Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.1072
Peer-review started: April 23, 2022
First decision: June 19, 2022
Revised: June 30, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 27, 2022
Processing time: 152 Days and 9.4 Hours
Tailgut cysts are defined as congenital cysts that develop in the rectosacral space from the residue of the primitive tail. As a congenital disease, caudal cysts are very rare, and their canceration is even rarer, which makes the disease prone to misdiagnosis and delayed treatment. We describe a case of caudal cyst with adenocarcinogenesis and summarize in detail the characteristics of cases with analytical value reported since 1990.
A 35-year-old woman found a mass in her lower abdomen 2 mo ago. She was asymptomatic at that time and was not treated because of the coronavirus disease 2019 pandemic. Two weeks ago, the patient developed abdominal distension and right waist discomfort and came to our hospital. Except for the high level of serum carcinoembryonic antigen, the medical history and laboratory tests were not remarkable. Magnetic resonance imaging showed a well-defined, slightly lobulated cystic-solid mass with a straight diameter of approximately 10 cm × 9 cm in the presacral space, slightly high signal intensity on T2-weighted imaging, and moderate signal intensity on T1-weighted imaging. The mass was completely removed by laparoscopic surgery. Histopathological examination showed that the lesion was an intestinal mucinous adenocarcinoma, and the multidisciplinary team decided to implement postoperative chemotherapy. The patient recovered well, the tumor marker levels returned to normal, and tumor-free survival has been achieved thus far.
The case and literature summary can help clinicians and researchers develop appropriate exa
Core Tip: Tailgut cysts (TGCs) are rare congenital cysts of the retrorectal space. We report a case of TGC with adenocarcinogenesis and review the literature on caudal cyst adenocarcinogenesis. Magnetic resonance imaging is the most valuable tool for diagnosis and differential diagnosis, and preoperative biopsy is not worth advocating. Early MDT plays an important role in the accurate diagnosis and selection of the most appropriate personalized treatment. Complete resection of TGCs is the key to avoiding postoperative recurrence. We recommend MDT with complete surgical resection as the core and discuss the advantages and disadvantages of various diagnostic and treatment strategies.
- Citation: Wang YS, Guo QY, Zheng FH, Huang ZW, Yan JL, Fan FX, Liu T, Ji SX, Zhao XF, Zheng YX. Retrorectal mucinous adenocarcinoma arising from a tailgut cyst: A case report and review of literature. World J Gastrointest Surg 2022; 14(9): 1072-1081
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/1072.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.1072
Tailgut cysts (TGCs) are congenital cysts that develop in the retrorectal-presacral space from the remnants of the primitive embryonic hindgut[1,2]. These rare cysts are more common in women. Patients may present with lower abdominal pain and perianal lesions. Due to the risk of complications, such as recurrent perianal suppuration and malignant changes, surgical treatment is necessary. TGCs with malignant transformation are extremely rare[3]. The types of malignant transformation include adenocarcinoma, neuroendocrine tumor, and carcinoid. Most of these tumors are more inert than other epithelial malignant tumors; however, a small number of them are aggressive and resistant to treatment. Adenocarcinoma caused by TGCs is very rare, with only 28 cases with clinical details having been reported in the medical literature since 1990. In this paper, we report a new case and summarize the clinical and pathological features of adenocarcinoma from TGCs with a literature review. The reported cases were retrieved from the PubMed and Reference Citation Analysis (https://www.referencecitationanalysis.com/) database, and case summary information was carefully extracted from each article searched by PubMed (Table 1). To the best of our knowledge, a summary of adenocarcinogenesis of TGCs has not been reported before. Here, we focus on the regular characteristics of malignant transformation of TGCs to facilitate clinical diagnosis and treatment.
