Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1230
Peer-review started: July 20, 2022
First decision: September 12, 2022
Revised: September 17, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: November 27, 2022
Processing time: 127 Days and 18.8 Hours
The prognostic value of quantitative assessments of the number of retrieved lymph nodes (RLNs) in gastric cancer (GC) patients needs further study.
To discuss how to obtain a more accurate count of metastatic lymph nodes (MLNs) based on RLNs in different pT stages and then to evaluate patient prognosis.
This study retrospectively analyzed patients who underwent GC radical surgery and D2/D2+ LN dissection at the Cancer Hospital of Harbin Medical University from January 2011 to May 2017. Locally weighted smoothing was used to analyze the relationship between RLNs and the number of MLNs. Restricted cubic splines were used to analyze the relationship between RLNs and hazard ratios (HRs), and X-tile was used to determine the optimal cutoff value for RLNs. Patient survival was analyzed with the Kaplan-Meier method and log-rank test. Finally, HRs and 95% confidence intervals were calculated using Cox proportional hazards models to analyze independent risk factors associated with patient outcomes.
A total of 4968 patients were included in the training cohort, and 11154 patients were included in the validation cohort. The smooth curve showed that the number of MLNs increased with an increasing number of RLNs, and a nonlinear relationship between RLNs and HRs was observed. X-tile analysis showed that the optimal number of RLNs for pT1-pT4 stage GC patients was 26, 31, 39, and 45, respectively. A greater number of RLNs can reduce the risk of death in patients with pT1, pT2, and pT4 stage cancers but may not reduce the risk of death in patients with pT3 stage cancer. Multivariate analysis showed that RLNs were an independent risk factor associated with the prognosis of patients with pT1-pT4 stage cancer (P = 0.044, P = 0.037, P = 0.003, P < 0.001).
A greater number of RLNs may not benefit the survival of patients with pT3 stage disease but can benefit the survival of patients with pT1, pT2, and pT4 stage disease. For the pT1, pT2, and pT4 stages, it is recommended to retrieve 26, 31 and 45 LNs, respectively.
Core Tip: The prognostic value of quantitative assessments of the number of retrieved lymph nodes (RLNs) in gastric cancer (GC) patients needs further study. The purpose of this study was to discuss how to obtain a more accurate count of metastatic LNs based on RLNs according to different pT stages and then to evaluate the prognosis of patients. Our results showed that the optimal number of RLNs for pT1-pT4 stage GC patients were 26, 31, 39 and 45, respectively. A greater number of RLNs can reduce the risk of death in patients with pT1, pT2, and pT4 stage cancers but may not pT3 stage.
- Citation: Wang H, Yin X, Lou SH, Fang TY, Han BL, Gao JL, Wang YF, Zhang DX, Wang XB, Lu ZF, Wu JP, Zhang JQ, Wang YM, Zhang Y, Xue YW. Metastatic lymph nodes and prognosis assessed by the number of retrieved lymph nodes in gastric cancer. World J Gastrointest Surg 2022; 14(11): 1230-1249
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1230.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1230
Gastric cancer (GC) is the sixth most common malignant tumor in the world, with more than 860000 deaths each year[1]. The depth of tumor invasion - lymph node (LN) metastasis - distant metastasis (TNM) staging system issued by the Union for International Cancer Control and the American Joint Committee on Cancer (AJCC) is the global standard for GC staging[2,3]. LN metastasis of tumor cells is one of the most common forms of GC metastasis[4,5]. Therefore, surgeons performed LN dissection based on the perigastric lymphatic pathways to control metastasis. Karpeh et al[6] found that compared with the location of LN metastasis, the number of metastatic LNs (MLNs) was more important in determining the prognosis of GC patients. The AJCC 8th edition staging system divided GC patients into stages pN3a and pN3b according to MLNs based on pN3 stage, which was effective in clinical applications for evaluating patient prognosis. Therefore, accurate assessment of MLNs is critical for determining the prognosis of GC patients.
Radical gastrectomy and LN dissection are necessary for the long-term survival of GC patients[7]. For the evaluation of MLNs, sufficient numbers of retrieved LNs (RLNs) need to be acquired during surgery and confirmed by postoperative pathological examination[8]. At present, D2/D2 + LN dissection is the standard lymphadenectomy for GC[9]. Compared with D1, expanded LN dissection may effectively control LN metastasis to prolong patient survival[10,11] and clear potential metastatic LNs[12]. Smith et al[13] found that for pT1/2N0 patients, every 10 additional RLNs may be associated with a 7.6% increase in overall survival (OS). However, the linear relationship shows that MLNs are positively correlated with RLNs[14-17], indicating that insufficient RLNs may lead to stage migration. The pN stage determined by RLNs might thus be affected and differ from the actual pN stage, which causes errors in subsequent treatment and assessment of prognosis[18]. Furthermore, a previous study showed that evaluating the optimal number of RLNs based on pT staging can not only enhance the accuracy of staging but also better predict patient prognosis[13]. In this context, we analyzed RLNs according to a more accurate pT stage based on clinical application and discussed how to obtain accurate MLNs through RLNs for precise staging and the influence of RLNs on patient prognosis.
This study retrospectively analyzed patients who underwent radical GC surgery in the Gastrointestinal Surgery Department of the Cancer Hospital Affiliated to Harbin Medical University from January 2011 to May 2017. We analyzed the suitable RLNs in pT1-pT4 stages based on pT stage and explored their relationship with long-term patient survival.
This study retrospectively analyzed patients who underwent radical GC surgery and D2/D2 + LN dissection at the Affiliated Tumor Hospital of Harbin Medical University from January 2011 to May 2017. The diagnosis of GC was based on tissue samples obtained from preoperative gastroscopy, which were further confirmed by professional pathologists through tissue collected during surgery. The surgical method and LN dissection were performed in accordance with the Japanese GC Treatment Guidelines (Fifth Edition)[19].
The exclusion criteria for this study were as follows: (1) Tumor located in the whole stomach; (2) Preoperative chemotherapy; (3) Patients with a history of other malignant tumors; and (4) Remnant GC. The clinicopathological data of the patients were stored in the GC information management system v1.2 of the Affiliated Tumor Hospital of Harbin Medical University (copyright number 2013SR087424, http://www.sgihmu.com), including sex, age, tumor location, tumor size, histological type, pT stage, pN staging, etc. The above content was in compliance with the eighth edition of AJCC regulations[3].
Oxaliplatin + capecitabine (XELOX) or oxaliplatin + S-1 (SOX) are the primary treatment options for patients in pathological stages II to III. Due to the long time span, to ensure the accuracy of this study, we included only patients who received complete chemotherapy at our institution, for a total of 1119 patients. The remaining patients were not included in the postoperative chemotherapy patient group because these patients did not complete all postoperative chemotherapy regimens in our institution, and most of the patients returned to local hospitals for treatment after surgery and did not have complete chemotherapy records.
All patients were followed up after surgery: Stage I patients every 12 mo, stage II patients every 6 mo, and stage III patients every 3-6 mo. Follow-up was conducted by telephone, fax, e-mail, or in the outpatient complex building of the Affiliated Tumor Hospital of Harbin Medical University. Follow-up included complete blood cell analysis, biochemical examination, tumor markers, gastroscopy, and abdominal ultrasonography, and some patients underwent computed tomography (CT)/positron emission tomography-CT examination according to their condition.
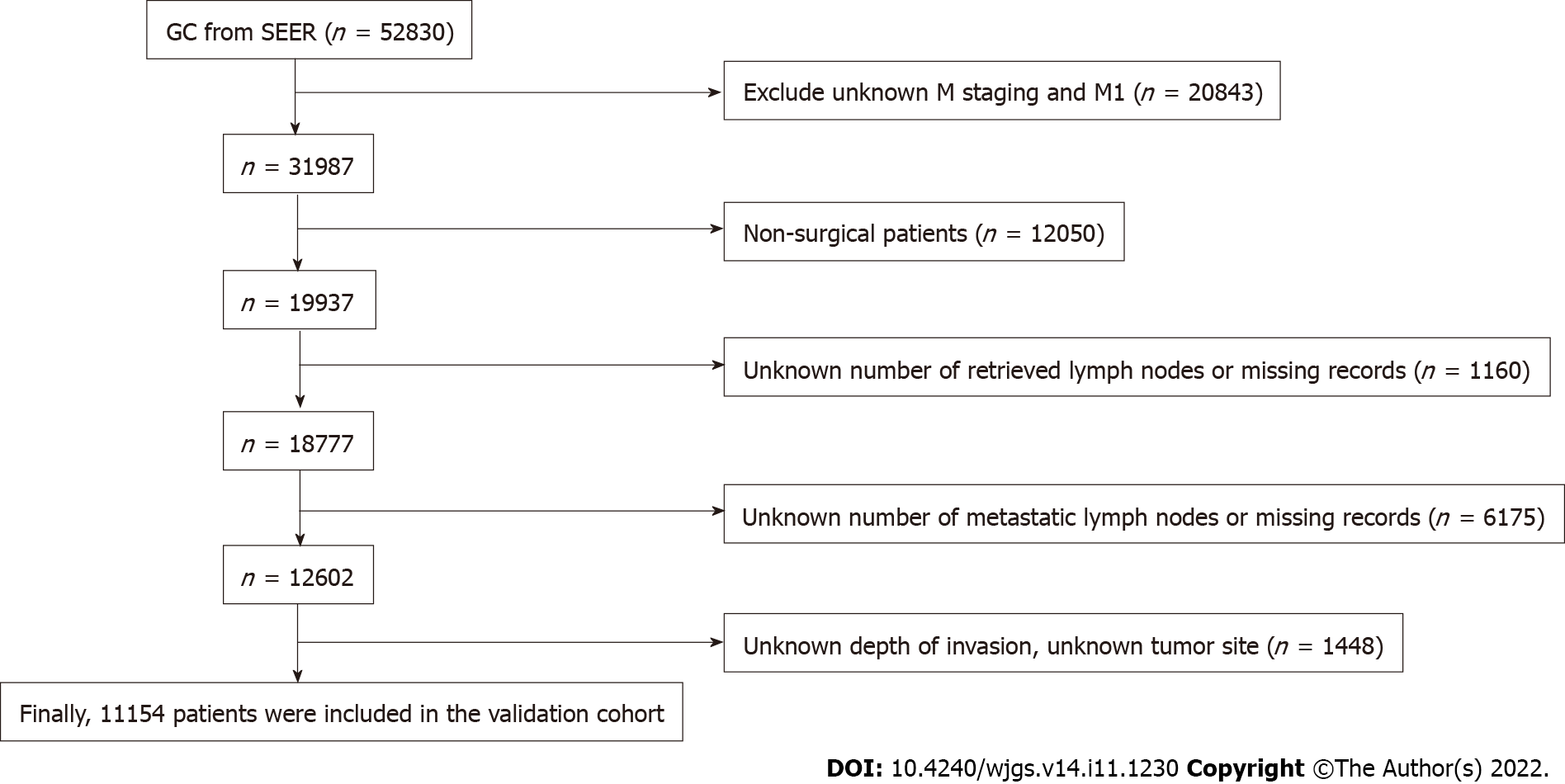
Data for the validation cohort were obtained from the National Cancer Institute Surveillance, Epidemiology, and End Results Program (http://seer.cancer.gov/) provided by SEER*Stat software. We included patients diagnosed with GC between 2010 and 2016 to ensure a minimum follow-up of 5 years. Patients with incomplete or missing records of tumor invasion depth, LNs status, and distant metastasis status were excluded, and then pT staging and pN staging were reverified according to the eighth edition of the AJCC staging manual. The screening process is shown in Figure 1.
