Published online Feb 27, 2022. doi: 10.4240/wjgs.v14.i2.107
Peer-review started: September 3, 2021
First decision: October 2, 2021
Revised: October 13, 2021
Accepted: January 14, 2022
Article in press: January 14, 2022
Published online: February 27, 2022
Processing time: 172 Days and 4 Hours
Mirizzi syndrome (MS) remains a challenging biliary disease, and its low rate of preoperative diagnosis should be resolved. Moreover, technological advances have not resulted in decisive improvements in the surgical treatment of MS. Complex bile duct lesions due to MS make surgery difficult, especially when the laparoscopic approach is adopted. The safety and long-term effect of MS treatment need to be guaranteed in terms of preoperative diagnosis and surgical strategy.
To analyze preoperative diagnostic methods and the safety, effectiveness, prognosis and related factors of surgical strategies for different types of MS.
The clinical data of MS patients who received surgical treatment from January 1, 2010 to December 31, 2020 were retrospectively reviewed. Patients with malignancies, choledochojejunal fistula, lack of data and lost to follow-up were excluded. According to preoperative imaging examination records and documented intraoperative findings, the clinical types of MS were determined using the Csendes classification. The safety, effectiveness and long-term prognosis of surgical treatment in different types of MS, and their interactions with the clinical characteristics of patients were summarized.
Sixty-six patients with MS were included (34 males and 32 females). Magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) showed specific imaging features of MS in 58 cases (87.9%), which was superior to ultrasound scan (USS) in the diagnosis of MS and more sensitive to subtle biliary lesions than USS. The overall laparoscopic surgery completion rate was 53.03% (35/66), where the completion rates of MS type I, II and III were 69.05% (29/42), 42.86% (6/14) and zero (0/10), respectively. Thirty-one patients (46.97%) underwent laparotomy or conversion to laparotomy including 11 cases of iatrogenic bile duct injury which occurred in type I patients, and 25 of these patients underwent bile duct exploration, repair and T-tube drainage. In addition, 25 patients underwent intraoperative choledochoscopy and T-tube cholangiography. Overall, 21 cases (31.8%) were repaired by simple suturing, and 14 cases (21.2%) were repaired using the remaining gallbladder wall patch in the subtotal cholecystectomy. The ascendant of the Csendes classification types led to an increase in surgical complexity reflected by increased operation time, bleeding volume and cost. Gender, acute abdominal pain and measurable stone size had no effect on Csendes type of MS or final surgical approach. Age had no effect on the classification of MS, but it influenced the final surgical approach, hospital stay and cost. A total of 66 patients obtained a relatively high preoperative diagnostic rate and underwent surgery safely without serious complications, and no mortality was observed during the follow-up period of 36.5 ± 26.5 mo (range 13-76, median 22 mo).
MRI/MRCP can improve the preoperative diagnosis of MS. The Csendes classification can reflect the difficulty of treatment. The surgical strategies including laparoscopic surgery for MS should be formulated based on full evaluation and selection.
Core Tip: Accurate preoperative diagnosis is a prerequisite for rational selection of surgical strategies for Mirizzi syndrome (MS). Preoperative images combined with findings during intraoperative exploration to determine the classification of MS is the basis for confirming the surgical approach. The present study revealed that magnetic resonance imaging is an effective and reliable preoperative diagnostic method for MS. Laparoscopic surgery can be used in most patients with MS type I and II following detailed evaluation, while type III and IV patients require laparotomy or conversion surgery. Our results verified that disease classification can reflect the difficulty of MS surgery.
- Citation: Lai W, Yang J, Xu N, Chen JH, Yang C, Yao HH. Surgical strategies for Mirizzi syndrome: A ten-year single center experience. World J Gastrointest Surg 2022; 14(2): 107-119
- URL: https://www.wjgnet.com/1948-9366/full/v14/i2/107.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i2.107
Mirizzi syndrome (MS) is a special clinical complication of cholecystolithiasis. It refers to a series of symptoms caused by compression of the common bile duct (CBD) or hepatic duct with or without varying degrees of cholecystobiliary fistula, which results from the impaction of stones in the Hartmann pouch or cystic duct of the gallbladder and/or tissue inflammation and edema[1].
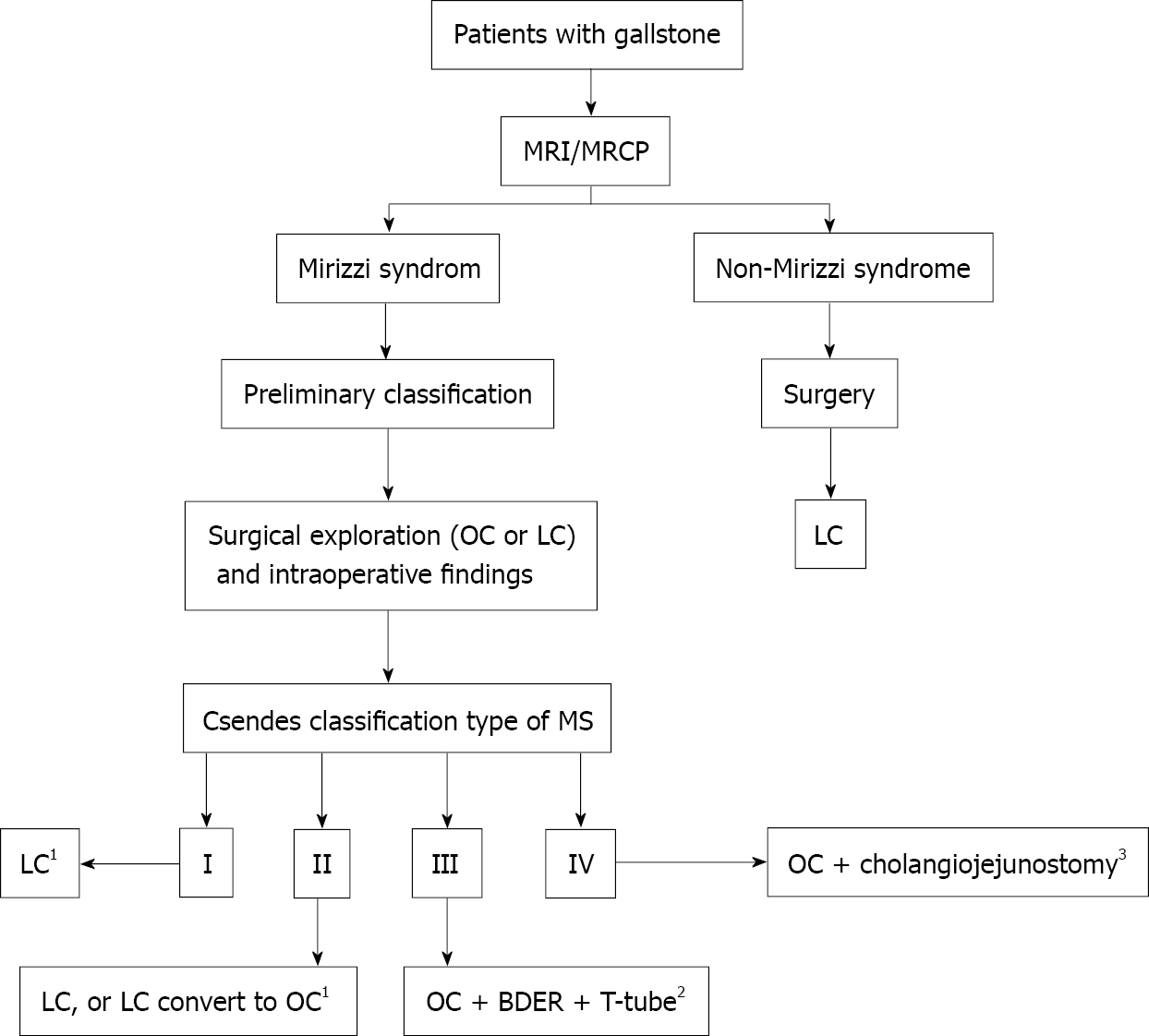
