Published online Jun 27, 2021. doi: 10.4240/wjgs.v13.i6.585
Peer-review started: January 18, 2021
First decision: April 19, 2021
Revised: April 24, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: June 27, 2021
Processing time: 150 Days and 23.7 Hours
Chylous ascites is a rare complication in colorectal surgery with limited evidence.
To systematically review all available evidence to describe the incidence, clinical presentation, risk factors and management strategies.
The systematic review was performed through PubMed, MEDLINE, EMBASE and Cochrane and cross-checked up to November 2020. The data collated included: Demographics, indications (benign vs malignant), site of disease, surgical approach, extent of lymphadenectomy, day to and method of diagnosis of chylous ascites and management strategies.
A total of 28 studies were included in the final analysis (426 cases). Patient age ranged from 31 to 89 years. All except one case were performed for malignancy. Of the 426 cases, 195 were right-colonic, 121 left-colonic, 103 pelvic surgeries and 7 others. The majority were diagnosed during the same inpatient stay by recognition of typical drain appearance and increased volume. Three cases were diagnosed during outpatient visits with increased abdominal distention and subsequently underwent paracentesis. Most cases were managed successfully non-operatively (fasting with prolonged drainage, total parenteral nutrition, somatostatin analogues or a combination of these). Only three cases required surgical intervention after failing conservative management and subsequently resolved completely. Risk factors identified include: Right-colonic surgery/ tumour location, extent of lymphadenectomy and number of lymph nodes harvested.
Chylous ascites after colorectal surgery is a relatively rare complication. Whilst the majority of cases resolved without surgical intervention, preventative measures should be undertaken such as meticulous dissection and clipping of lymphatics during lymphadenectomy to prevent morbidity.
Core Tip: Chylous ascites is a rare entity in colorectal surgery. However, observed rates have increased in recent years in association with increasingly aggressive D3 lymphadenectomy and does carry significant risk of morbidity from malnutrition, electrolytes imbalances and immunosuppression secondary to protein and lymphocyte loss. Therefore, great care should be applied to dissection and control of lymphatics around major vasculature.
- Citation: Ng ZQ, Han M, Beh HN, Keelan S. Chylous ascites in colorectal surgery: A systematic review. World J Gastrointest Surg 2021; 13(6): 585-596
- URL: https://www.wjgnet.com/1948-9366/full/v13/i6/585.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i6.585
Chylous ascites (CA) or chyle leak (CL) after colorectal surgery remains a relatively rare complication with estimated incidence of 1%-6.5%[1,2]. It usually occurs after unrecognized trauma to the lymphatics during surgery. It has been reported in the literature with surgical procedures where close dissection to the lymphatic system is performed such as: Thoracic surgeries, pancreatic resections, retroperitoneal lymph node dissection, abdominal aortic aneurysm repair, pelvic surgery in gynaecology, living donor nephrectomy and liver transplant[3]. The incidence of CA/CL in abdominal surgery is approximately 1%-11%[4]. This condition was extremely rare in the open era of colorectal surgery.
The rapid advancement in technology and surgical techniques have enabled more aggressive minimally invasive lymphadenectomy in cancer surgery in the hope of achieving R0 resection for better overall and disease-free survivals[5,6]. This is reflected in the increased uptake in complete mesocolic resection (CME) or D3 lymphadenectomy in colorectal surgery. As a result, it is thought that this is an important risk factor for CA/CL[1]. Its cause, preventive measures and new strategies for management are not well elucidated in the literature. Therefore, the aim of this study was to perform a systematic review on all evidence on CA/CL after colorectal surgery to describe its incidence, clinical presentation, risk factors and management options.
