Published online Mar 27, 2022. doi: 10.4240/wjgs.v14.i3.221
Peer-review started: September 1, 2021
First decision: November 7, 2021
Revised: November 14, 2021
Accepted: March 5, 2022
Article in press: March 5, 2022
Published online: March 27, 2022
Processing time: 204 Days and 23.4 Hours
Complete mesocolic excision (CME) with central vascular ligation (CVL) was proposed by Hohenberger in 2009. The CME principle has gradually become the technical standard for colon cancer surgery. How to achieve CME with CVL in laparoscopic right hemicolectomy (LRH) is controversial, and a unified standard approach is not yet available. In recent years, the authors’ team has integrated the theory of membrane anatomy, tried to combine the cephalic approach with the classic medial approach (MA) for technical optimization, and proposed a cranial-medial mixed dominant approach (CMA).
To explore the feasibility of operational approaches for LRH with CME.
In this retrospective cohort study, the clinical data of 57 patients with right-sided colon cancer (TNM stage I, II, or III) who underwent LRH with CME from January 2016 to June 2020 were collected and summarized. There were 31 patients in the traditional MA group and 26 in the CMA group.
There were no significant differences in baseline data between the two groups. The operation was shorter and the number of lymph nodes dissected was higher in the CMA group than in the MA group, but there was no significant difference in the number of positive lymph nodes, intraoperative blood loss, postoperative exhaust time, feeding time, postoperative hospital stay or postoperative complication incidence.
Our study shows that the CMA is a safe and feasible procedure for LRH with CME and has a unique advantage.
Core Tip: This work presents the combination of the cranial approach and the classic medial approach and optimization of the combined approach to propose a cranial-medial mixed dominant approach (CMA) based on embryonic development and membrane anatomy. Our study shows that the CMA is a safe and feasible procedure for laparoscopic right hemicolectomy with complete mesocolic excision and has a unique advantage.
- Citation: Lin L, Yuan SB, Guo H. Does cranial-medial mixed dominant approach have a unique advantage for laparoscopic right hemicolectomy with complete mesocolic excision? World J Gastrointest Surg 2022; 14(3): 221-235
- URL: https://www.wjgnet.com/1948-9366/full/v14/i3/221.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i3.221
Since Heald[1] proposed the total mesorectal excision (TME) principle in 1982, TME has become the international gold standard for rectal cancer[2]. In 1991, Jacobs et al[3] first reported laparoscopic colorectal cancer resection. A similar concept of complete mesocolic excision (CME) with central vascular ligation (CVL) was proposed by Hohenberger et al[4] in 2009 based on the concepts of TME. The CME principle has gradually become the technical standard for colon cancer surgery[5,6]. The National Comprehensive Cancer Network (NCCN) guidelines for colon cancer recommended laparoscopic surgery for patients with curable colon cancer[7] for years, but it is generally considered that laparoscopic right hemicolectomy (LRH) is relatively complex and difficult[8]. How to achieve CME with CVL in LRH has been controversial, and a unified standard approach is not yet available. Before this procedure can be generally recommended, a consensus is needed on how the operation can be carried out optimally. However, quite a few approaches have been proposed[9-11]. In recent years, the authors’ team has integrated the theory of embryonic development and membrane anatomy, combined the cranial approach with the classic medial approach (MA) and optimized the combined approach to propose a cranial-medial mixed dominant approach (CMA). This approach allows better control of surgical risks, is more compliant with CME requirements, and is more standardized and reproducible.
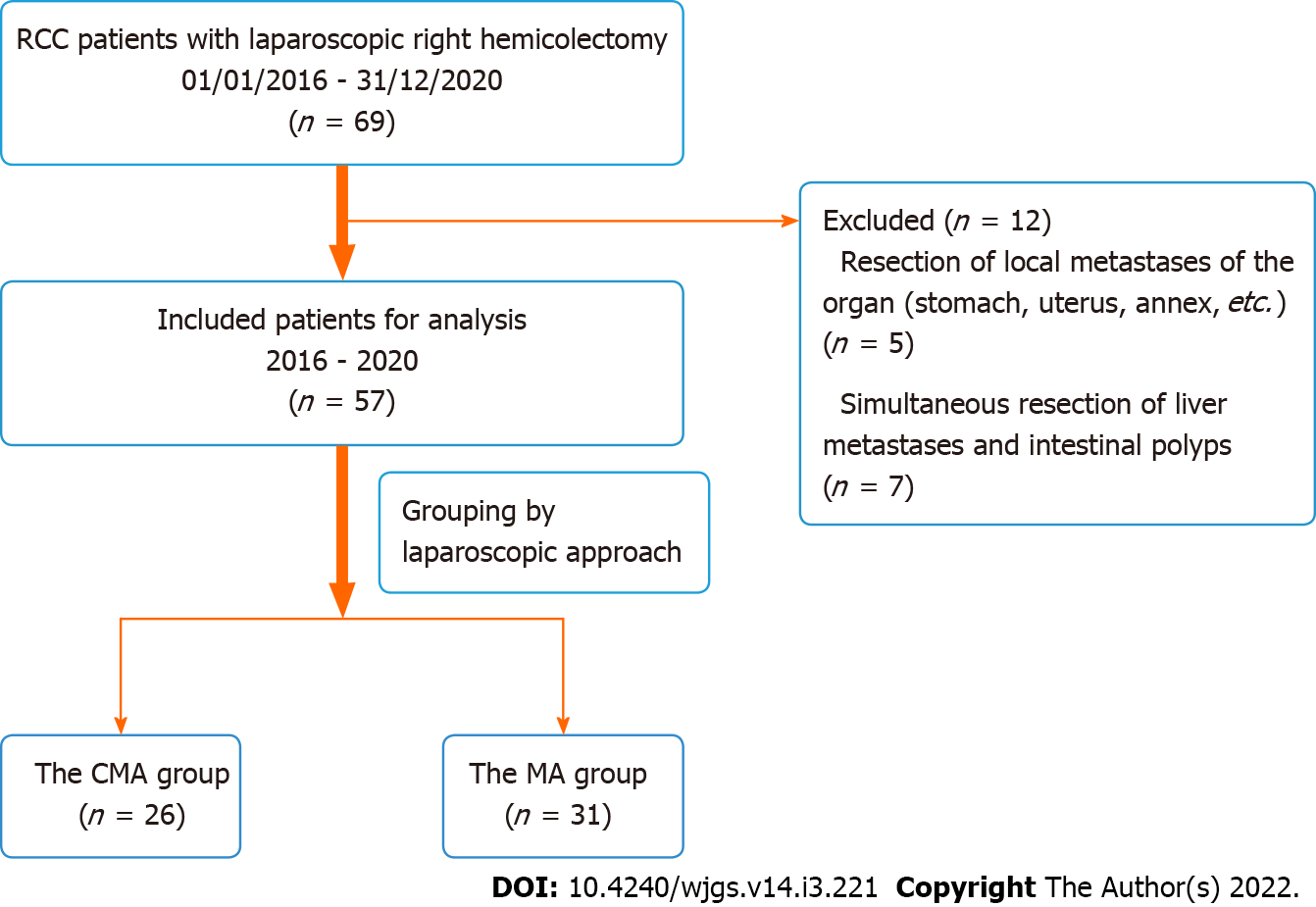