OS was defined as the follow-up time from the time of operation to the time of death or the last date of follow-up. If the patient was alive at the last follow-up, it was included in this study, expressed by the mean ± SD and the 5-year survival rate. The relationship between RLNs and MLNs at each stage was analyzed using locally weighted smoothing (LOESS)[19]. The relationship between RLNs and hazard ratios (HRs) at each stage, pT1-pT4, was assessed by a restricted cubic spline model[20]. X-tile software was used to calculate the optimal cutoff value of RLNs for the prognosis of pT1-pT4 GC (X-Tile version 3.6.1 Yale University, New Haven, CT)[21], and then the Kaplan-Meier method and log-rank test were used to evaluate the effect of the best cutoff value of the number of RLNs in each stage, pT1-pT4, on prognosis. The chi-square test was used to analyze the relationship between the optimal cutoff value of RLNs in each stage, pT1-pT4, and the clinicopathological characteristics of patients. HRs and 95% confidence intervals were calculated using a Cox proportional hazards model. In all analyses, P < 0.05 was considered statistically significant. All analyses were performed using R software (version 4.1.2) and SPSS (version 25 for Windows).
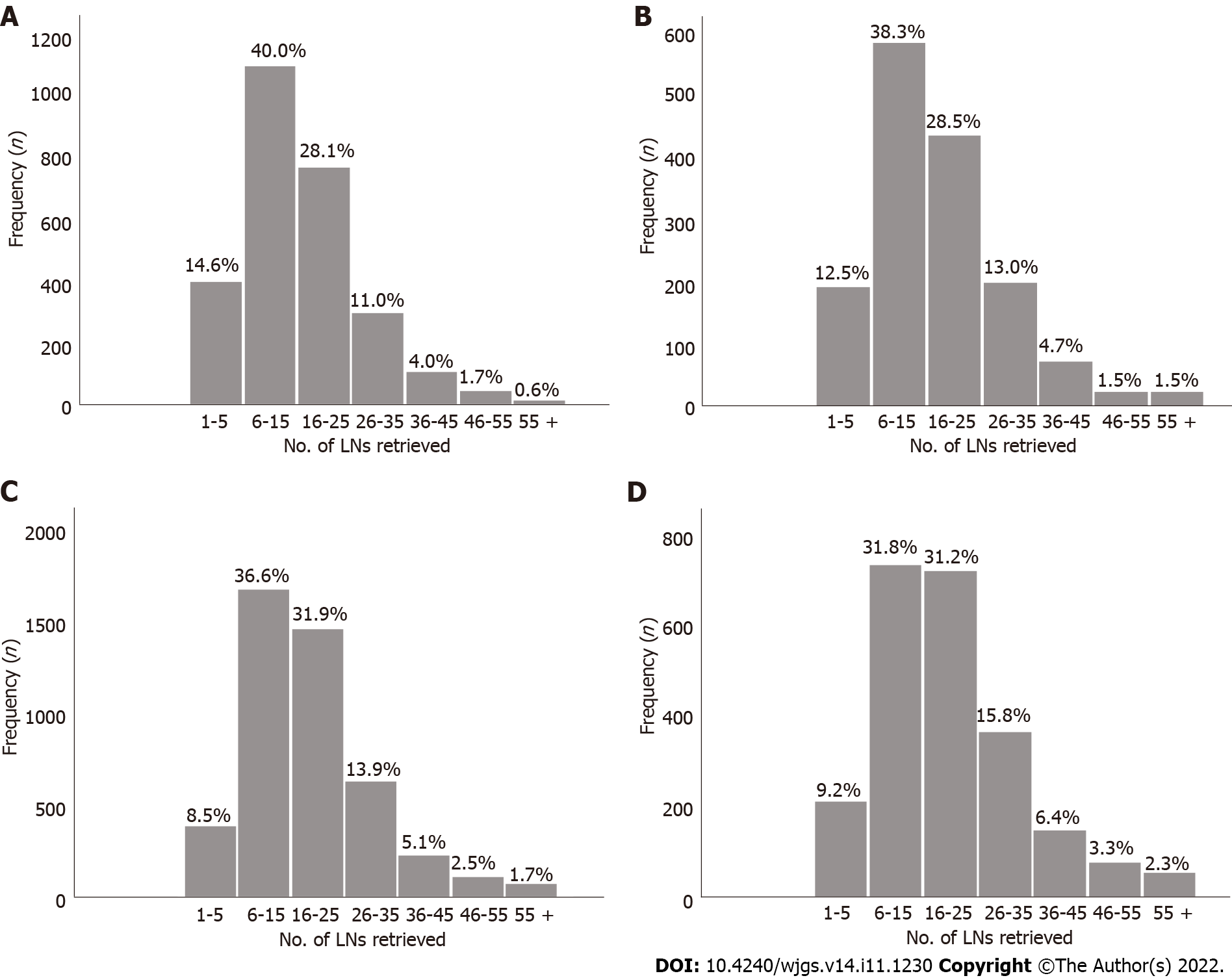
Ultimately, at our institution, a total of 4968 patients were included in the study as a training cohort (Table 1). Among them, there were 1106 patients in the pT1 stage, 745 patients in the pT2 stage, 1583 patients in the pT3 stage, and 1534 patients in the pT4 stage. In the entire cohort, the median number of RLNs was 27 (range 1-95), with 2062 pN0 stage patients, 927 pN1 stage patients, 893 pN2 stage patients, and 1086 pN3 stage patients according to postoperative pathological examinations.
