Published online May 27, 2022. doi: 10.4240/wjgs.v14.i5.452
Peer-review started: October 24, 2021
First decision: December 27, 2021
Revised: January 17, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: May 27, 2022
Processing time: 212 Days and 15.9 Hours
Neoadjuvant chemotherapy (NACT) combined with surgery is regarded as an effective treatment for advanced gastric cancer (AGC). Laparoscopic surgery represents the mainstream of minimally invasive surgery. Currently, surgeons focus more on surgical safety and oncological outcomes of laparoscopic gastrectomy after NACT. Thus, we sought to evaluate short- and long-term outcomes between laparoscopic total gastrectomy (LTG) and open total gas
To compare the short and long-term outcomes between LTG and OTG for AGC after NACT.
We retrospectively collected the clinicopathological data of 136 patients who accepted gastrectomy after NACT from June 2012 to June 2019, including 61 patients who underwent LTG and 75 who underwent OTG. Clinicopathological characteristics between the LTG and OTG groups showed no significant difference. SPSS 26.0, R software, and GraphPad PRISM 8.0 were used to perform statistical analyses.
Of the 136 patients included, eight acquired pathological complete response, and the objective response rate was 47.8% (65/136). The LTG group had longer operation time (P = 0.015), less blood loss (P = 0.003), shorter days to first flatus (P < 0.001), and shorter postoperative hospitalization days (P < 0.001). LTG spent more surgical cost than OTG (P < 0.001), while total hospitalized cost of LTG was less than OTG (P < 0.001). 21 (28.0%) patients in the OTG group and 14 (23.0%) in the LTG group had 30-d postoperative complications, but there was no significant difference between the two groups (P = 0.503). The 3-year overall survival (OS) rate was 60.6% and 64.6% in the LTG and OTG groups, respectively [hazard ratio (HR) = 0.859, 95% confidence interval (CI): 0.522-1.412, P = 0.546], while the 3-year disease-free survival (DFS) rate was 54.5% and 51.8% in the LTG and OTG group, respectively (HR = 0.947, 95%CI: 0.582-1.539, P = 0.823). Multivariate cox analysis showed that body mass index and pTNM stage were independent risk factors for OS while vascular invasion and pTNM stage were independent risk factors for DFS (P < 0.05).
After NACT, LTG shows comparable 30-d postoperative morbidity as well as 3-year OS and DFS rate to OTG. We recommend that experienced surgeons select LTG other than OTG for proper AGC patients after NACT.
Core Tip: Neoadjuvant chemotherapy (NACT), defined as chemotherapy before surgery, is currently a hot research topic of perioperative therapy for advanced gastric cancer. In this study, we focused on the short- and long-term outcomes between laparoscopic total gastrectomy (LTG) and open total gastrectomy (OTG) after NACT. We found that the LTG group had longer operation time, less blood loss, shorter time to first flatus, and shorter postoperative hospitalization days. LTG showed comparable 30-d postoperative morbidity as well as 3-year overall survival and disease-free survival rate to OTG. Based on our results, we recommend that experienced surgeons select LTG for proper patients after NACT.
- Citation: Cui H, Zhang KC, Cao B, Deng H, Liu GB, Song LQ, Zhao RY, Liu Y, Chen L, Wei B. Short and long-term outcomes between laparoscopic and open total gastrectomy for advanced gastric cancer after neoadjuvant chemotherapy. World J Gastrointest Surg 2022; 14(5): 452-469
- URL: https://www.wjgnet.com/1948-9366/full/v14/i5/452.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i5.452
Gastric cancer (GC) is the fifth most prevalent malignant tumor and its tumor-related death ranks fourth according to the updated database of GLOBOCAN in 2020[1]. In China, it is the second most lethal tumor[2]. Perioperative integrated therapy is gradually taken into account in the treatment of GC. Neoadjuvant chemotherapy (NACT), as a crucial part of integrated therapy, is currently a hot research topic. Unlike postoperative chemotherapy, NACT puts chemotherapy prior to surgery, which brings advantages as follows: (1) More possibility of reducing tumor stages and increasing R0 resection rate[3]; (2) Better tolerance to chemotherapy before surgery; (3) Identical surgical safety compared with surgery-first therapy[4,5]; (4) High complete rate of total chemotherapy; and (5) Potential survival benefit relative to other interventional treatments. After MAGIC study[6] first proved the surgical safety and long-term survival benefit of perioperative chemotherapy, more prospective randomized clinical trials like FLOT4[7], RESOLVE[8], and RESONANCE[9] sprung up and acquired the initial conclusion that NACT showed superiority in terms of pathological complete response (pCR) rate and long-term survival. This contributed to its further clinical utilization.
Laparoscopy is a representative of minimally invasive surgery techniques in the 21st century. Since Kitano et al[10] reported the first laparoscopic gastrectomy in 1994, laparoscopy has emerged as a standard surgical approach especially for distal gastrectomy proved by several high-quality trials[11,12].
Laparoscopic total gastrectomy (LTG) was carried out relatively late due to its complex surgical procedure and anastomotic technical difficulty. Although LTG has been proved safer than open total gastrectomy (OTG) for clinical stage I GC by CLASS-02 study[13], the option of LTG is still conservative in the treatment of advanced GC (AGC). At present, a multitude of retrospective articles conducted in experienced medical centers demonstrated comparable short- and long-term outcomes between LTG and OTG[14,15], but prospective studies have not acquired final results.
Currently, surgical safety and oncological outcomes after NACT have gradually attracted surgeons' attention. Based on standardization of NACT for AGC in Western countries, which was advised by European guidelines, van der Wielen et al[16] conducted STOMACH trial as the first multi-institutional RCT study which demonstrated the comparable complication rate and non-inferiority of 1-year overall survival (OS) and disease-free survival (DFS) between LTG and OTG after NACT in Western countries[16]. However, it is still unclear whether LTG has superior short and long-term outcomes compared with OTG or not for AGC patients who accepted NACT in China. As minimally invasive surgery is gaining popularization and great importance is attached to NACT in China, more studies should be conducted for the proper application of LTG after NACT.
This is a retrospective study conducted at the General Surgery Department of the Chinese PLA General Hospital. Clinical and pathological data of patients with AGC who accepted NACT before LTG or OTG plus D2 lymphadenectomy from June 2012 to June 2019 were collected. The eligible criteria were: (1) Clinical tumor stage II-III (including Bulky N or large type 3-4) proved by endoscopic ultrasonography, abdominal computed tomography (CT), and positron emission tomography-CT (PET-CT); (2) Histologically proved gastric adenocarcinoma by preoperative gastroscopy and biopsy; (3) Ages ranging from 18 to 75 years; (4) ASA score ≤ III; (5) Integrated clinical and pathological data; and (6) No conversion to OTG in the LTG group. All patients accepted LTG or OTG followed by NACT (chemotherapeutic regimen: SOX, XELOX, SF, or DCF) according to the consultation of a multi-disciplinary team.