According to the clinical manifestations, pathophysiological changes and imaging features, MS is divided into different types. The Csendes classification is most commonly used in classification of clinical types of MS[2]. This classification divides MS into four main types. Type I: The Hartmann pouch or cystic duct is impacted by gallstones accompanied by compression of the CBD, without fistula formation. Type II: A fistula is formed and the eroded circumference of the CBD is less than one third of that in type I. Type III: The fistula erodes two thirds of the circumference of the CBD. Type IV: Cholecystobiliary fistula involves the whole circumference of the CBD.
Advances in medical technology, such as laparoscopy and robotic surgery, have brought hepatobiliary surgery into a new era. Even so, MS is still a dilemma for surgeons because the low incidence rate leads to difficulty in accumulating personal experience, and is associated with a high conversion rate, and a high risk of operative complications, particularly bile duct injury (BDI)[3,4].
Accurate diagnosis is a prerequisite for the correct treatment of MS. An inaccurate diagnosis usually results in misjudgment during surgery, increases the incidence of BDI, and finally leads to worse clinical consequences. MS has long been a dilemma for surgeons, especially when laparoscopic surgery is performed[5,6]. The diagnosis and management of MS are still challenging[7,8].
Even in patients with a definite diagnosis, many difficulties and risks still need to be overcome in dealing with MS. The erosion of structures, changes in anatomy, dense adhesions and fibrotic lesions caused by stone incarceration and local inflammation increase the difficulty of surgery, the risk of bleeding and the probability of BDI[9]. In view of the above reasons, it is believed that laparoscopic surgery is not the best treatment method for MS, even if it is not a contraindication[10,11].
Endoscopic retrograde cholangiopancreatography (ERCP) can provide more accurate biliary images, and establish diagnosis before operation, and intervene on the combined CBD stones simultaneously. However, its inherent disadvantages limit its application in the comprehensive treatment of MS[1,12,13], such as the treatment of the diseased gallbladder, and cholecystectomy still needs to be performed at the same time or delayed.
Due to the high cost and low popularization, requirements for the operating skills of surgeons, and complications similar to laparoscopic surgery, robotic surgery is not a practical means to treat MS[14-16]. Moreover, it is usually performed in combination with ERCP when dealing with MS, which increases the requirements for facilities and personnel[17,18].
This study retrospectively reviewed the experience of the surgical treatment of MS in our hospital over the past ten years. This experience is mainly based on the strategy that magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) is used as an essential preoperative diagnostic method, combined with the findings of intraoperative exploration to determine the surgical plan in MS patients, without ERCP preoperatively or intraoperatively. The strategy was safe and effective, even though ERCP was not routinely performed. It can be implemented in hospitals with basic facilities and medical qualifications. It is especially suitable for promotion in areas with insufficient medical resources.
We conducted a retrospective study involving patients diagnosed with MS who were treated by surgery at the Chengdu First People’s Hospital. Data were collected from the case database in our hospital.
All patients diagnosed with MS from January 1, 2010 to December 31, 2020 were enrolled in this study. The inclusion criteria were: (1) Over 18 years old; (2) MS patients without intrahepatic bile duct stones and choledocholithiasis; and (3) The results of related imaging and detailed intraoperative exploration were recorded. The exclusion criteria were: (1) Patients with hepatobiliary malignancies; (2) Patients complicated by choledochojejunal fistula; (3) Data were missing and could not be classified; and (4) Patients lost to follow-up.
According to preoperative imaging examination and intraoperative findings, the clinical types of MS was determined using the Csendesclassification[2].
The study was reviewed and approved by the Institutional Review Board of Chengdu First People’s Hospital(Chengdu Integrated TCM & Western Medicine Hospital). All patients and/or their guardians signed an informed consent before surgery, which met the ethical requirements. Due to the retrospective design of the study, informed consent was waived by the ethics committee for this study.
All patients were followed up in the outpatient department until to June 30, 2021. At least one liver function and ultrasound scan (USS) examination of the hepatobiliary system was completed during the follow-up period after discharge. Before extubation, T-tube cholangiography was performed routinely in patients with T-tube placement, and MRI/MRCP was adopted if necessary. The patients with a percutaneous transhepatic cholangio pancreatic drainage (PTCD) tube were treated in the same way as those with a T-tube. Whether the patients would receive subsequent treatment was determined according to the review results.