| Characteristics | Training cohort | Validation cohort | P value |
| n = 4968 | n = 11154 | ||
| Sex | < 0.001 | ||
| Male | 3634 (73.1) | 7214 (64.7) | |
| Female | 1334 (26.9) | 3940 (35.3) | |
| Age (yr) | < 0.001 | ||
| ≤ 60 | 2845 (57.3) | 3418 (30.6) | |
| > 60 | 2123 (42.7) | 7736 (69.4) | |
| Tumor location | < 0.001 | ||
| Upper third | 552 (11.1) | 3954 (35.4) | |
| Middle third | 811 (16.3) | 1248 (11.2) | |
| Lower third | 3605 (72.6) | 5952 (53.4) | |
| Tumor size (mm) | < 0.001 | ||
| ≤ 50 | 3225 (64.9) | 6813 (61.1) | |
| > 50 | 1743 (35.1) | 4341 (38.9) | |
| Histological type | < 0.001 | ||
| Well -moderately differentiated | 2056 (41.4) | 3402 (30.5) | |
| Poorly-undifferentiated | 2204 (44.4) | 4197 (37.6) | |
| Signet ring cell | 397 (8.0) | 1899 (17.0) | |
| Others | 311 (6.3) | 1656 (14.8) | |
| pT stage | < 0.001 | ||
| pT1 | 1106 (22.3) | 2746 (24.6) | |
| pT2 | 745 (15.0) | 1534 (13.8) | |
| pT3 | 1583 (31.9) | 4570 (41.0) | |
| pT4 | 1534 (30.9) | 2304 (20.7) | |
| pN stage | < 0.001 | ||
| pN0 | 2062 (41.5) | 5411 (48.5) | |
| pN1 | 927 (18.7) | 2039 (18.3) | |
| pN2 | 893 (18.0) | 1768 (15.9) | |
| pN3 | 1086 (21.9) | 1936 (17.4) | |
| pTNM | < 0.001 | ||
| I | 1445 (29.1) | 3476 (31.2) | |
| II | 1383 (27.8) | 3821 (34.3) | |
| III | 2140 (43.1) | 3857 (34.6) | |
| RLNs, median (range) | 27 (1-95) | 16 (1-90) | |
| Chemotherapy | < 0.001 | ||
| No/unknown | 3769 (75.9) | 5191 (46.5) | |
| Yes | 1199 (22.5) | 5963 (53.5) |
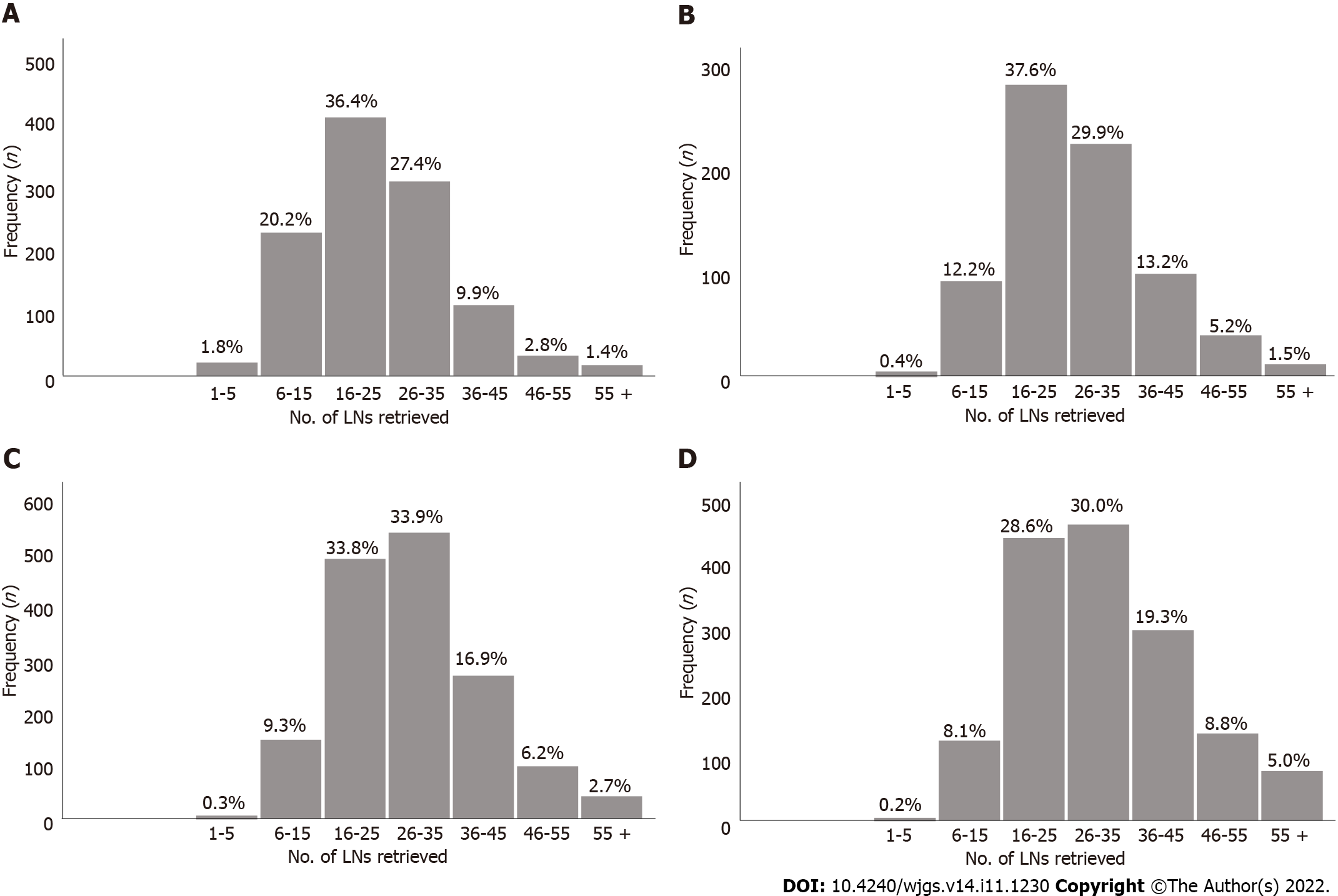
For the Surveillance, Epidemiology, and End Results (SEER) database, after excluding patients according to the exclusion criteria, 11154 patients were finally included in the study as a validation cohort (Figure 1). Among them, there were 2746 pT1 patients, 1534 pT2 patients, 4570 pT3 patients, and 2304 pT4 patients. In the entire validation cohort, the median number of RLNs was 16 (range 1-90), with 5411 pN0 stage patients, 2039 pN1 stage patients, 1768 pN2 stage patients, and 1936 pN3 stage patients according to postoperative pathological examinations (Table 1).
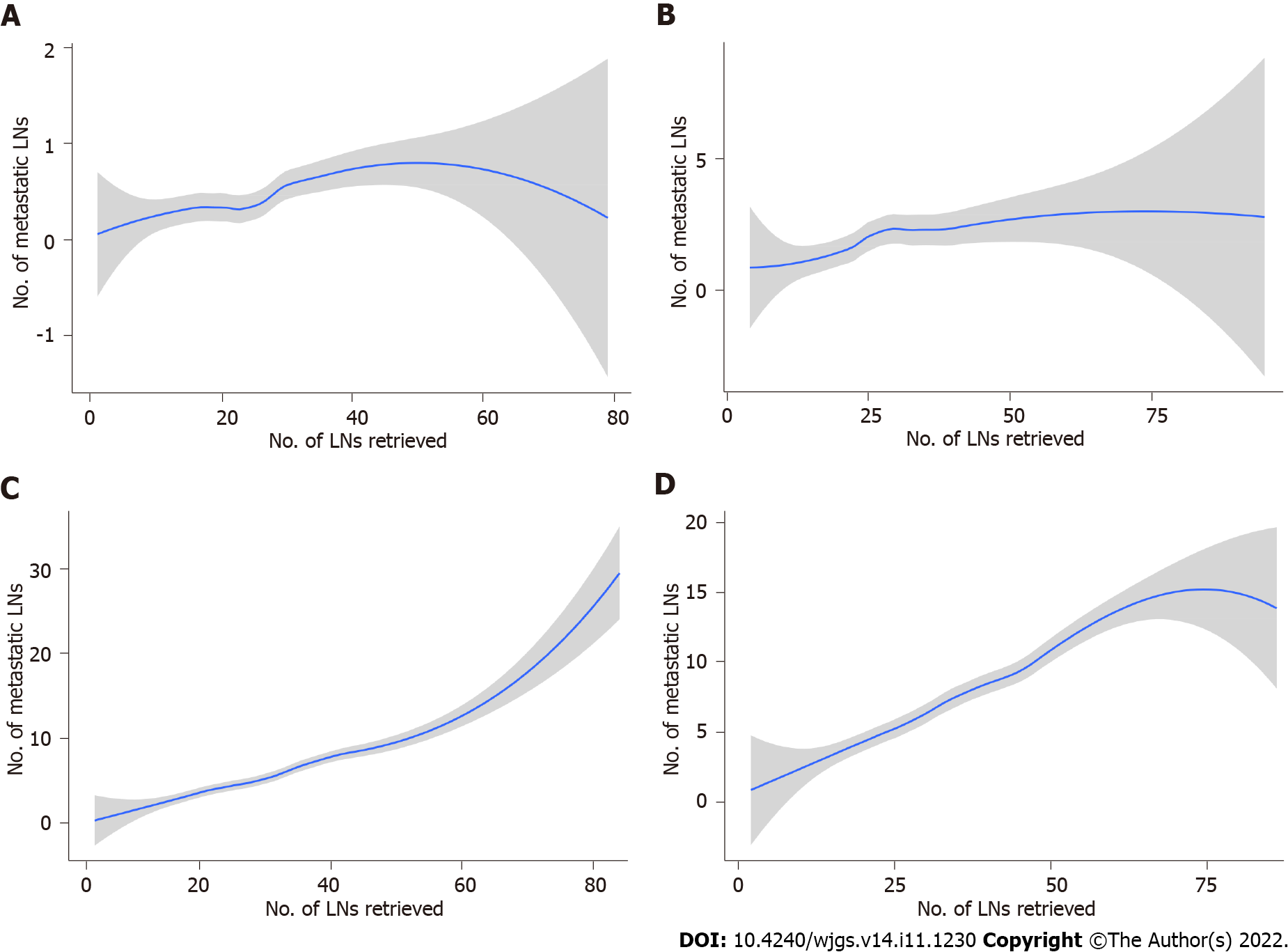
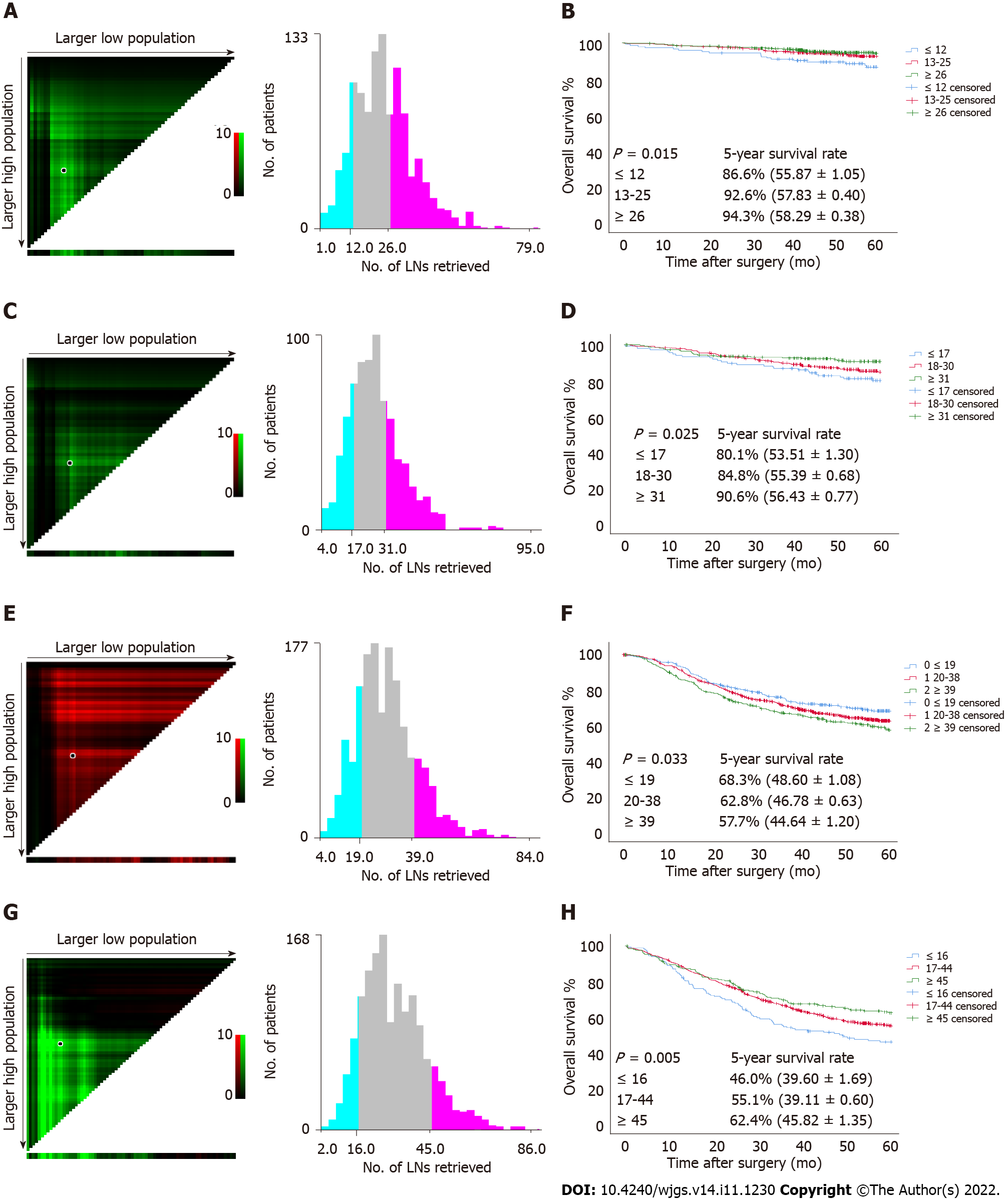
The absolute and relative frequencies of RLNs in each subgroup at the pT1-pT4 stages in the training cohort are shown in Figure 2, and the absolute and relative frequencies of RLNs in each subgroup at the pT1-pT4 stages in the validation cohort are shown in Figure 3. In the training cohort, for pT1, 16 or more LNs were enucleated in 77.9% of patients, with a median of 23 (range 1-79) of 26862 RLNs, for pT2, 16 or more LNs were enucleated in 87.4% of patients, with a median of 25 (range 4-95) of 20193 RLNs, for pT3, 16 or more LNs were enucleated in 90.4% of patients, with a median of 28 RLNs of 46501(range 4-84), for pT4, 91.7% of patients had 16 or more enucleated LNs, there were 47936 RLNs, and the median was 29 (range 2-86). The LOESS nonlinear trend showed that MLNs in each subgroup showed an upward trend with increasing RLNs (Figures 4A-D), whereas for the pT1 stage, the nonlinear trend indicated that when the number of RLNs exceeded approximately 50, the MLNs decreased with increasing RLNs.