Surgical procedures were conducted according to Japanese Gastric Cancer Treatment Guidelines[17]. D2 lymphadenectomy was performed, including resection of No. 1, 2, 3a, 4sa, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, 11d, and 12a. Dissection of No. 10 lymph nodes was performed when a tumor was located in the upper stomach invading the greater curvature. Roux-en-Y reconstruction was achieved after tumor dissection. One month after surgery, residual adjuvant chemotherapy was carried out under the guidance of surgeons with rich experience.
We retrospectively collected clinicopathologic indicators including blood loss, operation time, time to first flatus (days), postoperative hospitalization days, surgical and hospitalized cost, retrieved lymph nodes, tumor length, etc. The 30-d morbidity and mortality were recorded from case report form and its severe degree was assessed in accordance with the Clavien-Dindo classification[18]. We defined Clavien-Dindo classification ≥ IIIa as severe complication.
Follow-up started 3 mo after operation by outpatient visit or telephone until patients’ death. Frequency of adjuvant chemotherapy, survival status, and recurrence or not were mentioned during inquiries. If patients dropped out, the time of last accessible follow-up or last discharge was defined as cutoff value.
We used SPSS statistical package, version 26 (IBM software), R software, and GraphPad PRISM 8.0 software to perform statistical analyses. Continuous variables are described as mean ± SD for normal distributions, while medians and interquartile ranges are used to represent skew distributions. Comparison tests were performed by the Student’s t test and Mann–Whitney U test as appropriate. Categorical variables are described as frequencies with percent, and Chi square test was performed to demonstrate difference of categorical variables between two groups. Moreover, the difference of perioperative laboratorial index between two groups is vividly presented by line chart and box diagram.
To show long-term oncological outcomes, overall survival and disease-free survival were analyzed using Kaplan-Meier method and log-rank test was used to determine significance. We used univariate cox analyses to explore the related indexes and put indicators with P < 0.10 into multivariate analysis. Multivariate analyses, with backward variable selection, were conducted using the Cox proportional hazards regression model. All tests were two-sided and statistical significance was set at P < 0.05.
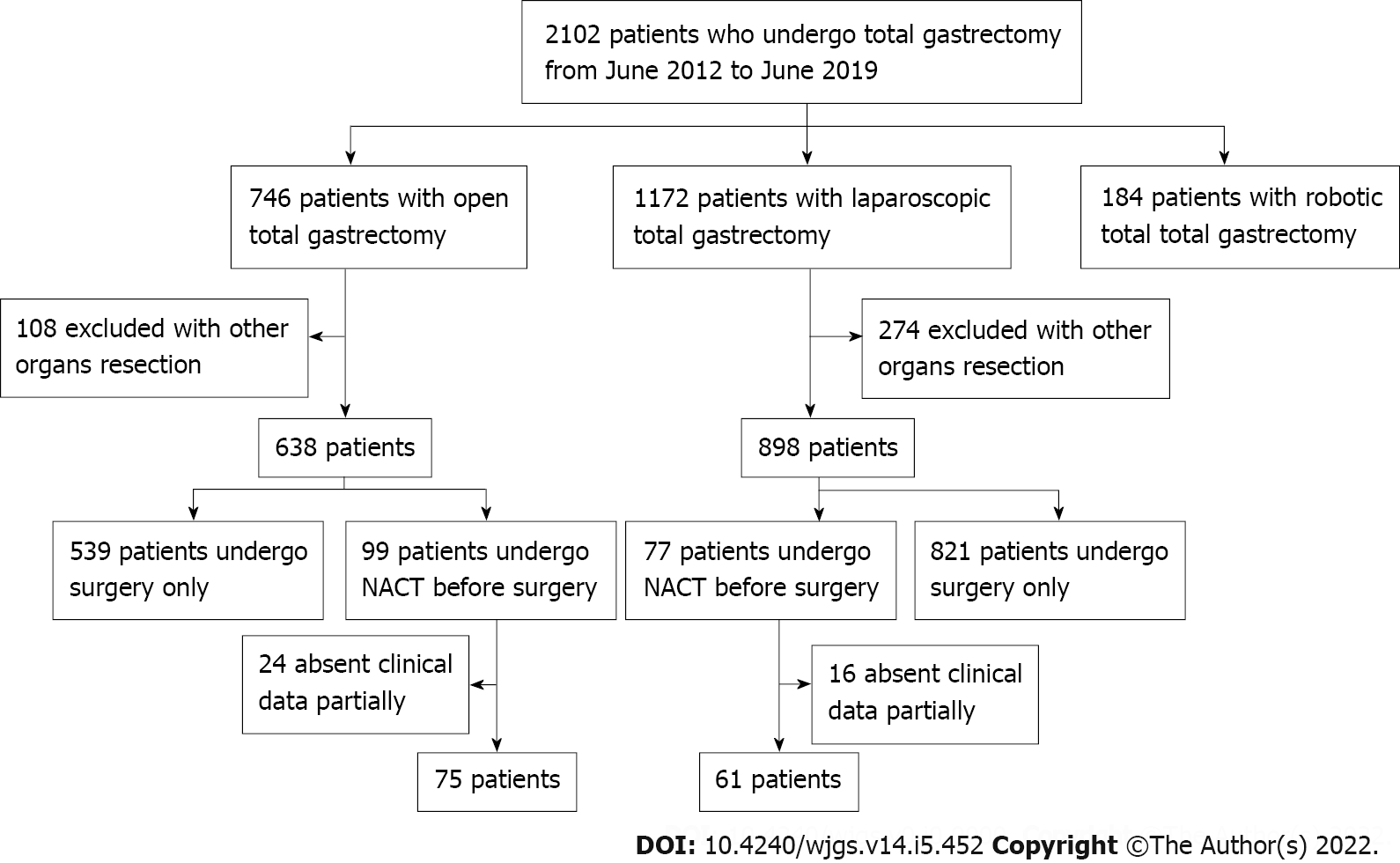
We collected the clinical data of 2102 patients who underwent total gastrectomy from June 2012 to June 2019 at the Chinese PLA General Hospital. After screening as described in Figure 1, 136 patients were included into this case-control study with 61 patients in NACT-LTG group and 75 patients in NACT-OTG group. Clinicopathologic characteristics of patients in the two groups are summarized in Tables 1 and 2. Groups were comparable according to sex, age, body mass index (BMI), comprehensive complication index score, proportion of previous abdominal surgery, tumor diameter, clinical and pathologic TNM stage, tumor location, nerve or vascular invasion, and histological type with no significant difference.
| Clinical characteristic | LTG group (n = 61) | OTG group (n = 75) | P value |
| Gender | 0.821 | ||
| Male | 47 | 59 | |
| Female | 14 | 16 | |
| Age (yr) | 57.56 ± 10.35 | 56.84 ± 11.95 | 0.712 |
| BMI (kg/m2) | 22.81 ± 2.67 | 23.67 ± 3.31 | 0.099 |
| CCI score, n (%) | 0.982 | ||
| 0-2 | 43 | 53 | |
| > 2 | 18 | 22 | |
| History of abdominal surgery | 0.179 | ||
| No | 54 | 60 | |
| Yes | 7 | 15 | |
| Clinical tumor stage | |||
| cT | 0.695 | ||
| T2 | 1 | 6 | |
| T3 | 22 | 23 | |
| T4 | 38 | 46 | |
| cN | 0.191 | ||
| N0 | 7 | 4 | |
| N+ | 54 | 71 | |
| cTNM | 0.468 | ||
| II | 5 | 9 | |
| III | 56 | 66 | |
| Historical factor | 0.088 | ||
| 2012-2015 | 22 | 38 | |
| 2016-2019 | 39 | 37 |
| Pathological characteristic | LTG group (n = 61) | OTG group (n = 75) | P value |
| Tumor diameter, cm (median, IQR) | 4.0 (2.5-6.5) | 4.0 (2.0-6.0) | 0.366 |
| Site of tumor | 0.244 | ||
| Upper 1/3 | 30 | 27 | |
| Middle 1/3 | 21 | 29 | |
| Diffused | 10 | 19 | |
| ypT | 0.751 | ||
| T0 | 1 | 7 | |
| T1 | 5 | 5 | |
| T2 | 10 | 14 | |
| T3 | 34 | 30 | |
| T4 | 11 | 19 | |
| ypN | 0.190 | ||
| N0 | 19 | 35 | |
| N1 | 14 | 11 | |
| N2 | 12 | 11 | |
| N3 | 16 | 18 | |
| ypTNM | 0.300 | ||
| 0 | 1 | 7 | |
| I | 8 | 17 | |
| II | 22 | 16 | |
| III | 29 | 34 | |
| IV | 1 | 1 | |
| Nerve invasion | 0.545 | ||
| Yes | 20 | 21 | |
| No | 41 | 54 | |
| Vascular invasion | 0.982 | ||
| Yes | 18 | 22 | |
| No | 43 | 53 | |
| Differentiation | 0.616 | ||
| Well/moderate | 27 | 30 | |
| Poor/undifferentiated | 34 | 45 |
All the 136 patients accepted NACT before surgery. Among them, 113 patients adopted SOX regimen (48 in LTG group and 65 in OTG group), 17 used XELOX regimen (8 in LTG group and 9 in OTG group), and 6 accepted other regimens like DCF and SF; no significant difference was found in the utilization of chemotherapy regimen between the two groups (P = 0.143). Cycles of NACT was determined mainly by patients’ chemotherapeutic reaction and tumor response, with no significant difference between the two groups (P = 0.467). We recorded adverse events during chemotherapy by patients’ self-report and laboratorial index, and classified severe degree via CTCAE version 4.0. We found that patients in the two groups had comparable adverse events with no significant difference (P = 0.535). The LTG group had significantly longer chemotherapy–surgical procedure interval compared with the OTG group (5.07 ± 1.67 wk vs 4.55 ± 1.33 wk; P = 0.047). There was no significant difference in adjuvant therapy between the two groups (P = 0.545) (Table 3).
| Variable | LTG group (n = 61) | OTG group (n = 75) | P value |
| Number of cycles of NACT | 0.467 | ||
| 1-2 | 13 | 12 | |
| 3-4 | 45 | 59 | |
| > 4 | 3 | 4 | |
| NACT regimen | 0.143 | ||
| SOX | 48 | 65 | |
| XELOX | 8 | 9 | |
| Other | 5 | 1 | |
| Clinical response | 0.659 | ||
| CR | 1 | 7 | |
| PR | 28 | 29 | |
| SD | 28 | 34 | |
| PD | 4 | 5 | |
| Adverse effects after NACT | 0.535 | ||
| Grade 0 | 13 | 17 | |
| Grade I | 16 | 21 | |
| Grade II | 17 | 23 | |
| Grade III | 11 | 12 | |
| Grade IV | 4 | 2 | |
| Chemotherapy–surgical procedure interval (wk) | 5.07 ± 1.67 | 4.55 ± 1.33 | 0.047 |
| Adjuvant therapy | 0.545 | ||
| Yes | 52 | 61 | |
| No | 9 | 14 |
Clinical response was another factor defined in accordance with RECIST criteria[19]. In this study, 8 (5.9%) patients achieved a completed response while 57 (41.9%) had a partial response. However, other patients did not have obvious downstage after NACT and were defined as stable disease (62 patients) and progressive disease (9 patients).
Of 58 (95.1%) patients in the LTG group and 74 (98.7%) patients in the OTG group acquired R0 resection (P = 0.471). Compared with the OTG group, the LTG group had longer operation time (255.66 ± 40.10 min vs 238.59 ± 40.30 min, P = 0.015) and less blood loss [150 (100-300) mL vs 200 (200-300) mL, P = 0.003]. The number of retrieved lymph nodes was similar between the two groups (33.38 ± 13.26 in LTG group vs 34.75 ± 16.69 in OTG group, P = 0.603).
Regarding postoperative recovery, we found that the LTG group showed advantages of enhanced recovery after surgery in comparison with the OTG group with regard to days to first flatus (4.36 ± 1.28 d vs 5.41 ± 1.16 d, P < 0.001) and postoperative hospitalization days (9.48 ± 3.98 d vs 11.89 ± 3.36 d, P < 0.001).
Perioperative expenditure was another concern to evaluate cost-effectiveness of different surgical approaches. In this study, even though LTG spent more surgical cost than OTG (P < 0.001), LTG seemed more economical compared with OTG in terms of total hospitalized cost (P < 0.001). Specific indicators mentioned above are presented in Table 4.
| Variable | LTG group (n = 61) | OTG group (n = 75) | P value |
| Surgical time, min | 255.66 ± 40.10 | 238.59 ± 40.30 | 0.015 |
| Blood loss, mL (median, IQR) | 150 (100-300) | 200 (200-300) | 0.003 |
| Blood loss (mL), n (%) | 0.003 | ||
| < 200 | 31 | 13 | |
| 200-400 | 20 | 51 | |
| > 400 | 10 | 11 | |
| Retrieved lymph nodes, n | 33.38 ± 13.26 | 34.75 ± 16.69 | 0.603 |
| No. 10 lymph nodes dissection | 0.339 | ||
| No | 41 | 56 | |
| Yes | 20 | 19 | |
| Extent of resection | 0.471 | ||
| R0 | 58 | 74 | |
| R1/R2 | 3 | 1 | |
| Time to first flatus, d | 4.36 ± 1.28 | 5.41 ± 1.16 | 0.000 |
| Postoperative stay, d | 9.48 ± 3.98 | 11.89 ± 3.36 | 0.000 |
| Surgery costs, $ | 5419.99 ± 1315.39 | 4162.36 ± 791.93 | 0.000 |
| Hospitalization costs, $ (median, IQR) | 13105.92 (11713.18-14640.53) | 14873.96 (13501.66-17131.31) | 0.000 |
| Total complication rate (%) | 14 (23.0) | 21 (28.0) | 0.503 |
| Clavien-Dindo classification | |||
| Grade II | 12 | 19 | |
| Peritoneal infection | 2 | 2 | |
| Lymphatic leakage | 2 | 0 | |
| Anastomotic leakage | 1 | 0 | |
| Pancreatic fistula | 1 | 1 | |
| Ileus | 1 | 2 | |
| Cardiac failure | 1 | 0 | |
| Hypoproteinemia | 2 | 8 | |
| Anemia | 2 | 2 | |
| Cholecystitis | 0 | 1 | |
| Incision infection | 0 | 2 | |
| Pneumonia | 0 | 1 | |
| Grade IIIa | 1 | 2 | |
| Deep venous thrombosis | 1 | 0 | |
| Pleural effusion | 0 | 1 | |
| Anastomotic leakage | 0 | 1 | |
| Grade V | 1 | 0 | |
| Septic shock | 1 | 0 | |
| Severe complication rate (%) | 2 (3.3) | 2 (2.7) | 1.000 |
In subgroup analysis, we compared the difference between the LTG and OTG groups on the basis of different pathological tumor stages. After balancing the baseline characteristics, similar results were obtained like above in ypTNM 0-II patients (Table 5). Whereas, for patients with ypTNM III-IV, no significant difference was observed on surgical time (P = 0.332) or blood loss (P = 0.159) between the two groups (Table 6).
| Variable | LTG group (n = 31) | OTG group (n = 40) | P value |
| Gender | 0.841 | ||
| Male | 25 | 33 | |
| Female | 6 | 7 | |
| Age (yr) | 59.10 ± 10.51 | 57.63 ± 11.16 | 0.574 |
| BMI (kg/m2) | 22.58 ± 2.77 | 23.72 ± 2.93 | 0.102 |
| CCI score | 0.594 | ||
| 0-2 | 22 | 26 | |
| > 2 | 9 | 14 | |
| Tumor diameter, cm (median, IQR) | 3.00 (2.20-4.50) | 2.30 (1.42-4.00) | 0.158 |
| Surgical time, min | 260.97 ± 37.20 | 237.93 ± 35.51 | 0.010 |
| Blood loss, mL (median, IQR) | 150 (100-200) | 200 (200-300) | 0.002 |
| Blood loss (mL), n (%) | 0.000 | ||
| 0-200 | 19 | 5 | |
| 200-400 | 9 | 31 | |
| > 400 | 3 | 4 | |
| Retrieved lymph nodes, n | 34.00 ± 15.11 | 36.38 ± 17.64 | 0.552 |
| Time to first flatus, d | 4.32 ± 1.28 | 5.45 ± 1.24 | 0.000 |
| Postoperative stay, d | 8.94 ± 3.63 | 11.65 ± 3.03 | 0.001 |
| Surgery costs, $ | 5641.18 ± 1351.17 | 4163.48 ± 627.86 | 0.000 |
| Hospitalization costs, $ | 13389.70 ± 2254.38 | 15024.88 ± 23358.95 | 0.004 |
| Total complication rate (%), C-D classification | 5 (16.1) | 9 (22.5) | 0.503 |
| II | 4 | 8 | |
| IIIa | 0 | 1 | |
| V | 1 | 0 | |
| Severe complication rate (%) | 1(3.2) | 1 (2.5) | 1.000 |
| Variable | LTG group (n = 30) | OTG group (n = 35) | P value |
| Gender | 0.931 | ||
| Male | 22 | 26 | |
| Female | 8 | 9 | |
| Age (yr) | 55.97 ± 10.10 | 55.94 ± 12.90 | 0.993 |
| BMI (kg/m2) | 23.03 ± 2.60 | 23.63 ± 3.73 | 0.468 |
| CCI score | 0.514 | ||
| 0-2 | 21 | 27 | |
| > 2 | 9 | 8 | |
| Tumor diameter, cm | 5.5 (3.5-8.0) | 5.0 (4.0-8.0) | 0.916 |
| Surgical time, min | 250.17 ± 42.99 | 239.34 ± 45.69 | 0.332 |
| Blood loss, mL (median, IQR) | 200 (100-350) | 300 (200-400) | 0.159 |
| Blood loss (mL), n (%) | 0.404 | ||
| 0-200 | 12 | 8 | |
| 200-400 | 11 | 20 | |
| > 400 | 7 | 7 | |
| Retrieved lymph nodes, n | 32.73 ± 11.24 | 32.89 ± 15.58 | 0.965 |
| Time to first flatus, d | 4.40 ± 1.30 | 5.37 ± 1.09 | 0.002 |
| Postoperative stay, d | 10.03 ± 4.30 | 12.17 ± 3.73 | 0.036 |
| Surgery costs, $ | 4793.57 (4032.20-6242.77) | 3871.55 (3686.28-4416.86) | 0.000 |
| Hospitalization costs, $ | 13190.05 (12036.98-14591.47) | 15263.28 (13162.85-17143.01) | 0.000 |
| Total complication rate (%), C-D classification | 9 (30.0) | 12 (34.3) | 0.647 |
| II | 8 | 11 | |
| IIIa | 1 | 1 | |
| Severe complication rate (%) | 1 (3.3) | 1 (2.9) | 1.000 |
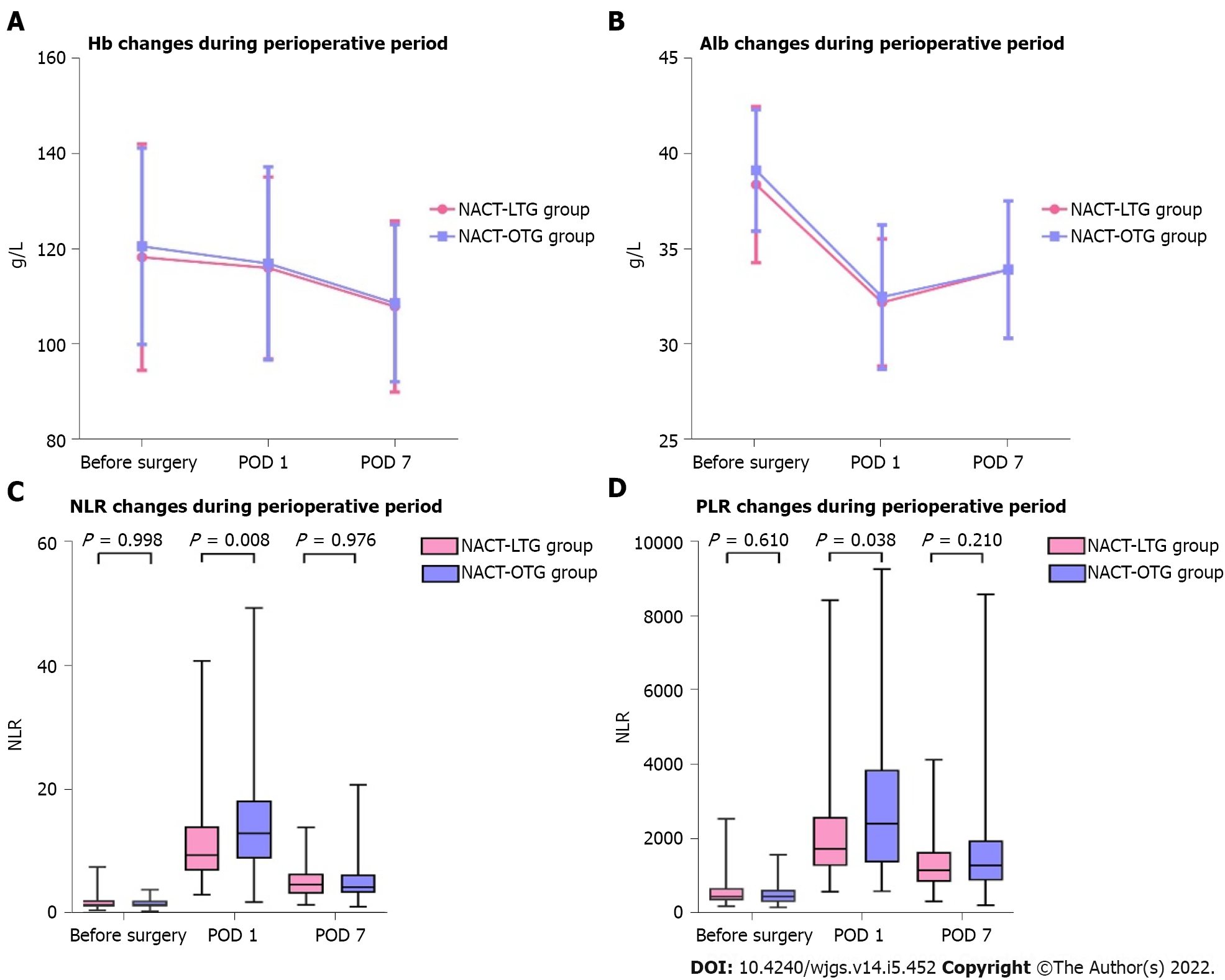
We selected partial laboratorial indexes like hemoglobin (Hb) and albumin (Alb) in the perioperative period to figure out the changes of perioperative nutritional status between LTG and OTG. In spite of different timelines including before surgery, postoperative day 1 (POD 1), and POD 7, there were no significant difference in Hb or Alb between the two groups.
Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were also calculated through laboratory tests. In this study, except for a higher NLR in the OTG group compared with the LTG group at POD 1 (P = 0.008) and PLR in the OTG compared with the LTG group at POD 1 (P = 0.038), no significant difference was observed between the two groups in other periods. Visualized comparison is depicted in Figure 2.
Of the 136 patients who underwent surgery after NACT, 21 (28.0%) in the OTG group and 14 (23.0%) in the LTG group developed Grade II or above postoperative complications evaluated by the Clavien-Dindo classification, with no significant difference between the two groups (P = 0.503). Two (3.3%) patients who underwent LTG had severe complications, wherein one patient died because of septic shock at POD 3. The rate of severe complications after OTG (2/75, 2.7%) did not differ significantly from that in the LTG group (P = 1.000). Table 4 gives the detailed items of complications.
Subgroup analysis showed that regardless of ypTNM 0-II or ypTNM III-IV patients, there was no significant difference in overall or severe complication rate between the two groups (P > 0.05) (Tables 5 and 6).
Of the 136 patients included, 127 (93.4%) completed follow-up. The last follow-up day was December 30, 2021. The median follow-up period was 69 (range, 1–112) mo. The 3-year OS rate was 60.6% and 64.6% in the LTG and OTG groups, respectively [hazard ratio (HR) = 0.859, 95% confidence interval (CI): 0.522-1.412], which demonstrated no significant difference between the two groups (log-rank χ2 = 0.364, P = 0.546). The 3-year DFS rate was 54.5% and 51.8% in the LTG and OTG groups, respectively (HR = 0.947, 95%CI: 0.582-1.539), which presented no significant difference (log-rank χ2 = 0.05, P = 0.823). Kaplan-Meier curves are shown in Figure 3.
Additionally, we set up two subgroups according to different ypTNM stages to explore the oncological impact of the two surgical approaches. For ypTNM 0-II patients, there was no significant difference in 3-year OS rate (P = 0.264) or DFS rate (P = 0.262) between LTG and OTG, neither were the subgroup of ypTNM III-IV patients (P > 0.05).These results illustrated the similar long-term outcomes between LTG and OTG after NACT no matter what ypTNM stage was. Kaplan-Meier curves for different subgroups are shown in Supplementary Figure 1.
Multivariate Cox analyses are shown in Tables 7 and 8. In the univariate analysis, BMI, pTNM stage, tumor diameter, estimated blood loss, and vascular and nerve invasion were significantly correlated with OS (P < 0.10), and pTNM stage, tumor diameter, estimated blood loss, and vascular invasion were significantly correlated with DFS (P < 0.10). In the multivariate analysis, BMI and pTNM stage were independent risk factors for OS while vascular invasion and pTNM stage were independent risk factors for DFS (P < 0.05). Historical factor was not significantly associated with OS or DFS (P > 0.05).
| Factor | Univariate analysis | P value | Multivariate analysis | P value | ||
| HR | 95%CI | HR | 95%CI | |||
| Sex | 0.127 | |||||
| Male | 1.000 | |||||
| Female | 1.541 | 0.885-2.684 | ||||
| Age | 0.647 | |||||
| < 65 | 1.000 | |||||
| ≥ 65 | 1.129 | 0.671-1.900 | ||||
| BMI (kg/m2) | 0.091 | 0.049 | ||||
| < 25 | 1.000 | 1.000 | ||||
| ≥ 25 | 0.601 | 0.333-1.086 | 0.547 | 0.300-0.998 | ||
| Surgical approach | 0.549 | |||||
| Laparoscopy | 1.000 | |||||
| Open | 1.164 | 0.708-1.914 | ||||
| CCI score | 0.438 | |||||
| 0-2 | 1.000 | |||||
| ≥ 2 | 1.225 | 0.733-2.049 | ||||
| pTNM stage | 0.000 | 0.006 | ||||
| 0-II | 1.000 | 1.000 | ||||
| III-IV | 2.632 | 1.569-4.413 | 2.224 | 1.258-3.930 | ||
| Tumor diameter (cm) | 0.039 | 0.153 | ||||
| ≤ 3 | 1.000 | 1.000 | ||||
| > 3 | 1.838 | 1.031-3.277 | 1.577 | 0.844-2.945 | ||
| Operation time (min) | 0.483 | |||||
| ≤ 240 | 1.000 | |||||
| > 240 | 1.192 | 0.730-1.948 | ||||
| Estimated blood loss (mL) | 0.074 | 0.588 | ||||
| ≤ 200 | 1.000 | 1.000 | ||||
| > 200 | 1.559 | 0.958-2.536 | 1.154 | 0.688-1.935 | ||
| Vascular invasion | 0.008 | 0.062 | ||||
| No | 1.000 | 1.000 | ||||
| Yes | 1.987 | 1.200-3.289 | 1.712 | 0.974-3.010 | ||
| Nerve invasion | 0.079 | 0.567 | ||||
| No | 1.000 | 1.000 | ||||
| Yes | 1.580 | 0.949-2.632 | 0.838 | 0.456-1.537 | ||
| Differentiation | 0.261 | |||||
| Well/moderate | 1.000 | |||||
| Poor/undifferentiated | 1.335 | 0.806-2.212 | ||||
| Complications | 0.662 | |||||
| No | 1.000 | |||||
| Yes | 1.131 | 0.651-1.968 | ||||
| Historical factor | 0.861 | |||||
| 2012-2015 | 1.000 | |||||
| 2016-2019 | 0.957 | 0.587-1.560 | ||||
| Factor | Univariate analysis | P value | Multivariate analysis | P value | ||
| HR | 95%CI | HR | 95%CI | |||
| Sex | 0.259 | |||||
| Male | 1.000 | |||||
| Female | 0.851 | 0.642-1.127 | ||||
| Age | 0.267 | |||||
| < 65 | 1.000 | |||||
| ≥ 65 | 1.326 | 0.806-2.181 | ||||
| BMI (kg/m2) | 0.706 | |||||
| < 25 | 1.000 | |||||
| ≥ 25 | 0.706 | 0.403-1.237 | ||||
| Surgical approach | 0.825 | |||||
| Laparoscopy | 1.000 | |||||
| Open | 0.947 | 0.582-1.539 | ||||
| CCI score | 0.707 | |||||
| 0-2 | 1.000 | |||||
| ≥ 2 | 1.104 | 0.660-1.847 | ||||
| pTNM stage | 0.000 | 0.022 | ||||
| 0-II | 1.000 | 1.000 | ||||
| III-IV | 2.418 | 1.471-3.973 | 1.854 | 1.095-3.140 | ||
| Tumor diameter (cm) | 0.022 | 0.200 | ||||
| ≤ 3 | 1.000 | 1.000 | ||||
| > 3 | 1.954 | 1.100-3.470 | 1.484 | 0.812-2.710 | ||
| Operation time (min) | 0.710 | |||||
| ≤ 240 | 1.000 | |||||
| > 240 | 1.095 | 0.679-1.765 | ||||
| Estimated blood loss (mL) | 0.024 | 0.204 | ||||
| ≤ 200 | 1.000 | 1.000 | ||||
| > 200 | 1.730 | 1.075-2.785 | 1.379 | 0.840-2.263 | ||
| Vascular invasion | 0.001 | 0.020 | ||||
| No | 1.000 | 1.000 | ||||
| Yes | 2.245 | 1.378-3.659 | 1.824 | 1.101-3.022 | ||
| Nerve invasion | 0.203 | |||||
| No | 1.000 | |||||
| Yes | 1.387 | 0.838-2.295 | ||||
| Differentiation | 0.283 | |||||
| Well/moderate | 1.000 | |||||
| Poor/undifferentiated | 1.311 | 0.800-2.148 | ||||
| Complications | 0.751 | |||||
| No | 1.000 | |||||
| Yes | 1.093 | 0.631-1.894 | ||||
| Historical factor | 0.691 | |||||
| 2012-2015 | 1.000 | |||||
| 2016-2019 | 1.102 | 0.683-1.779 | ||||
The application of NACT to AGC rapidly increased because of its potential oncological benefit[20]. At present, surgeons focus mainly on the impact of NACT on gastrectomy[16,21]. In this study, we reported mono-institutional retrospective outcomes aiming to evaluate surgical safety and oncological efficacy between LTG and OTG after NACT in China, which could provide a reference to the reasonable utilization of minimally invasive surgery for AGC patients who accepted NACT.
NACT before surgery has several advantages over surgery first for AGC, such as tumor regression, better tolerance, and improved R0 resection. Previous studies which consisted of over 100 cases of NACT showed that pCR rate ranged from 5%-17.2%[22]. In the present research, 8 (5.9%) patients achieved a pathologic complete response while 65 (47.8%) gained an objective response that was consistent with the results mentioned above. Better chemotherapeutic response was the crucial premise of radical gastrectomy. In this study, 58 (95.1%) patients in the LTG group and 74 (98.7%) in the OTG group achieved R0 resection, and no significant difference (P = 0.471) was found between the two groups. These results indicated that LTG could ensure considerable R0 resection in comparison to OTG after NACT.
Perioperative laboratorial indexes could evaluate the extent of surgical damage and nutritional status, and even might predict prognosis[23]. In our series, no significant difference was observed in Alb and Hb between LTG and OTG at three time points, including before surgery, POD 1, and POD 7. The incidence of hypoproteinemia seemed lower in the LTG group (3.3%) compared with the OTG group (10.7%), but the difference was not significant (P = 0.190), which indicated that LTG after NACT did not obviously improve postoperative nutritional status with advantages of minimally invasive surgery. NLR and PLR were regarded as potential markers to predict further prognosis[24]. Our results found no significant difference in PLR or NLR between the LTG and OTG groups before surgery and at POD 7, which implied that LTG and OTG after NACT had analogical long-term outcomes up to a point. However, higher NLR and PLR were observed at POD 1 in the OTG group than in the LTG group. We attributed this interesting phenomenon to stronger stress response at early period after OTG[25], which might elevate inflammation and suppress inherit immunity, leading to higher NLR and PLR. Hence, most studies selected pre-operation as a factor rather than other time points[26].
Adhesion of tissues, lack of anatomical layer, and peri-gastric edema and fibrosis might occur after NACT, which increased the surgical difficulty. Laparoscopy has several advantages like delicate manipulation, regional amplification, faster recovery, and damage control that might reduce the surgical risk of NACT. Li et al[21] found that laparoscopic distal gastrectomy had remarkably lower postoperative morbidity compared with open distal gastrectomy (20% vs 46%, P = 0.007) for patients with AGC who received NACT[21]. In this study, our perioperative clinical indicators showed that LTG offered benefits of less blood loss (P = 0.003), shorter days to first flatus, and shorter postoperative hospitalization dasy (P < 0.001) compared with OTG, which illuminated specific superiority of minimally invasive surgery. LTG also could achieve adequate lymph nodes dissection with a comparable number of retrieved lymph nodes between LTG and OTG (33.38 ± 13.26 vs 34.75 ± 16.69, P = 0.603). Meanwhile, an interesting phenomenon was found that LTG cost more on operation and less on total hospitalization than OTG, which was similar to the results of the studies by Tegels et al[27] and Hoya et al[28]. Gosselin-Tardif et al[29] also found that the application of laparoscopic gastrectomy was more cost-effective compared with open gastrectomy in Canadians. We reckon that the fact that expensive disposable surgical instruments mostly relied on import might elevate surgical cost in LTG, but fast postoperative recovery could offset deviations by reducing other costs, which suggested LTG as a probable cost-effective alternative surgical approach after NACT.
In terms of perioperative complications, CLASS-02 trial conducted in China demonstrated that LTG performed by experienced surgeons had acceptable postoperative morbidity (19.1%) for clinical stage I GC[13]. STOMACH trial showed no significant difference in the rate of postoperative complications between OTG (42.9%) and LTG (34.0%) in LTG after NACT in Western countries (P = 0.408). Wang et al[30] demonstrated that LTG had comparable safety to OTG after NACT in the perioperative period and patients in the LTG group could benefit from less intravenous patient-controlled analgesia (IV-PCA) use[30]. Back to our study, we found that LTG did not significantly increase or decrease 30-d postoperative complications compared with OTG after NACT (overall morbidity of LTG vs OTG: 23.0% vs 28.0%, P = 0.503; severe morbidity of LTG vs OTG: 3.3% vs 2.7%, P = 1.000), which was similar to the results of the studies mentioned above. These results still existed in different ypTNM stage patients. Thus, we consider that the application of LTG after NACT could be safe and feasible whatever tumor stage was and we recommend to initiate prospective studies to give high-grade evidence in East Asia.
Long-term outcomes were inevitable to evaluate oncological benefit caused by different surgical approaches. The studies by Gambhir et al[14] and Komatsu et al[31] both pointed out a comparable long-term survival between LTG and OTG, nevertheless it remained uncertain between the LTG and OTG group after NACT. Our results of follow-up focused on 3-year OS and DFS rates showed no significant difference between the two groups (LTG compared to OTG: 3-year OS: 60.6% vs 64.6%, P = 0.546; 3-year DFS: 54.5% vs 51.8%, P = 0.823). Subgroup analysis according to different ypTNM stages also showed no significant difference in 3-year OS or DFS rate. These findings suggested that patients with LTG after NACT had similar oncological benefits compared with those in the OTG group irrespective of stage, and LTG after NACT could be regarded as an alternative surgical approach with acceptable short and long-term outcomes.
Our study has several limitations. Principally, this is not a prospective study which lacked of authentic evidence-based support and existed selection bias. Under the trend of climbing application of NACT as a promising treatment for AGC in East Asia[32], large-scale retrospective or even multi-institutional RCT studies are required to better understand the association between LTG and OTG after NACT. Moreover, small sample size increased the probability of type II error and reduced the power of test. To decrease such impact, we combined patients with adjacent ypTNM stages into one group to ensure enough sample size in subgroup analysis. Third, although SOX regimen was the main NACT treatment in our study, other regimens like XELOX and DCF were also used for a small portion of appropriate patients, which may slightly influence short or long-term outcomes. In addition, even the baseline characteristics of patients included in this study were comparable between the LTG and OTG groups, some potential imbalance caused by unknown indicators may affect the validity of results.
To sum up, this study suggested that there are no significant disparities between LTG and OTG in postoperative complication rates, 3-year OS rates, and 3-year DFS rates after NACT for AGC patients. LTG performed by experienced surgeons after NACT has several advantages including less blood loss, faster postoperative recovery, and less hospitalized cost, which could be regarded as an alternative surgical approach with its safety, feasibility, and comparable oncological benefits at any ypTNM stage.
Neoadjuvant chemotherapy (NACT) combined with surgery is regarded as an effective treatment for advanced gastric cancer (AGC). Laparoscopic surgery represents the mainstream of minimally invasive surgery.
Currently, surgeons focus more on surgical safety and oncological outcomes of laparoscopic gastrectomy after NACT.
We sought to evaluate short- and long-term outcomes between laparoscopic total gastrectomy (LTG) and open total gastrectomy (OTG) after NACT.
We retrospectively collected the clinicopathological data of 136 patients who accepted gastrectomy after NACT from June 2012 to June 2019, including 61 patients in the LTG group and 75 patients in the OTG group. Clinicopathological characteristics between the LTG and OTG groups showed no significant difference. We compared the perioperative indexes and long-term outcomes between the LTG and OTG groups after NACT. SPSS 26.0, R software, and GraphPad PRISM 8.0 were used to perform statistical analyses.
In this study, we found that LTG had longer operation time, less blood loss, shorter days to first flatus, and shorter postoperative hospitalization days compared with OTG. LTG showed comparable 30-d postoperative morbidity as well as 3-year OS and DFS rate to OTG.
This study suggested that there are no significant disparities between LTG and OTG in postoperative complication rates, 3-year OS rates, and 3-year DFS rates after NACT for AGC patients. LTG performed by experienced surgeons after NACT has several advantages including less blood loss, faster postoperative recovery, and less hospitalized cost, which could be regarded as an alternative surgical approach with its safety, feasibility, and comparable oncological benefits at any ypTNM stage.
We recommend that experienced surgeons could select LTG for proper patients after NACT. Large-scale retrospective or even multi-institutional RCT studies are required to better understand the association between LTG and OTG after NACT.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68628] [Article Influence: 13725.6] [Reference Citation Analysis (201)] |
| 2. | Sun D, Cao M, Li H, He S, Chen W. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin J Cancer Res. 2020;32:129-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Yu JH, Wang ZZ, Fan YC, Liu MX, Xu K, Zhang N, Yao ZD, Yang H, Zhang CH, Xing JD, Cui M, Su XQ. Comparison of neoadjuvant chemotherapy followed by surgery vs. surgery alone for locally advanced gastric cancer: a meta-analysis. Chin Med J (Engl). 2021;134:1669-1680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Umeda S, Kanda M, Nakanishi K, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Shimizu D, Kobayashi D, Tanaka C, Fujiwara M, Murotani K, Kodera Y. Short-term outcomes of gastrectomy after neoadjuvant chemotherapy for clinical stage III gastric cancer: propensity score-matched analysis of a multi-institutional database. Surg Today. 2021;51:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Fujisaki M, Mitsumori N, Shinohara T, Takahashi N, Aoki H, Nyumura Y, Kitazawa S, Yanaga K. Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc. 2021;35:1682-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4739] [Article Influence: 237.0] [Reference Citation Analysis (7)] |
| 7. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2086] [Cited by in RCA: 1819] [Article Influence: 259.9] [Reference Citation Analysis (0)] |
| 8. | Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, Wang X, Wu A, Li G, Su X, Xiao G, Cui M, Wu D, Wu X, Zhou Y, Zhang L, Dang C, He Y, Zhang Z, Sun Y, Li Y, Chen H, Bai Y, Qi C, Yu P, Zhu G, Suo J, Jia B, Li L, Huang C, Li F, Ye Y, Xu H, Yuan Y, E JY, Ying X, Yao C, Shen L, Ji J; RESOLVE study group. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 303] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 9. | Wang X, Li S, Sun Y, Li K, Shen X, Xue Y, Liang P, Li G, Chen L, Zhao Q, Fu W, Liang H, Xin H, Suo J, Fang X, Zheng Z, Xu Z, Chen H, Zhou Y, He Y, Huang H, Zhu L, Yang K, Ji J, Ye Y, Zhang Z, Li F, Wang X, Tian Y, Park S. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer. 2021;21:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 11. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 80.0] [Reference Citation Analysis (1)] |
| 12. | Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Ito S, Takagi M, Takagane A, Teshima S, Koeda K, Nunobe S, Yoshikawa T, Terashima M, Sasako M. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 13. | Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 14. | Gambhir S, Inaba CS, Whealon M, Sujatha-Bhaskar S, Pejcinovska M, Nguyen NT. Short- and long-term survival after laparoscopic versus open total gastrectomy for gastric adenocarcinoma: a National database study. Surg Endosc. 2021;35:1872-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Oh Y, Kim MS, Lee YT, Lee CM, Kim JH, Park S. Laparoscopic total gastrectomy as a valid procedure to treat gastric cancer option both in early and advanced stage: A systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | van der Wielen N, Straatman J, Daams F, Rosati R, Parise P, Weitz J, Reissfelder C, Diez Del Val I, Loureiro C, Parada-González P, Pintos-Martínez E, Mateo Vallejo F, Medina Achirica C, Sánchez-Pernaute A, Ruano Campos A, Bonavina L, Asti ELG, Alonso Poza A, Gilsanz C, Nilsson M, Lindblad M, Gisbertz SS, van Berge Henegouwen MI, Fumagalli Romario U, De Pascale S, Akhtar K, Jaap Bonjer H, Cuesta MA, van der Peet DL. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer. 2021;24:258-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 17. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1415] [Article Influence: 283.0] [Reference Citation Analysis (2)] |
| 18. | Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, Sasako M. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 660] [Article Influence: 60.0] [Reference Citation Analysis (1)] |
| 19. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22820] [Article Influence: 1342.4] [Reference Citation Analysis (1)] |
| 20. | Das M. Neoadjuvant chemotherapy: survival benefit in gastric cancer. Lancet Oncol. 2017;18:e307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Li Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, Ren H, Su X, Ji J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2019;154:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 22. | Petrelli F, Ghidini M, Barni S, Sgroi G, Passalacqua R, Tomasello G. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: A systematic review and meta-analysis. Gastric Cancer. 2019;22:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Wang H, Jiang J, Cao X, Liu Q. Early decrease in postoperative serum albumin predicts severe complications in patients with colorectal cancer after curative laparoscopic surgery. World J Surg Oncol. 2018;16:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 25. | Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004;18:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, Mori S, Sasaki K, Omoto I, Kurahara H, Maemura K, Okubo K, Uenosono Y, Ishigami S, Natsugoe S. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19:672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 27. | Tegels JJ, Silvius CE, Spauwen FE, Hulsewé KW, Hoofwijk AG, Stoot JH. Introduction of laparoscopic gastrectomy for gastric cancer in a Western tertiary referral centre: A prospective cost analysis during the learning curve. World J Gastrointest Oncol. 2017;9:228-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Hoya Y, Taki T, Tanaka Y, Yano H, Hirabayashi T, Okamoto T, Kashiwagi H, Yanaga K. Disadvantage of operation cost in laparoscopy-assisted distal gastrectomy under the national health insurance system in Japan. Dig Surg. 2010;27:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Gosselin-Tardif A, Abou-Khalil M, Mata J, Guigui A, Cools-Lartigue J, Ferri L, Lee L, Mueller C. Laparoscopic versus open subtotal gastrectomy for gastric adenocarcinoma: cost-effectiveness analysis. BJS Open. 2020;4:830-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Lei X, Liu Z, Shan F, Ying X, Li Z, Ji J. Short-term outcomes of laparoscopic versus open total gastrectomy after neoadjuvant chemotherapy: a cohort study using the propensity score matching method. J Gastrointest Oncol. 2021;12:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Komatsu S, Kosuga T, Kubota T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Ichikawa D, Otsuji E. Comparison of short- and long-term outcomes following laparoscopy and open total gastrectomy for gastric cancer: a propensity score-matched analysis. Am J Transl Res. 2020;12:2225-2233. [PubMed] |
| 32. | Terashima M, Yoshikawa T, Boku N, Ito S, Tsuburaya A, Iwasaki Y, Fukagawa T, Tokunaga M, Sano T, Sasako M; Stomach Cancer Study Group, Japan Clinical Oncology Group. Current status of perioperative chemotherapy for locally advanced gastric cancer and JCOG perspectives. Jpn J Clin Oncol. 2020;50:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakano H, Japan; Otowa Y, Japan; Quartuccio N, Italy S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR