balanced regarding demographic, therapeutic, and prognostic characteristics. After PSM, the 5-year PFS rates were 18.0% for irinotecan-based NC and 26.0% for oxaliplatin-based NC, while the 5-year OS rates were 49.7% for irinotecan-based NC and 46.8% for oxaliplatin-based NC. Intraoperative blood loss (201 vs 264 mL, P = 0.024), operation time (188 vs 208 min, P = 0.012), and postoperative complications (28.6% vs 42.3%, P = 0.019) all favored the irinotecan-based NC group. In the multivariable analysis, carbohydrate antigen 19-9 [hazard ratio (HR) = 1.52, 95% confidence interval (CI): 1.03-2.24], RAS mutation (HR = 1.47, 95%CI: 1.13-1.91), response to NC (HR = 1.83, 95%CI: 1.21-2.76), tumor size > 5 cm (HR = 1.48, 95%CI: 1.06-2.06), and tumor number > 1 (HR = 1.45, 95%CI: 1.08-2.15) were independently associated with the PFS.
In patients with CRLM, the PFS and OS are similar between irinotecan- and oxaliplatin-based NC. On the other hand, irinotecan-based NC is superior to oxaliplatin-based NC in terms of shorter operation time, smaller intraoperative blood loss, and fewer postoperative complications.
This retrospective cohort analysis was the first to compare the OS and PFS of irinotecan-based NC vs oxaliplatin-based NC in patients with CRLM. Even though these results can help determine the best options for patients with CRLM, multicenter randomized controlled trials would be required for confirmation. In addition, future studies could examine different dosing strategies in patients with CRLM.
We acknowledge the help of Xiao-Luan Yan, who made substantial contributions to the acquisition of the data, and Li-Jun Wang, Da Xu, and Yan-Yan Wang, who made substantial contributions to the analysis and interpretation of the data. All these contributors were involved in drafting the manuscript but did not meet the criteria for authorship. We thank Pfizer Medical Teams’ support.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21462] [Article Influence: 1951.1] [Reference Citation Analysis (6)] |
| 2. | Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3:50-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 3. | Leung U, Gönen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, D'Angelica MI. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated With Resection. Ann Surg. 2017;265:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Breitenstein S, DeOliveira ML, Raptis DA, Slankamenac K, Kambakamba P, Nerl J, Clavien PA. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg. 2010;252:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Liu W, Zhang W, Xu Y, Li YH, Xing BC. A Prognostic Scoring System to Predict Survival Outcome of Resectable Colorectal Liver Metastases in this Modern Era. Ann Surg Oncol. 2021;28:7709-7718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, Mentha G, Terris B. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 773] [Article Influence: 35.1] [Reference Citation Analysis (1)] |
| 7. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, Risio M, Muratore A, Capussotti L, Curley SA, Abdalla EK. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 962] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 8. | Samura H, Oki E, Okumura H, Yoshida T, Kai S, Kobayashi K, Kinjo T, Mori S, Tohyama T, Ohgaki K, Kawanaka H, Makiyama A, Ureshino N, Kotaka M, Shimose T, Ando K, Saeki H, Baba H, Maehara Y, Mori M. A phase I/II study of S-1 and irinotecan (IRIS) combined with cetuximab in patients with RAS wild-type metastatic colorectal cancer (KSCC1401). Cancer Chemother Pharmacol. 2020;86:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN; Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 10. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-18; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2856] [Article Influence: 105.8] [Reference Citation Analysis (1)] |
| 11. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 969] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 12. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2407] [Cited by in RCA: 2395] [Article Influence: 92.1] [Reference Citation Analysis (1)] |
| 13. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3005] [Cited by in RCA: 2836] [Article Influence: 109.1] [Reference Citation Analysis (1)] |
| 14. | Behrenbruch C, Prabhakaran S, Udayasiri D, Hollande F, Michael M, Hayes I, Heriot A, Knowles B, Thomson B. Survival benefit of neoadjuvant chemotherapy and surgery versus surgery first for resectable colorectal liver metastases: a cohort study. ANZ J Surg. 2021;91:1196-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Bacalbasa N, Balescu I, Cretoiu D, Halmaciu I, Dimitriu M, Socea B, Diaconu C, Iliescu L, Savu C, Filipescu A, Stoica C, Stiru O. Determination of whether HIPEC is beneficial in patients with synchronous peritoneal and liver metastases from colorectal cancer (Review). Exp Ther Med. 2021;22:1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Predescu D, Boeriu M, Constantin A, Socea B, Costea D, Constantinoiu S. Pregnancy and Colorectal Cancer, from Diagnosis to Therapeutical Management - Short Review. Chirurgia (Bucur). 2020;115:563-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P, Jaeck D. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 353] [Article Influence: 19.6] [Reference Citation Analysis (2)] |
| 18. | Pathak S, Tang JM, Terlizzo M, Poston GJ, Malik HZ. Hepatic steatosis, body mass index and long term outcome in patients undergoing hepatectomy for colorectal liver metastases. Eur J Surg Oncol. 2010;36:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Wicherts DA, de Haas RJ, Sebagh M, Ciacio O, Lévi F, Paule B, Giacchetti S, Guettier C, Azoulay D, Castaing D, Adam R. Regenerative nodular hyperplasia of the liver related to chemotherapy: impact on outcome of liver surgery for colorectal metastases. Ann Surg Oncol. 2011;18:659-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Soubrane O, Brouquet A, Zalinski S, Terris B, Brézault C, Mallet V, Goldwasser F, Scatton O. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P, Eng C, Hoff PM, Vauthey JN, Wolff RA, Kopetz S. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, Brezault C, Soubrane O, Abdalla EK, Vauthey JN, Mentha G, Terris B. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Park J, South Korea; Socea B, Romania S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