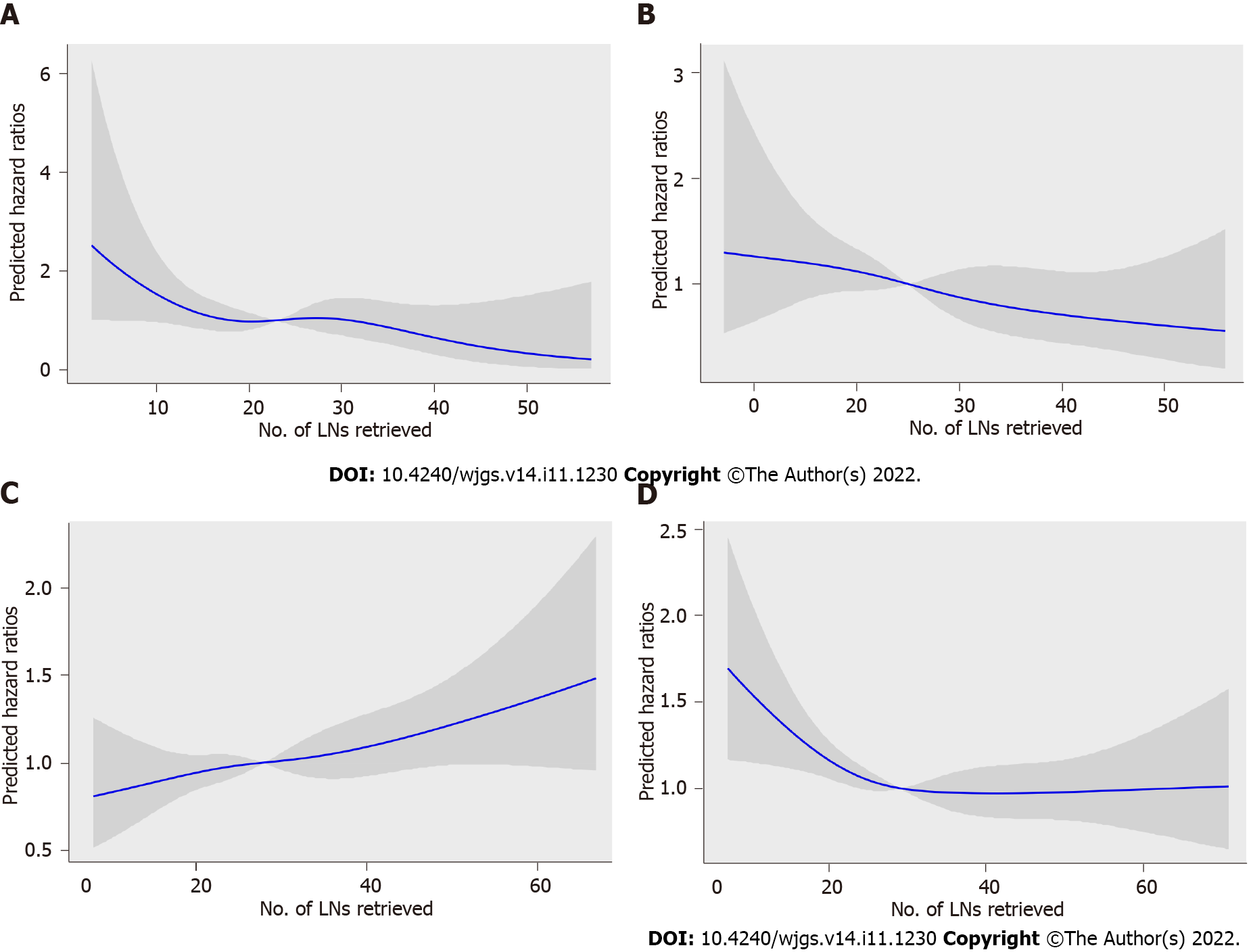
To assess the relationship between RLNs and mortality risk, we performed a restricted cubic spline model analysis (Figures 5A-D). For pT1, pT2, and pT4 stages, the smooth curve shows that HRs decrease with the increase in RLNs. For pT3, the smooth curve shows that HRs increase with the increase in RLNs. The results showed that the number of LNs retrieved may affect patient survival. However, the trend in HRs and RLNs in the pT3 stage was opposite that in the pT1 stage, pT2 stage, and pT4 stage. To further verify the effect of RLNs on patient survival, every 10 LNs was taken as the cutoff point. That is, fewer than 5 LNs were removed, and 6-15 LNs were removed until more than 55 LNs were retrieved. Table 2 lists the 5-year survival rates based on RLNs in each subgroup, increasing at intervals of every 10 LNs. For patients with pT1, pT2, and pT4 stage cancers, adding RLNs prolonged the 5-year patient survival rate, but for patients with pT3 stage cancer, adding RLNs did not prolong the 5-year patient survival rate.
| pT stage | No. of retrieved lymph nodes | P value | |||||||||||||
| 1-5 (No., %) | 6-15 (No., %) | 16-25 (No., %) | 26-35 (No., %) | 36-45 (No., %) | 46-55 (No., %) | 55 + (No., %) | |||||||||
| pT1 | 20 | 90.0 | 223 | 89.1 | 403 | 92.5 | 303 | 94.4 | 110 | 91.0 | 31 | 100.0 | 16 | 100.0 | 0.210 |
| pT2 | 3 | 66.7 | 86 | 82.1 | 280 | 84.3 | 223 | 86.4 | 98 | 91.3 | 39 | 87.1 | 11 | 100.0 | 0.371 |
| pT3 | 4 | 50.0 | 148 | 70.0 | 486 | 64.8 | 531 | 61.7 | 267 | 60.3 | 98 | 62.4 | 42 | 48.5 | 0.172 |
| pT4 | 3 | 33.3 | 124 | 45.9 | 439 | 51.0 | 460 | 58.3 | 296 | 55.2 | 135 | 67.4 | 77 | 56.1 | 0.005 |
Since a nonlinear relationship between RLNs and HRs was observed in each subgroup at the pT1-pT4 stages, we analyzed survival differences among these patients by X-tile software (Figure 6). The results showed that for the pT1 stage, the best cutoff values for RLNs were 12 and 26, for the pT2 stage, the best cutoff values for RLNs were 17 and 31, or pT3, the best cutoff values for RLNs were 19 and 39, and for pT4, the best cutoff values for RLNs were 16 and 45. After that, subgroup survival analysis was performed according to the best cutoff alue of RLNs in each substage. Increasing RLNs can improve prognosis of patients with pT1, pT2, and pT4 stages hile may not improve prognosis of patients with pT3 stage. In addition, chi-square analysis showed that for pT1 stage and pT3 stage cancers, with the increase in RLNs, the proportion of patients younger than 60 years old gradually increased, and there was a statistically significant correlation (P < 0.001, P = 0.002). For stages pT1, pT3, pT4, pN stage increased with the optimal cutoff value of the number of removed LNs, and there was a statistically significant association (P < 0.001, P < 0.001, P < 0.001) (Table 3).
| Characteristics | pT1 (1106), RLNs | P value | pT2 (745), RLNs | P value | pT3 (1583), RLNs | P value | pT4 (1534), RLNs | P value | ||||||||
| ≤ 12 | 13-25 | ≥ 26 | ≤ 17 | 18-30 | ≥ 31 | ≤ 19 | 20-38 | ≥ 39 | ≤ 16 | 17-44 | ≥ 45 | |||||
| Sex | 0.114 | 0.803 | 0.006 | 0.132 | ||||||||||||
| Male | 112 | 353 | 320 | 109 | 274 | 188 | 230 | 677 | 240 | 119 | 851 | 161 | ||||
| Female | 31 | 150 | 140 | 32 | 80 | 62 | 73 | 295 | 68 | 43 | 286 | 74 | ||||
| Age (yr) | < 0.001 | 0.699 | 0.002 | 0.273 | ||||||||||||
| ≤ 60 | 74 | 302 | 323 | 80 | 214 | 152 | 137 | 523 | 183 | 82 | 637 | 138 | ||||
| > 60 | 69 | 201 | 137 | 61 | 140 | 98 | 166 | 449 | 125 | 80 | 500 | 97 | ||||
| Tumor location | 0.003 | 0.216 | 0.036 | 0.025 | ||||||||||||
| Upper third | 17 | 24 | 19 | 17 | 34 | 15 | 54 | 139 | 36 | 34 | 137 | 26 | ||||
| Middle third | 14 | 59 | 68 | 17 | 40 | 37 | 63 | 164 | 68 | 25 | 211 | 45 | ||||
| Lower third | 112 | 420 | 373 | 107 | 280 | 198 | 186 | 669 | 204 | 103 | 789 | 164 | ||||
| Tumor size (mm) | 0.005 | 0.004 | < 0.001 | < 0.001 | ||||||||||||
| ≤ 50 | 139 | 477 | 417 | 129 | 287 | 196 | 202 | 514 | 147 | 87 | 549 | 81 | ||||
| > 50 | 4 | 26 | 43 | 12 | 67 | 54 | 101 | 458 | 161 | 75 | 588 | 154 | ||||
| Histological type | 0.008 | 0.689 | 0.878 | 0.145 | ||||||||||||
| Well-moderately differentiated | 67 | 273 | 229 | 67 | 160 | 104 | 125 | 378 | 116 | 73 | 391 | 73 | ||||
| Poorly-undifferentiated | 39 | 153 | 158 | 63 | 148 | 113 | 122 | 426 | 141 | 75 | 631 | 135 | ||||
| Signet ring cell | 14 | 36 | 43 | 6 | 24 | 17 | 36 | 113 | 32 | 7 | 57 | 12 | ||||
| Others | 23 | 41 | 30 | 5 | 22 | 16 | 20 | 55 | 19 | 7 | 58 | 15 | ||||
| pN stage | < 0.001 | 0.128 | < 0.001 | < 0.001 | ||||||||||||
| pN0 | 125 | 43 | 374 | 85 | 195 | 127 | 112 | 241 | 62 | 54 | 220 | 37 | ||||
| pN1 | 15 | 49 | 45 | 32 | 80 | 57 | 86 | 206 | 54 | 41 | 240 | 22 | ||||
| pN2 | 3 | 22 | 28 | 21 | 50 | 40 | 68 | 237 | 64 | 42 | 275 | 43 | ||||
| pN3 | 0 | 2 | 13 | 3 | 29 | 26 | 37 | 288 | 128 | 25 | 402 | 133 | ||||
| pTNM | 0.014 | 0.045 | < 0.001 | 0.003 | ||||||||||||
| I | 140 | 479 | 419 | 85 | 195 | 127 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| II | 3 | 24 | 40 | 53 | 130 | 97 | 198 | 447 | 116 | 43 | 201 | 31 | ||||
| III | 0 | 0 | 1 | 3 | 29 | 26 | 105 | 525 | 192 | 119 | 936 | 204 | ||||
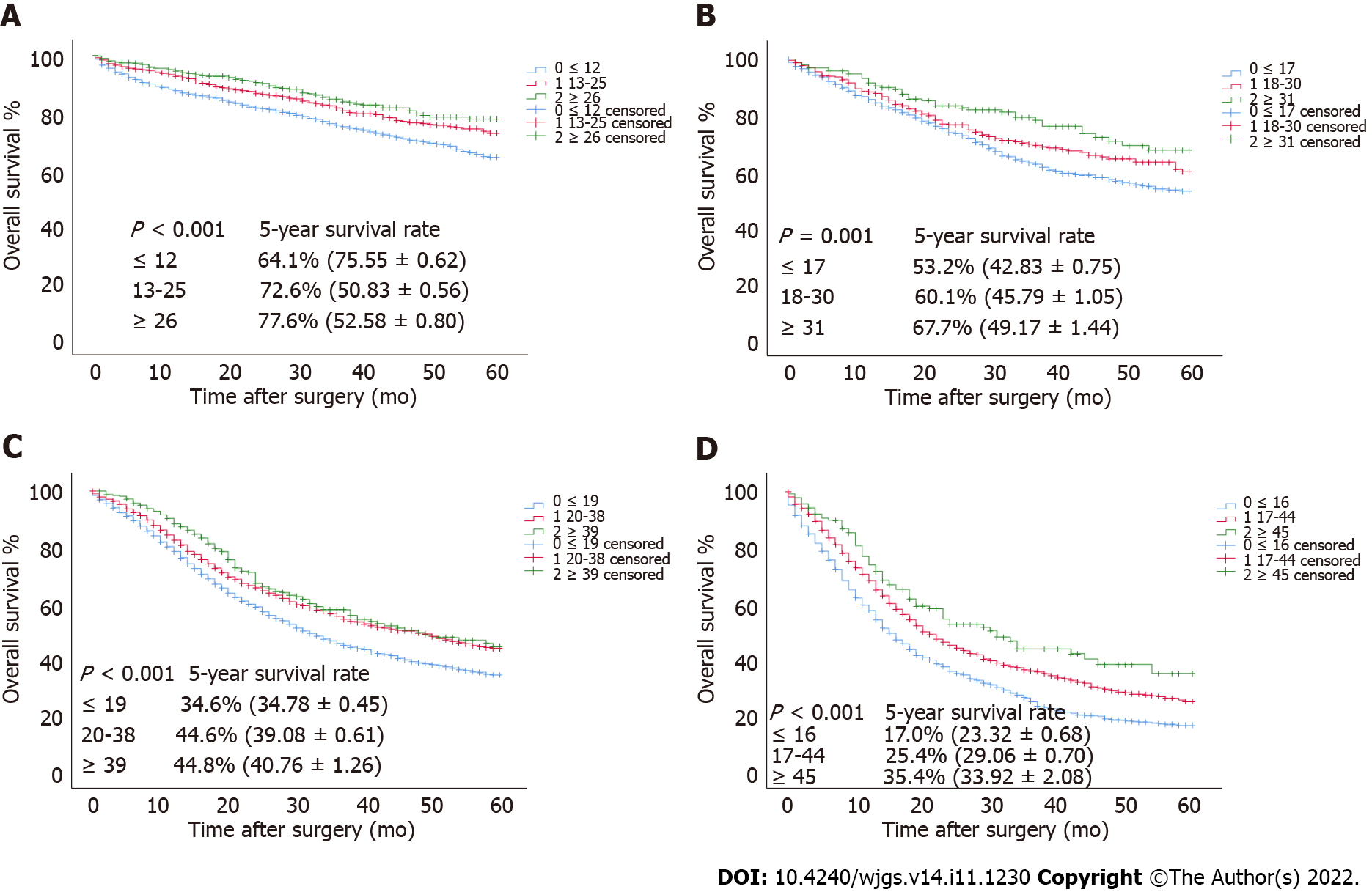
To verify the relationship between the optimal cutoff value of RLNs in this study and the long-term survival of patients, we used the SEER validation cohort to validate the pT1-pT4 subgroup (Figure 7). Increasing RLNs can improve prognosis of patients with pT1-pT4 stages. Chi-square analysis found that for pT1-pT4, with the increase in RLNs, the proportion of patients less than 60 years old gradually increased, and pN stage increased with the optimal cutoff value for the number of removed LNs, and there was a statistically significant association (Table 4).
| Characteristics | pT1 (2746), RLNs | P value | pT2 (1534), RLNs | P value | pT3 (4570), RLNs | P value | pT4 (2304), RLNs | P value | ||||||||
| ≤ 12 | 13-24 | ≥ 25 | ≤ 17 | 18-30 | ≥ 31 | ≤ 19 | 20-38 | ≥ 39 | ≤ 16 | 17-44 | ≥ 45 | |||||
| Sex | 0.521 | 0.263 | 0.033 | 0.668 | ||||||||||||
| Male | 727 | 678 | 288 | 584 | 305 | 121 | 1988 | 1012 | 223 | 576 | 630 | 82 | ||||
| Female | 428 | 439 | 186 | 316 | 138 | 70 | 775 | 469 | 103 | 448 | 511 | 57 | ||||
| Age (yr) | 0.018 | 0.049 | 0.006 | 0.054 | ||||||||||||
| ≤ 60 | 278 | 305 | 145 | 252 | 133 | 64 | 869 | 499 | 130 | 306 | 384 | 53 | ||||
| > 60 | 877 | 810 | 329 | 648 | 410 | 127 | 1894 | 982 | 196 | 718 | 757 | 86 | ||||
| Tumor location | 0.008 | 0.001 | < 0.001 | < 0.001 | ||||||||||||
| Upper third | 354 | 382 | 140 | 348 | 168 | 54 | 1391 | 709 | 93 | 159 | 143 | 13 | ||||
| Middle third | 134 | 139 | 81 | 93 | 59 | 39 | 188 | 146 | 54 | 109 | 172 | 34 | ||||
| Lower third | 667 | 596 | 253 | 459 | 216 | 98 | 1184 | 626 | 179 | 756 | 826 | 92 | ||||
| Tumor size (mm) | 0.575 | 0.009 | < 0.001 | 0.002 | ||||||||||||
| ≤ 50 | 966 | 934 | 387 | 695 | 314 | 132 | 1581 | 749 | 149 | 443 | 417 | 46 | ||||
| > 50 | 189 | 183 | 87 | 205 | 129 | 59 | 1182 | 432 | 177 | 581 | 724 | 93 | ||||
| Histological type | 0.648 | 0.945 | 0.951 | 0.193 | ||||||||||||
| Well-moderately differentiated | 538 | 502 | 217 | 304 | 138 | 67 | 782 | 427 | 86 | 169 | 147 | 25 | ||||
| Poorly-undifferentiated | 316 | 314 | 141 | 304 | 158 | 62 | 1212 | 628 | 144 | 397 | 469 | 52 | ||||
| Signet ring cell | 187 | 193 | 64 | 123 | 58 | 26 | 406 | 228 | 51 | 238 | 288 | 37 | ||||
| Others | 114 | 108 | 52 | 169 | 89 | 36 | 363 | 198 | 45 | 220 | 237 | 25 | ||||
| pN stage | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||||||
| pN0 | 1008 | 885 | 369 | 547 | 255 | 96 | 1196 | 526 | 94 | 253 | 166 | 16 | ||||
| pN1 | 115 | 148 | 53 | 216 | 91 | 39 | 664 | 275 | 48 | 236 | 135 | 19 | ||||
| pN2 | 28 | 63 | 30 | 106 | 52 | 31 | 561 | 311 | 55 | 298 | 212 | 21 | ||||
| pN3 | 4 | 21 | 22 | 31 | 45 | 25 | 342 | 369 | 129 | 237 | 628 | 83 | ||||
| pTNM | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||||||
| I | 1123 | 1033 | 422 | 547 | 255 | 96 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| II | 32 | 82 | 46 | 322 | 143 | 70 | 1860 | 801 | 142 | 180 | 130 | 13 | ||||
| III | 0 | 2 | 6 | 31 | 45 | 25 | 903 | 680 | 184 | 844 | 1011 | 126 | ||||
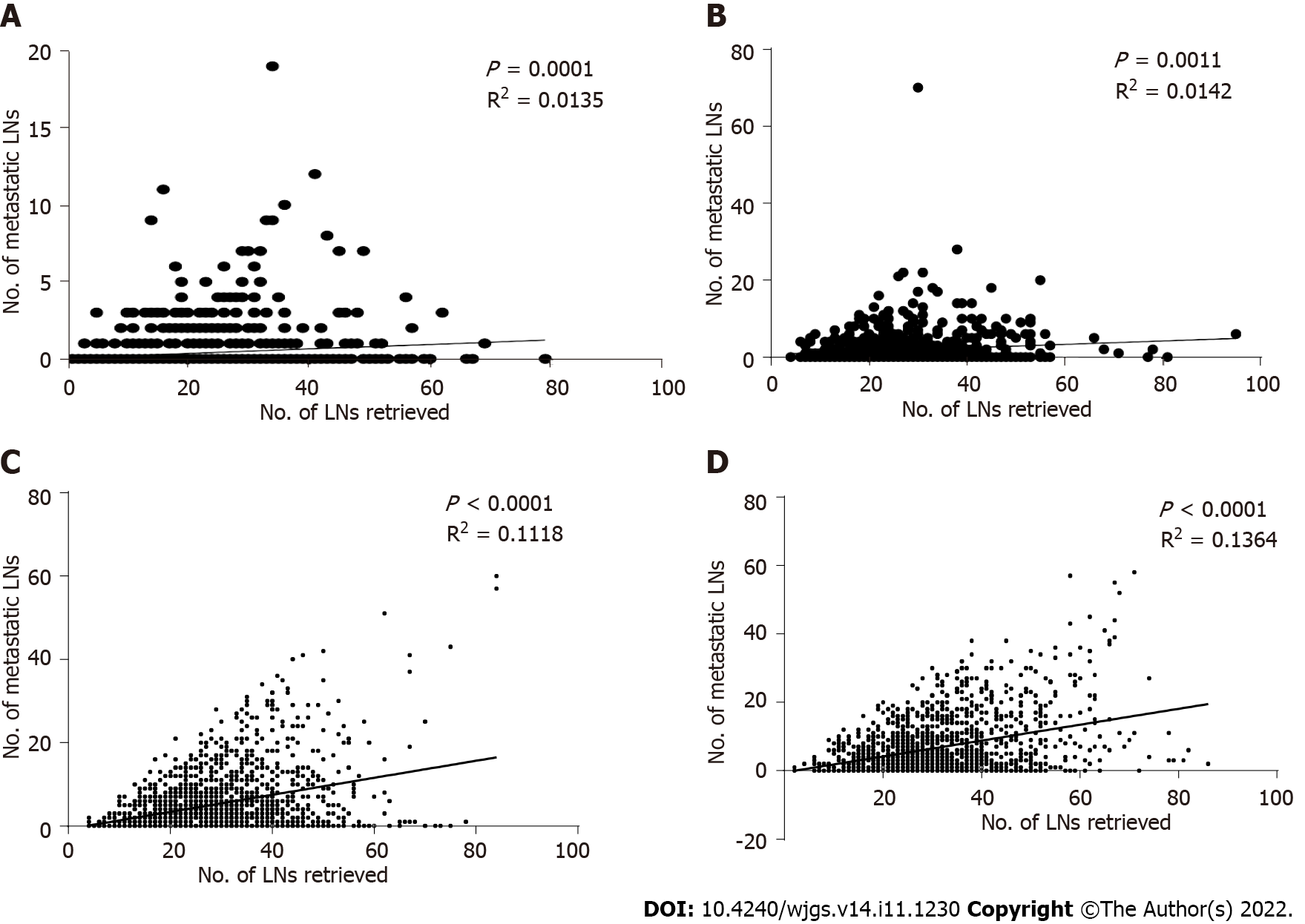
For the pT1-pT4 stages, a scatter plot and linear regression showed that the number of positive LNs detected by pathology increased with the number of LNs removed during surgery, and this result was statistically significant (P = 0.0001, R2 = 0.0135; P = 0.0011, R2 = 0.0142; P < 0.0001, R2 = 0.1118; P < 0.0001, R2 = 0.1364) (Figures 8A-D).
Finally, multivariate analysis showed that age, tumor location, MLNs, and RLNs were independent risk factors associated with the prognosis of patients with pT1 stage cancer. Age, MLNs, and RLNs were independent risk factors associated with the prognosis of patients with pT2 stage cancer. Age, tumor size, MLNs, and RLNs were independent risk factors associated with the prognosis of patients with pT3 stage cancer. Age, tumor size, MLNs, and RLNs were independent risk factors associated with the prognosis of patients with pT4 stage cancer (Table 5).
| Characteristics | Multivariate analysis, pT1 | Multivariate analysis, pT2 | Multivariate analysis, pT3 | Multivariate analysis, pT4 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | - | - | - | - | ||||
| Male | ||||||||
| Female | ||||||||
| Age | 1.056 (1.030-1.082) | < 0.001 | 1.048 (1.024-1.072) | < 0.001 | 1.016 (1.007-1.025) | < 0.001 | 1.021 (1.013-1.029) | < 0.001 |
| Tumor location | 0.034 | - | 0.122 | - | ||||
| Upper third | 1 | 1 | ||||||
| Middle third | 0.384 (0.151-0.972) | 0.043 | 0.828 (0.623-1.100) | 0.192 | ||||
| Lower third | 0.413 (0.209-0.815) | 0.011 | 0.783 (0.619-0.989) | 0.040 | ||||
| Tumor size (mm) | - | - | < 0.001 | < 0.001 | ||||
| ≤ 50 | 1 | 1 | ||||||
| > 50 | 1.435 (1.201-1.715) | 1.422 (1.209-1.671) | ||||||
| Histological type | - | - | 0.260 | - | ||||
| Well-moderately differentiated | 1 | |||||||
| Poorly-undifferentiated | 1.133 (0.934-1.374) | 0.204 | ||||||
| Signet ring cell | 1.305 (0.993-1.374) | 0.056 | ||||||
| Others | 1.037 (0.993-1.716) | 0.851 | ||||||
| MLNs | 1.224 (1.133-1.322) | < 0.001 | 1.067 (1.049-1.086) | < 0.001 | 1.063 (1.052-1.073) | < 0.001 | 1.053 (1.044-1.063) | < 0.001 |
| RLNs | 0.976 (0.954-0.999) | 0.044 | 0.979 (0.960-0.999) | 0.037 | 0.988 (0.979-0.996) | 0.003 | 0.974 (0.967-0.981) | < 0.001 |
| Chemotherapy | - | - | - | - | ||||
| Yes | ||||||||
| No/unknown | ||||||||
In the SEER validation cohort, sex, age, tumor location, MLNs, and RLNs were associated with prognosis in patients with pT1 stage independent risk factors. Age, tumor location, tumor size, MLNs, RLNs and chemotherapy were independent risk factors associated with the prognosis of patients with pT2 stage cancer. Age, tumor size, MLNs, and RLNs were independent risk factors associated with the prognosis of patients with pT3 stage cancer. Age, tumor location, tumor size, MLNs, and RLNs were independent risk factors associated with the prognosis of patients with pT4 stage cancer (Table 6).
| Characteristics | Multivariate analysis, pT1 | Multivariate analysis, pT2 | Multivariate analysis, pT3 | Multivariate analysis, pT4 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | 0.001 | - | - | - | ||||
| Male | 1 | |||||||
| Female | 0.712 (0.596-0.851) | |||||||
| Age | 1.044 (1.035-1.052) | < 0.001 | 1.032 (1.024-1.040) | < 0.001 | 1.018 (1.014-1.022) | < 0.001 | 1.018 (1.014-1.022) | < 0.001 |
| Tumor location | < 0.001 | < 0.001 | - | 0.007 | ||||
| Upper third | 1 | 1 | 1 | |||||
| Middle third | 0.491 (0.364-0.661) | < 0.001 | 0.671 (0.496-0.908) | 0.010 | 0.883 (0.718-1.085) | 0.235 | ||
| Lower third | 0.636 (0.534-0.758) | < 0.001 | 0.603 (0.501-0.726) | < 0.001 | 1.122 (0.963-1.308) | 0.140 | ||
| Tumor size (mm) | - | 0.004 | < 0.001 | < 0.001 | ||||
| ≤ 50 | 1 | 1 | 1 | |||||
| > 50 | 1.323 (1.091-1.604) | 1.172 (1.079-1.274) | 1.285 (1.157-1.427) | |||||
| Histological type | - | - | - | - | ||||
| Well-moderately differentiated | ||||||||
| Poorly-undifferentiated | ||||||||
| Signet ring cell | ||||||||
| Others | ||||||||
| MLNs | 1.111 (1.088-1.135) | < 0.001 | 1.022 (1.013-1.030) | < 0.001 | 1.024 (1.021-1.027) | < 0.001 | 1.035 (1.030-1.039) | < 0.001 |
| RLNs | 0.978 (0.969-0.986) | < 0.001 | 0.981 (0.973-0.990) | < 0.001 | 0.986 (0.983-0.990) | < 0.001 | 0.973 (0.969-0.978) | < 0.001 |
| Chemotherapy | - | 0.002 | - | - | ||||
| Yes | 1 | |||||||
| No/unknown | 1.323 (1.110-1.577) | |||||||
In clinical practice, pT stage according to the depth of tumor invasion can effectively assess patient prognosis, and the risk of LN metastasis increases as pT stage increases[13,22,23]. Smith et al[13] analyzed the optimal number of RLNs by pT staging and found that for the pN0 and pN1 stages of different pT stages, increasing RLNs could prolong prognosis and improve stage migration, and when RLNs reached 40, prognosis could be significantly improved. Chinese GC patients are mostly in the advanced stage, and the frequency of LN metastasis is high. For different pT stages, RLNs ≤ 15 cannot achieve accurate staging of pN0 and pN1 stages[24]. However, for patients with extensive LN metastasis (pN2-pN3), the appropriate number of RLNs cannot be effectively determined. In addition, although the LN metastasis rate can help to avoid stage migration, it is suitable for the removal of less than 15 LNs or D1 resection[22,25], whereas our study mostly focuses on D2 resection of 16 LNs. Therefore, pT stage was used as the basis to assess the number of RLNs in this study, which could be used to accurately assess patient prognosis. For patients with few RLNs, we suggest that more attention is needed, and active treatment may improve the prognosis of such patients.
Although early GC has a better prognosis, patient prognosis of patients still differs significantly. When accompanied by lymphatic and vascular invasion, the prognosis of early GC is still poor, and the risk of LN metastasis is high[26,27]. Osumi et al[26] found that the frequency of LNs also increased with increasing macroscopic tumor diameter. In addition, Choi et al[28] performed a more detailed grouping of pN staging according to the location of LN metastasis and achieved good applicability. In this study, we found that 16% of pT1 stage GC patients developed LN metastasis, and 18% of pT1 stage GC patients in the SEER validation cohort developed LN metastasis. This proportion is also consistent with the proportion of LN metastases found in 11% of pT1 GC patients by Yoshikawa et al[29]. For pT2 stage cancer, 45.4% of the patients in the database of this study had LN metastasis, and 41.9% of the patients in the SEER validation cohort had LN metastasis, which indicates that pT1 and pT2 GC are in earlier stages. The smooth curve shows that for pT1 stage and pT2 stage cancer, MLNs and RLNs have a positive trend, but for pT1 stage cancer, when RLNs are approximately 50, the number of MLNs shows a downward trend, which may be related to the lower risk of LN metastasis in early GC. This finding also means that increasing the numbers of RLNs may not result in more MLNs. It is still necessary to accurately evaluate LN status.
Minimally invasive surgeries, such as laparoscopy, are mostly used in early GC, which is beneficial to enhance patients’ postoperative recovery. In a laparoscopy-related study, Lee et al[30] found no significant difference in OS between laparoscopic surgery and traditional open surgery for early GC and no significant difference in the number of LNs removed (laparotomy: 36.4 vs laparoscopy: 36). An et al[31] found no significant difference in disease-free survival between laparoscopic and open surgery for early-stage GC, whereas there was still no significant difference in the number of LNs removed (laparotomy: 24 vs laparoscopic: 26). These results support the hypothesis that, regardless of the indications for minimally invasive treatment, sufficient LNs still need to be removed in patients with early-stage GC, independent of the technique employed. Our smooth curve findings also support this hypothesis, which is consistent with previous studies[12-14]. For early-stage GC, we found that removal of more than 26 LNs can significantly improve patient prognosis, and the 5-year survival rate of patients when RLNs were appropriately increased to 46 was 100%. The applicability of the cutoff values of our RLNs has been well validated in the SEER database, which also includes people of different races, such as white, black, and Asian individuals. This finding also shows that the cutoff value of RLNs in this study had good applicability and clinical potential.
For GC patients at the pT3 stage, both the smooth curve and the survival curve indicate that increasing numbers of RLNs may not prolong patient long-term survival, and the 5-year survival rate of cases with more than 39 RLNs is lower than those with less than 19 RLNs (57.7% vs 68.3%), which is contrary to the conclusion of the SEER database validation cohort. Chi-square analysis of the difference between the database in this study and the SEER database found that for pT3 stage patients, regardless of the training cohort or validation cohort, there was a statistically significant correlation between the number of RLNs and age. In the training cohort, the proportion of young GC patients increased significantly with the number of RLNs, whereas the opposite was true in SEER. Relevant studies have shown that GC is more aggressive among young patients and that the prognosis is worse[32,33]. In addition, a large number of perigastric LNs are associated with antitumor immunity. When tumors are detected by the immune system, it can lead to local LN enlargement[34,35], and extensive LN dissection may compromise the patients’ immune system function[36]. In addition, there is stage migration in patients in pT3, and we cannot determine whether the poorer prognosis of patients with higher RLNs is because the discovery of more MLNs masks the actual therapeutic benefit of LN dissection. Therefore, both of the above factors may be responsible for this opposite survival trend.
For GC patients at the pT4 stage, both the smooth curve and the survival curve indicate that increasing numbers of RLNs may prolong patients’ long-term survival, which is consistent with previous studies on RLNs[37,38]. However, we found that the survival rate of patients with RLNs ≥ 55 was lower than that of patients with RLNs ≤ 55. Since only 77 patients had RLNs ≤ 55, we think this finding may be due to the small sample size, which also needs to be expanded for verification. Nevertheless, the trend in the survival curves suggested that an increase in RLNs can improve prognosis, and it was well validated in SEER, which also suggested that the increase in RLNs could help improve the prognosis of patients with pT4 stage disease. Clearly, increasing the number of RLNs is particularly important for local control in advanced stages of the disease. In the AJCC 8th edition staging system, when patients with pT4a or pT4b stage have LN metastases, the final pTNM stage is classified as stage III. Although treatment methods have been improved, the prognosis of stage III GC is still poor[39]. Zhang et al[40] found that for patients in the T4 stage, if the number of MLNs was ≥ 21, the prognosis was similar to that at stage IV. In this study, the smooth curve shows that MLNs increase with RLNs, which also means that there may be high-risk patients in pT4 stage with a similar prognosis to stage IV. Therefore, increasing the number of RLNs may guarantee accurate TNM staging and can help differentiate such high-risk patients. We also found that if 45 LNs are removed, the long-term survival may be prolonged significantly, which is also suitable for GC patients of different regions and races in the SEER database. However, the cutoff value for RLNs is different from that in Zhang et al[38] (45 vs 31). Zhang et al[38] included only patients without LN metastasis, and we think that it may have caused the difference found in the included samples. Chi-square analysis found that when RLNs were ≥ 45, the proportion of patients in pN3 stage increased significantly, and linear regression showed that there was a significant correlation between RLNs and MLNs, all of which indicated that some patients in pT4 stage had low to high TNM stage. Therefore, the increase in RLNs is helpful for accurate staging and local control of LNs, but this finding also needs to be confirmed by follow-up studies.
There were some limitations in this study. First, as a retrospective study, we included patients from 2011 to 2017. Due to the longer time span, some clinical information was missing from our study, such as carcinoembryonic antigen, programmed cell death-1, and other clinical information, and it may be difficult to assess the connection between clinicopathological features and RLNs. Second, assessing patient sensitivity to chemotherapy using RLNs also deserves further study. Therefore, we will supply clinical information in future clinical studies.
Our study shows that RLNs are an independent risk factor associated with the prognoses of pT1-pT4 stage GC patients. The mortality risk of patients with an increasing number of RLNs is not constant. For patients with pT1, pT2, and pT4 stage cancers, increasing the number of RLNs can prolong patient long-term survival. However, for patients with pT3 stage cancer, adding RLNs may not improve their long-term survival. For pT1 stage patients, it is recommended to retrieve at least 26 LNs. For pT2 stage patients, it is recommended to retrieve at least 31 LNs. For pT4 stage patients, it is recommended to retrieve 45 LNs.
Gastric cancer (GC) is the sixth most common malignant tumor in the world. The number of metastatic lymph nodes (MLNs) was more important in determining the prognosis of GC patients. For the evaluation of MLNs, sufficient numbers of retrieved lymph nodes (RLNs) need to be acquired during surgery and confirmed by postoperative pathological examination. RLNs based on pT staging can not only enhance the accuracy of staging but also better predict patient prognosis. However, the prognostic value of quantitative assessments of the number of RLNs in GC patients needs further study.
Assessing whether RLNs have prognostic significance for GC of different pT stages will provide a basis for clinicians to treat and predict the prognosis of GC patients.
To discuss how to obtain a more accurate count of MLNs based on RLNs in different pT stages and then to evaluate patient prognosis.
This study retrospectively analyzed patients who underwent GC radical surgery and D2/D2 + LN dissection at the Cancer Hospital of Harbin Medical University from January 2011 to May 2017. Locally weighted smoothing was used to analyze the relationship between RLNs and the number of MLNs. Restricted cubic splines were used to analyze the relationship between RLNs and hazard ratios (HRs), and X-tile was used to determine the optimal cutoff value for RLNs. Patient survival was analyzed with the Kaplan-Meier method and log-rank test. Finally, HRs and 95% confidence intervals were calculated using Cox proportional hazards models to analyze independent risk factors associated with patient outcomes.
A total of 4968 patients were included in the training cohort, and 11154 patients were included in the validation cohort. The smooth curve showed that the number of MLNs increased with an increasing number of RLNs, and a nonlinear relationship between RLNs and HRs was observed. X-tile analysis showed that the optimal number of RLNs for pT1-pT4 stage GC patients was 26, 31, 39, and 45, respectively. A greater number of RLNs can reduce the risk of death in patients with pT1, pT2, and pT4 stage cancers but may not reduce the risk of death in patients with pT3 stage cancer. Multivariate analysis showed that RLNs were an independent risk factor associated with the prognosis of patients with pT1-pT4 stage cancer (P = 0.044, P = 0.037, P = 0.003, P < 0.001).
A greater number of RLNs may not benefit the survival of patients with pT3 stage disease but can benefit the survival of patients with pT1, pT2, and pT4 stage disease. For the pT1, pT2, and pT4 stages, it is recommended to retrieve 26, 31 and 45 LNs respectively.
Due to the longer time span, some clinical information was missing from our study, such as tumor markers and other clinical information. Therefore, we focused on the relationship between RLNs and some clinicopathological features in the future, as well as the evaluation of the sensitivity of RLNs to different chemotherapy regimens.
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1834] [Article Influence: 262.0] [Reference Citation Analysis (0)] |
| 2. | He X, Wu W, Lin Z, Ding Y, Si J, Sun LM. Validation of the American Joint Committee on Cancer (AJCC) 8th edition stage system for gastric cancer patients: a population-based analysis. Gastric Cancer. 2018;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4671] [Article Influence: 519.0] [Reference Citation Analysis (4)] |
| 4. | Vos EL, Nakauchi M, Gönen M, Castellanos JA, Biondi A, Coit DG, Dikken JL, D'Ugo D, Hartgrink H, Li P, Nishimura M, Schattner M, Song KY, Tang LH, Uyama I, Vardhana S, Verhoeven RHA, Wijnhoven BPL, Strong VE. Risk of Lymph Node Metastasis in T1b Gastric Cancer: An International Comprehensive Analysis from the Global Gastric Group (G3) Alliance. Ann Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Shen KH, Wu CW, Lo SS, Hsieh MC, Hsia CY, Chiang SC, Lui WY. Factors correlated with number of metastatic lymph nodes in gastric cancer. Am J Gastroenterol. 1999;94:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than Number? Ann Surg. 2000;232:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 266] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21:274s-275s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Jiang L, Yao Z, Zhang Y, Hu J, Zhao D, Zhai H, Wang X, Zhang Z, Wang D. Comparison of lymph node number and prognosis in gastric cancer patients with perigastric lymph nodes retrieved by surgeons and pathologists. Chin J Cancer Res. 2016;28:511-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1408] [Article Influence: 281.6] [Reference Citation Analysis (2)] |
| 10. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1335] [Article Influence: 83.4] [Reference Citation Analysis (1)] |
| 11. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, van Elk PJ, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H, Sasako M. Extended lymph node dissection for gastric cancer: who may benefit? J Clin Oncol. 2004;22:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 657] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 13. | Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 486] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 14. | Macalindong SS, Kim KH, Nam BH, Ryu KW, Kubo N, Kim JY, Eom BW, Yoon HM, Kook MC, Choi IJ, Kim YW. Effect of total number of harvested lymph nodes on survival outcomes after curative resection for gastric adenocarcinoma: findings from an eastern high-volume gastric cancer center. BMC Cancer. 2018;18:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Callahan MA, Christos PJ, Gold HT, Mushlin AI, Daly JM. Influence of surgical subspecialty training on in-hospital mortality for gastrectomy and colectomy patients. Ann Surg. 2003;238:629-36; discussion 636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Feng F, Zheng G, Guo X, Liu Z, Xu G, Wang F, Wang Q, Guo M, Lian X, Zhang H. Impact of body mass index on surgical outcomes of gastric cancer. BMC Cancer. 2018;18:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Hayashi S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. Number of retrieved lymph nodes is an independent prognostic factor after total gastrectomy for patients with stage III gastric cancer: propensity score matching analysis of a multi-institution dataset. Gastric Cancer. 2019;22:853-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107:2143-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Goh EH, Jiang L, Hsu JP, Tan LWL, Lim WY, Phoon MC, Leo YS, Barr IG, Chow VTK, Lee VJ, Lin C, Lin R, Sadarangani SP, Young B, Chen MI. Epidemiology and Relative Severity of Influenza Subtypes in Singapore in the Post-Pandemic Period from 2009 to 2010. Clin Infect Dis. 2017;65:1905-1913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Wang P, Deng J, Sun Z, Wang W, Wang Z, Xu H, Zhou Z, Liang H. Proposal of a novel subclassification of pN3b for improvement the prognostic discrimination ability of gastric cancer patients. Eur J Surg Oncol. 2020;46:e20-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 3077] [Article Influence: 146.5] [Reference Citation Analysis (8)] |
| 22. | Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW, Lah KH, Lee JH, Choi SH, Min JS. Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg. 2002;26:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Shen JY, Kim S, Cheong JH, Kim YI, Hyung WJ, Choi WH, Choi SH, Wang LB, Noh SH. The impact of total retrieved lymph nodes on staging and survival of patients with pT3 gastric cancer. Cancer. 2007;110:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Zhao L, Han W, Yang X, Zhao D, Niu P, Gao X, Wu Z, Zhang X, Li Z, Ji G, Chen Y. Exceeding 30 ELNs is strongly recommended for pT3-4N0 patients with gastric cancer: A multicenter study of survival, recurrence, and prediction model. Cancer Sci. 2021;112:3266-3277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. Metastatic gastric lymph node rate is a significant prognostic factor for resectable stage IV stomach cancer. J Am Coll Surg. 1997;185:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Osumi H, Kawachi H, Murai K, Kusafuka K, Inoue S, Kitamura M, Yoshio T, Kakusima N, Ishihara R, Ono H, Yamamoto N, Sugino T, Nakatsuka S, Ida S, Nunobe S, Bando E, Omori T, Takeuchi K, Fujisaki J. Risk stratification for lymph node metastasis using Epstein-Barr virus status in submucosal invasive (pT1) gastric cancer without lymphovascular invasion: a multicenter observational study. Gastric Cancer. 2019;22:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Takeuchi A, Ojima T, Katsuda M, Hayata K, Goda T, Kitadani J, Tominaga S, Fukuda N, Nakai T, Yamaue H. Venous Invasion Is a Risk Factor for Recurrence of pT1 Gastric Cancer with Lymph Node Metastasis. J Gastrointest Surg. 2022;26:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Choi YY, An JY, Katai H, Seto Y, Fukagawa T, Okumura Y, Kim DW, Kim HI, Cheong JH, Hyung WJ, Noh SH. A Lymph Node Staging System for Gastric Cancer: A Hybrid Type Based on Topographic and Numeric Systems. PLoS One. 2016;11:e0149555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Is D2 Lymph node dissection necessary for early gastric cancer? Ann Surg Oncol. 2002;9:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Lee JH, Nam BH, Ryu KW, Ryu SY, Kim YW, Park YK, Kim S. Comparison of the long-term results of patients who underwent laparoscopy vs open distal gastrectomy. Surg Endosc. 2016;30:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | An JY, Heo GU, Cheong JH, Hyung WJ, Choi SH, Noh SH. Assessment of open versus laparoscopy-assisted gastrectomy in lymph node-positive early gastric cancer: a retrospective cohort analysis. J Surg Oncol. 2010;102:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Lee JH, Ryu KW, Lee JS, Lee JR, Kim CG, Choi IJ, Park SR, Kook MC, Kim YW, Bae JM. Decisions for extent of gastric surgery in gastric cancer patients: younger patients require more attention than the elderly. J Surg Oncol. 2007;95:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg. 2007;94:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | He WZ, Xie QK, Hu WM, Kong PF, Yang L, Yang YZ, Jiang C, Yin CX, Qiu HJ, Zhang HZ, Zhang B, Xia LP. An increased number of negative lymph nodes is associated with a higher immune response and longer survival in colon cancer patients. Cancer Manag Res. 2018;10:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Degiuli M, Arolfo S, Evangelista A, Lorenzon L, Reddavid R, Staudacher C, De Nardi P, Rosati R, Elmore U, Coco C, Rizzo G, Belluco C, Forlin M, Milone M, De Palma GD, Rega D, Delrio P, Guerrieri M, Ortenzi M, Muratore A, Marsanic P, Restivo A, Deidda S, Zuin M, Pucciarelli S, De Luca R, Persiani R, Biondi A, Roviello F, Marrelli D, Sgroi G, Turati L, Morino M. Number of lymph nodes assessed has no prognostic impact in node-negative rectal cancers after neoadjuvant therapy. Results of the "Italian Society of Surgical Oncology (S.I.C.O.) Colorectal Cancer Network" (SICO-CCN) multicentre collaborative study. Eur J Surg Oncol. 2018;44:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Zhou J, Zhang WW, Wu SG, He ZY, Sun JY, Wang Y, Chen QH. The impact of examined lymph node count on survival in squamous cell carcinoma and adenocarcinoma of the uterine cervix. Cancer Manag Res. 2017;9:315-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Zhao B, Zhang J, Chen X, Sun T, Wang Z, Xu H, Huang B. The retrieval of at least 25 Lymph nodes should be essential for advanced gastric cancer patients with lymph node metastasis: A retrospective analysis of single-institution database study design: Cohort study. Int J Surg. 2017;48:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Zhang N, Bai H, Deng J, Wang W, Sun Z, Wang Z, Xu H, Zhou Z, Liang H. Impact of examined lymph node count on staging and long-term survival of patients with node-negative stage III gastric cancer: a retrospective study using a Chinese multi-institutional registry with Surveillance, Epidemiology, and End Results (SEER) data validation. Ann Transl Med. 2020;8:1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1504] [Article Influence: 150.4] [Reference Citation Analysis (1)] |
| 40. | Zhang Z, Huang JY, Wang PL, Hou WB, Yin SC, Xu HM. Should All Stage N3b Patients with Advanced Gastric Cancer Be Considered Equivalent? J Gastrointest Surg. 2019;23:1742-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kawabata H, Japan; Lieto E, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