| Case | Ref. | Sex | Age | Clinical symptoms | Duration | Size (mm) | MRI/CT | Biopsy | Invasion | Position S4+/S3- | CEA/CA199 | Surgery planning |
| 1 | Baverez et al[19], 2021 | F | 57 | Perianal suppuration | 5 yr | 55 | +/+ | + | Anal canal and perianal skin | - | 30/UN | Abdominoperineal resection |
| 2 | Wang et al[3], 2020 | F | 50 | Irregular defecation | 2 wk | 90 × 80 | +/+ | - | - | - | 79.89/57.60 | Trans-sacrococcygeal approach |
| 3 | Rachel et al[20], 2019 | F | 73 | Anal abscess and associated fistula | 40 yr | 56 × 46 | +/+ | + | Anal canal and perianal skin | - | UN | Trans-sacrococcygeal approach |
| 4 | Martins et al[4], 2020 | F | 54 | Pelvic and perineal pain | 1-2 mo | 50 × 35 | +/+ | + | Sacrum | - | UN | Trans-sacrococcygeal approach |
| 5 | Li et al[21], 2019 | M | 33 | - | - | 80 × 59 | +/- | - | - | - | 26.97/106.50 | Trans-sacrococcygeal approach |
| 6 | Şahin et al[22], 2020 | F | 55 | Swelling of the buttocks | 6 mo | 21 × 16 | +/- | - | - | - | -/204 | Trans-sacrococcygeal approach |
| 7 | Almeida Costa and Rio[23], 2018 | F | 53 | Defecation and lower abdominal pain | UN | UN | +/+ | - | Sacrum | + | UN | Trans-sacrococcygeal approach |
| 8 | Zhao et al[11], 2015 | F | 44 | Pelvic and perineal pain | 6 mo | 100 | -/+ | + | Rectum and surrounding | + | +/UN | Partial resection and drainage of the pelvic tumor |
| 9 | Chhabra et al[8], 2013 | F | 56 | Hematuria | 1 yr | 46 × 37 | -/+ | + | - | - | -/UN | Trans-sacrococcygeal approach |
| 10 | Jarboui et al[24], 2008 | F | 49 | Pelvic and perineal pain | 6 mo | 150 | -/+ | - | - | + | UN | Laparotomy |
| 11 | Tampi et al[2], 2007 | F | 57 | Low backache | 6 mo | 120 × 100 × 80 | -/+ | - | Liver | + | -/- | Laparotomy |
| 12 | Andea and Klimstra[25], 2005 | F | 47 | Gluteal pain | 3 mo | 40 × 40 | UN/UN | - | - | UN | -/UN | UN |
| 13 | Cho et al[26], 2005 | F | 40 | Perianal pain | 1 mo | 100 × 80 × 70 | +/ | + | Sacrum | - | 159/2270 | Abdominoperineal resection and partial sacrectomy |
| 14 | Kanthan et al[12], 2004 | F | 76 | Perianal pain | UN | 65 × 45 × 35 | -/+ | + | - | - | UN | Trans-sacrococcygeal approach |
| 15 | Moreira et al[13], 2001 (case-1) | F | 64 | Constipation and frequent urination | 2 mo | 120 × 100 | +/UN | - | - | UN | UN | UN |
| 16 | Moreira et al[13], 2001 (case-2) | F | 68 | Rectal “fullness” | 2 yr | 180 × 40 | +/+ | - | - | UN | UN | UN |
| 17 | Schwarz et al[14], 2000 | M | 47 | Bilateral flank pain, constipation | 3 mo | 160 | -/+ | - | - | - | 46/- | Abdominoperineal resection and partial sacrectomy |
| 18 | Prasad et al[27], 2000 | F | 36 | - | UN | 95 × 92 × 88 | +/+ | UN | - | UN | UN | UN |
| 19 | Sauer et al[28], 2000 | F | 58 | Recurrent perianal fistulas | 17 | 55 × 40 × 35 | +/+ | - | - | + | 6.7/42 | Laparotomy |
| 20 | Graadt van Roggen et al[7], 1999 | F | 43 | - | - | 130 | +/- | - | UN | + | +/UN | UN |
| 21 | Maruyama et al[29], 1998 | F | 66 | Perianal pain | 6 mo | 100 × 90 | +/+ | - | - | 3.8/- | Trans-sacrococcygeal approach | |
| 22 | Lim et al[10], 1998 | F | 40 | Urinary frequency and constipation | 8 mo | 250 × 100 × 100 | +/- | - | - | UN | -/- | Laparotomy |
| 23 | Yamaguchi et al[30], 2001 | M | 32 | Anal fistula | 4 yr | UN | +/+ | - | Rectum | UN | UN | Pelvic evisceration |
| 24 | Liessi et al[31], 1995 | M | 50 | UN | UN | UN | +/+ | UN | Sacrum | UN | UN | Trans-sacrococcygeal approach |
A 35-year-old Chinese woman complained of a lower abdominal mass for 2 mo and abdominal distension for 2 wk.
The patient accidentally found a mass in her lower abdomen in May 2020 with no related clinical symptoms. She delayed hospitalization for 2 mo due to the coronavirus disease 2019 pandemic. Two months later, due to abdominal distension and right waist discomfort, the patient went to the gynecology clinic to seek medical help. Since the onset of the disease, the patient has had no dysuria or menstrual changes.
The patient’s past medical history included a loop electrosurgical excision procedure for cervical erosion 10 years ago.
No family history was identified.
Physical examination showed that the patient's abdomen was flat and soft, with no abnormal bulge, tenderness, or rebound pain. A cystic-solid mass of approximately 10 cm, which was painless and could not be pushed, was palpated slightly higher than the pubic bone. Gynecological bimanual examination showed no abnormalities of the vagina, cervix, or uterus.
Laboratory studies were normal except for an elevation in serum carcinoembryonic antigen (CEA) to 132.69 ng/mL.
