Published online Aug 27, 2022. doi: 10.4240/wjgs.v14.i8.788
Peer-review started: February 11, 2022
First decision: April 19, 2022
Revised: April 30, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: August 27, 2022
Processing time: 193 Days and 21.7 Hours
In recent years, the incidence of types II and III adenocarcinoma of the esophagogastric junction (AEG) has shown an obvious upward trend worldwide. The prognostic prediction after radical resection of AEG has not been well established.
To establish a prognostic model for AEG (types II and III) based on routine markers.
A total of 355 patients who underwent curative AEG at The First Affiliated Hospital of Anhui Medical University from January 2014 to June 2015 were retrospectively included in this study. Univariate and multivariate analyses were performed to identify the independent risk factors. A nomogram was constructed based on Cox proportional hazards models. The new score models was analyzed by C index and calibration curves. The receiver operating characteristic (ROC) curve was used to compare the predictive accuracy of the scoring system and tumor-node-metastasis (TNM) stage. Overall survival was calculated using the Kaplan-Meier curve amongst different risk AEG patients.
Multivariate analysis showed that TNM stage (hazard ratio [HR] = 2.286, P = 0.008), neutrophil-to-lymphocyte ratio (HR = 2.979, P = 0.001), and body mass index (HR = 0.626, P = 0.026) were independent prognostic factors. The new scoring system had a higher concordance index (0.697), and the calibration curves of the nomogram were reliable. The area under the ROC curve of the new score model (3-year: 0.725, 95% confidence interval [CI]: 0.676-0.777; 5-year: 0.758, 95%CI: 0.708-0.807) was larger than that of TNM staging (3-year: 0.630, 95%CI: 0.585-0.684; 5-year: 0.665, 95%CI: 0.616-0.715).
Based on the serum markers and other clinical indicators, we have developed a precise model to predict the prognosis of patients with AEG (types II and III). The new prognostic nomogram could effectively enhance the predictive value of the TNM staging system. This scoring system can be advantageous and helpful for surgeons and patients.
Core Tip: Based on the serum markers and other clinical indicators, we developed a precise model to predict the prognosis of patients with adenocarcinomas of the esophagogastric junction (types II and III). This scoring system can be advantageous for surgeons and patients.
- Citation: Wei ZJ, Qiao YT, Zhou BC, Rankine AN, Zhang LX, Su YZ, Xu AM, Han WX, Luo PQ. Model established based on blood markers predicts overall survival in patients after radical resection of types II and III adenocarcinoma of the esophagogastric junction. World J Gastrointest Surg 2022; 14(8): 788-798
- URL: https://www.wjgnet.com/1948-9366/full/v14/i8/788.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i8.788
Adenocarcinomas of the esophagogastric junction (AEG), which are located within 5 cm of the esophagogastric junction, are classified into three subgroups: Types I, II, and III. Type I AEG (adenocarcinoma of the distal esophagus) is most prevalent in Western countries; types II and III AEG are more prevalent than type I in Asia and are mostly treated as gastric cancer[1,2]. The incidence rate of AEG has significantly increased over the past two decades and is increasing more rapidly than any other type of neoplasm[3,4].
Surgery is considered the only curative treatment for patients with AEG; however, the survival rate is not good even with surgery[5].
At present, many studies are exploring non-invasive and sensitive biomarkers that can accurately predict the prognosis of patients with AEG. Among these, carcinoembryonic antigen (CEA) has been used for the early diagnosis of cancer[6]. Cancer-related systemic inflammatory responses, such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), play an important role in the progression and outcome of tumors[7,8]. Patients with a high NLR have a poor prognosis[9]. Malnutrition is also related with the prognosis of patients; however, few studies have assessed the predictive value of inflammatory, nutritional, and blood tumor markers for overall survival (OS) in patients with AEG (types II and III)[10]. This research established a nomogram to explore the value of blood markers.