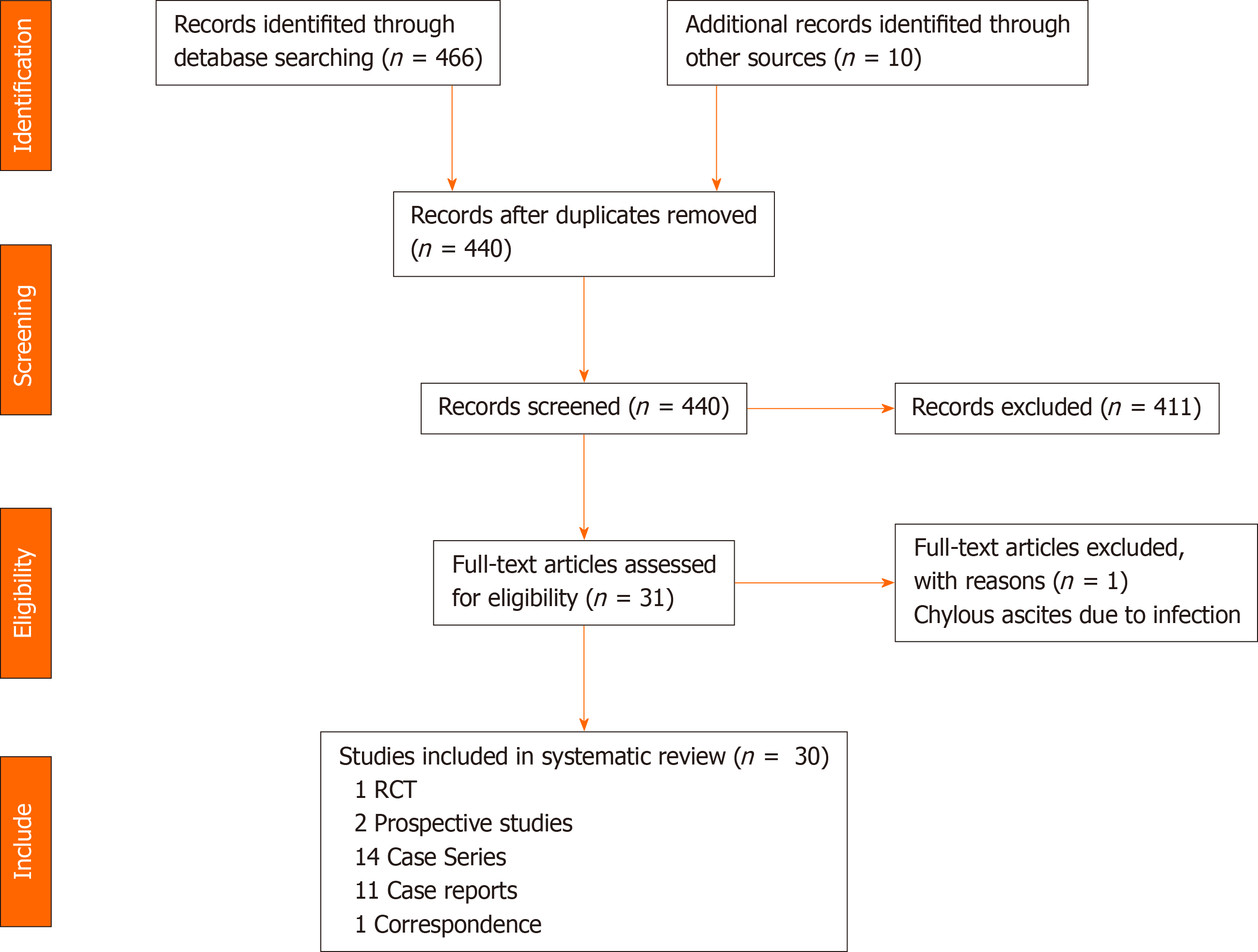
A systematic review of the literature was performed by searching PubMed, MEDLINE, EMBASE and Cochrane databases up to November 2020. The medical subject headings and keywords used individually or in combination were: “Chyle”, “chylous”, “leak”, “fistula”, “ascites”, “colon”, “rectal”, “colorectal”, “laparoscopic”, “complication”, “lymphatic”, “CME”, “D3”, “lymphadenectomy”, “somatostatin”, and “octreotide”. All references were searched and cross-checked. All foreign language articles including Chinese and Japanese publications were translated by medical personnel with proficiency in both the foreign language and English. The selection pathway is described as per the PRISMA flowchart in Figure 1.
Due to the rarity of the condition, all studies reporting post-operative CL/CA secondary to colorectal surgery including case reports and letters to editors were included in the systematic review for analysis. Studies were excluded if performed on paediatric populations < 16 years of age or if there was insufficient information for analysis.
A data pro forma was designed prior to data collection for uniformity. Two investigators (Ng ZQ and Han M) individually collected the data. Any difference in opinion was resolved through discussion among the investigators. The data collected included: Author, journal, year of publication, country, type of study, number of cases, age, gender, diagnosis (benign or malignant), type of surgery (laparoscopic or open, right or left colonic or rectal surgery, level of lymphadenectomy, specific intra-operative findings), drain left in-situ, days to diagnosis of CA/CL, method of diagnosis (drain appearance, laboratory testing and imaging), management utilized (non-operative vs operative) and long-term outcomes.
Meta-analysis was not undertaken due to marked heterogeneity of the data. A formal quality assessment of the articles was not undertaken as a significant proportion of the articles are case reports and case series.
For the uniformity of the data, the following definitions were set: (1) Right colonic surgery includes right hemicolectomy and right extended hemicolectomy; (2) Left colonic surgery includes left hemicolectomy, sigmoidectomy and anterior resection (above peritoneal reflection); (3) Pelvic surgery includes any colectomy with anastomosis below the peritoneal reflection and abdominoperineal resection; (4) The category of others includes those non-specified; (5) D2/D3 dissection definitions were based on the Japanese Society for Cancer of the Colon and Rectum guidelines[7]; and (6) CME was defined as “sharp dissection of the embryological mesocolic plane to create an intact envelope with high tie of colonic arteries and veins called central vascular ligation (CVL), ensuring maximum removal of lymphatic vessels and lymph nodes”[8].
