Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.1026
Peer-review started: February 22, 2022
First decision: April 13, 2022
Revised: April 13, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: September 27, 2022
Processing time: 212 Days and 3.9 Hours
Gastric cancer is a common malignant tumor. Early detection and diagnosis are crucial for the prevention and treatment of gastric cancer.
To develop a blood index panel that may improve the diagnostic value for discriminating gastric cancer and gastric polyps.
Thirteen tumor-related detection indices, 38 clinical biochemical indices and 10 cytokine indices were examined in 139 gastric cancer patients and 40 gastric polyp patients to build the model. An additional 68 gastric cancer patients and 22 gastric polyp patients were enrolled for validation. After area under the curve evaluation and univariate and multivariate analyses.
Five tumor-related detection indices, 12 clinical biochemical indices and 1 cytokine index showed significant differences between the gastric cancer and gastric polyp groups. Carbohydrate antigen (CA) 724, phosphorus (P) and ischemia-modified albumin (IMA) were included in the blood index panel, and the area under the curve (AUC) of the index panel was 0.829 (0.754, 0.905). After validation, the AUC was 0.811 (0.700, 0.923). Compared to the conventional index CA724, the blood index panel showed significantly increased diagnostic value.
We developed an index model that included CA724, P and IMA to discriminate the gastric cancer and gastric polyp groups, which may be a potential diagnostic method for clinical practice.
Core Tip: Early diagnosis and early treatment of gastric cancer is the key to improving the survival and cure rates of patients. Therefore, early detection and diagnosis are crucial for the prevention and treatment of gastric cancer. In this study, the we aimed to evaluate the diagnostic value of the blood index panel for gastric cancer.
- Citation: Guo GH, Xie YB, Zhang PJ, Jiang T. Blood index panel for gastric cancer detection. World J Gastrointest Surg 2022; 14(9): 1026-1036
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/1026.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.1026
Gastric cancer is a common malignant tumor that endangers human health, and it ranks second only to lung cancer in the number of deaths resulting from various malignant tumors[1]. The occurrence and development of gastric cancer is a multistage process involving changes at the gene and molecular levels. There is a period of precancerous lesions in the early stage of gastric cancer, and most of the pre
With further research, finding a simple, fast and easy dynamic observation method that can screen high-risk groups of gastric cancer (such as patients with atypical hyperplasia) would be beneficial for early diagnosis, and serum biomarkers (tumor markers, combined screening of cytokines and biochemical indicators) may be new targets for the early diagnosis of gastric cancer. Tumor markers reflect the occurrence and development of tumors and the degree of activation or inactivation of tumor-related genes. Since these substances are secreted by tumor cells and released into the blood and body fluids during tumor proliferation, they can be used to indicate the presence of tumors[6,7]. An ideal tumor marker has the characteristics of high sensitivity and high specificity, is present in body fluids, especially blood, and is easy to detect. In recent years, due to the rapid development of molecular bio
In our study, we examined 13 tumor-related indices, 38 clinical biochemical indices and 10 cytokines in gastric cancer and gastric polyp patients and aimed to develop an index panel that can improve the diagnostic value of discriminating gastric cancer and gastric polyp patients. This panel may become a detection method for clinical practice.
Signed informed consent was obtained, and this study was approved by the Ethics Committee of the First Center of Chinese PLA General Hospital. A total of 269 serum samples were collected from patients with gastric cancer and gastric polyps who were admitted to the First Center of Chinese PLA General Hospital. The inclusion criteria for gastric cancer and gastric polyps were as follows: (1) Primary; (2) Confirmed by pathological diagnosis; (3) No radiotherapy or chemotherapy before surgery; (4) Preoperative diagnosis with more than two imaging results; and (5) Complete medical records and follow-up data. The exclusion criteria were as follows: (1) Received radiotherapy, chemotherapy, and immunotherapy; (2) Immune system diseases; (3) Chronic wasting diseases and infectious diseases; and (4) Other types of malignant tumors. A total of 139 gastric cancer patients and 40 gastric polyp patients were enrolled for model building. An additional 68 gastric cancer patients and 22 gastric polyp patients were enrolled for validation. The two groups were age- and sex-matched. Three milliliters of fasting venous blood was collected from the subjects, incubated for 30 min, and centrifuged at 3500 r/min for 7 min to separate the serum, and the specimens without hemolysis or chyle were qualified and stored at -80 °C.