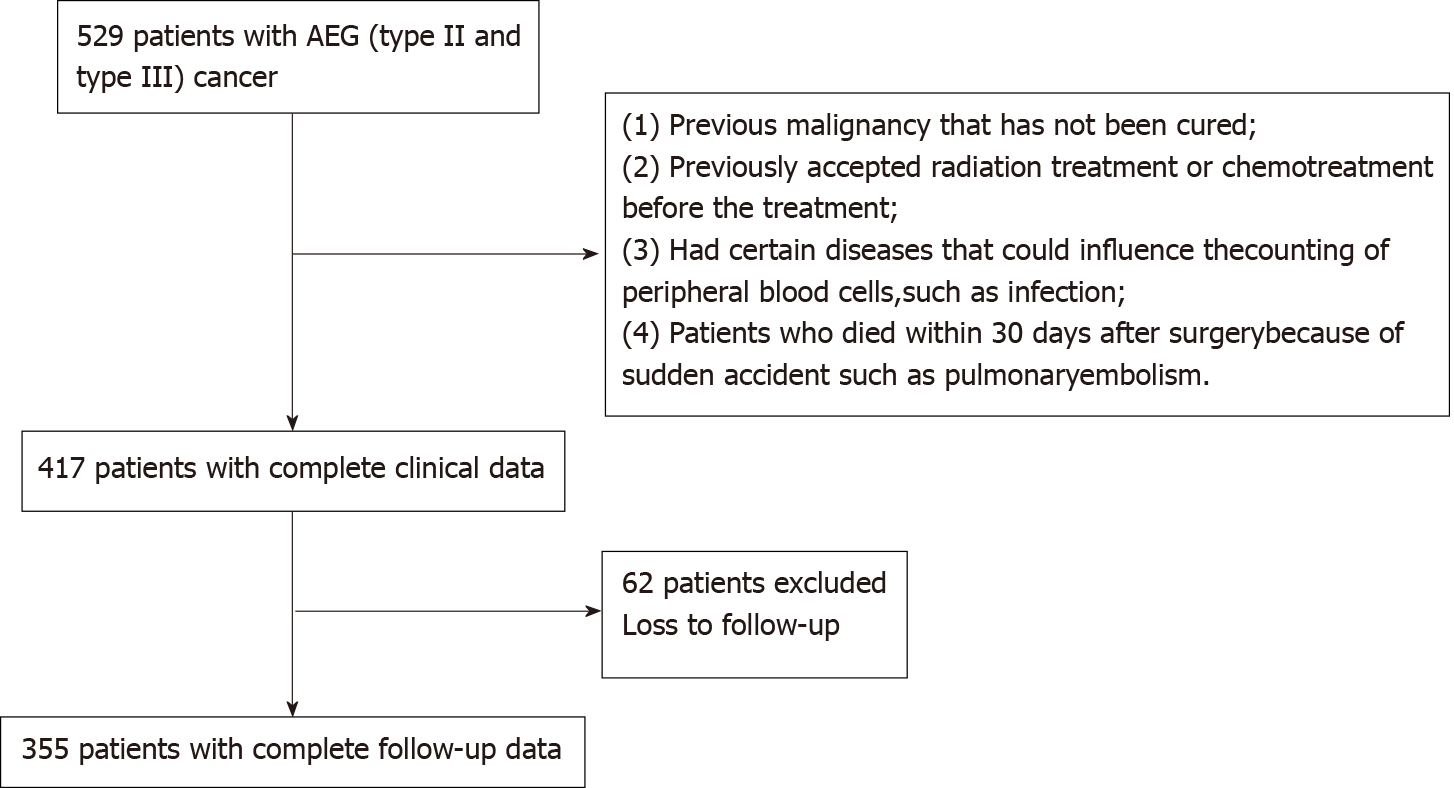
We collected blood and clinical data of patients with AEG (types II and III) who were hospitalized at the First Affiliated Hospital of Anhui Medical University between January 2014 and June 2015. Patients were analyzed retrospectively according to the inclusion and exclusion criteria. The inclusion criteria were as follows: (1) Patients confirmed with AEG (types II and III) by pathological diagnosis; (2) Radical resection of the tumor; (3) Absence of heart diseases or organ failure; and (4) Peripheral blood test results obtained within 1 wk before surgery. The exclusion criteria were as follows: (1) Previously untreated malignancy; (2) Previously accepted radiation treatment or chemotherapy before the treatment; (3) Presence of certain diseases, such as infection, which could influence the peripheral blood cell counts; (4) Patients who died within 30 d after surgery because of sudden accidents, such as pulmonary embolism; and (5) Patients with incomplete data. In accordance with the inclusion criteria, 440 patients with AEG were included in the study. Finally, a cohort of 355 patients was analyzed based on the exclusion criteria. The patient admission process is shown in Supplementary Figure 1. This study was conducted conforming to the TRIPOD guidelines. This study included 355 patients and the testing group, including 120 patients, who were hospitalized at the First Affiliated Hospital of Anhui Medical University between January 2018 and June 2018.
Data on patients’ demographic and clinicopathological features were gathered from the medical records of our hospital, including age, gender, body mass index (BMI), tumor size, differentiation grade, tumor-node-metastasis (TNM) stage, tumor location, surgery time, cancerous node, smoking, and comorbidities. The pathological tumor stage was categorized according to the 7th edition of the American Joint Committee on Cancer TNM staging system. The routine laboratory data evaluated were as follows: Neutrophil, lymphocyte, and platelet counts; prealbumin, albumin, hemoglobin, CEA, CA199, and fibrinogen levels.
Peripheral blood tests were performed within 1 wk before surgery, and the following indices were determined: NLR, PLR, and prognostic nutritional index (PNI). The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count, and the PLR was calculated by dividing the absolute platelet count by the absolute lymphocyte count. The PNI was calculated as serum albumin (g/L) + 5 × total lymphocyte count (109/L)[11]. The NLR, PLR, and PNI were grouped into low and high groups according to the Youden index (maximum [sensitivity + specificity-1])[12]. The BMI (kg/m2) was divided into the following three groups: < 18.5 (low group), 18.5-24.9 (normal group), and ≥ 25 (high group). The CEA, CA199, albumin and prealbumin levels were grouped based on their normal values.
All patients with Siewert type II/III AEG underwent radical surgery with celiac and mediastinal lymphadenectomy. All the patients underwent radical D2 lymphadenectomy. They received four to six cycles of first-line adjuvant combination chemotherapy after surgery with oxaliplatin plus 5-fluorouracil/leucovorin or a prodrug of 5-fluorouracil (capecitabine; CapeOX).
Multivariate and univariate survival analyses were performed using the Cox proportional hazard pattern. Harrell’s concordance index (C-index) was used in the nomogram to evaluate the model performance for the prognosis of patients with AEG. Calibration and receiver operating characteristic (ROC) curves were used to verify the accuracy of the new scoring system. Survival analysis was compared using Kaplan-Meier method, and the nomogram was constructed using the R package “rms,” “Hmisc,” “lattice,” “Formula,” and “foreign.” The data are presented using the Statistical Package for the Social Sciences software (16.0 version) and RStudio software (version 1.1.447- 2009-2018; RStudio, Inc.). A P value < 0.05 was considered statistically significant.
The baseline characteristics of 355 patients are presented in Table 1. Overall, 281 (79.1%) male and 74 (20.9%) female patients were included. The median age of the patients was 65 years (range, 29-85 years). The median follow-up period was 52 mo (range, 1.5-72 mo).
| Characteristic | Surviving | Dead |
| Gender | ||
| Male | 148 (78.3) | 134 (80.7) |
| Female | 41 (21.7) | 32 (19.3) |
| Age (yr) | 65.00 (60.00-71.00) | 63.00 (59.00-69.25) |
| Tumor size | 5.00 (4.00-7.00) | 4.00 (2.50-5.50) |
| TNM stage | ||
| I-II | 49 (25.9) | 105 (63.3) |
| III | 140 (74.1) | 61 (36.7) |
| Differentiation grade | ||
| Low | 59 (31.2) | 70 (42.2) |
| High | 130 (68.8) | 96 (57.8) |
| BMI (kg/m2) | 21.23 (19.88-23.85) | 22.96 (20.96-25.00) |
| Tumor location | ||
| Siewert II | 104 (55.0) | 98 (59.0) |
| Siewert III | 85 (45.0) | 68 (41.0) |
| NLR | 2.37 (1.61-3.62) | 2.20 (1.55-2.86) |
| PLR | 122.75 (87.98-182.94) | 108.03 (81.43-152.54) |
| CEA | 3.60 (1.95-9.30) | 2.20 (1.44-6.85) |
| CA199 | 10.34 (5.64-20.26) | 9.88 (5.75-16.88) |
| PNI | 48.80 (45.30-53.15) | 50.35 (47.20-53.45) |
| Albumin | 41.60 (38.40-44.80) | 42.40 (39.48-44.30) |
| Prealbumin | 187.00 (153.50-234.00) | 239.50 (201.75-264.25) |
| Neutrophil count | 3.41 (2.72-4.53) | 3.26 (2.38-4.48) |
| Platelet count | 188.00 (143.00-235.50) | 176.00 (145.00-219.50) |
| Lymphocyte count | 1.43 (1.10-1.82) | 1.63 (1.26-1.97) |
Table 2 shows the results of univariate risk factors. Age, prealbumin, TNM stage, tumor size, histological type, CEA, PNI, PLR, NLR, BMI, hemoglobin, and cancerous nodes were significant indicators. The variables with a P value < 0.05, as determined by the univariate analysis, were included in the multivariate analysis. Among them, TNM stage (hazard ratio [HR] = 2.286, P = 0.008), NLR (HR = 2.979, P = 0.001), and BMI (HR = 0.626, P = 0.026) were independent prognostic factors (Table 3).
| Characteristic | Coefficient | HR (95%CI) | P value |
| Gender (men/women as reference) | 0.078 | 1.081 (0.765, 1.528) | 0.660 |
| Age | 0.019 | 1.019 (1.002, 1.037) | 0.031 |
| NLR | 0.176 | 1.193 (1.112, 1.280) | < 0.001 |
| Tumor size | 0.178 | 1.195 (1.134, 1.260) | < 0.001 |
| TNM stage | 1.042 | 2.836 (2.046, 3.930) | < 0.001 |
| Histologic type | 0.390 | 1.477 (1.086, 2.009) | 0.013 |
| CA199 | 0.000 | 1.000 (0.998, 1.002) | 0.948 |
| PNI | -0.034 | 0.966 (0.940, 0.993) | 0.013 |
| PLR | 0.003 | 1.003 (1.001, 1.005) | 0.009 |
| Fibrinogen | 0.010 | 1.030 (0.970, 1.095) | 0.332 |
| Albumin | -0.289 | 0.557 (0.479, 1.008) | 0.056 |
| Prealbumin | -0.102 | 0.362 (0.271, 0.484) | < 0.001 |
| Surgery time | 0.017 | 1.017 (0.755, 1.369) | 0.912 |
| BMI | -0.580 | 0.560 (0.431, 0.727) | < 0.001 |
| Cancerous node | 0.219 | 1.245 (1.150, 1.347) | < 0.001 |
| Hemoglobin | -0.006 | 0.994 (0.988, 1.000) | 0.033 |
| Tumor location | 0.719 | 1.127 (0.855, 1.487) | 0.397 |
| Smoking | 0.006 | 0.994 (0.970, 1.019) | 0.624 |
| Comorbidities | 0.017 | 0.983 (0.953, 1.013) | 0.264 |
| Characteristic | Coefficient | HR (95%CI) | P value |
| TNM stage | 0.827 | 2.286 (1.236, 4.227) | 0.008 |
| BMI | -0.470 | 0.625 (0.413, 0.946) | 0.026 |
| NLR | 1.092 | 2.979 (1.565, 5.674) | 0.001 |
| CEA | 0.008 | 1.008 (0.997, 1.019) | 0.143 |
| Age | 0.031 | 0.970 (0.556, 1.691) | 0.914 |
| Tumor size | 0.143 | 1.154 (0.651, 2.045) | 0.624 |
| PNI | 0.347 | 1.415 (0.783, 2.557) | 0.250 |
| PLR | 0.040 | 1.041 (0.567, 1.912) | 0.897 |
| Hemoglobin | 0.197 | 0.821 (0.479, 1.408) | 0.474 |
| Prealbumin | 0.122 | 0.885 (0.496, 1.578) | 0.678 |
| Differentiation grade | 0.073 | 1.075 ( 0.630, 1.836) | 0.791 |
| Cancerous node | 0.084 | 1.088 (0.587, 2.016) | 0.789 |
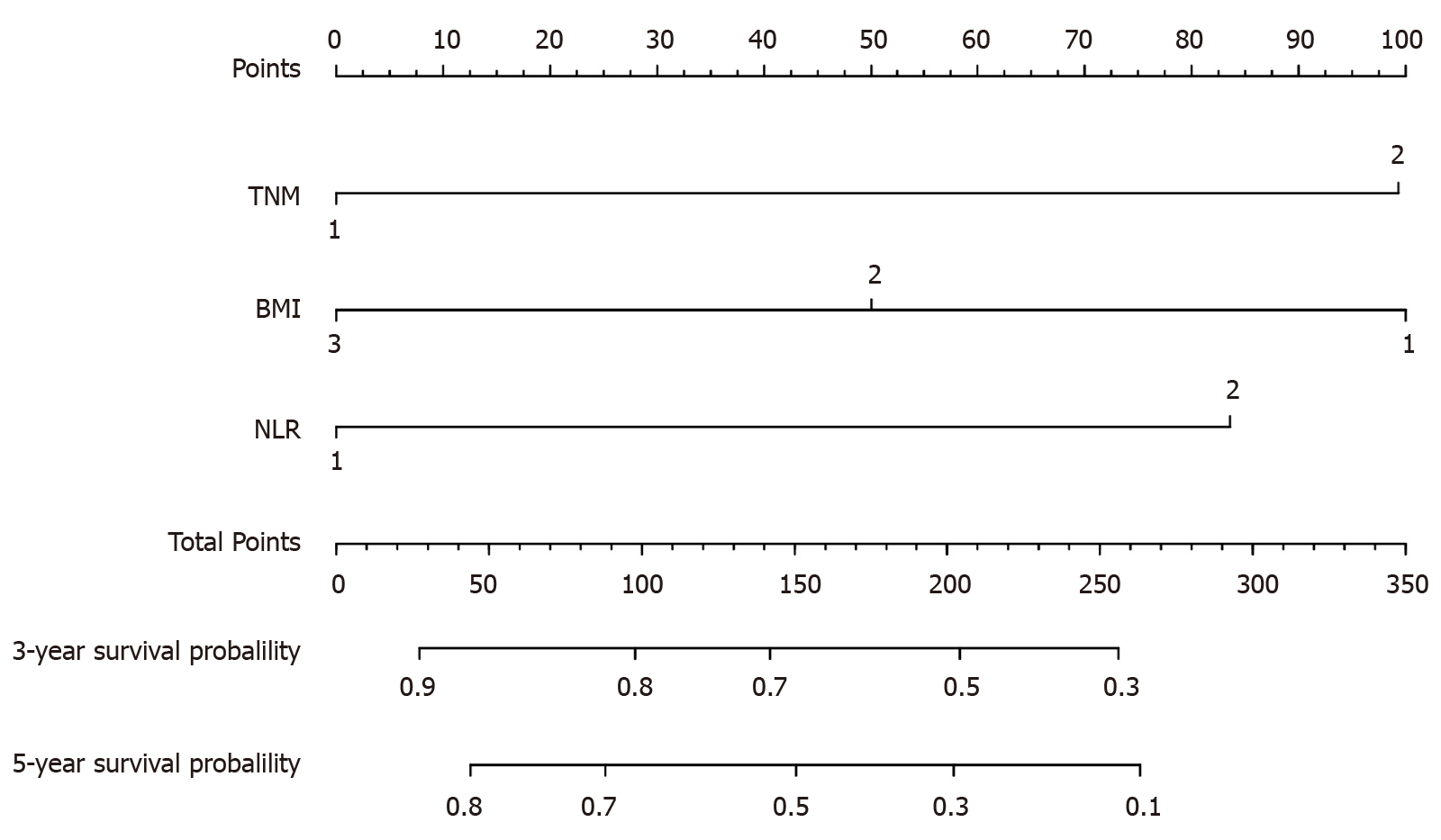
A model was constructed to predict OS of AEG patients based on the Cox analysis (Figure 1). Each subgroup variable was assigned a score. A scoring system was used to assign a score to each variable (Table 4). To apply the nomogram, a vertical line was delineated to indicate the row to assign point values for each variable. Subsequently, the corresponding scores were summed to obtain the total score. Finally, a vertical line from the total point was drawn to obtain the 3-year and 5-year survival probability.
| NLR | Points | TNM stage | Points | BMI | Points |
| Low (1) | 0 | I and II (1) | 0 | Low (1) | 0 |