A total of one randomized controlled trial[9], two prospective studies[10,11], 14 case series[1,12-21], 11 case reports[22-32], and one correspondence[33] were included in the final analysis, resulting in a total of 426 cases of CA/CL in the literature. From 2000 to 2010, 59 cases of CA/CL were reported. Between 2011 to 2020, there was a six-fold increase in number of cases reported (n = 367). 22 publications originated from the Asia pacific followed by five from Europe and one from the Middle East. The estimated incidence of CA/CL after colorectal surgery from these series is 5.5%.
Where age was reported, the mean ages of patients were between 57 and 65 years. The overall range of age was 31 to 89 years. Of the 426 cases of CA/CL, there were 195 right colonic surgeries, 121 Left colonic surgeries, 103 pelvic surgeries and 7 others or unspecified (Table 1).
| Ref. | No. of Cases | Age/gender | Diagnosis | Type of surgery | Level of lymphadenectomy |
| Giovannini et al[22], Italy, 2005 | 1 | 69/M | MA | Lap AR | D3 |
| Chan et al[30], Malaysia, 2006 | 1 | 32/M | MA | AR | N/A |
| Chi et al[21], China, 2006 | 10 | N/A | MA | Lap LAR (n = 3); Open LAR (n = 7) | N/A |
| Giovannini et al[34], Italy, 2008 | 1 | 60/M | MA | Laparotomy (previous endoscopic removal of R colon polyp) | Lymphadenectomy (not specified) |
| Lu et al[35], China, 2010 | 46 | 58.7 (mean) (24-83); M (n = 27) / F (n = 20) | MA | Laparoscopic (n = 28); Open (n = 18); R Hemicolectomy (n = 16); L Hemicolectomy (n = 7); AR (n = 23) | N/A |
| Sun et al[36], China, 2012 | 46 | 57.4 (mean); M (n = 29)/F (n = 17) | MA | R Hemicolectomy (n = 30); L Hemicolectomy (n = 16) | D3 |
| Feng et al[12], China, 2012 | 1 | N/A | MA | Lap R Hemicolectomy | CME |
| Nishigori et al[1], Japan, 2012 | 9 | Age Range 49-80; M (n = 4)/F (n = 5) | MA | Open R Hemicolectomy (n = 5); Open HAR (n = 1); Open sigmoidectomy (n = 1); Lap AR (n = 1); Lap HAR | D2/D3 |
| Nakayama et al[31], Japan, 2012 | 1 | 67/M | MA | LAR | Para-aortic lymphadenectomy |
| Bartolini et al[32], Italy, 2013 | 3 | 43/F; 78/F; 85/F | MA | Lap AR | N/A |
| Galketiya et al[23], Australia, 2013 | 1 | 76/F | MA | AR | N/A |
| Matsumura et al[24], Japan, 2013 | 2 | 64/F; 80/M | MA | Lap R hemicolectomy (n = 1); Lap HAR (n = 1) | D2; D3 |
| Matsuda et al[2], Japan, 2013 | 9 | Age Range 55-80; M (n = 7)/F (n = 2) | MA | Lap R hemicolectomy (n = 4); Lap AR (n = 5) | D3 |
| Han et al[14], China, 2013 | 4 | N/A | MA | Lap assisted radical R hemicolectomy (n = 4) | D3 |
| Shin et al[15], Korea, 2014 | 2 | N/A | MA | Lap Colectomy | D3 and CME |
| Soyer et al[25], Turkey, 2014 | 1 | 76/F | MA | Open R Hemicolectomy | D3 |
| Inada et al[16], Japan, 2014 | 1 | N/A | MA | Lap APR | D2 |
| Ha et al[26], Korea, 2015 | 1 | 65/M | MA | Lap AR | D2 |
| Lee et al[17], Korea, 2016 | 138 | 65.0 ± 11.8 | MA | Open (n = 6); Laparoscopic (n =132); R Hemicolectomy (n = 27); L Hemicolectomy (n = 4); AR (n = 40); LAR (n = 63); APR (2); Others (2) | D3 |
| Korkolis et al[27], Greece, 2017 | 1 | 44/M | MA | Lap LAR | D2 |
| Wang et al[6], China, 2017 | 22 | N/A | MA | Lap R hemicolectomy | CME |
| Pascual et al[33], Spain, 2017 | 1 | 57/F | B | Lap sigmoidectomy | N/A |
| Owada et al[28], Japan, 2018 | 1 | 60s/M | MA | Lap sigmoidectomy | D3 |
| Fujii et al[9], Japan, 2018 | 11 | N/A | MA | AR (Lap/Open) | D2 |
| Shimajiri et al[29], Japan, 2018 | 1 | 31/M | MA | Lap descending colectomy | D3 |
| An et al[19], Korea, 2018 | 6; 9 | N/A; N/A | MA; MA | Lap R hemicolectomy; Lap R hemicolectomy | CME; Non-CME |
| Suzuki et al[20], Japan, 2019 | 1 | N/A | MA | N/A | N/A |
| Lee et al[10], Korea, 2020 | 50 | N/A | MA | Lap R hemicolectomy | D3/D4 |
For right colonic surgeries, the extent of lymphadenectomy was not reported in 16 cases, 9 were non-CME dissections, none were D2 dissections and 168 were D3 dissections/CME. For left colonic surgeries, the extent of lymphadenectomy was not reported in 29 cases, 5 cases underwent D1 dissection, 7 cases were D2 dissections and 73 were D3 dissections/CME. For pelvic surgeries, the extent of lymphadenectomy was not reported in 10 cases, 2 cases were D2 dissections and 93 cases were D3 dissections/CME.
