Published online Dec 27, 2022. doi: 10.4240/wjgs.v14.i12.1320
Peer-review started: September 1, 2022
First decision: October 20, 2022
Revised: October 24, 2022
Accepted: December 1, 2022
Article in press: December 1, 2022
Published online: December 27, 2022
Processing time: 116 Days and 22.9 Hours
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract with an increasing incidence worldwide. Comprehensive therapy for CD focuses on symptom control and healing the intestinal mucosa to improve the quality of life and prevent complications. Surgical intervention plays a vital role in comprehensive therapy. However, deciding the optimal timing for surgical inter
Core Tip: Surgical intervention plays an important role in the comprehensive treatment of Crohn’s disease (CD). However, the timing of surgery has always been a major controversial point. This review focuses on the main surgical indications for CD and the clinical factors that may influence surgical timing decisions. We also emphasize the value of early surgery in treating CD.
- Citation: Xia K, Gao RY, Wu XC, Yin L, Chen CQ. Timing of individualized surgical intervention in Crohn’s disease . World J Gastrointest Surg 2022; 14(12): 1320-1328
- URL: https://www.wjgnet.com/1948-9366/full/v14/i12/1320.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i12.1320
Crohn’s disease (CD) is a chronic inflammatory bowel disease that can affect the entire digestive tract, especially the terminal ileum and proximal colon[1,2]. The course of CD is protracted, characterized by alternating active and remission stages. The epidemiologic patterns of CD depict that the prevalence and hospitalization rates are currently rising gradually worldwide, contributing to an increasing burden on healthcare systems[3-6]. The underlying cause of CD is still unknown but includes a variety of factors, including genetic susceptibility, environmental triggers, immune regulation, and gut microbial imbalance[7-9]. CD is prone to various complications due to penetrating and chronic intestinal inflammatory response, including intestinal obstruction, bowel perforation, fistula, or intra-abdominal abscess[10,11]. After diagnosis, approximately 50% and 70% of CD patients develop complications within 5 or 10 years, respectively[12,13].
Recently, the launch of new biological agents has breathed new life into the clinical treatment of CD, while surgical intervention still plays an indispensable role[14-16]. The cumulative surgery rate for CD patients is 16.6%, 35.4%, 53%, and 94.5% for 1, 5, 10, and 30 years, respectively, after the onset of the disease[17]. The choice of optimal timing for surgical intervention has always been a focus of controversy. Some scholars advocate for early surgical intervention if drugs fail to achieve good results. Nevertheless, the recurrence after surgery is almost inevitable, and approximately 40% of CD patients require reoperation[18]. Other scholars prefer to avoid early surgery only if it is necessary to resect the intestinal segments that cause complications following the principle of intestinal conservation. However, postoperative complications significantly increase due to poor nutritional status and severe abdominal infection[19]. This review mainly focuses on the choice of individualized surgical intervention timing for CD patients.
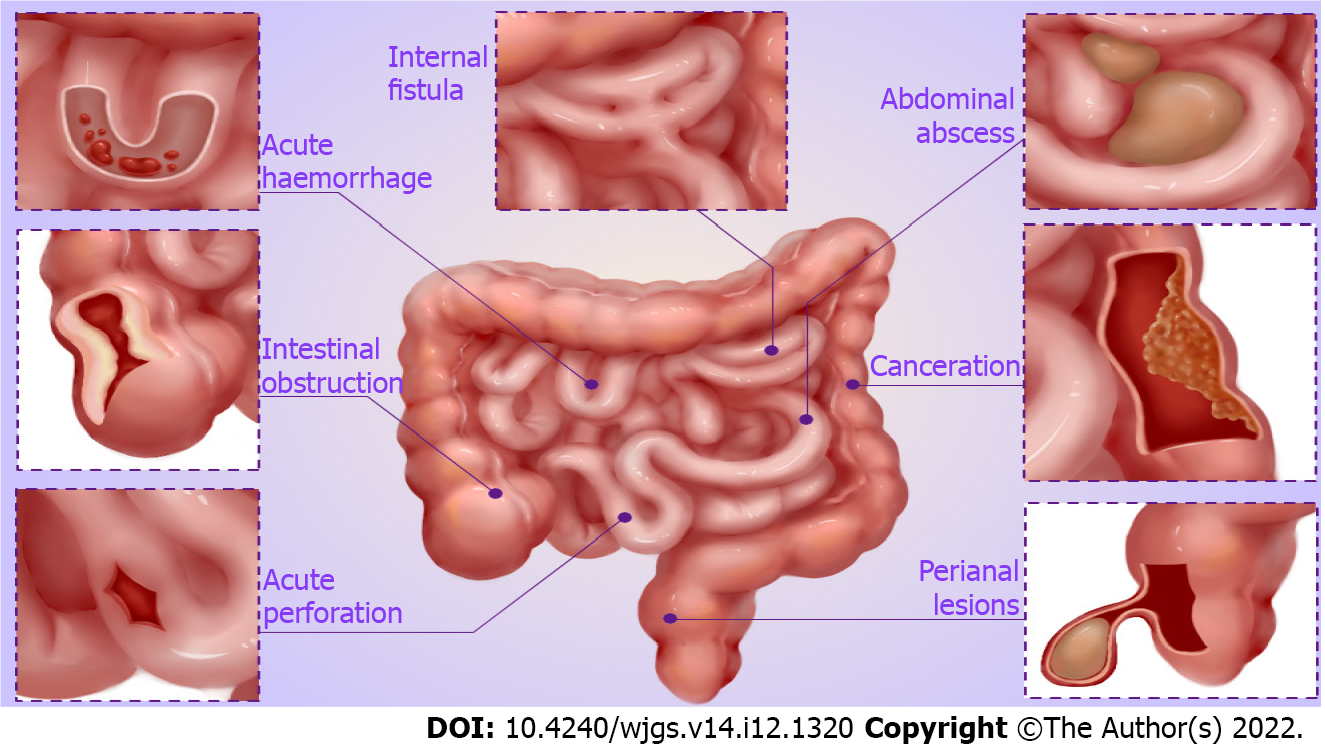
According to the relevant literature and clinical experience, we summarize the main surgical indications for CD, which involve serious complications of CD (Figure 1), failure of medical therapy, and growth retardation in children.
Intestinal obstruction: Intestinal obstruction is a common and serious complication of CD, especially fibrosis-associated intestinal stricture[20]. Lin et al[21] revealed that approximately 70% of CD patients inevitably develop fibrosis-associated intestinal stricture a decade following diagnosis. Medical treatment is frequently ineffective in patients who develop intestinal obstruction, and surgical resection is primarily required in that case[22,23]. Certainly, with the development of endoscopic technology, endoscopic balloon dilation is also an appropriate treatment option when the length of strictures is ≤ 5 cm, non-angulated, and with a sizeable intestinal cavity large enough to allow balloon dilators in the absence of contraindications such as the presence of fistula, abscess, or malignancy[24,25]. Furthermore, acute inflammatory obstruction can be frequently relieved by medical therapy. If conservative therapy is ineffective, surgical intervention should be considered to relieve the obstruction.
Intra-abdominal abscess: Intra-abdominal abscess is an important clinical complication of CD, the cause of which may be spontaneous or secondary to surgery[26,27]. The current first-line therapy for CD complicated by intra-abdominal abscess, is percutaneous abscess drainage with systemic antibiotics[28,29]. However, surgical intervention should be considered actively if the symptoms of sepsis do not improve after drainage, abscess ruptures with severe peritonitis, or multiple abscesses cannot be drained. Intestine resection appears to be inevitable in most CD patients presenting intra-abdominal abscess[30,31].
Fistula: Therapy for fistula has always been a complex clinical challenge. Simple enteral fistula without infection and clinical symptoms can be healed by a medical treatment such as enteral nutrition or biological agents[32,33]. For other complex enteral fistulae, including spontaneous enteroenteral or enteroexternal fistula formed after abscess drainage, the possibility of self-healing is low, and surgery should be adopted[34,35]. CD patients with severe fistula are often accompanied by loss of digestive fluid, resulting in disturbance of internal environmental balance, secondary infection, and malnutrition. Therefore, the infection should be readily controlled, and adequate nutritional support provided before elective surgery[36,37]. Yzet et al[38] recently reported successful cases of endoscopic treatment for enteroexternal fistula, which was feasible with short-term effectiveness.
Perianal lesions: Perianal lesions are common complications of CD, with perianal fistula and abscess being one of the most common[39,40]. The management of symptomatic simple perianal fistula and complex perianal fistula employs a multidisciplinary approach, which includes antibiotics, biological therapies, and surgery[41,42]. Furthermore, stem cell therapy is also an effective option for complex perianal fistula in CD patients[43,44]. As for the treatment of perianal abscess, surgical drainage and antibiotic therapy are preferred.
Perforation, massive bleeding, or canceration: The incidence of CD complicated by acute perforation is low. However, emergency surgical intervention is often required if it occurs[45]. When complicated by massive bleeding, the location of bleeding should be identified, and treatments such as drug, endoscopic, or interventional hemostasis should be actively adopted. Emergency surgery is required if the above treatments fail and massive bleeding continues[46,47]. In addition, CD complicated by canceration is an absolute indication for surgery[48].
Failure of medical therapy: Surgical intervention may be considered when drug therapy fails, and symptoms such as intolerance to severe side effects and ineffectiveness to various biological agents are difficult to control.
Growth retardation in children: Pediatric CD often presents as a triad of abdominal pain, diarrhea, and weight loss, characterized by growth retardation[49,50]. Therefore, the pediatric treatment of CD induces and maintains clinical remission of the disease and optimizes nutrition and growth as soon as possible[51]. Surgery should be performed before puberty for prepubertal or early pubertal patients with severe malnutrition resulting in growth arrest[52]. Since the rate of postoperative recurrence is still high, drug therapy is required to maintain remission after surgery[53].
Surgical intervention for CD aims to deal with complications and improve the quality of life of patients, as they tend to be in poor general conditions. Therefore, except for emergencies such as massive bleeding and acute perforation, adequate preoperative preparation should be completed to improve the efficacy of surgery. As a clinician, more attention should be paid to following the clinical factors to minimize perioperative complications.
Malnutrition is one of the prominent clinical manifestations of CD. Our team recently published a study indicating that CD patients were at higher nutritional risk than healthy people[54]. It can hinder wound healing and increase the risk of incision infection, hernia, and anastomotic leak[55]. Therefore, nutritional status is recognized as an independent risk factor for postoperative complications. Yamamoto et al[56] revealed that patients with preoperative low albumin levels (< 30 g/L) had a 2.6-fold increased incidence of postoperative complications, similar to that reported by Shah et al[57]. Another study indicated that preoperative optimization with nutritional support reduced the overall rate of postoperative complications of CD[58]. Thus, perioperative nutritional support is vital for CD patients, while enteral nutrition should be adopted when the intestinal state permits. Appropriate enteral nutrition can improve the nutritional status, protect the intestinal mucosal barrier, and induce clinical remission[59,60]. It is a well-established and recommended first-line induction therapy in pediatric CD with remission rates of up to 80%[61].
A recent study by Bachour et al[62] revealed that abdominal infection was associated with an increased risk of surgical postoperative recurrence of CD. Tzivanakis et al[63] indicated that the presence of preoperative abdominal abscess formation was identified as an independent predictor of anastomotic-associated complications. If the risk factor is present before surgery, the risk of anastomotic complications can be increased to 14%. Therefore, CD patients with abdominal abscesses can often be first managed with antibiotics and percutaneous drainage, while definitive surgical intervention should be performed after the infection has been controlled[64].
Whether preoperative CD treatment with tumor necrosis factor inhibitors (TNFis) increases the risk of postoperative complications remains controversial. TNFis may compromise immunity, collagen production, and angiogenesis, resulting in postoperative infective complications and altered wound healing[65,66]. In addition, TNF-α is a key cytokine in collagen production and angiogenesis, with animal studies confirming its role in wound healing[67]. However, previous studies have confirmed that preoperative TNFis exposure was not correlated with postoperative infectious complications[68-70] (Table 1).
| Ref. | Drugs | Type of study | Number of patients | Observations | Conclusion |
| Cohen et al[68], 2022 | TNFis | Prospective study | 947 | Postoperative infection rate | No correlation |
| Uchino et al[69], 2022 | TNFis | Retrospective study | 305 | Surgical mortality | No correlation |
| Abd El Aziz et al[70], 2022 | TNFis | Prospective study | 274 | Intra-abdominal septic complications | No correlation |
| Azzam et al[71], 2022 | Azathioprine | Retrospective study | 105 | Endoscopic recurrence rate | Negative correlation |
| Cosnes et al[72], 2005 | Azathioprine | Retrospective study | 2573 | Intestinal complications | No correlation |
| Nguyen et al[73], 2014 | Steroids | Retrospective study | 15495 | Postoperative sepsis and VTE | Positive correlation |
Azathioprine is commonly used as an immunosuppressant for treating CD and may not increase the risk of postoperative complications. Although azathioprine has demonstrated efficacy in preventing postoperative recurrence, there is no significant decrease in the need for surgery or intestinal complications from CD[71,72] (Table 1). Furthermore, CD patients are frequently treated with steroids before surgery. Nguyen et al[73] indicated that preoperative steroids were correlated with a higher risk of postoperative sepsis (Table 1). Therefore, steroids should be minimized or discontinued 6 mo before surgery.
Early surgery for CD is commonly performed within a short time after diagnosis, while the time frame is still inconclusive[74,75]. An et al[76] defined early surgery as patients who had undergone upfront surgery for CD due to an acute complication and those who underwent surgery within 6 mo of diagnosis. Interestingly, this study revealed that patients with ileocolonic CD may have a better prognosis if undergoing early surgical intervention, with fewer admissions to the hospital and reduced overall operation rates. Aratari et al[77] also defined early surgery when performed at the time of CD diagnosis, when these patients underwent surgery for the acute or subacute presentation of CD. Meanwhile, late surgery was defined as patients with an established diagnosis of CD who underwent surgery during the course of the disease on account of intestinal complications or refractoriness to medical therapy. Early surgery may significantly prolong the time of clinical recurrence of CD compared to late surgery. Considering the lack of evidence from these retrospective studies, the conclusions warrant further verification.
Early surgical intervention may benefit patients with localized CD, which refers to intestinal CD affecting < 30 cm in extent. This usually applies to an ileocaecal location but also isolated colonic disease, or conceivably to proximal small intestinal disease[78]. Ponsioen et al[79] indicated that early laparoscopic surgery for localized CD could improve the overall quality of life of patients and reduce the rate of recurrence and reoperation. A long-term follow-up study by Stevens et al[80] during the LIR! C-trial revealed that most patients with localized CD who underwent early surgery were free of anti-TNF treatment, and none required a second surgery. Conversely, almost half of the patients who underwent anti-TNF treatment moved on to a Crohn-related resection. Furthermore, de Groof et al[81] revealed that mean CD total direct healthcare costs per patient at 1 year were lower in the group who underwent early surgery compared with the anti-TNF group. Early surgical intervention is a reasonable and cost-effective treatment option for patients with localized CD.
China has a high incidence of hepatitis and tuberculosis. However, anti-TNF treatment may increase the risk of opportunistic infections[82,83]. Early surgery instead of anti-TNF treatment can reduce opportunistic infections. Additionally, early surgical resection of localized lesions may improve the response to postoperative anti-TNF treatment, the curative effect of which is better than that of the initial therapy[84,85].
CD is a refractory disease with a high misdiagnosis rate, a tendency for lifelong recurrence, and a high rate of operation and reoperation. Surgical intervention is a key part of the comprehensive treatment of CD. Inappropriate timing of surgery may lead to catastrophic postoperative complications, increasing the risk of surgery and prolonging hospital stays. Therefore, clinicians need to evaluate the severity and type of CD as well as the effectiveness of medical therapy and choose the timing of surgical intervention based on individual circumstances to ensure the maximum benefit for CD patients. Maybe in the future, with the deepening of multi-omics researches such as radiomics, metabolomics, and microbiomics, it will provide a more favorable basis for individualized timing of CD surgery and identify the early changes of CD related acute lesions.
We thank the medical teams of Diagnostic and Treatment Center for Refractory Diseases of Abdomen Surgery, Shanghai Tenth People’s Hospital, for their support for this subject.
| 1. | Cohen NA, Micic DM, Sakuraba A. Factors associated with poor compliance amongst hospitalized, predominantly adolescent pediatric Crohn's disease patients. Ann Med. 2022;54:886-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Narula N, Wong ECL, Dulai PS, Sengupta NK, Marshall JK, Colombel JF, Reinisch W. Comparative Efficacy and Rapidity of Action for Infliximab vs Ustekinumab in Biologic Naïve Crohn's Disease. Clin Gastroenterol Hepatol. 2022;20:1579-1587.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Buie MJ, Quan J, Windsor JW, Coward S, Hansen TM, King JA, Kotze PG, Gearry RB, Ng SC, Mak JWY, Abreu MT, Rubin DT, Bernstein CN, Banerjee R, Yamamoto-Furusho JK, Panaccione R, Seow CH, Ma C, Underwood FE, Ahuja V, Panaccione N, Shaheen AA, Holroyd-Leduc J, Kaplan GG; Global IBD Visualization of Epidemiology Studies in the 21st Century (GIVES-21) Research Group, Balderramo D, Chong VH, Juliao-Baños F, Dutta U, Simadibrata M, Kaibullayeva J, Sun Y, Hilmi I, Raja Ali RA, Paudel MS, Altuwaijri M, Hartono JL, Wei SC, Limsrivilai J, El Ouali S, Vergara BI, Dao VH, Kelly P, Hodges P, Miao Y, Li M. Global Hospitalization Trends for Crohn's Disease and Ulcerative Colitis in the 21st Century: A Systematic Review With Temporal Analyses. Clin Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 4. | Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, Wilson DC, Cameron F, Henderson P, Kotze PG, Bhatti J, Fang V, Gerber S, Guay E, Kotteduwa Jayawarden S, Kadota L, Maldonado D F, Osei JA, Sandarage R, Stanton A, Wan M; InsightScope Pediatric IBD Epidemiology Group, Benchimol EI. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology. 2022;162:1147-1159.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 396] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 5. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1601] [Article Influence: 106.7] [Reference Citation Analysis (3)] |
| 6. | Stöss C, Berlet M, Reischl S, Nitsche U, Weber MC, Friess H, Wilhelm D, Neumann PA. Crohn's disease: a population-based study of surgery in the age of biological therapy. Int J Colorectal Dis. 2021;36:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Michaud E, Waeckel L, Gayet R, Goguyer-Deschaumes R, Chanut B, Jospin F, Bathany K, Monnoye M, Genet C, Prier A, Tokarski C, Gérard P, Roblin X, Rochereau N, Paul S. Alteration of microbiota antibody-mediated immune selection contributes to dysbiosis in inflammatory bowel diseases. EMBO Mol Med. 2022;14:e15386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Ananthakrishnan AN, Kaplan GG, Bernstein CN, Burke KE, Lochhead PJ, Sasson AN, Agrawal M, Tiong JHT, Steinberg J, Kruis W, Steinwurz F, Ahuja V, Ng SC, Rubin DT, Colombel JF, Gearry R; International Organization for Study of Inflammatory Bowel Diseases. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022;7:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Kong C, Yan X, Liu Y, Huang L, Zhu Y, He J, Gao R, Kalady MF, Goel A, Qin H, Ma Y. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Signal Transduct Target Ther. 2021;6:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 10. | Xia K, Gao R, Wu X, Ruan Y, Wan J, Wu T, Wang F, Lin Y, Yin L, Chen C. Crohn's Disease Complicated by Rare Types of Intestinal Obstruction: Two Case Reports. Front Med (Lausanne). 2022;9:895202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Yao J, Jiang Y, Ke J, Lu Y, Hu J, Zhi M. A Validated Prognostic Model and Nomogram to Predict Early-Onset Complications Leading to Surgery in Patients With Crohn's Disease. Dis Colon Rectum. 2021;64:697-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn's disease complicated by strictures: a systematic review. Gut. 2013;62:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 13. | Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 715] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 14. | Sands BE, Irving PM, Hoops T, Izanec JL, Gao LL, Gasink C, Greenspan A, Allez M, Danese S, Hanauer SB, Jairath V, Kuehbacher T, Lewis JD, Loftus EV Jr, Mihaly E, Panaccione R, Scherl E, Shchukina OB, Sandborn WJ; SEAVUE Study Group. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn's disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet. 2022;399:2200-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 15. | Hindson J. First-line infliximab for children with Crohn's disease. Nat Rev Gastroenterol Hepatol. 2021;18:150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Bouhnik Y, Carbonnel F, Laharie D, Stefanescu C, Hébuterne X, Abitbol V, Nachury M, Brixi H, Bourreille A, Picon L, Bourrier A, Allez M, Peyrin-Biroulet L, Moreau J, Savoye G, Fumery M, Nancey S, Roblin X, Altwegg R, Bouguen G, Bommelaer G, Danese S, Louis E, Zappa M, Mary JY; GETAID CREOLE Study Group. Efficacy of adalimumab in patients with Crohn's disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67:53-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 17. | Gao X, Yang RP, Chen MH, Xiao YL, He Y, Chen BL, Hu PJ. Risk factors for surgery and postoperative recurrence: analysis of a south China cohort with Crohn's disease. Scand J Gastroenterol. 2012;47:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg. 2000;231:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 485] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 19. | Brouquet A, Blanc B, Bretagnol F, Valleur P, Bouhnik Y, Panis Y. Surgery for intestinal Crohn's disease recurrence. Surgery. 2010;148:936-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Ou W, Xu W, Liu F, Guo Y, Huang Z, Feng T, Liu CY, Du P. Increased expression of yes-associated protein/YAP and transcriptional coactivator with PDZ-binding motif/TAZ activates intestinal fibroblasts to promote intestinal obstruction in Crohn's disease. EBioMedicine. 2021;69:103452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Lin XX, Qiu Y, Zhuang XJ, Liu F, Wu XM, Chen MH, Mao R. Intestinal stricture in Crohn's disease: A 2020 update. J Dig Dis. 2021;22:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Schulberg JD, Wright EK, Holt BA, Hamilton AL, Sutherland TR, Ross AL, Vogrin S, Miller AM, Connell WC, Lust M, Ding NS, Moore GT, Bell SJ, Shelton E, Christensen B, De Cruz P, Rong YJ, Kamm MA. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn's disease strictures (STRIDENT): an open-label, single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7:318-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Hayashi Y, Nakase H. The Molecular Mechanisms of Intestinal Inflammation and Fibrosis in Crohn's Disease. Front Physiol. 2022;13:845078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Bettenworth D, Gustavsson A, Atreja A, Lopez R, Tysk C, van Assche G, Rieder F. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn's Disease. Inflamm Bowel Dis. 2017;23:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 25. | Chen M, Shen B. Endoscopic Therapy in Crohn's Disease: Principle, Preparation, and Technique. Inflamm Bowel Dis. 2015;21:2222-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Levartovsky A, Barash Y, Ben-Horin S, Ungar B, Soffer S, Amitai MM, Klang E, Kopylov U. Machine learning for prediction of intra-abdominal abscesses in patients with Crohn's disease visiting the emergency department. Therap Adv Gastroenterol. 2021;14:17562848211053114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Clancy C, Boland T, Deasy J, McNamara D, Burke JP. A Meta-analysis of Percutaneous Drainage Versus Surgery as the Initial Treatment of Crohn's Disease-related Intra-abdominal Abscess. J Crohns Colitis. 2016;10:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Celentano V, Giglio MC, Pellino G, Rottoli M, Sampietro G, Spinelli A, Selvaggi F; Italian Society of Colorectal Surgery SICCR. High complication rate in Crohn's disease surgery following percutaneous drainage of intra-abdominal abscess: a multicentre study. Int J Colorectal Dis. 2022;37:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Collard MK, Benoist S, Maggiori L, Zerbib P, Lefevre JH, Denost Q, Germain A, Cotte E, Beyer-Berjot L, Corté H, Desfourneaux V, Rahili A, Duffas JP, Pautrat K, Denet C, Bridoux V, Meurette G, Faucheron JL, Loriau J, Souche R, Vicaut E, Panis Y, Brouquet A. A Reappraisal of Outcome of Elective Surgery After Successful Non-Operative Management of an Intra-Abdominal Abscess Complicating Ileocolonic Crohn's Disease: A Subgroup Analysis of a Nationwide Prospective Cohort. J Crohns Colitis. 2021;15:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Waked B, Holvoet T, Geldof J, Baert F, Pattyn P, Lobatón T, Hindryckx P. Conservative management of spontaneous intra-abdominal abscess in Crohn's disease: Outcome and prognostic factors. J Dig Dis. 2021;22:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Buisson A, Cannon L, Umanskiy K, Hurst RD, Hyman NH, Sakuraba A, Pekow J, Dalal S, Cohen RD, Pereira B, Rubin DT. Factors associated with anti-tumor necrosis factor effectiveness to prevent postoperative recurrence in Crohn's disease. Intest Res. 2022;20:303-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Klek S, Sierzega M, Turczynowski L, Szybinski P, Szczepanek K, Kulig J. Enteral and parenteral nutrition in the conservative treatment of pancreatic fistula: a randomized clinical trial. Gastroenterology. 2011;141:157-163, 163.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Kucharski MA, Wierzbicka A, Tsibulski A, Sotiri E, Dobrowolska A, Mańkowska-Wierzbicka D. Parenteral and Enteral Nutrition: A Bridge to Healing and Biological Therapy in a Patient With Enterocutaneous Fistula and Sepsis Complicated Crohn's Disease. JPEN J Parenter Enteral Nutr. 2021;45:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Gong J, Wei Y, Gu L, Li Y, Guo Z, Sun J, Ding C, Zhu W, Li N, Li J. Outcome of Surgery for Coloduodenal Fistula in Crohn's Disease. J Gastrointest Surg. 2016;20:976-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Ghimire P. Management of Enterocutaneous Fistula: A Review. JNMA J Nepal Med Assoc. 2022;60:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Papa A, Lopetuso LR, Minordi LM, Di Veronica A, Neri M, Rapaccini G, Gasbarrini A, Papa V. A modern multidisciplinary approach to the treatment of enterocutaneous fistulas in Crohn's disease patients. Expert Rev Gastroenterol Hepatol. 2020;14:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Abdalla S, Benoist S, Maggiori L, Zerbib P, Lefevre JH, Denost Q, Germain A, Cotte E, Beyer-Berjot L, Corte H, Desfourneaux V, Rahili A, Duffas JP, Pautrat K, Denet C, Bridoux V, Meurette G, Faucheron JL, Loriau J, Guillon F, Vicaut E, Panis Y, Brouquet A; GETAID Chirurgie group. Impact of preoperative enteral nutritional support on postoperative outcome in patients with Crohn's disease complicated by malnutrition: Results of a subgroup analysis of the nationwide cohort registry from the GETAID Chirurgie group. Colorectal Dis. 2021;23:1451-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Yzet C, Brazier F, Sabbagh C, Le Mouel JP, Hakim S, Nguyen-Khac E, Fumery M. Endoscopic Treatment of Enterocutaneous Fistulas in Crohn's Disease. Dis Colon Rectum. 2022;65:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Flacs M, Collard M, Doblas S, Zappa M, Cazals-Hatem D, Maggiori L, Panis Y, Treton X, Ogier-Denis E. Preclinical Model of Perianal Fistulizing Crohn's Disease. Inflamm Bowel Dis. 2020;26:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Mak WY, Mak OS, Lee CK, Tang W, Leung WK, Wong MTL, Sze ASF, Li M, Leung CM, Lo FH, Lam BCY, Chan KH, Shan EHS, Tsang SWC, Hui AJ, Chow WH, Chan FKL, Sung JJY, Ng SC. Significant Medical and Surgical Morbidity in Perianal Crohn's Disease: Results from a Territory-Wide Study. J Crohns Colitis. 2018;12:1392-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | de Las Casas SG, Alvarez-Gallego M, Martínez JAG, Alcolea NG, Serrano CB, Jiménez AU, Arranz MDM, Martín JLM, Migueláñez IP. Management of perianal fistula in inflammatory bowel disease: identification of prognostic factors associated with surgery. Langenbecks Arch Surg. 2021;406:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, Löwenberg M, Dijkstra G, Oldenburg B, de Boer NKH, van der Marel S, Bodelier AGL, Jansen JM, Haans JJL, Theeuwen R, de Jong D, Pierik MJ, Hoentjen F. Ustekinumab for Crohn's Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J Crohns Colitis. 2020;14:33-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 43. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Diez MC, Tagarro I, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2018;154:1334-1342.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 44. | Schwandner O. Stem cell injection for complex anal fistula in Crohn's disease: A single-center experience. World J Gastroenterol. 2021;27:3643-3653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (7)] |
| 45. | Chiarello MM, Pepe G, Fico V, Bianchi V, Tropeano G, Altieri G, Brisinda G. Therapeutic strategies in Crohn's disease in an emergency surgical setting. World J Gastroenterol. 2022;28:1902-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Goldstone RN, Steinhagen RM. Abdominal Emergencies in Inflammatory Bowel Disease. Surg Clin North Am. 2019;99:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | De Simone B, Davies J, Chouillard E, Di Saverio S, Hoentjen F, Tarasconi A, Sartelli M, Biffl WL, Ansaloni L, Coccolini F, Chiarugi M, De'Angelis N, Moore EE, Kluger Y, Abu-Zidan F, Sakakushev B, Coimbra R, Celentano V, Wani I, Pintar T, Sganga G, Di Carlo I, Tartaglia D, Pikoulis M, Cardi M, De Moya MA, Leppaniemi A, Kirkpatrick A, Agnoletti V, Poggioli G, Carcoforo P, Baiocchi GL, Catena F. WSES-AAST guidelines: management of inflammatory bowel disease in the emergency setting. World J Emerg Surg. 2021;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Porter RJ, Arends MJ, Churchhouse AMD, Din S. Inflammatory Bowel Disease-Associated Colorectal Cancer: Translational Risks from Mechanisms to Medicines. J Crohns Colitis. 2021;15:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 49. | Agrawal M, Spencer EA, Colombel JF, Ungaro RC. Approach to the Management of Recently Diagnosed Inflammatory Bowel Disease Patients: A User's Guide for Adult and Pediatric Gastroenterologists. Gastroenterology. 2021;161:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 50. | Heuschkel R, Salvestrini C, Beattie RM, Hildebrand H, Walters T, Griffiths A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Zimmerman L, Bousvaros A. The pharmacotherapeutic management of pediatric Crohn's disease. Expert Opin Pharmacother. 2019;20:2161-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Fehmel E, Teague WJ, Simpson D, McLeod E, Hutson JM, Rosenbaum J, Oliver M, Alex G, King SK. The burden of surgery and postoperative complications in children with inflammatory bowel disease. J Pediatr Surg. 2018;53:2440-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Yerushalmy-Feler A, Assa A. Pharmacological Prevention and Management of Postoperative Relapse in Pediatric Crohn's Disease. Paediatr Drugs. 2019;21:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Xia K, Gao R, Wu X, Sun J, Wan J, Wu T, Fichna J, Yin L, Chen C. Characterization of Specific Signatures of the Oral Cavity, Sputum, and Ileum Microbiota in Patients With Crohn's Disease. Front Cell Infect Microbiol. 2022;12:864944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 55. | Yamamoto T, Shimoyama T, Umegae S, Kotze PG. Impact of Preoperative Nutritional Status on the Incidence Rate of Surgical Complications in Patients With Inflammatory Bowel Disease With Vs Without Preoperative Biologic Therapy: A Case-Control Study. Clin Transl Gastroenterol. 2019;10:e00050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Yamamoto T, Allan RN, Keighley MR. Risk factors for intra-abdominal sepsis after surgery in Crohn's disease. Dis Colon Rectum. 2000;43:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 270] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 57. | Shah RS, Bachour S, Jia X, Holubar SD, Hull TL, Achkar JP, Philpott J, Qazi T, Rieder F, Cohen BL, Regueiro MD, Lightner AL, Click BH. Hypoalbuminaemia, Not Biologic Exposure, Is Associated with Postoperative Complications in Crohn's Disease Patients Undergoing Ileocolic Resection. J Crohns Colitis. 2021;15:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 58. | Yamamoto T, Nakahigashi M, Shimoyama T, Umegae S. Does preoperative enteral nutrition reduce the incidence of surgical complications in patients with Crohn's disease? Colorectal Dis. 2020;22:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Svolos V, Hansen R, Nichols B, Quince C, Ijaz UZ, Papadopoulou RT, Edwards CA, Watson D, Alghamdi A, Brejnrod A, Ansalone C, Duncan H, Gervais L, Tayler R, Salmond J, Bolognini D, Klopfleisch R, Gaya DR, Milling S, Russell RK, Gerasimidis K. Treatment of Active Crohn's Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology. 2019;156:1354-1367.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 60. | Sohouli MH, Fatahi S, Farahmand F, Alimadadi H, Seraj SS, Rohani P. Meta-analysis: efficacy of exclusive enteral nutrition as induction therapy on disease activity index, inflammation and growth factors in paediatric Crohn's disease. Aliment Pharmacol Ther. 2022;56:384-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 61. | Di Caro S, Fragkos KC, Keetarut K, Koo HF, Sebepos-Rogers G, Saravanapavan H, Barragry J, Rogers J, Mehta SJ, Rahman F. Enteral Nutrition in Adult Crohn's Disease: Toward a Paradigm Shift. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Bachour SP, Shah RS, Rieder F, Qazi T, Achkar JP, Philpott J, Lashner B, Holubar SD, Lightner AL, Barnes EL, Axelrad J, Regueiro M, Click B, Cohen BL. Intra-abdominal septic complications after ileocolic resection increases risk for endoscopic and surgical postoperative Crohn's disease recurrence. J Crohns Colitis. 2022;16:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Tzivanakis A, Singh JC, Guy RJ, Travis SP, Mortensen NJ, George BD. Influence of risk factors on the safety of ileocolic anastomosis in Crohn's disease surgery. Dis Colon Rectum. 2012;55:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 64. | Feagins LA, Holubar SD, Kane SV, Spechler SJ. Current strategies in the management of intra-abdominal abscesses in Crohn's disease. Clin Gastroenterol Hepatol. 2011;9:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Byrne LW, McKay D. Does perioperative biological therapy increase 30-day post-operative complication rates in inflammatory bowel disease patients undergoing intra-abdominal surgery? Surgeon. 2021;19:e153-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | de Buck van Overstraeten A, Wolthuis A, D'Hoore A. Surgery for Crohn's disease in the era of biologicals: a reduced need or delayed verdict? World J Gastroenterol. 2012;18:3828-3832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Jensen JS, Petersen NB, Biagini M, Bollen P, Qvist N. Infliximab treatment reduces tensile strength in intestinal anastomosis. J Surg Res. 2015;193:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Cohen BL, Fleshner P, Kane SV, Herfarth HH, Palekar N, Farraye FA, Leighton JA, Katz JA, Cohen RD, Gerich ME, Cross RK, Higgins PDR, Tinsley A, Glover S, Siegel CA, Bohl JL, Iskandar H, Ji J, Hu L, Sands BE. Prospective Cohort Study to Investigate the Safety of Preoperative Tumor Necrosis Factor Inhibitor Exposure in Patients With Inflammatory Bowel Disease Undergoing Intra-abdominal Surgery. Gastroenterology. 2022;163:204-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 69. | Uchino M, Ikeuchi H, Horio Y, Kuwahara R, Minagawa T, Kusunoki K, Goto Y, Beppu N, Ichiki K, Ueda T, Nakajima K, Ikeda M. Association between preoperative biologic use and surgical morbidity in patients with Crohn's disease. Int J Colorectal Dis. 2022;37:999-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Abd El Aziz MA, Abdalla S, Calini G, Saeed H, Stocchi L, Merchea A, Colibaseanu DT, Shawki S, Larson DW. Postoperative Safety Profile of Minimally Invasive Ileocolonic Resections for Crohn's Disease in the Era of Biologic Therapy. J Crohns Colitis. 2022;16:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Azzam N, AlRuthia Y, Al Thaher A, Almadi M, Alharbi O, Altuwaijri M, Alshankiti S, Alanazi M, Alanazi A, Aljebreen A, Regueiro M. Rate and risk factors of postoperative endoscopic recurrence of moderate- to high-risk Crohn's disease patients - A real-world experience from a Middle Eastern cohort. Saudi J Gastroenterol. 2022;28:201-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 492] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 73. | Nguyen GC, Elnahas A, Jackson TD. The impact of preoperative steroid use on short-term outcomes following surgery for inflammatory bowel disease. J Crohns Colitis. 2014;8:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | Maruyama BY, Ma C, Panaccione R, Kotze PG. Early Laparoscopic Ileal Resection for Localized Ileocecal Crohn's Disease: Hard Sell or a Revolutionary New Norm? Inflamm Intest Dis. 2022;7:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Luglio G, Kono T. Surgical Techniques and Risk of Postoperative Recurrence in CD: A Game Changer? Inflamm Intest Dis. 2022;7:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | An V, Cohen L, Lawrence M, Thomas M, Andrews J, Moore J. Early surgery in Crohn's disease a benefit in selected cases. World J Gastrointest Surg. 2016;8:492-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Aratari A, Papi C, Leandro G, Viscido A, Capurso L, Caprilli R. Early versus late surgery for ileo-caecal Crohn's disease. Aliment Pharmacol Ther. 2007;26:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1509] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
| 79. | Ponsioen CY, de Groof EJ, Eshuis EJ, Gardenbroek TJ, Bossuyt PMM, Hart A, Warusavitarne J, Buskens CJ, van Bodegraven AA, Brink MA, Consten ECJ, van Wagensveld BA, Rijk MCM, Crolla RMPH, Noomen CG, Houdijk APJ, Mallant RC, Boom M, Marsman WA, Stockmann HB, Mol B, de Groof AJ, Stokkers PC, D'Haens GR, Bemelman WA; LIR!C study group. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol. 2017;2:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 80. | Stevens TW, Haasnoot L, D’Haens GR, Buskens C, de Groof EJ, Eshuis EJ, Gardenbroef TJ, Mol B, Stokkers PCF, Bemelman WA, Ponsioen CY. OP03 Reduced need for surgery and medical therapy after early ileocaecal resection for Crohn’s disease: Long-term follow-up of the LIR! J Crohns Colitis. 2020;14:S003-S004. [DOI] [Full Text] |
| 81. | de Groof EJ, Stevens TW, Eshuis EJ, Gardenbroek TJ, Bosmans JE, van Dongen JM, Mol B, Buskens CJ, Stokkers PCF, Hart A, D'Haens GR, Bemelman WA, Ponsioen CY; LIR!C study group. Cost-effectiveness of laparoscopic ileocaecal resection versus infliximab treatment of terminal ileitis in Crohn's disease: the LIR! Gut. 2019;68:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 82. | Su X, Zheng L, Zhang H, Shen T, Liu Y, Hu X. Secular Trends of Acute Viral Hepatitis Incidence and Mortality in China, 1990 to 2019 and Its Prediction to 2030: The Global Burden of Disease Study 2019. Front Med (Lausanne). 2022;9:842088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 83. | Dong Z, Wang QQ, Yu SC, Huang F, Liu JJ, Yao HY, Zhao YL. Age-period-cohort analysis of pulmonary tuberculosis reported incidence, China, 2006-2020. Infect Dis Poverty. 2022;11:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 84. | Van Assche G, Lewis JD, Lichtenstein GR, Loftus EV, Ouyang Q, Panes J, Siegel CA, Sandborn WJ, Travis SP, Colombel JF. The London position statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organisation: safety. Am J Gastroenterol. 2011;106:1594-602; quiz 1593, 1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Bemelman WA, Warusavitarne J, Sampietro GM, Serclova Z, Zmora O, Luglio G, de Buck van Overstraeten A, Burke JP, Buskens CJ, Colombo F, Dias JA, Eliakim R, Elosua T, Gecim IE, Kolacek S, Kierkus J, Kolho KL, Lefevre JH, Millan M, Panis Y, Pinkney T, Russell RK, Shwaartz C, Vaizey C, Yassin N, D'Hoore A. ECCO-ESCP Consensus on Surgery for Crohn's Disease. J Crohns Colitis. 2018;12:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jha P, United States; Zha B, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