Continuous variables are presented as mean ± SD, and categorical variables are presented as frequencies and percentages. The comparison of rates among different groups was based on counting data χ2 test. The mean number of different groups was compared by variance analysis. Statistical analyses were performed using SPSS 19 (IBM Corp., Armonk, NY, United States). A two-sided P < 0.05 was considered statistically significant.
Sixty-six patients with MS were included, 34 males (51.5%) and 32 females (48.5%), which is approximately 0.6% of the patients who underwent cholecystectomy in our hospital during the same period. Their age ranged from 18 to 83 years (48.1 ± 15.0, median 47 years). Forty-eight patients (72.7%) with acute abdominal pain and 18 patients (27.3%) without acute abdominal pain were admitted through different routes.
Thirty-nine patients (59.1%) had at least one previous admission according to the available medical records. The upper limit of the normal reference value for total bilirubin detection in our hospital is 28 μmol/L. According to this standard, 35 patients (53.0%) also had jaundice at the time of admission, and 6 of these patients (1 with type II and 5 with type III) underwent preoperative PTCD because of severe comorbidities (hypertension in 1, diabetes in 2 and lung disease in 3) and received general anesthesia surgery after their comorbidities were controlled. The demographic data of the MS patients included in this study are shown in Table 1.
| Category | ||
| Male/Female | 34/32 | |
| Age (yr) | 48.1 ± 15.0, 18-83, 47 | |
| Admission route (Emergency/Outpatient) | 48/18 | |
| Previous admissions | 2.24 ± 0.96, 1-3, 3 | |
| Months from discovery of gallstone to this admission | 17.8 ± 4.51, 9-22, 21 | |
| Confirmed episodes of abdominal pain | 2.15 ± 1.04, 1-6, 2 | |
| Total bilirubin (μmol/L) | ≤ 28 | 31 (47.0%) |
| 28-56 | 27 (40.9%) | |
| > 56 | 8 (12.1%) | |
| Postoperative pathologicalresults ofgallbladder | Acute inflammation | 24 (36.4%) |
| Acute inflammation and gangrene | 8 (12.1%) | |
| Acute suppurative inflammation | 9 (13.6%) | |
| Chronic inflammation | 12 (18.2%) | |
| Chronic suppurative inflammation | 5 (7.6%) | |
| Xanthogranuloma | 8 (12.1%) | |
| Preoperative PTCD | 6 (9.1%) | |
| Preoperative treatment time (d) | 6.35 ± 3.28, 2-20, 6 | |
| Postoperative treatment time (d) | 7.36 ± 3.66, 3-19, 6.5 | |
| Total hospitalization time (d) | 13.76 ± 5.41, 6-31, 13 | |
| Hospitalization cost (CNY Yuan) | 24549 ± 6536, 13596-40815, 23044 | |
ERCP was not performed in any of the 66 MS patients, and USS and MRI/MRCP were performed in all the patients. USS showed bile duct dilatation in 13 cases (19.7%), bile duct compression in 11 cases (16.7%), and the others showed no specific signs. All patients underwent MRI/MRCP at the same time. The results showed that 58 cases (87.9%) had special imaging features of MS, including stones in the Hartmann pouch or cystic duct, extrinsic compression of the bile duct, dilatation of the bile duct and obvious inflammatory changes in Calot’s triangle. MRI/MRCP was superior to USS in the diagnosis of MS (Fisher’s exact test, χ2 = 5.873, P = 0.023). It seemed that serious biliary changes (type II and type III) could be easily identified by USS, especially when combined with higher bilirubin levels. MRI/MRCP was more sensitive to subtle biliary lesions than USS, even without jaundice (Table 2).
| Imaging examination | Type I | Type II | Type III | Statistics | Total bilirubin (μmol/L) | Statistics | |||
| ≤ 28 | 28-56 | > 56 | |||||||
| USS | + | 10 | 8 | 6 | χ2 = 12.00; P = 0.002 | 11 | 9 | 4 | χ2 = 0.760; P = 0.684 |
| - | 32 | 6 | 4 | 20 | 18 | 4 | |||
| MRI/MRCP | + | 34 | 14 | 10 | χ2 = 5.202; P = 0.074 | 23 | 27 | 8 | χ2 = 10.28; P = 0.006 |
| - | 8 | 0 | 0 | 8 | 0 | 0 | |||
According to preoperative imaging examinations and intraoperative findings, 42 patients were classified as Csendes type I, 14 patients were classifies as type II, and 10 patients were classified as type III. None of the patients had type IV disease. Taking laparoscopic surgery as the standard, the overall completion rate was 53.03% (35/66), where the completion rates in type I, II and III were 69.05% (29/42), 42.86% (6/14) and zero (0/10), respectively. Different Csendes types had different degrees of jaundice (χ2 = 51.417, P = 0.000), and the different types ultimately required different surgical methods, as laparoscopic surgery alone could not be performed in all MS patients (Table 3). The ascendant in the type of Csendes classification led to increased surgical complexity (Table 3). Thus, the higher the classification degree, the more difficult the surgery. This was reflected in increased operation time, bleeding volume and treatment cost, which were statistically significant (Table 3). The hospitalization time increased in different Csendes types, but the differences were not statistically significant.
| Type I | Type II | Type III | Type IV | Statistics | ||
| n (%) | 42 (63.64%) | 14 (21.21%) | 10 (15.15%) | 0 | ||
| Total bilirubin (μmol/L) | ≤ 28 | 29 | 2 | 0 | - | χ2 = 51.42; P = 0.000 |
| 28-56 | 13 | 11 | 3 | - | ||
| > 56 | 0 | 1 | 7 | - | ||
| Surgical methods | LC | 29 | 62 | 0 | - | χ2 = 29.91; P = 0.000 |
| LC convert to OC | 2 | 32 | 0 | - | ||
| LC convert to OC + BDER + T-tube | 71,2 | 43 | 83 | - | ||
| OC | 0 | 12 | 0 | - | ||
| OC + BDER + T-tube | 41,2 | 0 | 23 | - | ||
| Hospitalization time (d) | 12.8 ± 4.8; 6-25, 12.5 | 15.1 ± 6.2; 8-26, 13.5 | 15.9 ± 6.1; 8-31, 15 | - | F = 1.981; P = 0.146 | |
| Treatment cost (CNY Yuan) | 23037 ± 5522; 13596-40815, 21963 | 24916 ± 7146; 15108-36557, 23593 | 30387 ± 6865; 17161-40568, 28624 | - | F = 5.909; P = 0.004 | |
| Operative time (minutes) | 154.4 ± 91.1; 50-395, 122.5 | 230.4 ± 133.7; 80-480, 175 | 219.0 ± 122.2; 95-520, 177.5 | - | F = 3.486; P = 0.037 | |
| Bleeding volume (mL) | 96.6 ± 81.5; 20-340, 60 | 191.4 ± 123.3; 30-390, 180 | 163.5 ± 114.3; 25-400, 140 | - | F = 5.919; P = 0.004 | |
Gender, acute abdominal pain and the measurable stone size had no effect on Csendes type of MS and final surgical method. Preoperative treatment time did not affect the final surgical method (χ2 = 5.950, P = 0.295). However, the longer the preoperative treatment time, the longer the overall length of hospital stay (F = 19.70, P = 0.000) and the higher the overall cost (F = 6.778, P = 0.002).
Age had no effect on the classification of MS, but it did influence the final surgical method. The laparoscopic surgery completion rates in different age groups (< 45 year, 45-60 year and > 60 year) were 58.06% (18/31), 52.94% (9/17) and 47.06% (8/17), respectively (χ2 = 16.06, P = 0.042). In addition, hospital stay (F = 5. 654, P = 0.002) and hospitalization cost (F = 7.400, P = 0.008) in patients over 60 years old were both significantly higher than those in patients under 60 years old.
Three-port laparoscopic surgery was used routinely. Four-port laparoscopic surgery as an alternative technique was performed when necessary. The right subcostal incision was the standard approach for laparotomy or conversion. Impacted stones varied in size from 0.5 cm to 6 cm, resulting in different fistulas accompanied by local inflammation and fibrotic adhesions. The upper and lower bile ducts of these lesions were dilated to varying degrees (Table 4). Six patients with preoperative PTCD underwent intraoperative cholangiography through the PTCD tube to achieve the correct anatomical identification. Thirty-six patients underwent retrograde cholecystectomy to obtain correct anatomical identification. Due to improper operation when separating Calot’s triangle, such as vigorous tearing, 11 cases of iatrogenic BDI occurred in type I patients. Twenty-one cases (31.8%) were repaired by simple suturing, and 14 cases (21.2%) were repaired using the remaining gallbladder wall patch in subtotal cholecystectomy (STC). The excess gallbladder wall can be resected after satisfactory repair to avoid the formation of residual gallbladder. A T-tube should be placed in patients with obvious compression of the bile duct, severe scar fibrosis and unsatisfactory repair. The T-tube was generally placed below the bile duct repair site, one short arm placed upward to the repair site to play a supporting role, and the PTCD tube placed above the repair site. T-tubes were placed in 25 patients (37.9%), including 3 type III patients through the fistula, and in the other 22 cases through the bile duct incision. Twenty-five patients underwent intraoperative choledochoscopy and T-tube cholangiography to further clarify the condition of the bile duct and ensure no residual stones before the end of surgery. A Winslow foramen drainage tube was also routinely placed in all patients before the end of surgery. The operation time varied, but the total bleeding volume was acceptable and no patients required intraoperative blood transfusion (Table 4).
| Category | n = 66 | |
| Final surgical approach | 3-port laparoscopic surgery | 24, 36.4% |
| 4-port laparoscopic surgery | 11, 16.7% | |
| Right subcostal incision | 31, 46.9% | |
| Maximum diameter of stone (cm) | 2.15 ± 1.17, 0.5-6, 2 | |
| Fistula size (mm) | Longitudinal diameter | 4.1 ± 1.0, 2-6, 4 |
| Transverse diameter | 4.5 ± 1.4, 2-8, 4 | |
| Diameter of extra hepatic bile duct (mm) | Maximum 14 ± 2.8, 10-22, 14 | |
| Minimum 8.4 ± 1.8, 6-12, 8 | ||
| Iatrogenic BDI | 11, 16.7% (11 in type I) | |
| Retrograde resection of gallbladder | 36, 54.5% | |
| BDER (35, 53%) | Simple suture repair | 21, 31.8% (11 in type I, 10 in type II) |
| STC and repair using gallbladder wall | 14, 21.2% (4 in Type II,10 in type III) | |
| T-tube (25, 37.9%) (14-22 Fr, 18 Fr) | Transfistula 3 (in type III) | |
| Transbiliary incision 22 | ||
| Cholangiography (25, 37.9%) | Trans-PTCD | 61 |
| Trans-T-tube | 25 | |
| Choledochoscopy (25, 37.9%) | Trans-fistula | 3 |
| Trans-cystic duct | 2 | |
| Trans-biliary incision | 20 | |
| Operative time (min) | 180 ± 110, 50-520, 140 | |
| Bleeding volume (mL) | 127 ± 104, 87.5, 20-400 | |
A total of 66 patients were followed up for 36.5 ± 26.5 mo (range 13-76, median 22 mo). All Winslow foramen drainage tubes were removed 3-25 d after surgery according to the recovery, drainage characteristics, combined with liver function and USS results. If a T-tube was placed, it was removed 1.5 to 6 mo after cholangiography if liver function tests were normal.
Incision infection occurred in 7 patients, mainly in those who underwent open surgery or conversion. Overall, incision infection was mild and healed after local drainage and oral antibiotic treatment. Bile leakage occurred in 9 cases during the perioperative period accompanied by different degrees of localized peritonitis, which were resolved by strengthening drainage, delayed extubation and symptomatic treatment. Postoperative bleeding occurred in 4 patients, mainly manifested as bloody drainage (2 cases of abdominal bloody drainage and 2 cases of bloody bile), which lasted three to four days in the week after surgery. It was estimated that the average daily volume did not exceed 60 mL, and the patients recovered following conservative hemostasis treatment without reoperation or interventional therapy. Five patients were considered to have acute cholangitis due to abnormal liver function and fever. These patients recovered after liver protection and anti-infection treatment. Fourteen patients had a transient elevation in transaminase and/or bilirubin based on preoperative liver function, they gradually recovered and were discharged after symptomatic treatment. One patient with preoperative Csendes type III had elevated transaminase repeatedly with normal bilirubin after discharge. USS showed dilation of the right intrahepatic bile duct and MRI/MRCP showed slight constriction of the right hepatic duct with dilation of the right intrahepatic bile duct, which was considered to be compression caused by inflammation and edema. The transaminase level and imaging results gradually returned to normal after oral liver protective drug treatment. Five patients had residual or recurrent stones in the CBD during the follow-up period, and the stones were successfully removed (3 cases by choledochoscopyvia the T-tube sinus, and 2 cases by ERCP). Postoperative pneumonia occurred in 3 patients who had preoperative lung diseases, these patients recovered after treatment according to advice provided by the Respiratory Department. Seven cases had different degrees of gastrointestinal dysfunction which normalized after symptomatic treatment.
By the end of the follow-up period, no residual gallbladder was confirmed by imaging examination and no reoperations were necessary. No patients died during the follow-up period (Table 5).
| Postoperative complications | n (%) |
| Incision infection | 7 (10.6) |
| Bile leakage | 9 (13.6) |
| Bloody drainage | 4 (6.1) |
| Cholangitis | 5 (7.6) |
| Abnormal liver function | 14 (21.2) |
| Biliary stricture | 1 (1.5) |
| Residual or recurrent stone | 5 (7.6) |
| Pneumonia | 3 (4.5) |
| Gastrointestinal dysfunction | 7 (10.6) |
The first accurate description and report of MS was by the Argentine surgeon Mirizzi in 1948[1]. Since then, different MS classification criteria have emerged to aid surgical decision-making[3,19-23]. Among them, the Csendes classification with four types[2] is the most commonly used in clinical practice, which includes the presence or absence of gallbladder bile duct fistula and its degree.
The incidence of MS is relatively low, and usually accounts for less than 5% of gallstone patients[13,24,25]. The proportion of patients with each type of MS is also different, and gradually decreases from type I to type IV[23,26-29]. The proportion of patients with type I is 35% to 77%, and the proportion with type IV is usually less than 5%. Safioleas et al[26], Kwon and Inui[29] reported the diagnosis and treatment of 24 cases of MS in 8 years and 27 cases of MS in 20 years, respectively, and found no type IV patients. Cui et al[27] reported 198 cases of MS in 6 years, of which type I accounted for 59.1% and type IV accounted for 3.1%. Kamalesh et al[28] reported 20 cases in 7 years, of which type I accounted for 35% and type IV accounted for 5%.
As MS has no specific symptoms other than those observed in patients with gallstones, the preoperative diagnosis rate of MS is low, and it is confirmed by further exploration when iatrogenic BDI occurs during surgery. In various studies, the preoperative diagnosis rate of MS ranged from 30% to 83%[26,29].
At present, USS is still the first choice for the diagnosis of cholecystolithiasis, but the accuracy of USS for the diagnosis of MS is insufficient. As a basic and routine examination method, USS cannot objectively and comprehensively judge the condition of the bile duct preoperatively and most MS patients have no specific clinical manifestations other than the symptoms associated with gallstones; thus, the preoperative diagnosis of MS is not easy using USS[1,3,30]. Although Joseph et al[31] reported that the "Tri-duct sign" represented by the cystic duct, common hepatic duct and portal vein dilatation is helpful in the diagnosis of MS, the clinical typical "Tri-duct sign" is rare and it is affected by the experience of ultrasound examiners , limited understanding of MS and insufficient vigilance. Therefore, in order to improve the diagnostic accuracy, other methods such as CT, ERCP and MRI/MRCP are also used in the preoperative diagnosis of MS. Most studies have demonstrated that CT is not better than USS in the diagnosis of MS, and it is not a deterministic method. ERCP has been used to show the anatomical structure of the bile duct accurately, for removal of coexisting common duct stones and placement of a biliary stent, which is a great help for surgeons in managing MS. It is been considered the gold standard for MS diagnosis due to the above-mentioned advantages[32-35]. However, ERCP has certain equipment requirements and a technical threshold, and not every hospital can carry out ERCP routinely. ERCP is an invasive method of examination and treatment, and associated with some complications[1,12]. In clinical practice, ERCP is not usually performed in patients with simple gallstones, and is only performed if MS is suspected, rather than as a routine method. Therefore, a reliable routine preferred method to diagnose MS is required. As a result, ERCP cannot be popularized in the clinic, especially in hospitals with scarce resources. Due to the specific conditions of our hospital, we cannot conveniently and routinely perform ERCP; thus, ERCP was not included in the diagnosis and treatment of MS in this study. When ERCP is unavailable, the difficulties faced by surgeons cannot be reduced[13].
MRI/MRCP has beneficial characteristics such as it is noninvasive, repeatable, and provides multi-layer clear imaging. It can fully display the number, size and distribution of stones, the shape of the bile duct, the level and degree of obstruction, gallbladder lesions and other details, and help to screen tumors[12,13]. It has become the most suitable method for the preoperative diagnosis of MS, and has practical significance in helping surgeons to manage MS. In the present study, the diagnostic rate of preoperative MRI/MRCP for MS was 87.9% (58/66), while the detection rate of USS for MS was only 36.4% (24/66). However, MRI/MRCP is still insufficient in defining Csendes classification as it cannot accurately judge the presence and degree of the fistula[12,36], which should be further determined by combining with intraoperative findings.
Due to stone compression, biliary stricture, fistula formation, inflammatory edema, fibrotic adhesions, intraoperative bleeding and other difficult conditions, MS has become an important cause of BDI. It was also considered a taboo in laparoscopic surgery and open operation was suggested. In 2016, Kumar et al[3] reported 169 patients with MS, including 34 (20%) with type I, 97 (57%) with type II, 28 (17%) was type III and 10 (6%) with type IV MS, who were treated surgically. An open surgery was performed in 146 (86%) cases. Laparoscopic surgery was attempted in only 23 (14%) cases and was successful in only 1 patient with type II. Other scholars have also made considerable efforts to perform laparoscopic surgery for MS, but mainly for Csendes type I and type II patients[7-11]. The results of our study also showed that in most Csendes type I and in some type II MS patients, laparoscopic cholecystectomy (LC) can be completed safely with an overall success rate of 53% (35/66) under comprehensive evaluation and careful dissection. Generally, after relieving compression and inflammatory adhesion of type I MS, the diameter of the bile duct can be restored. However, BDI cannot be completely avoided. A total of 11 cases of BDI occurred in this study, all in type I patients, which may be related to the characteristics of local lesions and the failure of surgeons to treat with caution. Fortunately, BDI was not severe and did not lead to ischemia and disconnection of the bile duct. However, the occurrence of BDI will eventually lead to a change in classification, that is, patients with type I will at least upgrade to type II accompanied by an increase in the complexity of the operation. This is an important reason why our success rate of laparoscopic surgery is lower than those in other studies[7,9], even though our study had a relatively high preoperative diagnosis rate. Similarly, it is necessary to avoid fistula enlargement in Csendes type II and type III patients caused by iatrogenic injury.
From a technical perspective, small bile duct fistulas can be repaired with intermittent absorbable sutures. Such patients can usually undergo cholecystectomy and bile duct repair under complete laparoscopy without T-tube drainage. Larger fistulas can be repaired using the retained gallbladder wall patch following STC and a T-tube ought to be placed. STC is emphasized if bile duct repair is required, which can be used to repair the CBD fistula in difficult circumstances[37].
If the laparoscopic repair is not satisfactory or the operation is difficult, it should be converted to open surgery. For patients with Csendes type III MS, the surgical plan should be chosen based on the preoperative evaluation, combined with the technical level and clinical experience of the surgical team, and laparoscopic surgery should not be performed. According to our results, open surgery or timely conversion to open surgery was preferred in 31 cases (46.97%) including type I patients. Although the surgical trauma increased, the overall postoperative outcomes were good with no long-term morbidity or mortality.
Whether open or laparoscopic surgery for MS is chosen, correct anatomical identification is very important. Intraoperative biliary imaging can be used to clarify anatomy and avoid BDI[12]. We performed intraoperative cholangiography (6 of them via the PTCD and T tube) and choledochoscopy in 25 patients (37.9%). These methods can not only help us confirm the correct anatomical structure, but also judge whether there are complicated bile duct stones, strictures and satisfactory repair. We suggest that intraoperative cholangiography should be a mandatory adjunct in difficult situations.
In 2018, Seah et al[30] reported 64 patients with MS treated at Singapore General Hospital, including 43 with type I, 18 with type II, and 3 with type III. The diagnostic rate of MS was 88.9% by preoperative MRI and was 11.4% by USS, which were similar to our results (87.9% by MRI and 36.4% by USS). Our study also showed similar results to their studies in the frequency of intraoperative choledochoscopy (37.9% vs 44.6%) and cholangiography (37.9% vs 46.2%). However, in their study, 57 patients (57/64, 89.1%) chose direct open surgery or conversion surgery with a higher T-tube placement rate (63.1%) and an overall complication rate of approximately 43.8%. In addition, a total of 10 patients (10/64, 15.6%) needed hepaticoenteric anastomosis, including 3 patients with type I MS. They came to a conclusion on this basis that a trial of laparoscopic dissection with low threshold for open conversion is recommended if suspicion is high.
In our study, cholangiojejunostomy was avoided. However, according to the results of other studies[3,23,25,30], cholangiojejunostomy is still a necessary surgical method for patients with a large biliary fistula, especially those with obvious local scarring, ischemia or a large longitudinal defect. Therefore, patients who require cholangiojejunostomy are mainly some type III patients and almost all type IV patients. In addition, the surgical approach is usually open or laparoscopic converted to open surgery. Although surgical technology has made great progress in recent years, including endoscopy, minimally invasive technology and robotics, it has not directly improved the surgical treatment of type IV MS[14-16,29]. Our study did not include type IV patients; thus, we have no direct experience in the surgical treatment of type IV patients. It may take a little longer before technical progress can be routinely applied to the treatment of MS.
Thus, patients with MS should be evaluated comprehensively based on MRI/MRCP. Open surgery or timely conversion to open surgery should be selected when preoperative evaluation or LC intraoperative exploration shows that laparoscopic surgery is unsuitable. Based on this study, a flowchart of surgical strategies for MS is presented as Figure 1.
This study also found that the cost, operative time and bleeding volume in patients with Csendes type I, type II and type III showed an increasing trend with statistical significance (Table 3). Thus, the classification can reflect the difficulty of treatment, indicating that we should avoid increasing the risk to patients due to a change in classification caused by iatrogenic BDI.
Surgery for MS patients should be carried out as soon as the diagnosis and classification are determined. This study confirmed that prolonging preoperative treatment time does not increase the success rate of MS laparoscopic surgery. On the contrary, the longer the preoperative treatment time, the longer the overall length of hospital stay and the higher the overall cost. The reason for this may be that preoperative treatment cannot change the existing lesions and type of MS, and it is difficult to eliminate local inflammatory edema and fibrotic adhesions in a short time. This study also confirmed that the presence or absence of acute abdominal pain had no effect on the classification of MS and the final surgical technique, and suggested that the preoperative treatment time should not be prolonged until the symptoms disappear.This study also found that the success rate of laparoscopic surgery in elderly patients was lower and the treatment cost was higher, which may be related to the longer course of disease, more serious inflammatory scar adhesions and bile duct compression in elderly patients. In addition, it does not rule out the selection bias caused by the subjective will of both surgeons and patients in clinical practice. The size of stones has no effect on the classification of MS and the final surgical technique, which may be because the inflammation, edema, adhesions and compression induced by stones play important roles in the pathogenesis of MS.
The main limitations of our study are its retrospective nature and small sample size. The operations were completed by different surgeons, which inevitably resulted in heterogeneity of the treatment process and consequences. As the published studies adopted incompletely consistent classification standards of MS, the final conclusions have not reached a consensus. In view of this, we only provide our own experience in the surgical treatment of MS. The conclusions in our study should be confirmed by further large sample prospective research.
In this study, a relatively high preoperative diagnosis rate was obtained in 66 patients with MS who underwent surgery safely without serious long-term complications. Based on our limited experience, we recommend that MRI/MRCP should be considered a routine and necessary examination before laparoscopic surgery for MS. On the basis of a full evaluation and careful selection, MS patients can be treated by laparoscopic surgery, especially Csendes type I and type II patients, and timely conversion to open surgery may also be necessary. For patients with Csendes type III, the surgical technique requires careful decision-making. The Csendes classification can reflect treatment difficulty in MS patients, and increased risk due to a change in type grade caused by iatrogenic BDI should be avoided. These findings also suggest that active treatment should be carried out for gallbladder stones to reduce the risk of progression to MS, and surgery should be performed as soon as possible once MS is diagnosed. Use of the above strategies can reduce surgical complications, avoid cholangiojejunostomy and obtain a better clinical prognosis.
Mirizzi syndrome (MS) has always been a challenge for surgeons and an important cause of bile duct injury (BDI). At present, this problem has still not been resolved. If we do not accurately understand the pathological characteristics and potential surgical risks of MS, this may lead to adverse clinical consequences.
The treatment methods and effects for MS are changeable according to the different classification types, and the risks are also variable. Whether laparoscopic surgery is suitable for the treatment of MS is also controversial.
This study is a retrospective analysis using data accumulated over a decade that aimed to summarize preoperative diagnostic methods and the safety, effectiveness, prognosis and related factors of surgical strategies including laparoscopic surgery for different types of MS.
Sixty-six patients who met the inclusion criteria were included in the study. The diagnostic methods, clinical classification, surgical approach, complications and long-term prognosis were analyzed.
Magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) is superior to ultrasound scan in the diagnosis of MS. The overall laparoscopic surgery completion rate was 53.03% (35/66). Thirty-one patients (46.97%, 31/66) underwent laparotomy or conversion to laparotomy, including 11 cases of iatrogenic BDI which occurred in type I patients. Overall, 35 patients (53.03%, 35/66) needed bile duct repair using different methods. Twenty-five patients underwent intraoperative choledochoscopy and T-tube cholangiography. A total of 66 patients obtained a relatively high preoperative diagnosis rate and underwent surgery safely without serious complications and no mortality was observed during the follow-up period.
MRI/MRCP can improve the preoperative diagnosis rate of MS. Laparoscopic surgery can be undertaken safely in some patients with MS, especially Csendes type I and type II patients, and the surgical technique should be carefully determined for Csendes type III patients. The Csendes classification can reflect treatment difficulty and was related to the length of hospital stay and cost. The risk to patients due to a change in Csendes classification caused by iatrogenic injury during surgery should be avoided.
Sixty-six patients completed diagnostic and treatment procedures by different medical groups within 10 years, which may have led to significant heterogeneity. Accurate conclusions should be confirmed by further large sample prospective studies.
| 1. | Beltrán MA. Mirizzi syndrome: history, current knowledge and proposal of a simplified classification. World J Gastroenterol. 2012;18:4639-4650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (3)] |
| 2. | Csendes A, Díaz JC, Burdiles P, Maluenda F, Nava O. Mirizzi syndrome and cholecystobiliary fistula: a unifying classification. Br J Surg. 1989;76:1139-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 213] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 3. | Kumar A, Senthil G, Prakash A, Behari A, Singh RK, Kapoor VK, Saxena R. Mirizzi's syndrome: lessons learnt from 169 patients at a single center. Korean J Hepatobiliary Pancreat Surg. 2016;20:17-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Valderrama-Treviño AI, Granados-Romero JJ, Espejel-Deloiza M, Chernitzky-Camaño J, Barrera Mera B, Estrada-Mata AG, Ceballos-Villalva JC, Acuña Campos J, Argüero-Sánchez R. Updates in Mirizzi syndrome. Hepatobiliary Surg Nutr. 2017;6:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Lai EC, Lau WY. Mirizzi syndrome: history, present and future development. ANZ J Surg. 2006;76:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Rust KR, Clancy TV, Warren G, Mertesdorf J, Maxwell JG. Mirizzi's syndrome: a contraindication to coelioscopic cholecystectomy. J Laparoendosc Surg. 1991;1:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Zhao J, Fan Y, Wu S. Safety and feasibility of laparoscopic approaches for the management of Mirizzi syndrome: a systematic review. Surg Endosc. 2020;34:4717-4726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 8. | Vezakis A, Davides D, Birbas K, Ammori BJ, Larvin M, McMahon MJ. Laparoscopic treatment of Mirizzi syndrome. Surg Laparosc Endosc Percutan Tech. 2000;10:15-18. [PubMed] |
| 9. | Chowbey PK, Sharma A, Mann V, Khullar R, Baijal M, Vashistha A. The management of Mirizzi syndrome in the laparoscopic era. Surg Laparosc Endosc Percutan Tech. 2000;10:11-14. [PubMed] |
| 10. | Yeh CN, Wang SY, Liu KH, Yeh TS, Tsai CY, Tseng JH, Wu CH, Liu NJ, Chu YY, Jan YY. Surgical outcome of Mirizzi syndrome: Value of endoscopic retrograde cholangiopancreatography and laparoscopic procedures. J Hepatobiliary Pancreat Sci. 2021;28:760-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Clemente G, Tringali A, De Rose AM, Panettieri E, Murazio M, Nuzzo G, Giuliante F. Mirizzi Syndrome: Diagnosis and Management of a Challenging Biliary Disease. Can J Gastroenterol Hepatol. 2018;2018:6962090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Chen H, Siwo EA, Khu M, Tian Y. Current trends in the management of Mirizzi Syndrome: A review of literature. Medicine (Baltimore). 2018;97:e9691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Weledji EP, Ndono DN, Zouna F. A case of obstructive jaundice without biliary stones in a low resource setting. Clin Case Rep. 2021;9:e04163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Higgins RM, Frelich MJ, Bosler ME, Gould JC. Cost analysis of robotic versus laparoscopic general surgery procedures. Surg Endosc. 2017;31:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Kane WJ, Charles EJ, Mehaffey JH, Hawkins RB, Meneses KB, Tache-Leon CA, Yang Z. Robotic compared with laparoscopic cholecystectomy: A propensity matched analysis. Surgery. 2020;167:432-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Aguayo E, Dobaria V, Nakhla M, Seo YJ, Hadaya J, Cho NY, Sareh S, Sanaiha Y, Benharash P. National trends and outcomes of inpatient robotic-assisted versus laparoscopic cholecystectomy. Surgery. 2020;168:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Lee KF, Chong CN, Ma KW, Cheung E, Wong J, Cheung S, Lai P. A minimally invasive strategy for Mirizzi syndrome: the combined endoscopic and robotic approach. Surg Endosc. 2014;28:2690-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Magge D, Steve J, Novak S, Slivka A, Hogg M, Zureikat A, Zeh HJ 3rd. Performing the Difficult Cholecystectomy Using Combined Endoscopic and Robotic Techniques: How I Do It. J Gastrointest Surg. 2017;21:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Beltran MA, Csendes A. Mirizzi syndrome and gallstone ileus: an unusual presentation of gallstone disease. J Gastrointest Surg. 2005;9:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Beltran MA, Csendes A, Cruces KS. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg. 2008;32:2237-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 21. | Nagakawa T, Ohta T, Kayahara M, Ueno K, Konishi I, Sanada H, Miyazaki I. A new classification of Mirizzi syndrome from diagnostic and therapeutic viewpoints. Hepatogastroenterology. 1997;44:63-67. [PubMed] |
| 22. | Solis-Caxaj CA. Mirizzi syndrome: diagnosis, treatment and a plea for a simplified classification. World J Surg. 2009;33:1783-4; author reply 1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Payá-Llorente C, Vázquez-Tarragón A, Alberola-Soler A, Martínez-Pérez A, Martínez-López E, Santarrufina-Martínez S, Ortiz-Tarín I, Armañanzas-Villena E. Mirizzi syndrome: a new insight provided by a novel classification. Ann Hepatobiliary Pancreat Surg. 2017;21:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Dorrance HR, Lingam MK, Hair A, Oien K, O'Dwyer PJ. Acquired abnormalities of the biliary tract from chronic gallstone disease. J Am Coll Surg. 1999;189:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Abou-Saif A, Al-Kawas FH. Complications of gallstone disease: Mirizzi syndrome, cholecystocholedochal fistula, and gallstone ileus. Am J Gastroenterol. 2002;97:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Safioleas M, Stamatakos M, Safioleas P, Smyrnis A, Revenas C, Safioleas C. Mirizzi Syndrome: an unexpected problem of cholelithiasis. Our experience with 27 cases. Int Semin Surg Oncol. 2008;5:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Cui Y, Liu Y, Li Z, Zhao E, Zhang H, Cui N. Appraisal of diagnosis and surgical approach for Mirizzi syndrome. ANZ J Surg. 2012;82:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Kamalesh NP, Prakash K, Pramil K, George TD, Sylesh A, Shaji P. Laparoscopic approach is safe and effective in the management of Mirizzi syndrome. J Minim Access Surg. 2015;11:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Kwon AH, Inui H. Preoperative diagnosis and efficacy of laparoscopic procedures in the treatment of Mirizzi syndrome. J Am Coll Surg. 2007;204:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Seah WM, Koh YX, Cheow PC, Chow PKH, Chan CY, Lee SY, Ooi LLPJ, Chung AYF, Goh BKP. A Retrospective Review of the Diagnostic and Management Challenges of Mirizzi Syndrome at the Singapore General Hospital. Dig Surg. 2018;35:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Joseph S, Carvajal S, Odwin C. Sonographic diagnosis of Mirizzi's syndrome. J Clin Ultrasound. 1985;13:199-201. [PubMed] [DOI] [Full Text] |
| 32. | Xu XQ, Hong T, Li BL, Liu W, He XD, Zheng CJ. Mirizzi syndrome: our experience with 27 cases in PUMC Hospital. Chin Med Sci J. 2013;28:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Yuan H, Yuan T, Sun X, Zheng M. A Minimally Invasive Strategy for Mirizzi Syndrome Type II: Combined Endoscopic With Laparoscopic Approach. Surg Laparosc Endosc Percutan Tech. 2016;26:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Lee KF. Mirizzi syndrome: a new approach to an old problem. Hepatobiliary Surg Nutr. 2018;7:56-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Kim PN, Outwater EK, Mitchell DG. Mirizzi syndrome: evaluation by MRI imaging. Am J Gastroenterol. 1999;94:2546-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Piccinni G, Sciusco A, De Luca GM, Gurrado A, Pasculli A, Testini M. Minimally invasive treatment of Mirizzi's syndrome: is there a safe way? Ann Hepatol. 2014;13:558-564. [PubMed] |
| 37. | Brunt LM, Deziel DJ, Telem DA, Strasberg SM, Aggarwal R, Asbun H, Bonjer J, McDonald M, Alseidi A, Ujiki M, Riall TS, Hammill C, Moulton CA, Pucher PH, Parks RW, Ansari MT, Connor S, Dirks RC, Anderson B, Altieri MS, Tsamalaidze L, Stefanidis D; and the Prevention of Bile Duct Injury Consensus Work Group. Safe Cholecystectomy Multi-society Practice Guideline and State of the Art Consensus Conference on Prevention of Bile Duct Injury During Cholecystectomy. Ann Surg. 2020;272:3-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Balakrishnan DS, Kotelevets SM S-Editor: Fan JR L-Editor: A P-Editor: Fan JR