11 articles reported the dissecting equipment used including ultrasonic coagulating shears, electrothermal bipolar vessel sealers and/or electrosurgery[1,2,10,13,17,22,24,25,27,28,34] (Table 2).
| Ref. | Type of equipment used |
| Giovannini et al[22] | Harmonic scalpel |
| Giovannini et al[34] | Wet bipolar forceps |
| Nishigori et al[1] | Electrosurgical knife and ultrasonically activated scalpel |
| Matsumura et al[24] | Ultrasound-coagulation and electrical scalpel |
| Matsuda et al[2] | Laparoscopic coagulating shears and hemoclips |
| Baek et al[13] | Electrosurgical bipolar vessel sealer (Ligasure) or vascular clips |
| Soyer et al[25] | Ligasure |
| Lee et al[17] | Electrothermal bipolar vessel sealer or ultrasonic coagulating shears |
| Korkolis et al[27] | Ultrasonic sealing scalpel |
| Owada et al[28] | Electric surgical knife and ultrasonically activated devise |
| Lee et al[10] | Endoshears |
There was no uniformity in the definition of CL/CA in four studies that defined it[1,2,13,17]. Most commonly, diagnosis was based on the appearance of typical non-infected, white milky fluid from surgical drains. Where the definition included volume of drain output or drain triglyceride measurements, these were not standardized (Table 3).
| Ref. | Definition |
| Matsuda et al[2] | White milky fluid in the drainage that contained high level so triglycerides (> 150 mg/dL) |
| Nishigori et al[1] | Non-infectious milky fluid in the drainage tubes |
| Baek et al[13] | Non-infectious extravasation of milky or creamy peritoneal fluid in the drain tubes with a volume of > 200 mL/d and a triglyceride level > 100 mg/dL |
| Lee et al[20] | Presence of noninfectious milky or creamy peritoneal fluid in the drainage tubes, at a volume of > 200 mL/d |
The vast majority of cases were diagnosed following the resumption of oral intake whilst inpatients where the drain output increased significantly and the appearance became milky. The day of onset following surgery ranged from 2 to 8 (Table 4).
| No. | Initial management | Number of cases | Successful | Surgery | Ref. |
| 1 | No treatment (“Healed spontaneously”) | 53 | Yes | No | [1,10,24] |
| 2 | Fasting only | 14 | Yes | No | [1,2] |
| 3 | Fasting + TPN | 1 | Yes | No | [32] |
| 4 | Fasting + TPN + Octreotide/somatostatin | 103 | Yes | No | [21,25,34-36] |
| 5 | Failed (fasting + TPN + somatostatin) + surgery | 1 | No | Yes | [35] |
| 6 | Octreotide | 1 | Yes | No | [13] |
| 6 | MCT only | 9 | Yes | No | [1,13] |
| 7 | MCT + TPN/fasting | 1 | Yes | No | [23] |
| 8 | Fat-free diet only | 1 | Yes | No | [31] |
| 9 | Low fat diet only | 5 | Yes | No | [24,28,32] |
| 10 | Low fat diet + somatostatin | 1 | Yes | No | [27] |
| 11 | Low fat diet + MCT + Fasting/TPN/somatostatin | 1 | No | Yes | [26] |
| 12 | No treatment-discharged homeParacentesis + fasting/TPN + low fat dietParacentesis + TPN + somatostatinMCT | 1 | No | Yes | [29] |
| 13 | “Conservative therapy” only | 160 | Yes | No | [6,17] |
| 14 | Failed “Conservative therapy” + surgery | 1 | No | Yes | [33] |
| 15 | Not available | 72 | N/A | N/A | [9,12-16,19,20,30] |
Only three cases were diagnosed during outpatient review due to increasing abdominal distention and pain with timing reported on post-operative day 14, 21 and 42 respectively[23,26]. Of these, two cases were laparoscopic anterior resections with D2 dissections and one case underwent anterior resection with extent of lymphadenopathy not reported; all cases were for malignancy. All three cases underwent computed tomography (CT) abdomen which revealed massive ascites. All underwent imaging-guided paracentesis which showed typical appearance of CA/CL.
Six studies identified potential risk factors for the occurrence of CA/CL after colorectal surgery[1,2,13,17,35,36]. However, several are contradictory as summarized in Table 5. Overall, it is believed that the tumour location (especially right-sided), the extent of lymphadenectomy and the number of lymph nodes harvested are important risk factors.
| Ref. | Risk factor | Not risk factor |
| Lu et al[35], 2010 | Right colectomy | - |
| Sun et al[36], 2012 | Tumour size; Tumour location; Number of lymph nodes harvested harvested | - |
| Nishigori et al[1], 2012 | Tumour location; Tumours fed by SMA; D3 Dissection | - |
| Matsuda et al[2], 2013 | - | Blood loss; Operative time; Extent of lymph node dissection; Number of lymph nodes harvested; Region of lymph node dissection |
| Baek et al[13], 2013 | Older age; Right colectomy; Transverse segmental colectomy; Shorter operative time; Lower blood loss; Operator difference | - |
| Lee et al[17], 2016 | Shorter operative time; Positive lymph node metastasis; Number of lymph node harvested; Tumour size | Tumour location; Type of surgery |
99.3% of cases were managed non-operatively. 12.4% of cases required no treatment and resolved without further intervention. The remainder of cases were managed with prolonged drainage, diet modification to low fat diet (LFD) or medium chain triglyceride (MCT), fasting, total parenteral nutrition (TPN), somatostatin analogues or a combination of these. Management strategies have been summarized in Table 4.
Three cases required surgical intervention after failing non-operative management.
The first case[26] was a 65-year-old male diagnosed with CA 14 d after laparoscopic anterior resection with D2 dissection. He failed conservative management with LFD and MCT for one week followed by TPN with somatostatin for four weeks. He subsequently underwent a midline laparotomy with high fat diet given prior to surgery. A small 1mm defect was found in a lymphatic branch of the left lumbar trunk which was sutured. The CL resolved postoperatively.
The second case[33] was a 57-year-old female diagnosed with CA on day 8 post-laparoscopic sigmoidectomy. She failed 8 wk of conservative management. Pre-operative lymphoscintigraphy showed CL without a localized site of leakage. She underwent midline laparotomy with the aid of indocyanine green (ICG) which demonstrated a clear area of lymphatic disruption. This was successfully closed with clips.
The final case had a protracted hospital admission[29]. The patient was a 31-year-old male who underwent laparoscopic descending colectomy with D3 dissection for a neuroendocrine tumour. On post-operative day 4 the drain appearance became milky and CT abdomen confirmed a large amount of ascites. The drain was removed on day 7 and CA recurred. He underwent twice weekly paracentesis and commenced fasting with TPN on day 26. LFD was recommenced on day 56. He represented on day 96 with further CA where he received paracentesis, restarted TPN and was placed on somatostatin. MCT were commenced on day 113. CL continued so he subsequently underwent a laparoscopic exploration which found the CL at the upper part of ischemia modified albumin. It was ligated and fibrin glue was sprayed over the ligation site. Leakage subsequently resolved.
Matsuda and colleagues reported the long-term impact of CA on disease-free survival[2]. Their 3-year disease-free survival was significantly lower in patients that suffered post-operative CA (76.2% vs 93.4%, P = 0.02) but the 3-year overall survival did not differ.
This systematic review has shown that chylous ascites or chyle leak after colorectal surgery is a relatively rare complication with incidence estimated to be around 5.5% from the identified series.
When it occurs, CA/CL has a significant impact on the post-operative recovery of patients. It may lead to malnutrition, electrolytes imbalances and immunosuppression secondary to protein and lymphocyte loss[3]. Additionally, there is concern for increased risk of disease recurrence following CA/CL[2].
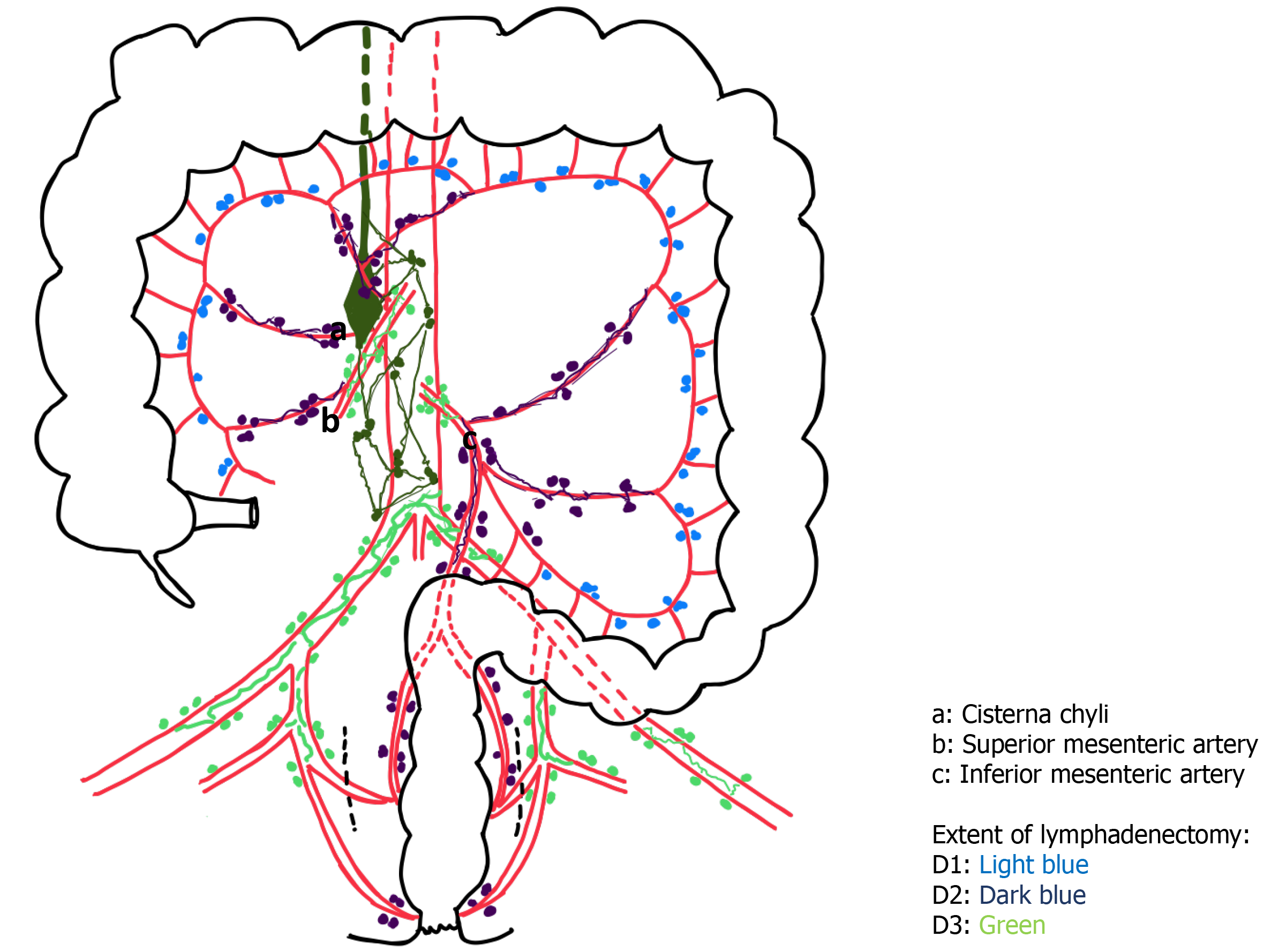
CA/CL has potential significance going forward as there has been recent considerable interest in CME or CVL for colonic cancers, mimicking the concept of total mesorectal excision for rectal cancers. The concept of CME/CVL mirrors the Japanese definition of D3 Lymphadenectomy which has been in practice in many East Asian centres[7]. This may explain why the majority of reported cases to date have stemmed from Japanese, Korean and Chinese centres[1,2,11,13,17,35]. A few studies have shown that the extent of lymphadenectomy (D3) and site of tumour (right-sided and fed by the superior mesenteric artery) are risk factors for CA/CL as these areas are rich in lymphatics (Figure 2) that can easily be injured during oncological clearance[1,13,35]. There has been the suggestion that the use of monopolar diathermy may not seal some of these lymphatic tubes. However, in this study the type of energy device used, whether ultrasonic shears or bipolar, did not seem to affect the occurrence of CA/CL. Smaller lymphatic tubes could easily be overlooked in the setting of pneumoperitoneum[3].
An interesting finding from this systematic review was the clinical presentation of CA. Most cases were detected during the same inpatient stay through typical drain appearance and increased volume. Most Western centres no longer place routine drains intra-operatively in uncomplicated elective right- and left-sided colonic surgeries[37]. As such, some cases of CL might be reabsorbed spontaneously without any clinical significance[13]. Where patient’s represent in the outpatient setting, CT abdomen will be useful for the exclusion of other complications such as abscess from anastomotic leak or haematoma. If large volume ascites is observed, imaging-guided paracentesis with drain insertion can be performed. The fluid can then be tested in the laboratory for triglyceride levels and/or evidence of chylomicrons if in any doubt.
The principles of management of CA/CL are similar to those previously described for CA/CL after living-donor nephrectomy by Ng and colleagues[3]. The main goal is to reduce mesenteric lymphatic flow to allow the injured site to heal. Early involve
The long-term outcome has only been reported in one study. Matsuda and colleagues showed that patients who developed post-operative CA has a shorted disease-free survival at 3-year compared to patients without CA. However, the 3-year overall survival rates were similar. This could be explained by the more advanced disease stage which requires more extensive lymphadenectomy. Alternatively, another theory postulates the dissemination of cancer cells with CA.
Chylous ascites or chyle leak after colorectal surgery is a rare complication especially in the Western world. Whilst the majority of cases resolve with conservative management, meticulous dissection and clipping of lymphatics especially during extended lymphadenectomy are vital to prevent this complication and its associated morbidity.
Chylous ascites or chyle leak after colorectal surgery remains a relatively rare complication.
There has been an increase in the uptake of complete mesocolic resection (CME) or D3 lymphadenectomy in colorectal surgery. As a result, it is thought that this is an important risk factor for chylous ascites or chyle leak.
The aim of this study was to perform a systematic review on all evidence on chylous ascites or chyle leak after colorectal surgery to describe its incidence, clinical presentation, risk factors and management options.
A systematic review of the literature was performed by searching PubMed, MEDLINE, EMBASE and Cochrane databases up to November 2020.
From 2000 to 2010, 59 cases of chylous ascites or chyle leak were reported. Between 2011 to 2020, there was a six-fold increase in number of cases reported. The estimated incidence of chylous ascites or chyle leak after colorectal surgery from these series is 5.5%.
Chylous ascites or chyle leak after colorectal surgery is a rare complication especially in the Western world. Whilst the majority of cases resolve with conservative management, meticulous dissection and clipping of lymphatics especially during extended lymphadenectomy are vital to prevent this complication and its associated morbidity.
Future colorectal trials should include this as a reported complication given the increasing number of CME and D3 Lymphadenectomy.
The authors would like to thank Dr. Abe T and Dr. Zhou M for their assistance in translating the Japanese and Chinese articles.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Hidaka E S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Nishigori H, Ito M, Nishizawa Y, Koyama A, Koda T, Nakajima K, Minagawa N, Kobayashi A, Sugito M, Saito N. Postoperative chylous ascites after colorectal cancer surgery. Surg Today. 2012;42:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Matsuda T, Fujita H, Kunimoto Y, Kimura T, Ogino K. Chylous ascites as a complication of laparoscopic colorectal surgery. Asian J Endosc Surg. 2013;6:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Ng ZQ, He B. A Proposed Classification System and Therapeutic Strategy for Chyle Leak After Laparoscopic Living-Donor Nephrectomy: A Single-Center Experience and Review of the Literature. Exp Clin Transplant. 2018;16:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Weniger M, D'Haese JG, Angele MK, Kleespies A, Werner J, Hartwig W. Treatment options for chylous ascites after major abdominal surgery: a systematic review. Am J Surg. 2016;211:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | De Simoni O, Barina A, Sommariva A, Tonello M, Gruppo M, Mattara G, Toniato A, Pilati P, Franzato B. Complete mesocolic excision vs conventional hemicolectomy in patients with right colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:881-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis. 2017;19:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (3)] |
| 7. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1423] [Article Influence: 237.2] [Reference Citation Analysis (3)] |
| 8. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-64; discussion 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1143] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 9. | Fujii S, Ishibe A, Ota M, Watanabe K, Watanabe J, Kunisaki C, Endo I. Randomized clinical trial of high vs low inferior mesenteric artery ligation during anterior resection for rectal cancer. BJS Open. 2018;2:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Lee J, Cho JR, Kim MH, Oh HK, Kim DW, Kang SB. Surgical outcomes according to the type of monopolar electrocautery device used in laparoscopic surgery for right colon cancer: a comparison of endo-hook vs endo-shears. Surg Endosc. 2020;34:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Jaswal VM, Babbar HS, Mann DS, Mahmood A. Maternal nutrition and development of intestinal functions: II--Effect of feeding high protein and high fat diets to lactating rats. Indian J Exp Biol. 1990;28:776-779. [PubMed] |
| 12. | Feng B, Sun J, Ling TL, Lu AG, Wang ML, Chen XY, Ma JJ, Li JW, Zang L, Han DP, Zheng MH. Laparoscopic complete mesocolic excision (CME) with medial access for right-hemi colon cancer: feasibility and technical strategies. Surg Endosc. 2012;26:3669-3675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Baek SJ, Kim SH, Kwak JM, Kim J. Incidence and risk factors of chylous ascites after colorectal cancer surgery. Am J Surg. 2013;206:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 14. | Han DP, Lu AG, Feng H, Wang PX, Cao QF, Zong YP, Feng B, Zheng MH. Long-term results of laparoscopy-assisted radical right hemicolectomy with D3 Lymphadenectomy: clinical analysis with 177 cases. Int J Colorectal Dis. 2013;28:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Shin JW, Amar AH, Kim SH, Kwak JM, Baek SJ, Cho JS, Kim J. Complete mesocolic excision with D3 Lymph node dissection in laparoscopic colectomy for stages II and III colon cancer: long-term oncologic outcomes in 168 patients. Tech Coloproctol. 2014;18:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Inada R, Yamamoto S, Oshiro T, Takawa M, Fujita S, Akasu T. Case-matched comparison of the short-term outcomes between laparoscopic and open abdominoperineal resection for rectal cancer. Surg Today. 2014;44:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Lee SY, Kim CH, Kim YJ, Kim HR. Chylous ascites after colorectal cancer surgery: risk factors and impact on short-term and long-term outcomes. Langenbecks Arch Surg. 2016;401:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Zhang C, Zhang D, Fu Z, Sun Y. Clinical outcome of laparoscopic complete mesocolic excision in the treatment of right colon cancer. World J Surg Oncol. 2017;15:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | An MS, Baik H, Oh SH, Park YH, Seo SH, Kim KH, Hong KH, Bae KB. Oncological outcomes of complete vs conventional mesocolic excision in laparoscopic right hemicolectomy. ANZ J Surg. 2018;88:E698-E702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Suzuki S, Fukunaga Y, Tamegai Y, Akiyoshi T, Konishi T, Nagayama S, Saito S, Ueno M. The short-term outcomes of laparoscopic-endoscopic cooperative surgery for colorectal tumors (LECS-CR) in cases involving endoscopically unresectable colorectal tumors. Surg Today. 2019;49:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Chi P, Lin HM, Xu ZB. [Comparison of surgical complication rate between laparoscopic and open radical resection for colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2006;9:221-224. [PubMed] |
| 22. | Giovannini I, Giuliante F, Chiarla C, Ardito F, Vellone M, Nuzzo G. Non-surgical management of a lymphatic fistula, after laparoscopic colorectal surgery, with total parenteral nutrition, octreotide, and somatostatin. Nutrition. 2005;21:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Galketiya K, Davis I. Chylous ascites complicating anterior resection for colorectal cancer. ANZ J Surg. 2013;83:391-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Matsumura A, Nishibeppu K, Matsuyama T, Ogino S, Takemura M, Mugitani T, Akami T, Shimode Y. [Two cases of chylous ascites after laparoscopic colorectal cancer surgery]. Gan To Kagaku Ryoho. 2013;40:1939-1941. [PubMed] |
| 25. | Soyer HV, Kayaalp C. Postoperative Chylous Ascites After Right Hemicolectomy. Eur J Surg Sci. 2014;5:111-113. |
| 26. | Ha GW, Lee MR. Surgical repair of intractable chylous ascites following laparoscopic anterior resection. World J Gastroenterol. 2015;21:6077-6081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Korkolis DP, Kapritsou M, Passas I, Kalafati M, Katsoulas T, Konstantinou EA. CHYLOUS ASCITES AFTER LAPAROSCOPIC LOW ANTERIOR COLORECTAL RESECTION FOR RECTOSIGMOID CARCINOMA: A CASE REPORT AND A LITERATURE REVIEW. Gastroenterol Nurs. 2017;40:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Owada Y, Kawasaki K, Asakura R, Yamada K, Hosono M, Okazaki T, Ienaga T. [A Case Report of Chylous Ascites after Laparoscopic Sigmoid Colectomy Treated with Conservative Therapy]. Gan To Kagaku Ryoho. 2018;45:1866-1868. [PubMed] |
| 29. | Shimajiri H, Egi H, Yamamoto M, Kochi M, Mukai S, Ohdan H. Laparoscopic management of refractory chylous ascites using fluorescence navigation with indocyanine green: A case report. Int J Surg Case Rep. 2018;49:149-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Chan KY, Teoh CM, Sukumar N. Chylous ascites after anterior resection for rectal carcinoma: a rare but significant incident. Asian J Surg. 2006;29:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Nakayama G, Morioka D, Murakami T, Takakura H, Miura Y, Togo S. Chylous ascites occurring after low anterior resection of the rectum successfully treated with an oral fat-free elemental diet (Elental(®)). Clin J Gastroenterol. 2012;5:216-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Bartolini I, Bechi P. Chylous ascites after laparoscopic anterior resection of the rectum. Surgery. 2013;153:875-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Pascual M, Pañella C, Pera M. Use of indocyanine green in the surgical treatment of chylous ascites after laparoscopic colectomy. Colorectal Dis. 2017;19:595-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Giovannini I, Giuliante F, Chiarla C, Giordano M, Ardito F, Vellone M, Sarno G, Nuzzo G. External lymphatic fistula after intra-abdominal lymphadenectomy for cancer. Treatment with total parenteral nutrition and somatostatin. Nutrition. 2008;24:1220-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Lu XR, Lin HM, Chi P. [Treatment of chyle leak following radical resection for colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:808-810. [PubMed] |
| 36. | Sun YW, Chi P, Lin HM, Lu XR, Huang Y, Xu ZB, Huang SH. [Risk factors of postoperative chyle leak following complete mesocolic excision for colon cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:328-331. [PubMed] |
| 37. | Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg. 2019;43:659-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1359] [Article Influence: 194.1] [Reference Citation Analysis (0)] |