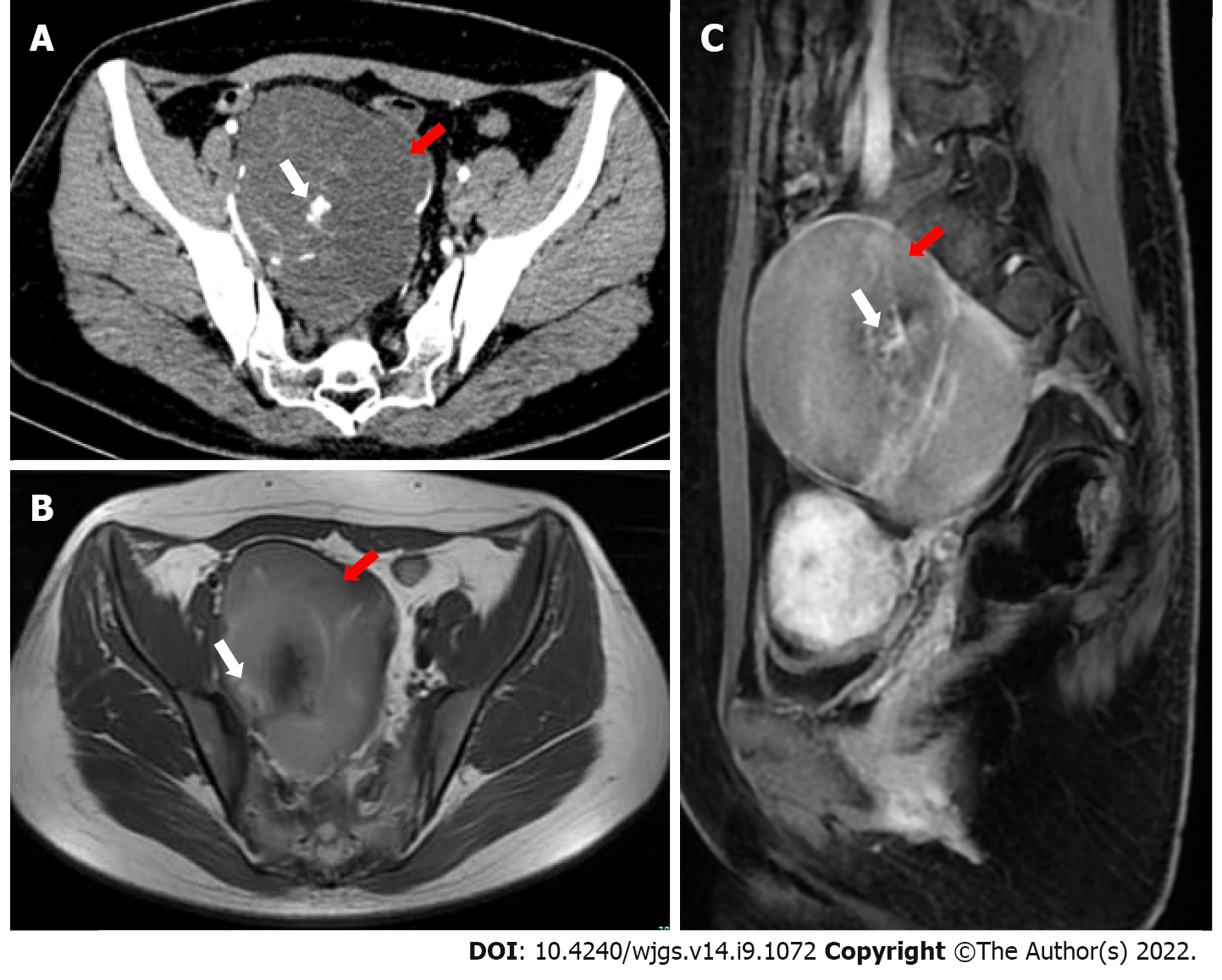
Gynecological B-mode ultrasonography examination showed that there was a cystic-solid mass close to the surface of the right ovary, mainly cystic, and the sound difference of the internal diaphragm was noisy. The appearance of thick and intense light spots followed by sound shadows, as well as a small blood flow signal in the solid part, allowed us to calculate a resistance index of 0.55. Computed tomography (CT) showed a cystic mass in the posterior rectal pelvis, extending to the level of the sacral promontory but not reaching the bony components of the sacrum or coccyx. The size of the mass was approximately 10 cm × 9 cm, and it showed polycystic changes with a septum and calcification. Contrast-enhanced CT indicated that the septum of the mass could be enhanced. Magnetic resonance imaging (MRI) showed a mass of abnormal signal on the right side of the pelvis measuring approximately 10 cm × 7 cm. Its borders were clear, with mixed high signal on T2-weighted imaging (T2WI) and localized lamellar low signal within. The right adnexal region was a cystic abnormal signal focus with a moderate signal on T1-weighted imaging (T1WI) and a slightly high signal on T2WI, with nodular ring reinforcement on an enhanced scan. No enlarged lymph nodes or abnormal masses were seen in the pelvis. There was also no abnormal signal in the pelvic wall tissue (Figure 1).