The 13 tumor-related indices included CEA, alpha fetoprotein (AFP), carbohydrate antigen 125 (CA125), CA199, CA153, CA724, cytokeratin fragment 211 (Cyfra211), ferritin (Ferr), neuron-specific enolase (NSE), squamous cell carcinoma (SCC), pepsinogen (PG) I, PG II, and PGI/II. The 38 clinical biochemical indices included alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), total bilirubin (TB), direct bilirubin (DB), total bile acid (TBA), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), glucose (GLu), urea nitrogen (UN), creatinine (Cr), uric acid (UA), cholesterol (CHO), triglyceride (TG), creatine kinase (CK), lactate dehydrogenase (LDH), isoenzyme of creatine kinase (CKMB), calcium (Ca), phosphorus (P), magnesium (Mg), potassium (K), sodium (Na), chlorine (Cl), carbon dioxide (CO2), lipoprotein a (LPa), high-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A1 (ApoA1), apoB, cysteine (CYS), sialic acid (SA), homocysteine (HCY), C-reactive protein (CRP), amylase (AMY), lipase (LPS), superoxide dismutase (SOD), and ischemia-modified albumin (IMA).
CEA, AFP, CA199, CA724, CA125, CA153, Cyfra211, Ferr, NSE, ALT, AST, TP, ALB, ALP, GGT, Glu, UN, CR, UA, CHO, TG, CK, Ca, P, Mg, K, Na, CL, CO2, HDL, LDL, CRP, AMY, and LPS detection kits, standards and controls were purchased from Roche Diagnostics Ltd. ApoA1, ApoB, CYS, Lp (a), and CKMB detection kits, standards and quality controls were purchased from Beijing Leadman Bio
The 10 cytokines included granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFNγ), interleukin-1β (IL-1β), IL-2, IL-4, IL-6, IL-8, IL-10, monocyte chemoattractant protein (MCP-1), and tumor necrosis factor α (TNFα) and were analyzed by a Luminex Instrument Model 200 Liquid Core Analyzer according to the instructions of the Human Cytokine/Chemokine Detection Kit. All reagents were equilibrated to room temperature (20 °C-25 °C) before the test. A schematic diagram of sample loading in a 96-well plate was drawn on paper (standards, 0, 3.2, 16, 80, 400, 2000, and 10000 ng/mL, QC I, QC II, sample), and duplicate wells were recommended. Then, 200 μL of assay buffer was added to each reaction well, which was sealed and shaken on a horizontal shaking instrument for 10 min (room temperature, 20 °C-25 °C). The excess assay buffer was blotted from the bottom with filter paper or paper towels. Then, 25 μL of analysis buffer was added to the background standard well, 25 μL of buffer was added to each sample well, 25 μL of each standard or quality control was added to the corresponding reaction well, and 25 μL of the appropriate matrix diluent was added to the background wells, standard wells, and quality control wells. When the analyte was serum or plasma, the serum matrix provided by the kit was used. When the analyte was tissue culture fluid or other supernatant, the corresponding medium was used as a diluent. A total of 25 μL of sample was added to the appropriate reaction well, the microspheres were mixed, and 25 μL of the mixed microspheres was added to each well. The wells were covered with parafilm and aluminum foil and incubated at room temperature (20 °C-25 °C) on a horizontal shaker for 1 h (when the test substance was serum or plasma, overnight incubation at 4 °C can improve the sensitivity). Then, the liquid was gently aspirated, the wells were washed with wash solution (200 μL/well) twice, the liquid was aspirated, and the washing solution at the bottom of the reaction plate was dried with filter paper or paper towel. The detection antibody was added (25 μL/well), and the plates were covered with parafilm and aluminum foil, shaken on a horizontal shaker and incubated at room temperature for 30 min. Streptavidin-PE (25 μL/well) was added, and the plates were covered with parafilm and aluminum foil, shaken on a horizontal shaker and incubated at room temperature for 30 min. Then, the liquid was gently aspirated, the wells were washed with wash solution (200 μL/well) twice, the liquid was aspirated, and the washing solution at the bottom of the reaction plate was blotted with filter paper or paper towel. Sheath fluid (100 μL/well) was added. The plates were covered with aluminum foil and shaken on a horizontal shaker for 5 min to resuspend the microspheres. The microspheres were read on a Luminex instrument, and the results were calculated.
SPSS 22.0 was used in this study. Measurement data are expressed as the median (25%, 75%). If the data were normally distributed, they were compared by two independent samples t tests. If not, they were compared by the rank sum test. The area under the curve (AUC) was used to evaluate the diagnostic value. Univariate and multivariate analyses were used to analyze the Exp (B) of the indices. Logistic regression analysis was used to build the index model. Z scores were used to compare the AUCs of the two groups.
As shown in Table 1, 13 tumor-related detection indices, including CEA, AFP, CA125, CA199, CA153, CA724, CY211, Ferr, NSE, SCC, PG I/II, PG II, and PG I, were compared between the gastric cancer and gastric polyp groups. Among the 13 tumor-related detection indices, CEA (P = 0.014), CA125 (P = 0.033), CA199 (P = 0.017), CA724 (P = 0.007) and PG I/II (P = 0.008) showed significant differences between the two groups, and the other 8 tumor-related detection indices (AFP, CA153, CY211, Ferr, NSE, SCC, PG II, and PG I) showed no significant differences.
| Indicator | Gastric polyp (n = 40) | Gastric cancer (n = 139) | P value |
| CEA | 1.16 (1.55, 2.11) | 1.11 (2.33, 5.11) | 0.014 |
| AFP | 1.64 (2.63, 3.62) | 1.43 (2.24, 3.23) | 0.499 |
| CA125 | 6.86 (9.91, 14.81) | 8.56 (13.73, 24.39) | 0.033 |
| CA199 | 4.8 (7.74, 13.91) | 5.07 (10.52, 29.36) | 0.017 |
| CA153 | 6.53 (9.3, 12.54) | 6.42 (9.03, 13.15) | 0.268 |
| CA724 | 0.84 (1.34, 3.68) | 1.43 (3.33, 11) | 0.007 |
| CY211 | 1.32 (1.67, 2.35) | 1.7 (2.47, 4.46) | 0.390 |
| Ferr | 63.86 (144.35, 268.48) | 26.19 (79.3, 174.4) | 0.176 |
| NSE | 8.39 (10.06, 11.87) | 7.55 (9.27, 11.57) | 0.732 |
| SCC | 0.43 (0.7, 1.08) | 0.5 (0.7, 1) | 0.247 |
| PG1/2 | 1.3 (4.31, 6.26) | 0.67 (2.98, 4.26) | 0.008 |
| PG2 | 7.65 (13.9, 29.68) | 9.9 (19.3, 32.4) | 0.199 |
| PG1 | 12.83 (58.5, 115.93) | 20.3 (53.8, 82) | 0.255 |
As shown in Table 2, 38 clinical biochemical indices, including ALT, AST, TP, ALB, TB, DB, TBA, ALP, GGT, GLu, UN, Cr, UA, CHO, TG, CK, LDH, CKMB, Ca, P, Mg, K, Na, Cl, CO2, LP (a), HDL, LDL, ApoA1, ApoB, CYS, SA, HCY, CRP, AMY, LPS, SOD and IMA, were compared between the gastric cancer and gastric polyp groups. ALB (P = 0.007), CHO (P = 0.035), TG (P = 0.017), Ca (P = 0.025), P (P = 0.008), Cl (P = 0.008), HDL (P = 0.004), LDL (P = 0.010), ApoA1 (P = 0.001), ApoB (P = 0.021), SOD (P = 0.001) and IMA (P = 0.001) showed significant differences between the two groups. The other 26 tumor-related detection indices, including ALT, AST, TP, TB, DB, TBA, ALP, GGT, GLu, UN, Cr, UA, CK, LDH, CKMB, Mg, K, Na, CO2, LP (a), CYS, SA, HCY, CRP, AMY and LPS, showed no significant differences.
| Indicator | Gastric polyp (n = 40) | Gastric cancer (n = 139) | P value |
| ALT | 11.73 (15.75, 19.35) | 10.7 (13.2, 18.3) | 0.322 |
| AST | 13.93 (17.85, 20.45) | 13.1 (15.6, 18.6) | 0.252 |
| TP | 64.73 (69.4, 72.3) | 61.9 (66.2, 69.4) | 0.095 |
| ALB | 38.9 (41.5, 43.8) | 36.5 (38.9, 41) | 0.007 |
| TB | 8.75 (11.8, 14.95) | 6.8 (9.4, 13.7) | 0.116 |
| DB | 2.33 (3.65, 4.7) | 2.4 (3.3, 4.9) | 0.248 |
| TBA | 2.65 (4.4, 5.98) | 2.6 (3.9, 7.4) | 0.622 |
| ALP | 44.65 (66.85, 77.48) | 56.2 (65.2, 81.9) | 0.076 |
| GGT | 13.13 (16.05, 27.43) | 13.3 (16.5, 24) | 0.773 |
| GLu | 4.74 (5.27, 5.6) | 4.72 (5, 5.49) | 0.627 |
| UN | 4.37 (5.22, 6.49) | 4.5 (5.21, 6.23) | 0.812 |
| Cr | 58.83 (65.3, 75.15) | 57.5 (68.2, 77.8) | 0.838 |
| UA | 261.1 (301.15, 371.9) | 228.4 (278.1, 330.5) | 0.117 |
| CHO | 3.99 (4.34, 5.18) | 3.56 (4.16, 4.68) | 0.035 |
| TG | 1.2 (1.46, 1.81) | 0.98 (1.25, 1.48) | 0.017 |
| CK | 37.68 (55.9, 82.83) | 38.6 (56.8, 76.1) | 0.740 |
| LDH | 139.65 (153.85, 174.43) | 118.1 (138, 158.9) | 0.792 |
| CKMB | 3.15 (6.7, 10.73) | 2.4 (6.2, 9.3) | 0.357 |
| Ca | 2.16 (2.26, 2.34) | 2.13 (2.19, 2.26) | 0.025 |
| P | 1.31 (1.53, 1.81) | 1.2 (1.36, 1.51) | 0.008 |
| Mg | 0.82 (0.87, 0.94) | 0.79 (0.85, 0.94) | 0.188 |
| K | 3.76 (4.05, 4.41) | 3.79 (3.99, 4.29) | 0.319 |
| Na | 141.23 (143.7, 146.35) | 141.3 (143.1, 144.5) | 0.579 |
| Cl | 104.6 (106.6, 108.38) | 103.3 (105.3, 106.9) | 0.008 |
| CO2 | 19.75 (22.15, 26.55) | 22.3 (24.9, 27.3) | 0.281 |
| LP (a) | 6.14 (17.34, 35.2) | 9.51 (14.82, 26.13) | 0.582 |
| HDL | 0.95 (1.12, 1.38) | 0.83 (1.03, 1.15) | 0.004 |
| LDL | 2.33 (2.77, 3.34) | 1.98 (2.4, 2.93) | 0.010 |
| ApoA1 | 1.08 (1.32, 1.59) | 0.96 (1.11, 1.24) | 0.001 |
| ApoB | 0.7 (0.84, 1.04) | 0.66 (0.77, 0.9) | 0.021 |
| CYS | 0.91 (1, 1.17) | 0.84 (0.96, 1.09) | 0.816 |
| SA | 53.85 (61.4, 65.38) | 55.8 (64.5, 70.6) | 0.179 |
| HCY | 9.85 (13.47, 16.5) | 10.63 (13.62, 17.74) | 0.414 |
| CRP | 0.43 (0.9, 3.78) | 0.7 (1.9, 5.4) | 0.702 |
| AMY | 47.2 (56.9, 77.23) | 40.9 (54.8, 68.1) | 0.433 |
| LPS | 28.25 (34.85, 44.13) | 28.2 (35.7, 44.5) | 0.291 |
| SOD | 141.33 (164.3, 189.5) | 108.3 (127.4, 157.4) | 0.001 |
| IMA | 62.73 (66, 69.35) | 56 (60.2, 63.6) | 0.001 |
As shown in Table 3, 10 tumor-related detection indices, including GM-CSF, IFNγ, IL-10, IL-1β, IL-2, IL-4, IL-6, IL-8, MCP-1, and TNFα, were compared between the gastric cancer and gastric polyp groups. Because IL-2 and IL-4 were lower than the detection limit in most samples, these two cytokine indices were deleted. After analysis, only TNFα (P = 0.001) showed a significant difference between the two groups, and the other 7 tumor-related detection indices, including GM-CSF, IFNγ, IL-10, IL-1β, IL-6, IL-8, and MCP-1, showed no significant differences.
| Indicator | Gastric polyp (n = 40) | Gastric cancer (n = 139) | P value |
| GM-CSF | 1.24 (2.7, 6.27) | 0.01 (0.53, 2.32) | 0.640 |
| IFNγ | 0.08 (0.25, 1.08) | 0.01 (0, 0.82) | 0.585 |
| IL-10 | 2.14 (3.39, 5.24) | 1.63 (4.06, 9.34) | 0.326 |
| IL-1β | 0.02 (0.31, 1.14) | 0.01 (0.08, 0.94) | 0.905 |
| IL-6 | 0.34 (0.94, 2.58) | 0.1 (1.98, 7.16) | 0.483 |
| IL-8 | 23.73 (51.11, 112.94) | 39.4 (62.55, 138.23) | 0.697 |
| MCP-1 | 321.54 (429.78, 594.82) | 310.31 (448.27, 612.02) | 0.993 |
| TNFα | 5.53 (7.09, 8.72) | 5.7 (9.87, 16.6) | 0.001 |
After comparing the tumor-related, clinical biochemical and cytokine indices between the gastric cancer and gastric polyp groups, the diagnostic value of the differential indices for discriminating between the gastric cancer and gastric polyp groups was evaluated. As shown in Table 4, the differential indices of CEA (P = 0.014), CA125 (P = 0.033), CA199 (P = 0.017), CA724 (P = 0.007), PG I/II (P = 0.008), ALB (P = 0.007), CHO (P = 0.035), TG (P = 0.017), Ca (P = 0.025), P (P = 0.008), Cl (P = 0.008), HDL (P = 0.004), LDL (P = 0.010), ApoA1 (P = 0.001), ApoB (P = 0.021), SOD (P = 0.001), IMA (P = 0.001) and TNFα (P = 0.001) were evaluated by the area under the curve. Only CA199 and CHO showed no significant differences. CEA, CA125, CA724, PG I/II, ALB, TG, Ca, P, Cl, HDL, LDL, ApoA1, ApoB, SOD, IMA and TNFα showed significant differences. The AUC of the best indicator, IMA, was 0.790 (0.705, 0.875). The P value was < 0.001. The AUC of the conventional index CA724 was 0.702 (0.614, 0.789). The P value was <0.001.
| Indicator | AUC | P value | Lower | Upper |
| CEA | 0.627 | 0.014 | 0.543 | 0.712 |
| CA125 | 0.637 | 0.008 | 0.546 | 0.729 |
| CA199 | 0.592 | 0.078 | 0.500 | 0.683 |
| CA724 | 0.702 | < 0.001 | 0.614 | 0.789 |
| PG1/2 | 0.628 | 0.014 | 0.517 | 0.738 |
| ALB | 0.687 | < 0.001 | 0.585 | 0.788 |
| CHO | 0.599 | 0.057 | 0.499 | 0.700 |
| TG | 0.655 | 0.003 | 0.561 | 0.748 |
| Ca | 0.640 | 0.007 | 0.534 | 0.746 |
| P | 0.668 | 0.001 | 0.566 | 0.769 |
| Cl | 0.635 | 0.009 | 0.537 | 0.733 |
| HDL | 0.648 | 0.004 | 0.551 | 0.746 |
| LDL | 0.633 | 0.010 | 0.532 | 0.735 |
| ApoA1 | 0.702 | 0.000 | 0.602 | 0.802 |
| ApoB | 0.609 | 0.036 | 0.505 | 0.714 |
| SOD | 0.755 | < 0.001 | 0.676 | 0.834 |
| IMA | 0.790 | < 0.001 | 0.705 | 0.875 |
| TNFα | 0.656 | 0.003 | 0.575 | 0d.736 |
After the diagnostic value evaluation of a single differential index for discriminating the gastric cancer and gastric polyp groups was performed, 16 indices, including CEA, CA125, CA724, PG I/II, ALB, TG, Ca, P, Cl, HDL, LDL, ApoA1, ApoB, SOD, IMA and TNFα, were further analyzed by univariate and multivariate analysis. As shown in Table 5, after the univariate analysis, the 3 indices Exp (B), CA724 (P = 0.03), P (P = 0.03) and IMA (P = 0.03) showed significant differences. The other indices (CEA, CA125, PG I/II, ALB, TG, Ca, Cl, HDL, LDL, ApoA1, ApoB, SOD and TNFα) showed no significant differences. Then, the 3 indices that showed significant differences were further analyzed by multivariate analysis. The Exp (B) of CA724, P and IMA was 1.17 (1.02, 1.34), 0.13 (0.03, 0.58), and 0.85 (0.78, 0.92), respectively.
| Indicator | Univariate analysis | Multivariate analysis | ||||||||
| Wals | P value | Exp (B) | Lower | Upper | Wals | P value | Exp (B) | Lower | Upper | |
| CEA | 1.02 | 0.31 | 1.04 | 0.97 | 1.11 | |||||
| CA125 | 1.53 | 0.22 | 0.99 | 0.98 | 1.01 | |||||
| CA724 | 4.50 | 0.03 | 1.18 | 1.01 | 1.38 | 5.21 | 0.02 | 1.17 | 1.02 | 1.34 |
| PG12 | 0.96 | 0.33 | 0.91 | 0.75 | 1.10 | |||||
| ALB | 0.01 | 0.93 | 0.99 | 0.85 | 1.16 | |||||
| TG | 0.79 | 0.37 | 0.64 | 0.23 | 1.72 | |||||
| Ca | 0.01 | 0.91 | 0.84 | 0.04 | 19.42 | |||||
| P | 4.45 | 0.03 | 0.15 | 0.03 | 0.88 | 7.05 | 0.01 | 0.13 | 0.03 | 0.58 |
| Cl | 2.73 | 0.10 | 0.85 | 0.71 | 1.03 | |||||
| HDL | 0.34 | 0.56 | 2.09 | 0.17 | 25.09 | |||||
| LDL | 0.10 | 0.76 | 0.84 | 0.27 | 2.60 | |||||
| ApoA1 | 2.42 | 0.12 | 0.09 | 0.00 | 1.86 | |||||
| ApoB | 0.39 | 0.53 | 4.36 | 0.04 | 45.13 | |||||
| SOD | 1.22 | 0.27 | 0.99 | 0.98 | 1.00 | |||||
| IMA | 4.50 | 0.03 | 0.89 | 0.79 | 0.99 | 14.77 | < 0.001 | 0.85 | 0.78 | 0.92 |
| TNFα | 3.07 | 0.08 | 1.08 | 0.99 | 1.19 | |||||
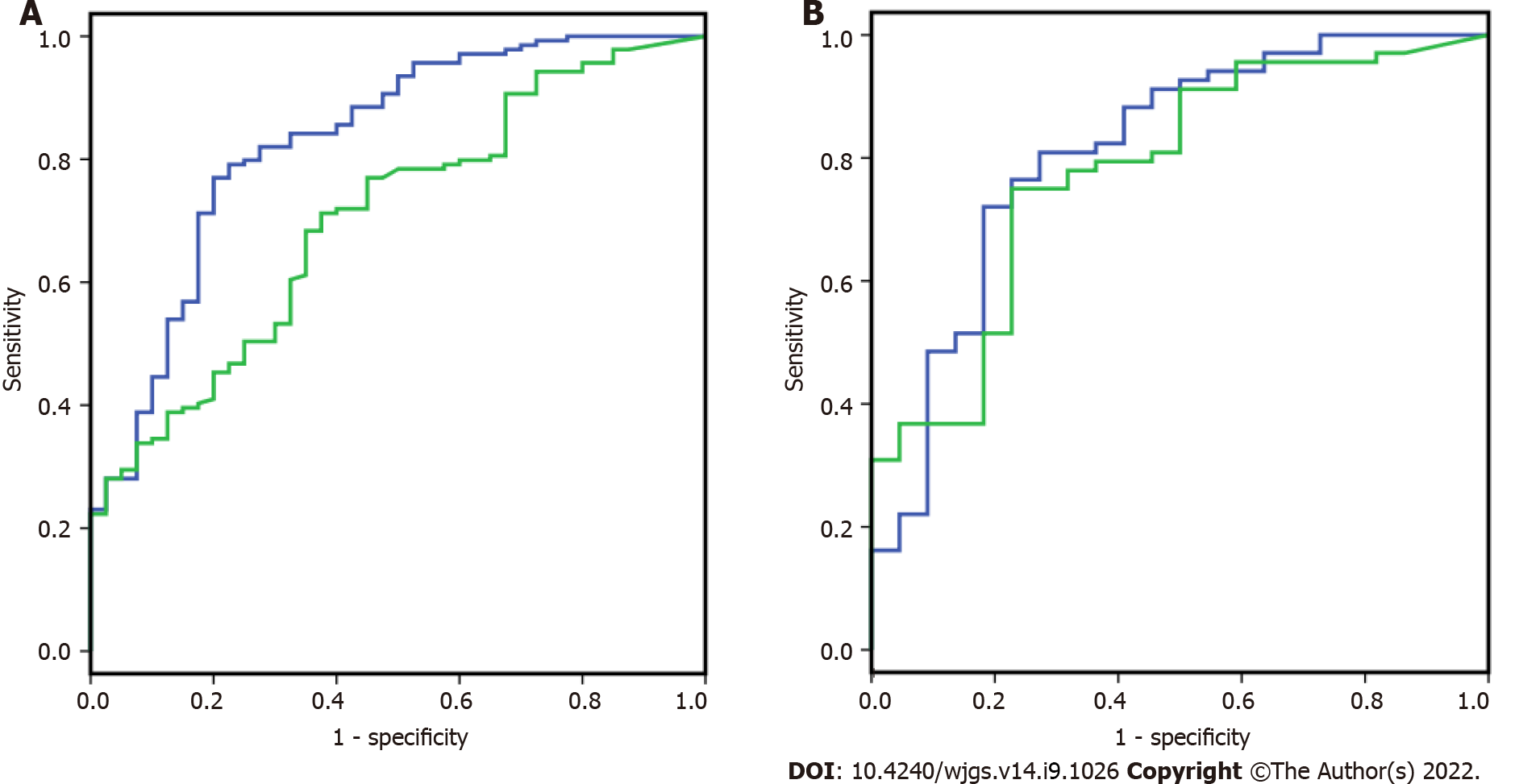
CA724, P and IMA were analyzed by logistic regression analysis to build a diagnostic index panel to differentiate the gastric cancer and gastric polyp groups. As shown in Figure 1A, for discriminating 139 gastric cancer and 40 gastric polyp patients, the AUC index panel was 0.829 (0.754, 0.905), and the conventional index CA724 was 0.704 (0.617, 0.791). The AUC of the index panel showed a significant increase compared to CA724 by z score statistics. After building the index model, as shown in Figure 1B, samples from independent individuals, including 68 gastric cancer patients and 22 gastric polyp patients, were used to validate the model. The AUC of the index panel and CA724 was 0.811 (0.700, 0.923), and that of the conventional index CA724 was 0.779 (0.668, 0.890).
The pepsinogen PG is a protein polypeptide chain composed of 375 amino acids, which can be divided into two categories according to biochemical and immunological properties: PG I and PG II. PG I is mainly synthesized by chief cells and cervical mucous cells, while PG II can be synthesized by gastric antrum mucous cells and proximal duodenal Brunner glands, in addition to chief cells and cervical mucous cells[16]. Synthesized PG I and PG II are mainly secreted into the gastric cavity, but a zymogen level of approximately 5% can be reversed and diffuse into the blood, which allows it to be detected in the blood. Studies have shown that the level of PG I can reflect the secretory function of gastric glands to a certain extent, and its level is positively correlated with the maximum secretion of gastric acid but negatively correlated with the degree of gastric body inflammation and atrophy[17]. An increase in the level of PG II suggests an inflammatory response in the gastric mucosa, while a decrease in the level of PG I suggests atrophy of the gastric corpus[13]. When the gastric mucosa atrophies and develops severe injury, the number of gastric glands and fundic glands will decrease or be replaced by pyloric glands, and the pyloric glands lack gastric chief cells and cervical mucous cells, which will lead to a decreases in the level of PG I and the ratio of PG I/II[18]. In our study, the result was 1.3 (4.31, 6.26) in the gastric polyp group and 0.67 (2.98, 4.26) in the gastric cancer group. The AUC was 0.628, which has certain clinical significance in the early diagnosis of gastric cancer.
Cytokines are important in the diagnosis of gastric cancer. Cytokines are small molecular proteins secreted by cells in response to various stimuli that can exert biological effects by binding to specific receptors on target cells[19]. Cytokine production and cellular immune function are important in the occurrence and development of tumors and have certain diagnostic and prognostic value in gastric di
There are still some limitations in this study. First, the detection indices were only examined in the gastric polyp and gastric cancer groups, and a healthy control group was not evaluated. Second, the stage of gastric cancer was not evaluated and should be evaluated in future studies. Third, the sample size of the gastric polyp group was relatively small, which may cause bias in this study.
In summary, we developed an index model that included CA724, P and IMA to distinguish between gastric cancer and gastric polyps. After validation, when compared to the conventional index CA724, the panel showed improvements in detecting gastric cancer and may be a potential discriminating method for use in clinical practice.
Early detection and diagnosis are crucial for the prevention and treatment of gastric cancer in clinical practice.
Blood index panels have been shown to improve the diagnostic value in many studies compared with single indices.
We aimed to develop a blood index panel that can improve the diagnostic value for discriminating gastric cancer and gastric polyps.
Tumor-related detection indices, clinical biochemical indices and cytokine indices were analyzed in samples from 139 gastric cancer patients and 40 gastric polyp patients for model building. An additional 68 gastric cancer patients and 22 gastric polyp patients were enrolled for validation.
Carbohydrate antigen (CA) 724, phosphorus (P) and ischemia-modified albumin were included in the blood index panel, and the area under the curve (AUC) index of the panel was 0.829 (0.754, 0.905). After validation, the AUC index was 0.811 (0.700, 0.923). Compared to the conventional CA724 used in the training and validation, the AUC index was 0.704 (0.617, 0.791) and 0.779 (0.668, 0.890). The blood index panel showed significantly increased diagnostic value.
We have developed a potential method for differentiating gastric cancer and gastric polyps based on a blood index panel. this tool may be helpful in clinical practice.
A healthy control group and stage of gastric cancer should be evaluated in future studies, and a larger sample size should be used.
| 1. | Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | den Hoed CM, Kuipers EJ. Gastric Cancer: How Can We Reduce the Incidence of this Disease? Curr Gastroenterol Rep. 2016;18:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Strong VE. Progress in gastric cancer. Updates Surg. 2018;70:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric Cancer: Where Are We Heading? Dig Dis. 2020;38:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 5. | Baretton GB, Aust DE. [Current biomarkers for gastric cancer]. Pathologe. 2017;38:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J Natl Cancer Inst. 2018;110:803-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 7. | Filik H, Avan AA. Electrochemical and Electrochemiluminescence Dendrimer-based Nanostructured Immunosensors for Tumor Marker Detection: A Review. Curr Med Chem. 2021;28:3490-3513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 9. | Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 386] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 10. | Gong X, Zhang H. Diagnostic and prognostic values of anti-helicobacter pylori antibody combined with serum CA724, CA19-9, and CEA for young patients with early gastric cancer. J Clin Lab Anal. 2020;34:e23268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Ding J, Zhang H, Wu Z. Study on the Differential Value of Tumor Marker CA724 on Primary Gastric Cancer. J Oncol. 2021;2021:2929233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Chen XZ, Zhang WK, Yang K, Wang LL, Liu J, Wang L, Hu JK, Zhang B, Chen ZX, Chen JP, Zhou ZG, Mo XM. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39:9031-9039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Zeyaullah M, AlShahrani AM, Ahmad I. Association of Helicobacter pylori Infection and Host Cytokine Gene Polymorphism with Gastric Cancer. Can J Gastroenterol Hepatol. 2021;2021:8810620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Perez-Perez GI, Garza-Gonzalez E, Portal C, Olivares AZ. Role of cytokine polymorphisms in the risk of distal gastric cancer development. Cancer Epidemiol Biomarkers Prev. 2005;14:1869-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Wong HL, Rabkin CS, Shu XO, Pfeiffer RM, Cai Q, Ji BT, Yang G, Li HL, Rothman N, Gao YT, Zheng W, Chow WH. Systemic cytokine levels and subsequent risk of gastric cancer in Chinese Women. Cancer Sci. 2011;102:1911-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Venerito M, Link A, Rokkas T, Malfertheiner P. Gastric cancer - clinical and epidemiological aspects. Helicobacter. 2016;21 Suppl 1:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Nunobe S, Ida S. Current status of proximal gastrectomy for gastric and esophagogastric junctional cancer: A review. Ann Gastroenterol Surg. 2020;4:498-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Yuan P, Lin L, Zheng K, Wang W, Wu S, Huang L, Wu B, Chen T, Li X, Cai L. Risk factors for gastric cancer and related serological levels in Fujian, China: hospital-based case-control study. BMJ Open. 2020;10:e042341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218-e228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 20. | Yoo SY, Lee SY, Yoo NC. Cytokine expression and cancer detection. Med Sci Monit. 2009;15:RA49-RA56. [PubMed] |
| 21. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 617] [Reference Citation Analysis (1)] |
| 22. | Loo SW, Pui TS. Cytokine and Cancer Biomarkers Detection: The Dawn of Electrochemical Paper-Based Biosensor. Sensors (Basel). 2020;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 724] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 24. | Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1464] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 25. | Salomon BL, Leclerc M, Tosello J, Ronin E, Piaggio E, Cohen JL. Tumor Necrosis Factor α and Regulatory T Cells in Oncoimmunology. Front Immunol. 2018;9:444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 26. | Philipp S, Sosna J, Adam D. Cancer and necroptosis: friend or foe? Cell Mol Life Sci. 2016;73:2183-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brochard C, France; Yumiba T, Japan S-Editor: Wang JL L-Editor: A P-Editor: Wang JL