| High (2) | 26 | III and IV (2) | 20 | Normal (2) | 58 |
| High (3) | 100 |
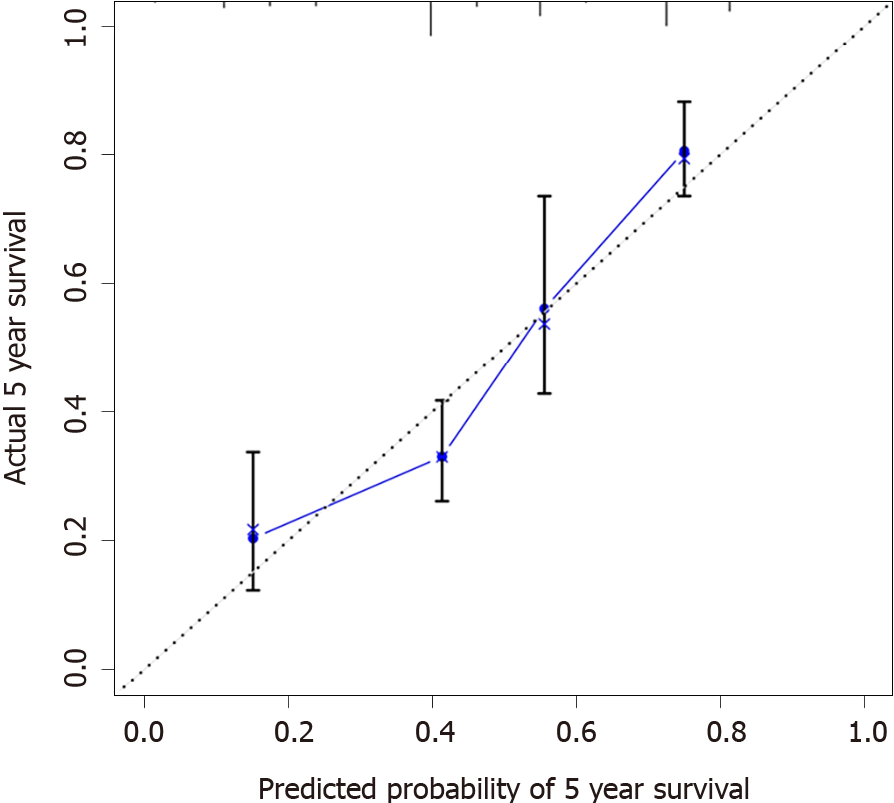
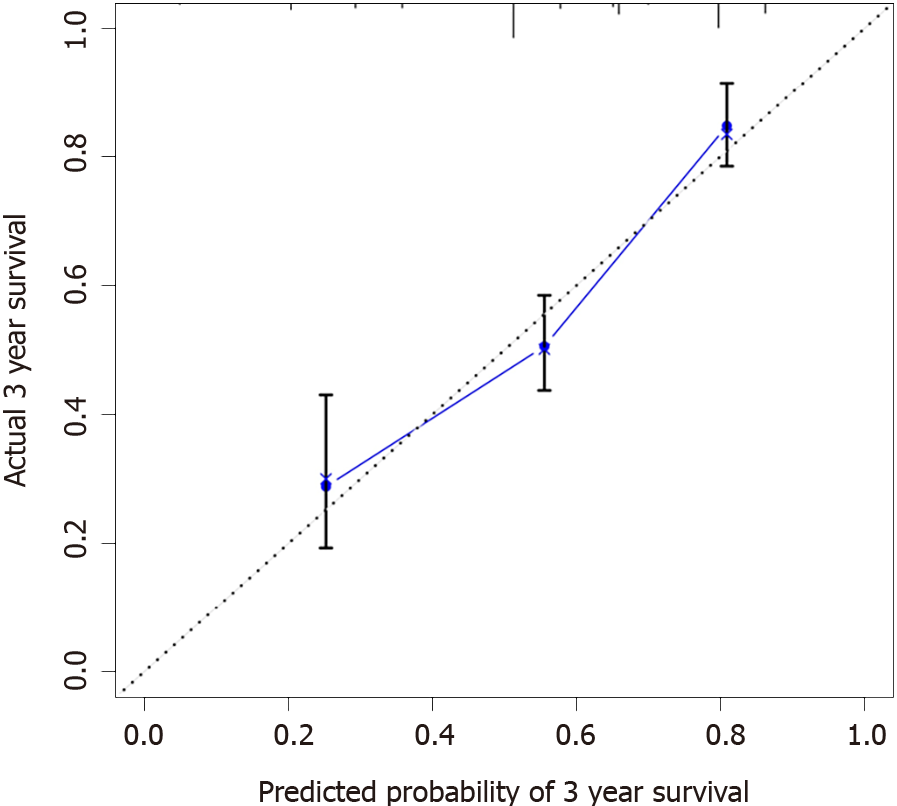
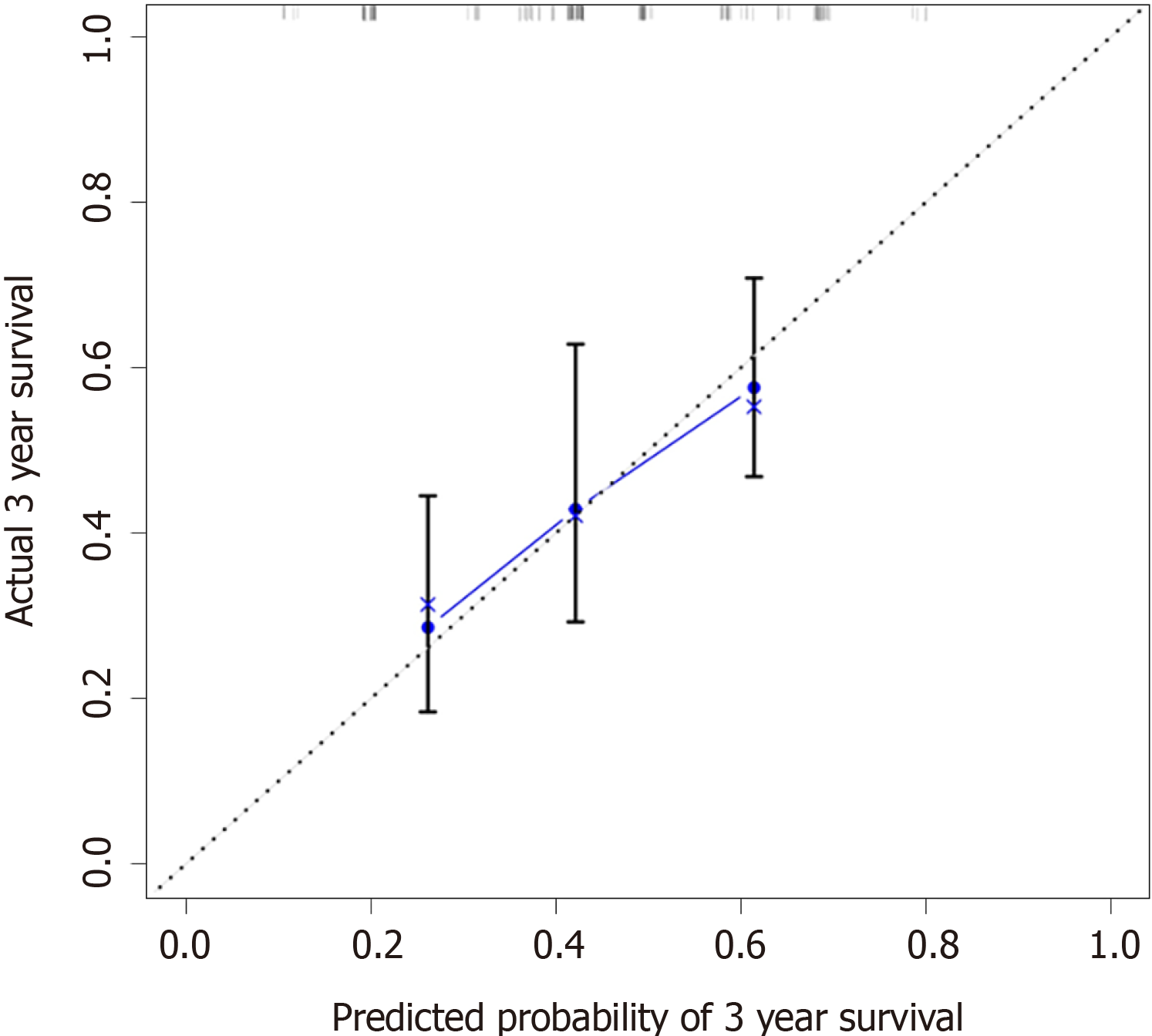
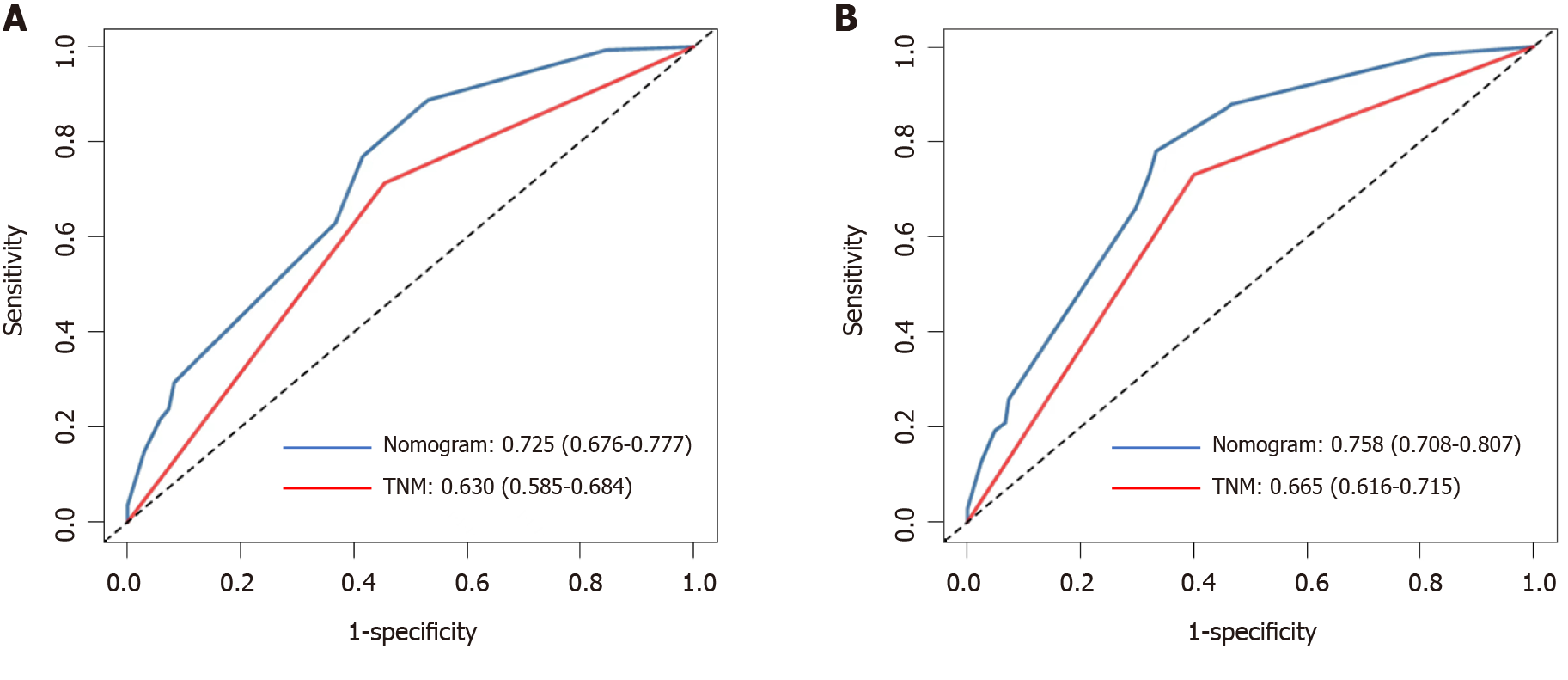
Calibration curves were used to verify the performance of the model in predicting OS of patients with AEG (Figures 2 and 3), and the results showed that the actual OS curve of the nomogram fits the predicted OS curve. Besides, the calibration curve in the testing group for 3-year OS was also good (Figure 4), and the C-index of the model was 0.697 (95% confidence interval [CI]: 0.660-0.734), indicating that this model was reliable. Besides, the area under the ROC curve (AUC) of the new score model (3-year: 0.725, 95%CI: 0.676-0.777; 5-year: 0.758, 95%CI: 0.708-0.807) was larger than that of the TNM stage (3-year: 0.630, 95%CI: 0.585-0.684; 5-year: 0.665, 95%CI: 0.616-0.715) (Figures 5 and 6), which indicated that the constructed nomogram was a reliable scoring system.
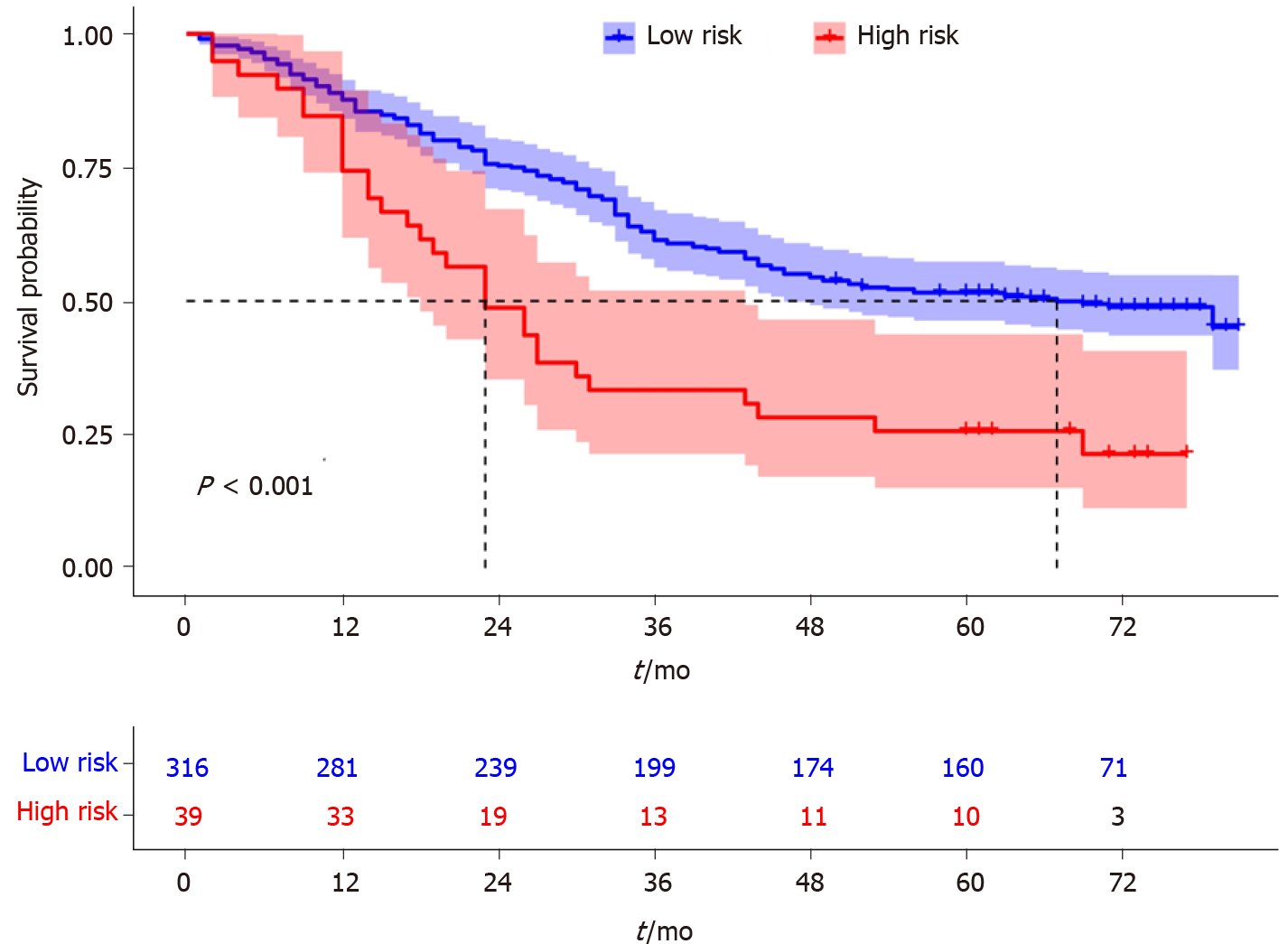
In addition, we divided the patients into two groups according to the total nomogram score (low-risk: < 58 and high-risk: ≥ 58) (Figure 7). The results showed that high-risk patients with AEG had a poor prognosis. The Kaplan-Meier curves indicated that the nomogram had excellent results in predicting survival.
Early detection of AEG is often difficult, owning to the limitations of diagnostic techniques, resulting in a poor prognosis. At present, the 5-year survival rate of patients with AEG is less than 30%[13]. The epidemiology, genetics, spread pattern, and prognosis of neoplasms in the esophagus, esophagogastric junction, and stomach remain unclear. The process of tumor development is complex. Gastroesophageal reflux disease and Helicobacter pylori have been reported as risk factors for AEG[14,15]. Therefore, many researchers have made significant contributions to improve the prognosis of AEG. Lymph node metastasis, tumor size, differentiation grade, and TNM stage have been defined as prognostic factors[16,17]. However, these prognostic factors are difficult to judge before surgery; therefore, research on prognostic serum markers has been widely conducted in recent years. To the best of our knowledge, this study is the first attempt to develop a prognostic nomogram that combines serum markers (including inflammatory markers, nutritional indices, and tumor markers) and clinicopathological characteristics to estimate the 3-year and 5-year survival probability, which was highly accurate in predicting the prognosis of patients with AEG (types II and III).
The multivariate analysis revealed that TNM stage, NLR, and BMI were important factors. Therefore, a model was built by these markers. Moreover, the calibration and ROC curves showed that the nomogram was reliable and precise.
In recent years, nomogram has been used to predict the prognosis of many cancers[18,19]. This model has been identified as a new standard that can integrate multiple predictive variables in a weighted manner and intuitively show the influence of variables on individual predictive values. Similar conclusions were obtained in the present study. The AUC of the nomogram was larger than that of TNM stage; therefore, the nomogram and TNM staging system can help in predicting the survival of patients with AEG. Furthermore, this nomogram can be applied in clinical practice to help surgeons evaluate the prognosis of patients and choose appropriate treatment.
Our nomogram contained three variables, and previous studies also got to the same conclusion[9,20]. Inflammatory indexes were related with the prognosis of gastrointestinal cancer patients[21]. This research found that NLR was an independent risk factor, and the possible mechanism is that systemic inflammation caused by tumors can release a large number of pro-inflammatory mediators, such as C-reactive protein, fibrinogen, vascular endothelial growth factor, and transforming growth factor-α. These factors stimulate the process of tumors[22]. Meanwhile, neutrophils could prevent natural killer cells and T cells in the system contacting and killing the tumor cells[23,24]. Therefore, the NLR should be included in the regular assessment index for patients with AEG.
As an independent prognostic indicator of tumor-related diseases, BMI has raised increasing concerns for researchers in recent years. BMI is related to the prognosis of breast carcinoma, non-small-cell lung cancer, and colorectal cancer, among others[25-27]. In this study, we found that BMI was significantly correlated with the prognosis of patients with AEG. However, the underlying mechanism remains unclear. Patients with AEG with a low BMI may have poor nutritional status and immune function[28]. This may have an adverse effect on disease progression; therefore, these patients may have a shorter OS.
Our research has two potential limitations. First, this study was a single-center study that did not include a sufficient number of cases to verify the results. Second, the included patients who underwent surgical resection for AEG cannot account for all patients with AEG.
TNM stage, NLR, and BMI are risk factors for the prognosis of patients with AEG. The novel nomogram accurately and reliably predicts the OS after radical resection of patients with AEG (types II and III). This may help clinicians formulate personalized treatment plans.
In recent years, the incidence of types II and III adenocarcinoma of the esophagogastric junction (AEG) has shown an obvious upward trend worldwide.
The prognostic prediction after radical resection of AEG has not been well established.
To establish a prognostic model for AEG (types II and III) based on routine markers.
The construction of the nomogram was based on Cox proportional-hazards models. The new score model was analyzed by C index and calibration curves. The receiver operating characteristic (ROC) curve was used to compare the predictive accuracy of the scoring system and tumor-node-metastasis (TNM) staging. Overall survival (OS) was calculated using the Kaplan-Meier curve amongst different risk AEG patients.
Multivariate analysis showed that TNM stage (hazard ratio [HR] = 2.286, P = 0.008), neutrophil-to-lymphocyte ratio (NLR) (HR = 2.979, P = 0.001), and body mass index (BMI) (HR = 0.626, P = 0.026) were independent prognostic factors. The new scoring system had a higher concordance index (0.697), and the calibration curves of the nomogram were reliable. The area under the ROC curve of the new score model (3-year: 0.725, 95% confidence interval [CI]: 0.676-0.777; 5-year: 0.758, 95%CI: 0.708-0.807) was larger than that of TNM staging (3-year: 0.630, 95%CI: 0.585-0.684; 5-year: 0.665, 95%CI: 0.616-0.715).
This model has been identified as a new standard that can integrate multiple predictive variables in a weighted manner and intuitively show the influence of variables on individual predictive values. To the best of our knowledge, this study is the first attempt to develop a prognostic nomogram that combines serum markers (including inflammatory markers, nutritional indices, and tumor markers) and clinicopathological characteristics to estimate the 3-year and 5-year survival probability, which is highly accurate in predicting the prognosis of patients with AEG (types II and III). TNM stage, NLR, and BMI were risk factors for the prognosis of patients with AEG and then a model was built which can predict the prognosis of patients.
The novel nomogram accurately and reliably predicts the OS after radical resection of patients with AEG (types II and III). This may help clinicians formulate personalized treatment plans.
| 1. | Kumamoto T, Kurahashi Y, Niwa H, Nakanishi Y, Okumura K, Ozawa R, Ishida Y, Shinohara H. True esophagogastric junction adenocarcinoma: background of its definition and current surgical trends. Surg Today. 2020;50:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Huang Q, Fan X, Agoston AT, Feng A, Yu H, Lauwers G, Zhang L, Odze RD. Comparison of gastro-oesophageal junction carcinomas in Chinese versus American patients. Histopathology. 2011;59:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Keeney S, Bauer TL. Epidemiology of adenocarcinoma of the esophagogastric junction. Surg Oncol Clin N Am. 2006;15:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Liu K, Yang K, Zhang W, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Schröder W, Bruns CJ. [Surgical strategies for carcinoma of the cardia (AEG type II) ]. Chirurg. 2019;90:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Liu XB, Gao ZY, Zhang QH, Pandey S, Gao B, Yang F, Tong Q, Li SB. Preoperative Neutrophil Lymphocyte Ratio Can Be Used as a Predictor of Prognosis in Patients With Adenocarcinoma of the Esophagogastric Junction: A Systematic Review and Meta Analysis. Front Oncol. 2020;10:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Chen Y, Jin M, Shao Y, Xu G. Prognostic Value of the Systemic Inflammation Response Index in Patients with Adenocarcinoma of the Oesophagogastric Junction: A Propensity Score-Matched Analysis. Dis Markers. 2019;2019:4659048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Zhang L, Su Y, Chen Z, Wei Z, Han W, Xu A. The prognostic value of preoperative inflammation-based prognostic scores and nutritional status for overall survival in resected patients with nonmetastatic Siewert type II/III adenocarcinoma of esophagogastric junction. Medicine (Baltimore). 2017;96:e7647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Grace EM, Shaw C, Lalji A, Mohammed K, Andreyev HJN, Whelan K. Nutritional status, the development and persistence of malnutrition and dietary intake in oesophago-gastric cancer: a longitudinal cohort study. J Hum Nutr Diet. 2018;31:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Park SH, Lee S, Song JH, Choi S, Cho M, Kwon IG, Son T, Kim HI, Cheong JH, Hyung WJ, Choi SH, Noh SH, Choi YY. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur J Surg Oncol. 2020;46:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1855] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 13. | Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer. 2010;13:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Chen XF, Zhang B, Chen ZX, Hu JK, Dai B, Wang F, Yang HX, Chen JP. Gastric tube reconstruction reduces postoperative gastroesophageal reflux in adenocarcinoma of esophagogastric junction. Dig Dis Sci. 2012;57:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Gao P, Cai N, Yang X, Yuan Z, Zhang T, Lu M, Jin L, Ye W, Suo C, Chen X. Association of Helicobacter pylori and gastric atrophy with adenocarcinoma of the esophagogastric junction in Taixing, China. Int J Cancer. 2022;150:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Chen J, Xia YJ, Liu TY, Lai YH, Yu JS, Zhang TH, Ooi S, He YL. Development and validation of a survival nomogram for patients with Siewert type II/III adenocarcinoma of the esophagogastric junction based on real-world data. BMC Cancer. 2021;21:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Zheng C, Feng X, Zheng J, Yan Q, Hu X, Feng H, Deng Z, Liao Q, Wang J, Li Y. Lymphovascular Invasion as a Prognostic Factor in Non-Metastatic Adenocarcinoma of Esophagogastric Junction After Radical Surgery. Cancer Manag Res. 2020;12:12791-12799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 19. | He Y, Mao M, Shi W, He Z, Zhang L, Wang X. Development and validation of a prognostic nomogram in gastric cancer with hepatitis B virus infection. J Transl Med. 2019;17:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Huang L, Wei ZJ, Li TJ, Jiang YM, Xu AM. A prospective appraisal of preoperative body mass index in D2-resected patients with non-metastatic gastric carcinoma and Siewert type II/III adenocarcinoma of esophagogastric junction: results from a large-scale cohort. Oncotarget. 2017;8:68165-68179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Gomes de Lima KV, Maio R. Nutritional status, systemic inflammation and prognosis of patients with gastrointestinal cancer. Nutr Hosp. 2012;27:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8428] [Article Influence: 526.8] [Reference Citation Analysis (9)] |
| 23. | Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J. 2013;27:3017-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Vernaci G, Dieci MV, Manfrin S, Mantiero M, Falci C, Faggioni G, Mioranza E, Menichetti A, Tasca G, Griguolo G, Miglietta F, Di Liso E, Saibene T, Michieletto S, Ghiotto C, Conte P, Guarneri V. BMI is an independent prognostic factor for late outcome in patients diagnosed with early breast cancer: A landmark survival analysis. Breast. 2019;47:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yendamuri S, Barbi J, Pabla S, Petrucci C, Punnanitinont A, Nesline M, Glenn ST, Depietro P, Papanicalou-Sengos A, Morrison C, Dy GK, Elkin PL. Body Mass Index Influences the Salutary Effects of Metformin on Survival After Lobectomy for Stage I NSCLC. J Thorac Oncol. 2019;14:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016;20:517-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Ji F, Liang Y, Fu S, Chen D, Cai X, Li S, Peng B, Liang L, Hua Y. Prognostic value of combined preoperative prognostic nutritional index and body mass index in HCC after hepatectomy. HPB (Oxford). 2017;19:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdellateif MS, Egypt; Tangsuwanaruk T, Thailand S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wu RR