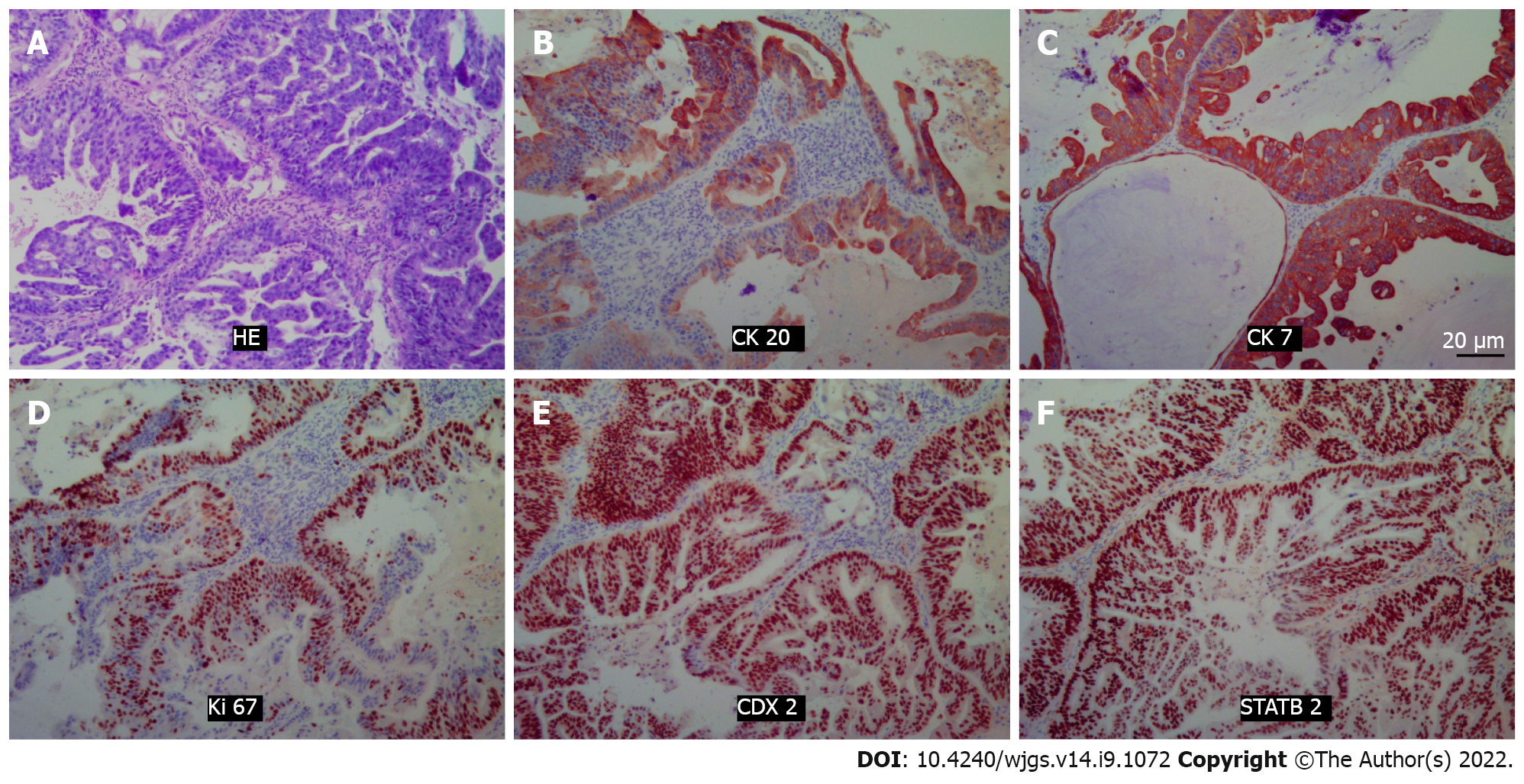
Histopathology revealed that the lesion was an intestinal mucinous adenocarcinoma, and the malignant transformation of an embryonic residual enterogenous cyst was considered. The results of pathological sections showed fibrous tissue with a cystic lining; the lesion was rich in cellular mucus and infiltrating the columnar epithelium, and it also showed high-grade atypical cell hyperplasia and mitotic activity. Morphologically, this was consistent with mucinous adenocarcinoma, intestinal type. Immunopathology showed that cytokeratin 20 (CK) 20, CK7, CDX2, and STATB2 were positive (Figure 2). After joint consultation with the Department of Pathology of University of California, Los Angeles, we diagnosed the patient with a TGC with adenocarcinogenesis.
After a multidisciplinary consultation and evaluation, laparoscopic surgery was performed under general anesthesia on July 14, 2020. During the operation, there was no obvious free fluid in the pelvis and no obvious abnormalities in the uterus, fallopian tubes, or ovaries.
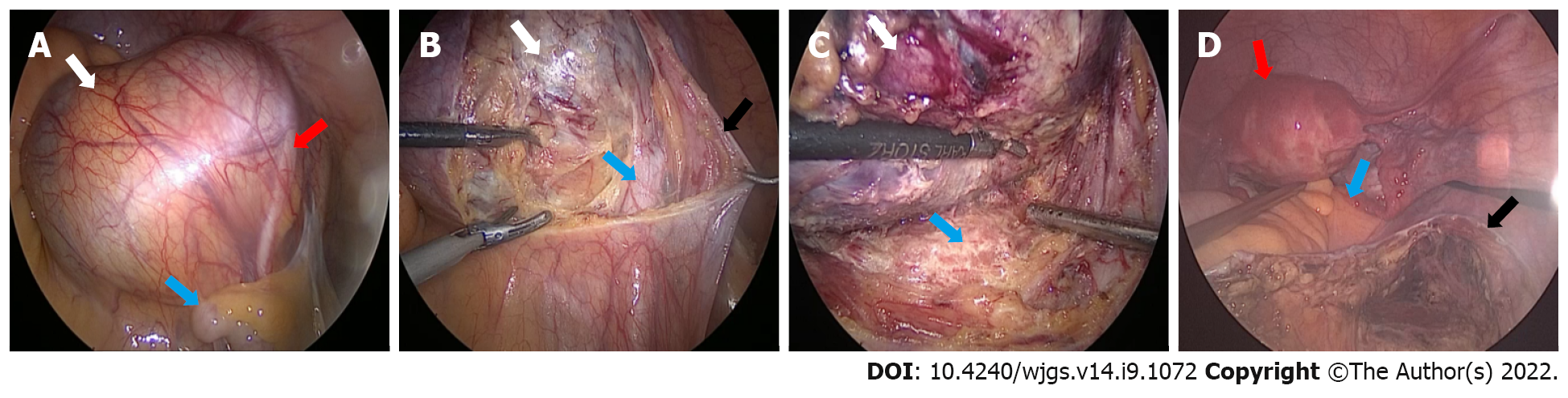
An enlarged cyst, swollen and measuring approximately 10 cm × 9 cm, was found behind the peritoneum in front of the sacrum near the right iliac vessel. Hyperplastic vessels were visible on the smooth surface of the swelling, the ureter was observed to pass through the surface, and the iliac vessels were visible below it, with no adhesion to the surrounding tissues of S2-S4.
The operation was performed by an experienced general surgeon and a gynecologist. With the help of laparoscopy, we successfully removed the cyst completely. After the cyst was removed from the abdominal cavity, we opened the cyst and found that its inner wall was rough; moreover, we found multiple tissue calcifications. The intraoperative frozen pathological results showed a retroperitoneal benign cyst and cyst wall fibrosis and calcification. After flushing the abdominal cavity and retroperitoneal space with distilled water, no residual cysts or enlarged lymph nodes were found, and the peritoneum was closed by suture (Figure 3).
The patient received six cycles of capecitabine and oxaliplatin (CapeOX) chemotherapy, and there were no grade 3-4 side effects during this treatment. After treatment, her CEA level decreased progressively and ultimately fell within the normal range, and no metastatic focus was found on CT. The patient received therapy with high compliance, expressed satisfaction with her recovery, and has been tumor-free for more than 18 mo.
TGCs are considered to be congenital cysts that develop in the rectosacral space from the residue of the primitive tail[1,2]. This incomplete degeneration of the extension of the tail from the posterior intestine of the embryo usually occurs at the 8th week of the embryonic stage[4]. The rectosacral space is a potential space located in the deep part of the pelvis, with the posterior rectal fascia in the front and the presacral fascia (Waldeyer fascia) in the back; this space extends upward to the peritoneum and downward to the level of the rectosacral fascia and perineal muscle[5]. The boundaries on both sides are roughly outlined by the ureter, iliac vessels, and sacral nerve roots[6]. This area includes the confluence of the embryonic hindgut, pelvis, and neuroectoderm, and consequently, there are many different tissue types that can lead to retrorectal tumors. Retrorectal tumors can be divided into congenital, inflammatory, neurogenic, and osteogenic tumors. Cystic congenital lesions consist of epidermoid cysts, dermoid cysts, TGCs, enterogenous cysts, teratomas, and teratocarcinomas[7]. Such lesions affect people of all ages from birth to adulthood and are more common in women. Sometimes, patients may have space-occupying symptoms due to the enlargement of deep pelvic masses[1,3]. Clinical manifestations are usually nonspecific, with half of the patients experiencing pain, perianal lesions, changes in defecation habits, dysuria, and neurological symptoms of the lower extremities and perineum[8]. Among congenital cystic lesions, the incidence of TGCs is relatively high, but the incidence of canceration is very rare.
The malignant transformation of TGCs into reported tumors includes adenocarcinoma, carcinoid, neuroendocrine carcinoma, endometrioid carcinoma, adenosquamous carcinoma, squamous cell carcinoma, and sarcoma[2]. Most of them are endocrine tumors and adenocarcinomas, while others, such as carcinoids, are rare. At present, approximately 28 cases of TGC adenocarcinoma have been reported, of which 24 with relatively complete data were retrieved. We describe a new case of TGC with mucinous cystadenocarcinoma and review the literature reports of TGCs with adenocarcinogenesis to provide a reference for diagnosis and treatment.
We summarize cases of TGC adenocarcinoma reported from 1990 to 2021, as the diagnostic and therapeutic approach is of limited interest due to the low prevalence and accuracy of diagnostic tools such as MRI and CT prior to 1990.