All the patients, both those in the CMA group and those in the MA group, were admitted to the Department of Gastrointestinal Surgery of Zhongshan Hospital of Xiamen University and underwent LRH with CME and CVL, which was performed by Professor Sibo Yuan. Between January 2016 and December 2020, adult patients who had a confirmed diagnosis of renal cell carcinoma (RCC), who underwent contrast-enhanced CT of the chest, abdomen, and pelvis for clinical staging (cTNM), and who underwent radical colectomy were selected from the database. The selection criteria were as follows: (1) Patients were 15 years of age or older, with no limitation on sex; (2) Patients had a confirmed diagnosis of clinical stage I, II, or III adenocarcinoma through biopsy of the right colon on colonoscopy, including the caecum, ascending colon, hepatic flexure, and proximal transverse colon; and (3) Patients underwent laparoscopic surgery at a scheduled time rather than emergency surgery due to severe obstruction or perforation. During 2016–2018, 36 patients underwent LRH with the traditional MA. From 2018 to 2020, 33 patients underwent treatment with the CMA. Twelve of the 69 patients were excluded from this study due to resection of local metastases of the organ (stomach, uterus, annex, etc.) and simultaneous resection of liver metastases and intestinal polyps, for which we could not assess the operative duration, postoperative recovery or other factors. Professor Yuan primarily used the MA before 2018 and proposed and primarily used the CMA after 2018 to complete LRH. Twenty-six patients were included in the CMA group, and 31 patients were included in the MA group after exclusion (Figure 1). Postoperative clinical tumour staging was based on the Union for International Cancer Control (UICC) cancer staging manual (version 6). Preoperative blood and albumin (ALB) transfusions were performed in cases of anaemia and hypoproteinaemia, respectively. The basic condition of the patients and the outcome data are shown in Table 1.
| Item | CMA group (n= 26) | MA group (n= 31) | P value |
| Age (yr) | 63.12 ± 13.65 | 61.35 ± 12.27 | 0.61 |
| Sex | 0.794 | ||
| Male | 14 | 18 | |
| Female | 12 | 13 | |
| BMI (kg/m2) | 21.42 ± 3.15 | 22.54 ± 3.43 | 0.209 |
| Tumour size (cm) | 5.18 ± 1.80 | 4.84 ± 2.06 | 0.52 |
| Previous abdominal surgery | 0.488 | ||
| Yes | 3 | 6 | |
| No | 23 | 25 | |
| Tumour location | 0.644 | ||
| Ileocecal junction | 7 | 6 | |
| Ascending colon | 11 | 12 | |
| Flexura hepatica coli | 8 | 13 | |
| Histological grade | 0.185 | ||
| Well | 0 | 1 | |
| Moderate | 18 | 26 | |
| Poor | 8 | 4 |
Dissociation of the right colon under laparoscopy was completed in both groups of patients (CMA and MA). Then, the surgeon made a small incision of approximately 4 cm on the right side of the abdomen to complete the anastomosis (routine end-side anastomosis), finally rearranging the bowel.
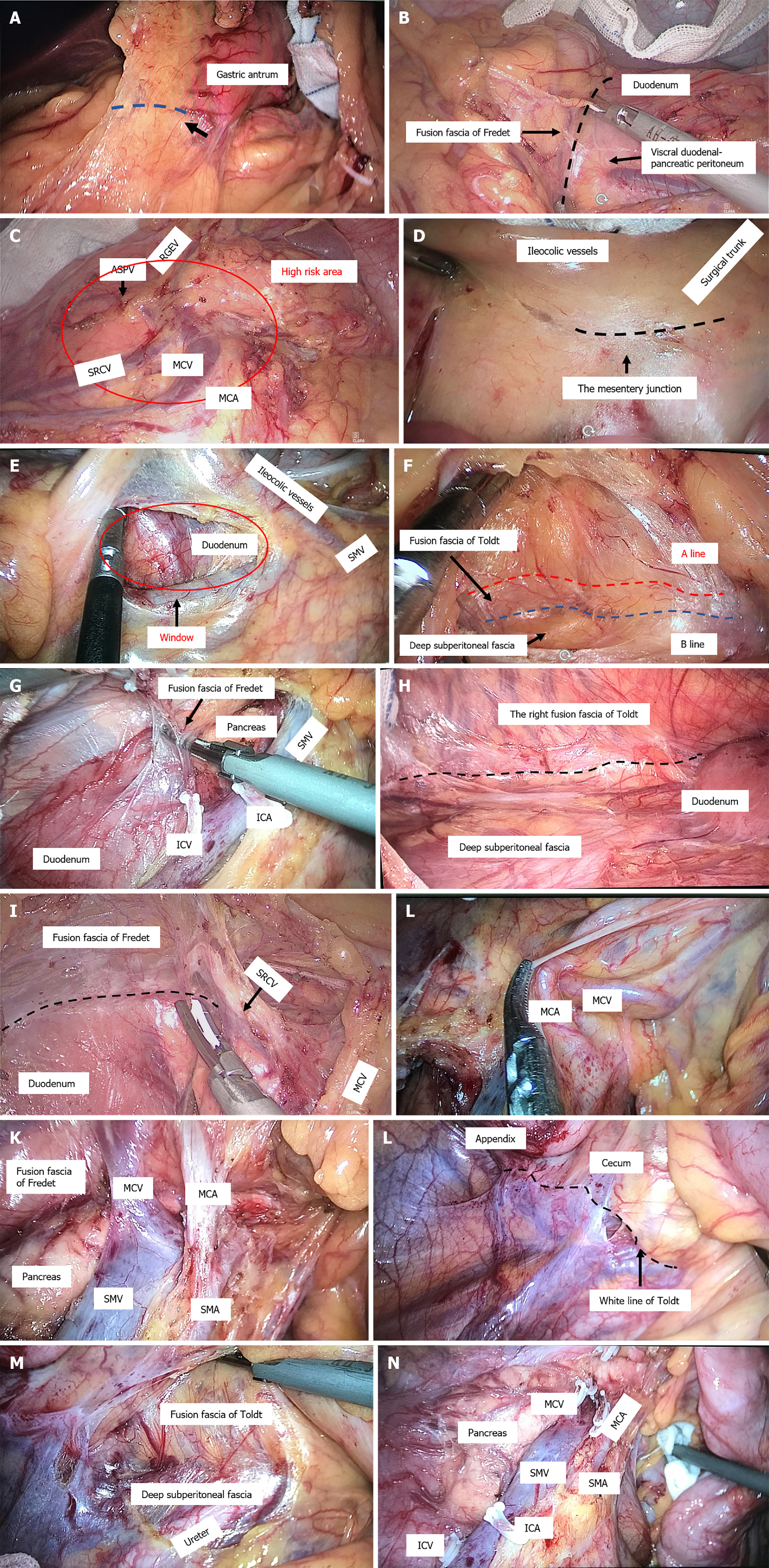
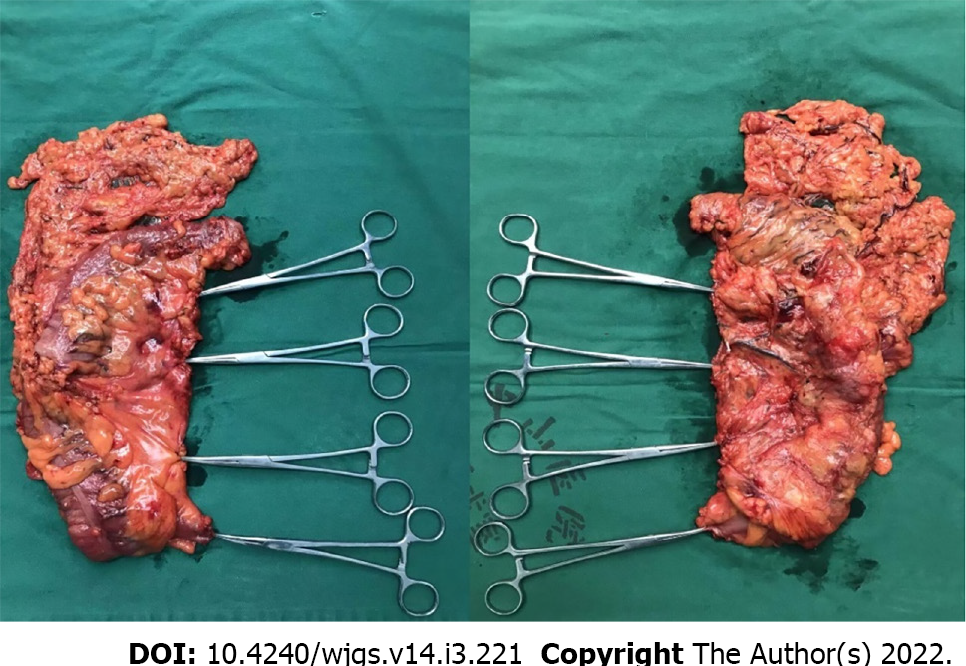
CMA: (1) Establishment of a laparoscopic system and intraperitoneal exploration: All patients were placed in the lithotomy position after the administration of general anaesthesia, with the left leg lowered as much as possible to avoid affecting the operation of the surgeon. Throughout the procedure, the surgeon stood on the left side of the patient, whereas the first assistant stood on the right side, and the second assistant held a mirror and stood between the legs of the patient. Five trocars were used (three 5 mm, one 12 mm, one 10 mm), with one observation and four operation ports. Among these, one observation port with a 10-mm trocar was located 2 cm lower than the umbilicus. One operation port with a 5-mm trocar was placed at Maxwell’s point. The second operation port with a 12-mm trocar was placed near the anti-Maxwell point. The third and fourth operation ports with 5-mm trocars were located approximately 2 cm lower than the edge of the rib arch across the left and right clavicular midline intersections (Figure 2). Laparoscopic exploration of the liver lobe, peritoneum, omentum, spleen, stomach, colon, pelvis, and small intestine was performed; the tumour location and size were evaluated to assess the extent of tumour invasion into the surrounding tissue and determine the scope of surgical resection. Then, the projection of the surgical trunk, the superior mesenteric artery (SMA) on the mesocolon and the root of the middle colic vessels were explored; (2) The greater omentum was split with an ultrasonic knife to the left of the superior edge of the transverse colon, the omental bursa was entered, and the greater omentum outside the gastric omental vascular arch (tumour of the ascending colon or ileocaecum) or inside the vascular arch (tumour of the hepatic curvature or right half of the transverse colon) was longitudinally cut off, revealing the right mesenteric fusion region of the transverse mesocolon, the mesogastrium and the underlying visceral duodenal-pancreatic peritoneum (also called the fusion fascia of Fredet)[12,13]; (3) Cephalic-approach procedure (CAP): The first assistant lifted the gastric body and pulled the mesogastrium upwards laterally, and the surgeon used the right hand to pull the transverse mesocolon downwards, which formed an antagonistic force and satisfactorily exposed the right fusion fascia area of the transverse mesocolon and the mesogastrium. The surgeon first dissected the fusion fascia in the innermost area adjacent to the gastric antrum (Figure 3A), entered the dorsal side of the fusion fascia of Fredet (Figure 3B), and then gently expanded the surgical plane between the fusion fascia of Fredet and the visceral duodenal-pancreatic peritoneum in a medial-to-lateral direction. After cleavage of the lateral “white line of Toldt” around the hepatic flexure, the fusion fascia was incised between the hepatic curvature of the colon and the second part of the duodenum and expanded downwards and slightly laterally, and the plane between the fusion fascia of Toldt and the subperitoneal deep fascia (Gerota fascia) near the lateral side of the second part of the duodenum was entered. Using the projection of the superior right colic vein (SRCV) on the fusion fascia of Fredet as a landmark, the surgical plane was expanded medially to expose the gastrocolic trunk of Henle (GCTH), and the nonvascularized mesocolic area was expanded on the left side of the root of the middle colonic vessels, completing the dissection of the surgical area of the GCTH[14,15] (SAGCTH), defined as the area of the superior mesenteric vein (SMV) located at the head of the pancreas and including the venous confluence of the right gastroepiploic vein (RGEV), anterosuperior pancreatic-duodenal vein (ASPV), and SRCV. Then, exposure was continue downwards to the second part of the duodenum, the head of the pancreas and the cranial root of the middle colic vessel; a piece of gauze was placed transversely at the lower edge as a landmark. In this procedure, the most important thing was to maintain the surgical plane between the fusion fascia of Fredet and the visceral duodenal-pancreatic peritoneum and to completely resect the fusion fascia of Fredet (Figure 3C); (4) Medial-approach procedure (MAP): The first assistant pulled up the mesocolon of the middle colic vascular area with the left hand, pulled the mesocolon of the ileocolic vascular area with the right hand, and exposed the projection of the surgical trunk[14,17] on the mesocolon. The surgeon incised the mesentery junction (the fusion point of the mesocolon, the visceral peritoneum, and the intestinal mesentery, approximately 3 cm below the projection of the ileocolic vessels to the confluence of the SMV) with an ultrasonic scalpel (Figure 3D and E), utilized the vapourization effect of the ultrasonic scalpel, sought the fusion fascia of Toldt and then entered the surgical plane between the fusion fascia of Toldt and subperitoneal deep fascia (Figure 3F); then, the surgeon slightly expanded the plane laterally to the white line of Toldt, down to the peritoneal reflexion area of the ileocaecum, and up to the lower margin of duodenum and cut off the right fusion fascia of Toldt at the third portion of the duodenum, where the fusion fascia of Toldt divided into the posterior pancreatic fascia of Treitz and the fusion fascia of Fredet. The dorsal side of the fusion fascia of Fredet was entered to reach a rendezvous of the surgical plane with that of the CAP (Figure 3G and H). The ileocolic artery (ICA) was used as a landmark, revealing the surgical trunk; the mesenteric radix was sharply dissected from the caudal side (small intestinal venous branch of the SMV) to the cranial side (the left root of the middle colic artery (MCA), with the projection of the gauze used as a landmark), and the roots of the vessels (ileocolic vessels, right colic artery, etc.) were ligated simultaneously; (5) Rendezvous of the surgical plane after the CAP and MAP. The rendezvous zone: (a) The nonvascularized mesocolic area on the left side of the root of the MCA was dissected to enter the ventral plane of the pancreas; and (b) The connecting line from the right side of the middle colic vessel to the GCTH was opened up, which connected the dorsal side of the fusion fascias of Fredet and Toldt. The root of the right branch of the MCA was ligated simultaneously; and (6) Cleavage of the lateral white line of Toldt was performed around the caecum (Figure 3L), along the ascending colon and around the hepatic flexure, connecting the posterior plane of the expanded fusion fascia of Toldt to complete the overall mobilization of the right colon (Figure 3M). The specimen from the operation was in Figure 4.
MA: First, we found the anatomic projection of the ileocolic vessel pedicle. We anatomized the SMV from the caudal side to the cranial side and ligated the roots of the vessels [ileocolic vein (ICV), ileocolic artery (ICA), RCV, right colic artery (RCA), etc.]. Then, we followed the fusion space of the hepatic flexure of the colon and completely dissected the colonic hepatic flexure (as mentioned above). Finally, we mobilized the right colon along with the expanded fusion fascia of Toldt.
Intraoperative data were obtained regarding the operative duration (duration of the total operation and the laparoscopic procedure), blood loss, specimen length, and number of resected and positive lymph nodes. Postoperative data, including exhaust time, liquid intake time, postoperative hospitalization (days), and postoperative complications, were recorded. Complications were graded according to the Clavien–Dindo classification[18]. Mortality and short-term postoperative complications within the first 30 postoperative days (or during the entire hospital stay if longer than 30 d) were recorded. Postoperative ileus was defined as no tolerance for solid food and no defecation by postoperative day 6[19]. Postoperative bleeding was defined as bleeding requiring at least one transfusion of packed red cells during surgery or in the subsequent 48 h.
All calculations and analyses were performed by SPSS software, version 22.0 (SPSS, Chicago, IL). Quantitative data are expressed as the mean ± SD. Student’s t test was used to compare the differences between the two groups; P < 0.05 was considered statistically significant.
Twenty-six and 31 patients were assigned to the MA and CMA groups, respectively (Table 1). There was no significant difference between the groups in sex, tumour location, tumour classification, laboratory results [carcinoembryonic antigen (CEA) level, haemoglobin (HB) level, white blood cell (WBC) count, ALB level, etc.] or body mass index.
The mean resection sample length in the MA group was 26.95 ± 6.18 cm, which was not different from that in the CMA group (27.926 ± 7.52 cm) (P = 0.598). The number of lymph nodes collected in the CMA group was 30.50 ± 15.31, which was significantly greater than that in the MA group (23.81 ± 9.06). The number of positive lymph nodes was similar in both groups. In the CMA group, the operative duration was 135.12 ± 17.47 min, and the laparoscopic procedure time was 69.73 ± 15.13 min, which were significantly lower (P < 0.05) than those in the MA group (150.61 ± 26.01 min and 84.81 ± 21.48 min, respectively). There was no significant difference in the intraoperative blood loss, feeding fluid time, exhaust time, length of hospital stay or postoperative laboratory results (seven days after the operation) between the two groups (P > 0.05) (Table 2).
| Item | CMA group (n= 26) | MA group (n= 31) | P value |
| Sample length (cm) | 26.95 ± 6.18 | 27.926 ± 7.52 | 0.598 |
| No. of lymph nodes collected | 30.50 ± 15.31 | 23.81 ± 9.06 | 0.046 |
| No. of positive lymph nodes | 2.15 ± 2.99 | 1.45 ± 2.32 | 0.323 |
| Nerve invasion | 0.524 | ||
| Yes | 20 | 26 | |
| No | 6 | 5 | |
| Vessel carcinoma embolus | 0.432 | ||
| Yes | 14 | 20 | |
| No | 12 | 11 | |
| Invasive depth | 0.021 | ||
| T1 | 2 | 1 | |
| T2 | 0 | 1 | |
| T3 | 8 | 1 | |
| T4 | 16 | 28 | |
| Lymph node metastasis | 0.658 | ||
| N0 | 13 | 19 | |
| N1 | 9 | 9 | |
| N2 | 4 | 3 | |
| pTNM | |||
| 0 | 0 | 1 | 0.339 |
| I | 1 | 0 | |
| II | 12 | 16 | |
| III | 11 | 14 | |
| IV | 2 | 0 | |
| Total operation time (min) | 135.12 ± 17.47 | 150.61 ± 26.01 | 0.01 |
| Laparoscopic procedure time (min) | 69.73 ± 15.13 | 84.81 ± 21.48 | 0.003 |
| Intraoperative blood loss (mL) | 48.46 ± 30.07 | 67.10 ± 87.88 | 0.309 |
| Exhaust time (d) | 3.81 ± 1.92 | 4.45 ± 1.15 | 0.123 |
| Liquid intake time (d) | 5.27 ± 1.87 | 4.81 ± 1.22 | 0.266 |
| Postoperative hospitalization (d) | 12.23 ± 2.23 | 11.29 ± 2.02 | 0.101 |
The incidence of complications in the CMA group was 23%, while that in the CA group was 13%, but the difference was not significant (P = 0.486). The 30 d mortality rate was 0 in both groups. However, there were 3 cases of lymphatic fistula in the CMA group, all of which were cured by conservative treatment (Table 3).
| Item | CMA group (n= 26) | MA group (n= 31) | P value |
| Complications | 6(23) | 4(13) | 0.486 |
| Anastomotic fistula | 0 | 0 | |
| Anastomotic stenosis | 0 | 0 | |
| Bleeding | 0 | 1 | |
| Lymphatic fistula | 3 | 1 | |
| Ileus | 2 | 0 | |
| Incisional hernia | 0 | 1 | |
| Acute urine retention | 0 | 0 | |
| Incision infection prevention | 1 | 1 | |
| Intra-abdominal infection | 0 | 0 | |
| Pulmonary infection | 0 | 0 |
Multiple cohort studies have confirmed the oncological effectiveness and surgical safety of CME with CVL[20-22], in which the embryologic tissue planes are resected along the entire enveloped mesocolon. There is a multicentre, prospective, randomized trial comparing conventional (laparoscopic) right hemicolectomy with robotic CME for patients with right-sided colon cancer at 4 centres in the UK currently underway, and we are very much looking forwards to its results[23]. Although there are still some doubts[8], laparoscopic CME has gradually become the technical standard for colon cancer[5]. However, there is no consensus on which standard surgical approach should be used to perform LRH with CME.
The representative approaches of LRH with CME include the MA, cephalic approach, caudal approach and other mixed approaches. European randomized controlled trials (RCTs) have suggested that[24] the MA has advantages in LRH and is both widely used in clinical practice and representative. However, Liang et al[9] suggested that the MA is difficult and commonly leads to bleeding due to variation in the surgical trunk and its branches. Matsuda et al[4] proposed a cranial-to-caudal approach in 2015 and considered that it is easy to expose the pancreas and the root of the middle colic vessels and facilitate lymph node dissection along the surgical trunk for advanced right-sided colon cancer. Zou et al[11] proposed a caudal-to-cranial approach and showed that it was easier to enter the dorsal side of the fusion fascia of Toldt. These approaches all have some limitations. In clinical practice, based on the universal principle of embryonic development and fusion fascia theory, is there a more optimized surgical approach?
In recent years, the authors' team has proposed and practised the CMA to perform LRH with CME, with satisfactory results. Compared with the MA group, the CMA group had obvious advantages in the total operative duration, laparoscopic procedure duration and the number of lymph nodes dissected, while the intraoperative blood loss and the incidence of postoperative complications were basically the same between the two groups.
The theoretical framework of the CMA is derived from four aspects. First, the fascia of the primitive gut (which develops into the mesogastrium, mesocolon, mesostenium, etc.) is continuous during embryonic development[25,26]. Second, during embryological development, the midgut loop rotates 270 counterclockwise around the primary SMA, and the greater omentum and transverse mesocolon overlay the frontal surface of the mesoduodenum[27-29]. The peritoneal membrane at the attachment site fuses and degenerates to form membranous connective tissue called the fusion fascia[29]. Third, the right fusion fascia of Toldt is divided into the posterior pancreatic fascia of Treitz dorsally and the anterior pancreatic fascia of Fredet ventrally at the second portion of the duodenum[13,17]. These fusion fascias are delineated by the posterior layer of the ascending mesocolon ventrally (the mesofascial interface) and by the prerenal fascia, representing the posterior parietal peritoneum covering the retroperitoneum (the retrofascial interface) dorsolaterally[28]. Finally, CME with CVL was defined as follows[4,13]: (1) Dissection between the right mesocolon and the retroperitoneum, following the embryological plane, the dorsal side of the fusion fascia of Toldt and the fusion fascia of Fredet (the retrofascial interface); (2) High ligation of ileocolic vessels, right colic vessels, and the right branches of middle colic vessels; and (3) Removal of a sufficient length of the colon.
In the CAP, after entering the omental bursa, we emphasized the anatomical function of the first cut of the ultrasonic knife and produced the bubble effect when dissecting the fusion fascia in the innermost area adjacent to the gastric antrum (Figure 2A). The bubble effect allows the “angel fair” to form and the surgical space to be confirmed; then, the fusion fascia of the dorsal leaf of the transverse mesocolon and the dorsal mesogastrium can be separated, easily exposing the surgical plane between the fusion fascia of Fredet and the visceral duodenal-pancreatic peritoneum and allowing entry. Garcia-Granero et al[14] indicated that the fusion fascia of Fredet should be removed completely. Mike and Kano[17,30] proposed that there are three fusion modes between the transverse mesocolon and mesoduodenum. That is, fusion between the ventral leaf of the transverse mesocolon and mesoduodenum, between the dorsal leaf of the transverse colon and mesoduodenum, and almost no fusion. We found that regardless of which mode was found, through the CAP, we could obtain a clear surgical plane and achieve a bloodless field.
The GCTH enters the SMV, dividing it into the distal “surgical trunk” and proximal “Henle’s trunk area” (SAGCTH). The difficulty of LRH lies in the SAGCTH. Due to the anatomy of this region, the risk of injury to the SMV and perioperative bleeding is considered to be high. Causes of bleeding or injury include vascular variations in the GCTH[31-33], improper traction during the operation, and an uneven pancreatic surface. In most cases, the GCTH is close to the lower edge of the pancreas, joining the SMV at the uncinate process of the pancreas. The right gastroepiploic vein is near the upper edge of the pancreatic head, sometimes closely associated with the pancreas, and the signs are difficult to identify. The course of the SRCV is special in that it bridges the gap between the transverse mesocolon and the mesogastrium before it merges into the GCTH[34], and inappropriate tension needs to be avoided in dissection of the SRCV. How can this anatomical region be dissected under laparoscopy? We suggest that the SRCV can be used as a landmark, as its inflow mode is relatively constant[35]. By tracking the direction of SRCV inflow into the GCTH from the outermost side of the pancreatic head and performing ligation at its root, the risk of bleeding caused by anatomical relationships and improper techniques can be avoided. In addition, the dorsal side of the transverse mesocolon can be fully exposed at the lower edge of the uncinate process to overcome the obstacle of the visual field under the traditional MA.
In the MAP, we first incised the mesocolon in the ileocolic area approximately 3 cm below the projection of ileocolic vessels to the confluence of the SMV, where a natural depression with colour distinction (yellow–white junction), which is the boundary between the intestinal mesentery and the right mesocolon, can be seen under high-definition laparoscopy. Some experts[36] have called this site the “trijunction”, i.e., the fusion point of the mesocolon, the visceral peritoneum, and the intestinal mesentery. Through the incision of this trijunction, we can enter the posterior space of the colon (the dorsal side of the fusion fascia of Toldt) behind the whole ascending colon and ileocecal part and can gently anatomize the whole plane of the posterior space of the colon. There is some controversy about the ideal surgical plane for colon separation. Zhang et al[37] considered the right retrocolic space to be ideal but did not define the level of the surgical plane. The separation plane should be behind the fusion fascia of Toldt, that is, between the fusion fascia of Toldt and the deep layer of the posterior subperitoneal fascia, as suggested by Mike M[17,30]. Based on autopsy experience, Culligan et al[38] proposed the view that the retrocolic space can be divided into two planes, the mesofascial plane and the retrofascial plane. Shinohara[16] pointed out the A line and the B line. The A line runs along the plane of the ventral side of the fusion fascia of Toldt without cutting it open. It does not affect the degree of lymph node dissection, but in most cases, the fusion fascia of Toldt is cut open, and it is easier to enter and expand the plane along the B line (dorsal side of the fusion fascia of Toldt). Therefore, he recommended dissociating along the B line. Our understanding is that we entered the mesofascial plane following the A line and the retrofascial plane following the B line. Coffey et al[39] suggested that the origin and termination of fascial lymphatics should be determined to partly address this question. A previous study[40] found that the fusion fascia of Toldt may serve a barrier function, as rarely in colorectal cancer does one observe the spread of colon cancer through the fascia into the retroperitoneum. Even where the mesocolon has been directly involved, spread through the fascia is unusual. Therefore, we agree with Mike M that complete removal of the fusion fascia of Toldt is necessary.
Coffey et al[41] proposed that attention should be given to maintenance of the surgical plane during LRH to meet the requirements of CME. How should the right plane be maintained? Our clinical viewpoint and theoretical basis are as follows: (1) In the process of embryonic development, the peritoneum and mesentery at the attachment site fuse and degenerate to form a single sheet of connective tissue called the fusion fascia at the end of intestinal rotation (the fusion fascias of Toldt and Fredet)[42,43], and the inside of the fusion fascia cannot be dissected by definition. It is easy to enter and expand the surgical plane behind the ascending colon from the dorsal side of the fusion fascia of Toldt; (2) The medial border of the fusion fascia of Fredet is the SMV and GCTH[13]. A safe surgical plane with better exposure can be obtained by entering from the dorsal side of the fusion fascia of Fredet, which can reduce the risk of injury to this area and especially prevent tearing and thus bleeding of the SMV, which can lead to life-threatening complications[43]; and (3) Although Shinohara[16] suggested that separation from the ventral side of the fusion fascia does not affect lymph node dissection, there is no evidence-based medical evidence that this procedure can ensure the integrity of lymphatic dissection. More importantly, this method can easily lead to fascia fragmentation and residue. Our conclusion is that to achieve CME in right-sided colon surgery, complete resection of the fusion fascias of Toldt and Fredet is necessary. How do we judge whether we entered the ventral side of the fusion fascia of Toldt under laparoscopy? First, the plane covered by the smooth, deep subperitoneal fascia (Gerota fascia) can be seen in the operation field, the reproductive vessels and peristaltic ureter can be seen behind this fascia, and the white line of Toldt can be seen faintly laterally. Second, a thin layer of relatively dense connective tissue membrane can be seen below the duodenum when the plane is expanded cephalad, and the duodenal wall can be seen vaguely behind this membrane. Third, the whole dissection process is bloodless. Bleeding indicates entry of the incorrect plane.
Where is the core anatomical area in the rendezvous process of the surgical plane of the CAP and MAP? Matsuda et al[10,44] noted that lymph node dissection around the middle colic vessels is technically demanding. The difficulty comes from the fusion of the transverse mesocolon in the middle colic vessel region with the greater omentum, pancreas and duodenum during embryonic development, forming a complex three-dimensional anatomical structure (Figure 2J). A substantial mesenteric tissue mass occurs at the root of the middle colic vessel region formed by midgut rotation during embryonic development. Although the fascia is contiguous, it is interrupted at points where vessels enter or leave the mesentery[39]. The position of the points is the edge of the envelope structure of the mesocolon. There is concentrated lymphatic flow and complex vascular variation at the lower edge of the uncinate process of the pancreas and the root of middle colic vessels[15,45-47]. Therefore, in LRH with CME, the dissection of the mesenteric area at the root of the middle colic vessels is the core anatomical area of the whole operation, and a simple approach such as the MA is difficult to complete. Under the CMA, we treated the cephalic part of the mesocolon of the middle colic vessel region first in the CAP, fully exposed the surgical plane behind the anterior pancreatic fascia to avoid pancreatic injury and safely exposed the GCTH and its branches; we exposed the mesenteric inner and lower boundaries of the SAGCTH and middle colic vessel region; and then we treated the caudal part of the middle colic vessel region to reach the rendezvous region of the surgical plane. Therefore, the mesentery in this area can be dissected in three dimensions to avoid residual mesenteric tissue, pancreatic injury, and injury to vessels such as the GCTH, which may lead to serious intraoperative bleeding.
Different researchers have different understandings of membrane anatomy but achieve the same result by different methods. Mike and Kano[17] have suggested that the membrane is continuous and that the membrane plane is continuous. Zhao et al[48] proposed the concept of a “mesenteric window”. After incising the inferior edge of the ileocolic vascular pedicle, we could easily enter the natural right retrocolic space and extend the space laterally and cranially. Shinohara[16] affirmed that the SRCV and its confluence with the GCTH constituted the rotation centre of the mesocolon during embryonic development. Coffey et al[39] considered that the central mechanism of fixation of the mesocolon and posterior abdominal wall, that is, the connection point of the mesentery and blood vessels, constitutes the "hilum" of the mesentery, which determines the medial boundary of dissection, just as right peritoneal reflection (the white line of Toldt) determines the lateral boundary. Garcia-Granero et al[14] found that the medial limit of the fascia of Fredet is represented by the SMV and GCTH, which is also the hilum of the mesocolon. The above research results strongly promote the accuracy of surgery in LRH. According to our understanding, the right mesocolon is fan-shaped, and the SMV axis is the core anatomical marker of the right mesocolon, which connects the mesenteric window and hilum. These two landmarks are the result of fusion of the gastrointestinal mesentery after rotation during embryonic development and are also the important theoretical basis of membrane anatomy for the CMA.
Although this study discusses the surgical approach, the ultimate pursuit of the surgeon is oncological benefits for the patient. An early study by West et al[49] suggested that attention should be given to the quality classification of surgical specimens in the surgical treatment of colon cancer, as colon cancer patients who undergo resection with an intact mesocolon achieve 15% better 5-year overall survival than those with defects in the mesocolic specimens. Xie et al[50] recommended that in gastrointestinal surgery, the mesentery should be removed completely to prevent cancer leakage. Benz et al[51] proposed a new classification system for CME in right-sided colon cancer, with the following distribution: type 0 (best), type I, type II, and type III (poorest). In type 0, the true CME specimen, the stalks of the ileocolic vessels and middle colic vessels are connected by tissue of the surgical trunk (lymphatic tissue package covering the SMV), and the mesocolic window has a complete medial frame of mesocolic tissue. Bertelsen et al[52] recently reported five-year outcomes for right-sided colon cancer across the capital region, demonstrating a significant reduction in recurrence in the CME group (9.7% vs 17.9%) and the potential for improved long-term outcomes after the resection of all UICC stage I-III right-sided colon adenocarcinomas. The original intention of presenting the CMA was to standardize the surgical procedure and to obtain better specimen quality.
The CMA is based on the theory of embryonic development and membrane anatomy, and the technical route itself weakens the vascular and lymphoid anatomy. The unique advantages of LRH with the CMA are as follows: (1) The team learning curve can be significantly shortened; (2) The operation can be performed with little to no bleeding, with a reduced probability of conversion to laparotomy and improved safety and efficiency; and (3) Higher-quality specimens can be obtained. Therefore, we believe that the CMA is the dominant approach for laparoscopic radical resection of the right colon. However, the CMA currently lacks RCT-based evidence and needs to be validated in further multicentre prospective studies.
Complete mesocolic excision (CME) with central vascular ligation (CVL) is the technical standard for colon cancer surgery. How to achieve CME with CVL in laparoscopic right hemicolectomy (LRH) is controversial. Several approaches have been proposed, but a unified standard approach is not yet available.
The authors' team has proposed and practised the cranial-medial mixed dominant approach (CMA) to perform LRH with CME for years. We would like to confirm that the CMA does have unique technical advantages through data rather than subjective opinionssby comparing it with the classic medial approach (MA).
To compare the CMA with the classic MA to prove that the CMA has unique advantages in performing LRH.
We compared the two groups (CMA and MA) by intraoperative data (operative duration, blood loss, specimen length, number of resected and positive lymph nodes, and postoperative data (exhaust time, liquid intake time, postoperative hospitalization, postoperative complications). Additionally, we described the procedure and technical points of the CMA in detail to facilitate the reader's understanding.
There were no significant differences in baseline data or the number of positive lymph nodes, intraoperative blood loss, postoperative exhaust time, feeding time, postoperative hospital stay or postoperative complication incidence between the two groups. The operation was shorter and the number of lymph nodes dissected was higher in the CMA group.
The CMA weakens the vascular and lymphoid anatomy and has unique advantages for LRH with CME and CVL.
More RCT-based evidence and further multicentre prospective studies are needed to validate the CMA.
| 1. | Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81:503-508. [PubMed] |
| 2. | Morino M, Parini U, Giraudo G, Salval M, Brachet Contul R, Garrone C. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg. 2003;237:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991;1:144-150. [PubMed] |
| 4. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-64; discussion 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1145] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 5. | Gouvas N, Agalianos C, Papaparaskeva K, Perrakis A, Hohenberger W, Xynos E. Surgery along the embryological planes for colon cancer: a systematic review of complete mesocolic excision. Int J Colorectal Dis. 2016;31:1577-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 7. | Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 576] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 8. | Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen M, Rasmussen LA, Jepsen LV, Kristensen B, Gögenur I; Copenhagen Complete Mesocolic Excision Study (COMES); Danish Colorectal Cancer Group (DCCG). Short-term outcomes after complete mesocolic excision compared with 'conventional' colonic cancer surgery. Br J Surg. 2016;103:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Liang JT, Lai HS, Lee PH. Laparoscopic medial-to-lateral approach for the curative resection of right-sided colon cancer. Ann Surg Oncol. 2007;14:1878-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Matsuda T, Iwasaki T, Mitsutsuji M, Hirata K, Maekawa Y, Tanaka T, Shimada E, Kakeji Y. Cranial-to-caudal approach for radical lymph node dissection along the surgical trunk in laparoscopic right hemicolectomy. Surg Endosc. 2015;29:1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Zou LN, Lu XQ, Wan J. Techniques and Feasibility of the Caudal-to-Cranial Approach for Laparoscopic Right Colectomy With Complete Mesenteric Excision. Dis Colon Rectum. 2017;60:e23-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Topographique DE. Anatomie humaine, descriptive et topographique. Journal of the American Medical Association. 1925;84:464-464. [DOI] [Full Text] |
| 13. | Rouvière H. Anatomie humaine, descriptive et topographique. Paris: Masson et cie, 1924. |
| 14. | Garcia-Granero A, Pellino G, Frasson M, Fletcher-Sanfeliu D, Bonilla F, Sánchez-Guillén L, Domenech Dolz A, Primo Romaguera V, Sabater Ortí L, Martinez-Soriano F, Garcia-Granero E, Valverde-Navarro AA. The fusion fascia of Fredet: an important embryological landmark for complete mesocolic excision and D3-lymphadenectomy in right colon cancer. Surg Endosc. 2019;33:3842-3850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | García-Granero Á, Sánchez-Guillén L, Fletcher-Sanfeliu D, Sancho-Muriel J, Alvarez-Sarrado E, Pellino G, Delgado-Moraleda JJ, Sabater Ortí L, Valverde-Navarro AA, Frasson M. Surgical anatomy of D3 lymphadenectomy in right colon cancer, gastrocolic trunk of Henle and surgical trunk of Gillot - a video vignette. Colorectal Dis. 2018;20:935-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Ignjatovic D, Spasojevic M, Stimec B. Can the gastrocolic trunk of Henle serve as an anatomical landmark in laparoscopic right colectomy? Am J Surg. 2010;199:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Mike M, Kano N. Laparoscopic surgery for colon cancer: a review of the fascial composition of the abdominal cavity. Surg Today. 2015;45:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1429] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 19. | van Bree SH, Bemelman WA, Hollmann MW, Zwinderman AH, Matteoli G, El Temna S, The FO, Vlug MS, Bennink RJ, Boeckxstaens GE. Identification of clinical outcome measures for recovery of gastrointestinal motility in postoperative ileus. Ann Surg. 2014;259:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Iversen ER, Kristensen B, Gögenur I; Danish Colorectal Cancer Group. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 21. | Zurleni T, Cassiano A, Gjoni E, Ballabio A, Serio G, Marzoli L, Zurleni F. Surgical and oncological outcomes after complete mesocolic excision in right-sided colon cancer compared with conventional surgery: a retrospective, single-institution study. Int J Colorectal Dis. 2018;33:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis. 2017;19:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (3)] |
| 23. | Rutgers M, Kahn J. Comparing standard laparoscopic hemicolectomy to CME radical right colectomy for patients with right sided colon cancer: a randomized controlled feasibility trial. Eur J Surg Oncol. 2020;46:e15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Rotholtz NA, Bun ME, Tessio M, Lencinas SM, Laporte M, Aued ML, Peczan CE, Mezzadri NA. Laparoscopic colectomy: medial vs lateral approach. Surg Laparosc Endosc Percutan Tech. 2009;19:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Coffey JC, Dillon M, Sehgal R, Dockery P, Quondamatteo F, Walsh D, Walsh L. Mesenteric-Based Surgery Exploits Gastrointestinal, Peritoneal, Mesenteric and Fascial Continuity from Duodenojejunal Flexure to the Anorectal Junction--A Review. Dig Surg. 2015;32:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Coffey JC, O'Leary DP. The mesentery: structure, function, and role in disease. Lancet Gastroenterol Hepatol. 2016;1:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 27. | Langman J, Sadler TW. Langman's medical embryology. Philadelphia, PA: Lippincott Williams & Wilkins, 2004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Jeong YJ, Cho BH, Kinugasa Y, Song CH, Hirai I, Kimura W, Fujimiya M, Murakami G. Fetal topohistology of the mesocolon transversum with special reference to fusion with other mesenteries and fasciae. Clin Anat. 2009;22:716-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Shinohara H, Kurahashi Y, Haruta S, Ishida Y, Sasako M. Universalization of the operative strategy by systematic mesogastric excision for stomach cancer with that for total mesorectal excision and complete mesocolic excision colorectal counterparts. Ann Gastroenterol Surg. 2018;2:28-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Mike M. Laparoscopic right colectomy. Operative maneuvers based on the fascial composition in the embryological standpoint. In: Kano N. Laparoscopic colorectal cancer surgery operative maneuvers based on the fascial composition in the embryological standpoint. Tokyo: Lgaku-Shoin, 2012: 116-133. |
| 31. | Kuzu MA, İsmail E, Çelik S, Şahin MF, Güner MA, Hohenberger W, Açar Hİ. Variations in the Vascular Anatomy of the Right Colon and Implications for Right-Sided Colon Surgery. Dis Colon Rectum. 2017;60:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Adamina M, Manwaring ML, Park KJ, Delaney CP. Laparoscopic complete mesocolic excision for right colon cancer. Surg Endosc. 2012;26:2976-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Voiglio EJ, Boutillier du Retail C, Neidhardt JP, Caillot JL, Barale F, Mertens P. Gastrocolic vein. Definition and report of two cases of avulsion. Surg Radiol Anat. 1998;20:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Strey CW, Wullstein C, Adamina M, Agha A, Aselmann H, Becker T, Grützmann R, Kneist W, Maak M, Mann B, Moesta KT, Runkel N, Schafmayer C, Türler A, Wedel T, Benz S. Laparoscopic right hemicolectomy with CME: standardization using the "critical view" concept. Surg Endosc. 2018;32:5021-5030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Garcia-Granero A, Sánchez-Guillén L, Frasson M, Sancho Muriel J, Alvarez Sarrado E, Fletcher-Sanfeliu D, Flor Lorente B, Pamies J, Corral Rubio J, Valverde Navarro AA, Martinez Soriano F, Garcia-Granero E. How to reduce the superior mesenteric vein bleeding risk during laparoscopic right hemicolectomy. Int J Colorectal Dis. 2018;33:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Xie D, Yu C, Gao C, Osaiweran H, Hu J, Gong J. An Optimal Approach for Laparoscopic D3 lymphadenectomy Plus Complete Mesocolic Excision (D3+CME) for Right-Sided Colon Cancer. Ann Surg Oncol. 2017;24:1312-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Zhang C, Ding ZH, Yu HT, Yu J, Wang YN, Hu YF, Li GX. Retrocolic spaces: anatomy of the surgical planes in laparoscopic right hemicolectomy for cancer. Am Surg. 2011;77:1546-1552. [PubMed] |
| 38. | Culligan K, Walsh S, Dunne C, Walsh M, Ryan S, Quondamatteo F, Dockery P, Coffey JC. The mesocolon: a histological and electron microscopic characterization of the mesenteric attachment of the colon prior to and after surgical mobilization. Ann Surg. 2014;260:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Coffey JC, Lavery I, Sehgal R, Walsh D. Mesenteric principles of gastrointestinal surgery; basic and applied science. New York, NY: CRC Press, 2017. |
| 40. | Gao Z, Ye Y, Zhang W, Shen D, Zhong Y, Jiang K, Yang X, Yin M, Liang B, Tian L, Wang S. An anatomical, histopathological, and molecular biological function study of the fascias posterior to the interperitoneal colon and its associated mesocolon: their relevance to colonic surgery. J Anat. 2013;223:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 41. | Coffey JC, Sehgal R, Culligan K, Dunne C, McGrath D, Lawes N, Walsh D. Terminology and nomenclature in colonic surgery: universal application of a rule-based approach derived from updates on mesenteric anatomy. Tech Coloproctol. 2014;18:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 42. | Tobin CE. The renal fascia and its relation to the transversalis fascia. Anatomical Record. 1944;89:295-311. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Woodburne R, Burkel W. The peritoneum. In: Woodburne R and Burkel W Essentials of human anatomy. New York: Oxford University Press, 1991: 436-446. |
| 44. | Matsuda T, Sumi Y, Yamashita K, Hasegawa H, Yamamoto M, Matsuda Y, Kanaji S, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y. Anatomy of the Transverse Mesocolon Based on Embryology for Laparoscopic Complete Mesocolic Excision of Right-Sided Colon Cancer. Ann Surg Oncol. 2017;24:3673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Jin G, Tuo H, Sugiyama M, Oki A, Abe N, Mori T, Masaki T, Atomi Y. Anatomic study of the superior right colic vein: its relevance to pancreatic and colonic surgery. Am J Surg. 2006;191:100-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Yada H, Sawai K, Taniguchi H, Hoshima M, Katoh M, Takahashi T. Analysis of vascular anatomy and lymph node metastases warrants radical segmental bowel resection for colon cancer. World J Surg. 1997;21:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Yamaguchi S, Kuroyanagi H, Milsom JW, Sim R, Shimada H. Venous anatomy of the right colon: precise structure of the major veins and gastrocolic trunk in 58 cadavers. Dis Colon Rectum. 2002;45:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 48. | Zhao LY, Liu H, Wang YN, Deng HJ, Xue Q, Li GX. Techniques and feasibility of laparoscopic extended right hemicolectomy with D3 lymphadenectomy. World J Gastroenterol. 2014;20:10531-10536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol. 2008;9:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 50. | Xie D, Liu L, Osaiweran H, Yu C, Sheng F, Gao C, Hu J, Gong J. Detection and Characterization of Metastatic Cancer Cells in the Mesogastrium of Gastric Cancer Patients. PLoS One. 2015;10:e0142970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Benz S, Tannapfel A, Tam Y, Grünenwald A, Vollmer S, Stricker I. Proposal of a new classification system for complete mesocolic excison in right-sided colon cancer. Tech Coloproctol. 2019;23:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Bertelsen CA, Neuenschwander AU, Jansen JE, Tenma JR, Wilhelmsen M, Kirkegaard-Klitbo A, Iversen ER, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Born PW, Kristensen B, Kleif J. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol. 2019;20:1556-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Egypt; Hegazy AA, Egypt S-Editor: Wang LL L-Editor: A P-Editor: Li X