First, we sorted out the historical process of a complete understanding of TGCs. Cancerous TGC was first reported in 1932, and Ballantyne reported the first case of adenocarcinoma with TGCs. That patient developed local recurrence, lung metastasis, and inguinal lymph node metastasis and died 8 mo after cyst resection. Subsequently, doctors began to pay attention to and share the diagnosis and treatment of this rare disease. Through the review of articles related to TGCs, we found that there were two related landmark systematic retrospective studies. The first one was conducted in 1987 when Hjermstad and Helwig[1] evaluated all the pathological specimens of posterior rectal cysts diagnosed by the Institute of Pathology of the Armed Forces of the United States during a period of 35 years, and 53 cases of "tailgut cysts" were selected[1]. Their screening criteria were that the cysts must be partially covered by a columnar or transitional epithelium, but there must be no myenteric plexus or serosa, nor can there be a complete muscular layer. Hjermstad and Helwig's study defined the pathological criteria for the diagnosis of TGCs, allowing doctors to unify the definition of the disease[1]. The second was a retrospective analysis of the clinical and pathological data of patients who underwent colectomy at Mayo Clinic in 2008, conducted by Mathis et al[9]. A total of 31 patients were diagnosed, including 28 females, with an average age of 52 years. The median diameter of the cyst was 4.4 cm. There were four patients with malignant transformation, comprising three cases of adenocarcinoma and one case of carcinoid, and the 5-year survival rate was 83%. The work of Mathis et al[9] provides a single-center clinical experiential basis for the treatment and prognosis of TGCs. At present, with the progress of medical technology, the surgical methods and chemotherapy schemes have changed, but their principle of complete resection of the tumor remains unaddressed.
The summary of cases of TGCs with adenocarcinogenesis showed that most of the patients were middle-aged adults with a female–male ratio of 10:1, which was much higher than the ratio of 3-4:1 in previous articles on caudal cysts. The clinical manifestations of TGCs are varied and nonspecific. However, by summarizing the cases of caudal cysts with adenocarcinoma, we found that half of the patients complained of an abdominal mass and pain, perianal disease, and changes in stool habits and stool characteristics, while other patients did not have any symptoms. TGC is a rare congenital retrorectal disease in which the residue of the fetal retroanal intestine grows in the retrorectal space. It should be noted that this gap is a potential space, and the mass has considerable room for growth. This can explain the late onset of the disease, and TGC canceration occurs during this process. It is suggested that TGCs should be regarded as a precancerous lesion to explain this phenomenon. More than half of the patients were diagnosed with TGCs within 1 year after the onset of symptoms, and most of them exhibited retrorectal masses by imaging examinations such as CT and MRI. Compared with CT, MRI has the ability of multiplanar imaging and better tissue contrast in presacral masses[10]. MR has more advantages in differential diagnosis. Regarding the differential diagnosis of presacral masses, anal gland cysts, repeated cysts, teratomas, epidermoid cyst chordomas, abscesses, metastatic tumors, and neurofibromas should be considered. Fat content on fat-saturated images indicates dermoid cysts[1]. In presacral cystic masses, epidermoid cysts, dermoid cysts, rectal repeated cysts, and meningoceles are usually monocular. Rectal repetitive cysts, which are located in front of the rectum, often communicate with the rectal cavity. In contrast, TGCs are usually polycystic and can be characterized by large cysts with small peripheral cysts. This polycystic change is very important. Regarding the MRI features of TGCs, low signal intensity is usually shown on T1WI, and high signal intensity is shown on T2WI. However, the internal signal intensity of T1WI and T2WI indicates the protein concentration in the lesion, which increases with age, and the cysts show high signal intensity on T1WI. However, the consistent feature is that most of the dominant cysts on T2-weighted sequences are hyperintense relative to the pelvic muscles. In addition, we are more concerned about the accuracy of MRI in predicting the nature of tumors. Cystic tumors with smooth, well-defined boundaries and no infiltrative or gadolinium enhancement are generally considered to be benign, whereas cysts with thickened and irregularly enhancing cyst wall boundaries, which may even be surrounded by inflammatory changes, are usually malignant.
MRI is the most valuable tool to meet the needs of diagnosis and differential diagnosis, to help improve preoperative assessments, to estimate the extent of the disease and malignant risk, and to determine the most appropriate treatment strategy. The effect of CT is not as accurate as that of MRI[3]. By pooling the literature, it was found that half of the cases had calcification and that the presence or absence of calcification was not of significant value in the diagnosis of benign or malignant lesions. Enhanced MRI and PET may be good examination methods for the diagnosis of malignant transformation and metastasis of TGCs, which is worth exploring in the future.
Preoperative biopsy of TGCs is considered unnecessary because it cannot confirm or even misconfirm the diagnosis of adenocarcinogenesis or tumor differentiation of TGCs[8]. Some authors believe that in the case of heterogeneous masses with elevated CEA, direct surgery should be performed without biopsy. However, in our statistical table, we can see that among three patients with benign lesions diagnosed by preoperative biopsy, one had a high CEA level (case 8)[11], one had a normal CEA level (case 9)[8], and one had an unknown CEA level (case 14)[12], but postoperative pathology confirmed adenocarcinoma. Therefore, biopsy can provide very limited help in diagnosing heterogeneous masses with normal CEA. Preoperative biopsies may pose major risks, such as malignant cell spillage or needle implantation. After such a biopsy, it is necessary to consider removing the tissue around the needle track during the operation, but in many cases, this is not easy to do. When we make the surgical plan, regardless of the biopsy results, we need to assume that this is a malignant lesion and adhere to the principle of complete resection. The accuracy of preoperative biopsy is in doubt, and this procedure may bring the risk of metastasis and increase the difficulty of operation. However, for patients who are unable or difficult to surgically remove the tumor, it is indeed a good method to determine the nature of the tumor through the pathological results of the biopsy and then perform surgical treatment after neoadjuvant chemotherapy.
Because cases of adenocarcinogenesis of TGCs are very rare, there are no guidelines to follow in the treatment of retrorectal tumors. In view of the strong positive expression of p53 and Ki-67 and the negative expression of p21 in the dysplastic epithelium of tailgut adenocarcinoma, it is speculated that the occurrence order of dysplasia and carcinoma is similar to that of colonic adenocarcinoma[13]. At present, the treatment mainly draws lessons from the clinical treatment guidelines for rectal adenocarcinoma, including European ESMO guidelines and American NCCN guidelines. It is suggested that multidisciplinary treatment should be adopted. Considering the postoperative pathological report and high CEA level, the present patient chose surgery and chemotherapy. The key to such operations is to remove the cyst wall completely. There are three common surgical approaches, namely, the anterior approach (abdomen), posterior approach (perineal approach), and combined abdominal perineal approach[4,14]. MRI will help to determine the margin of resection and identify the relationship between the tumor and the sacral level. For instance, if the tumor is below the middle of S3, the perineal approach can be considered[15]. All tumors extending above S4 usually require an abdominal or combined approach. For small lesions, the surgeon can also use a transvaginal approach. If malignant lesions are confirmed or suspected, the tumor tissue can be cleared more thoroughly via the combined abdominal perineal approach. Minimally invasive surgery has great advantages in the fine separation of anatomical hierarchy and reduction of complications[16,17]. In view of the leakage of the cancer and the large mass, it is recommended to use an endobag in the extraction of the specimen through a small incision in the abdominal wall. If there is no R0 resection or residual cyst wall and invasion of the tissue around the tumor leads to postoperative recurrence, comprehensive treatment schemes such as cytoreductive surgery, radiotherapy, chemotherapy, interventional therapy, and molecular targeted drug therapy are recommended. Considering that a small amount of leakage of TGC fluid during the operation might occur and that the postoperative pathology showed mucinous adenocarcinoma with high CEA, we chose to use CapeOX treatment to prevent recurrence. The reason for choosing CapeOX treatment is that it is feasible and widely used in malignant tumors of the digestive tract; the other reason is that the incidence of serious side effects of this regimen is low. In summary, complete resection of TGC masses during surgery is the key to avoiding postoperative recurrence and obtaining long-term survival for patients without metastasis[18].
Adenocarcinoma of TGCs is a very rare disease, and complete resection is still the gold standard. We do not recommend preoperative biopsies. Early MDT plays a significant role in the accurate diagnosis and selection of the most appropriate personalized treatment.
| 1. | Hjermstad BM, Helwig EB. Tailgut cysts. Report of 53 cases. Am J Clin Pathol. 1988;89:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 196] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Tampi C, Lotwala V, Lakdawala M, Coelho K. Retrorectal cyst hamartoma (tailgut cyst) with malignant transformation. Gynecol Oncol. 2007;105:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Wang M, Liu G, Mu Y, He H, Wang S, Li J. Tailgut cyst with adenocarcinoma transition: A rare case report. Medicine (Baltimore). 2020;99:e20941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Martins P, Canotilho R, Peyroteo M, Afonso M, Moreira A, de Sousa A. Tailgut cyst adenocarcinoma. Autops Case Rep. 2020;10:e2019115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Satyadas T, Davies M, Nasir N, Halligan S, Akle CA. Tailgut cyst associated with a pilonidal sinus: an unusual case and a review. Colorectal Dis. 2002;4:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Kildušis E, Samalavičius NE. Surgical management of a retro-rectal cystic hamartoma (tailgut cyst) using a trans-rectal approach: a case report and review of the literature. J Med Case Rep. 2014;8:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Graadt van Roggen JF, Welvaart K, de Roos A, Offerhaus GJ, Hogendoorn PC. Adenocarcinoma arising within a tailgut cyst: clinicopathological description and follow up of an unusual case. J Clin Pathol. 1999;52:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Chhabra S, Wise S, Maloney-Patel N, Rezac C, Poplin E. Adenocarcinoma associated with tail gut cyst. J Gastrointest Oncol. 2013;4:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 9. | Mathis KL, Dozois EJ, Grewal MS, Metzger P, Larson DW, Devine RM. Malignant risk and surgical outcomes of presacral tailgut cysts. Br J Surg. 2010;97:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Lim KE, Hsu WC, Wang CR. Tailgut cyst with malignancy: MR imaging findings. AJR Am J Roentgenol. 1998;170:1488-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Zhao XR, Gao C, Zhang Y, Yu YH. The Malignant Transformation of Retrorectal Cystic Hamartomas With Blood Irregular Antibodies Positive: A Case Report. Medicine (Baltimore). 2015;94:e2253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kanthan SC, Kanthan R. Unusual retrorectal lesion. Asian J Surg. 2004;27:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Moreira AL, Scholes JV, Boppana S, Melamed J. p53 Mutation in adenocarcinoma arising in retrorectal cyst hamartoma (tailgut cyst): report of 2 cases--an immunohistochemistry/immunoperoxidase study. Arch Pathol Lab Med. 2001;125:1361-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Schwarz RE, Lyda M, Lew M, Paz IB. A carcinoembryonic antigen-secreting adenocarcinoma arising within a retrorectal tailgut cyst: clinicopathological considerations. Am J Gastroenterol. 2000;95:1344-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Alsofyani TM, Aldossary MY, AlQahtani FF, Sabr K, Balhareth A. Successful excision of a retrorectal cyst through trans-sacral approach: A case report. Int J Surg Case Rep. 2020;71:307-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Rompen IF, Scheiwiller A, Winiger A, Metzger J, Gass JM. Robotic-Assisted Laparoscopic Resection of Tailgut Cysts. JSLS. 2021;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Inada R, Watanabe A, Toshima T, Katsura Y, Sato T, Sui K, Oishi K, Okabayashi T, Ozaki K, Shibuya Y, Matsumoto M, Iwata J. Laparoscopic Synchronous Resection for Descending Colon Cancer and Tailgut Cyst. Acta Med Okayama. 2021;75:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Baek SK, Hwang GS, Vinci A, Jafari MD, Jafari F, Moghadamyeghaneh Z, Pigazzi A. Retrorectal Tumors: A Comprehensive Literature Review. World J Surg. 2016;40:2001-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Baverez M, Thibaudeau E, Libois V, Kerdraon O, Senellart H, Raoul JL. Retrorectal Mucinous Adenocarcinoma Arising from a Tailgut Cyst: A Case Report. Case Rep Oncol. 2021;14:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Rachel F, Guzik A, Szczerba D, Kozieł K, Gutterch K. Tailgut cyst and a very rare case of a tailgut cyst with mucinous adenocarcinoma in a 73 year old woman treated for buttock abscess with fistula. Pol Przegl Chir. 2019;91:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Li W, Li J, Yu K, Zhang K. Retrorectal adenocarcinoma arising from tailgut cysts: a rare case report. BMC Surg. 2019;19:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Şahin S, Kepil N, Batur Ş, Çetin SE. Adenocarcinoma in a Tailgut Cyst: A Rare Case Report. Turk Patoloji Derg. 2020;36:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Almeida Costa NA, Rio G. Adenocarcinoma within a tailgut cyst. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Jarboui S, Jarraya H, Mihoub MB, Abdesselem MM, Zaouche A. Retrorectal cystic hamartoma associated with malignant disease. Can J Surg. 2008;51:E115-E116. [PubMed] |
| 25. | Andea AA, Klimstra DS. Adenocarcinoma arising in a tailgut cyst with prominent meningothelial proliferation and thyroid tissue: case report and review of the literature. Virchows Arch. 2005;446:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Cho BC, Kim NK, Lim BJ, Kang SO, Sohn JH, Roh JK, Choi ST, Kim SA, Park SE. A carcinoembryonic antigen-secreting adenocarcinoma arising in tailgut cyst: clinical implications of carcinoembryonic antigen. Yonsei Med J. 2005;46:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Prasad AR, Amin MB, Randolph TL, Lee CS, Ma CK. Retrorectal cystic hamartoma: report of 5 cases with malignancy arising in 2. Arch Pathol Lab Med. 2000;124:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Sauer J, Wolf HK, Junginger T. [Adenocarcinoma in a tail-gut cyst: a rare cause of recurrent perianal fistula]. Chirurg. 2000;71:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Maruyama A, Murabayashi K, Hayashi M, Nakano H, Isaji S, Uehara S, Kusuda T, Miyahara S, Kondo A, Yabana T. Adenocarcinoma arising in a tailgut cyst: report of a case. Surg Today. 1998;28:1319-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Yamaguchi K, Okushiba S, Katoh H, Shimizu M, Taneichi H. Tailgut cyst invaded by rectal cancer through an anal fistula: report of a case. Dis Colon Rectum. 2001;44:447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Liessi G, Cesari S, Pavanello M, Butini R. Tailgut cysts: CT and MR findings. Abdom Imaging. 1995;20:256-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bairwa DBL, India; Haddadi S, Algeria; Kawabata H, Japan; Nakaji K, Japan; Yap RVC, Philippines S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR