Published online Jun 27, 2021. doi: 10.4240/wjgs.v13.i6.563
Peer-review started: February 9, 2021
First decision: May 4, 2021
Revised: May 7, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: June 27, 2021
Processing time: 129 Days and 4.2 Hours
Hybrid endoscopic submucosal dissection (ESD) that comprises mucosal incision and partial submucosal dissection followed by snaring in a planned manner, has been developed for endoscopic resection of gastrointestinal neoplasms to overcome the technical barrier of ESD. Although the superiority of hybrid ESD with SOUTEN, a single multifunctional device, over conventional ESD has been indicated, the actual effect of snaring itself remains unclear since SOUTEN could be applied to hybrid ESD group, but not to the conventional ESD group, due to ethical issue in clinical practice.
To determine whether and how hybrid ESD was superior to conventional ESD in the endoscopic treatment of gastric lesions in an ex vivo porcine model basic study.
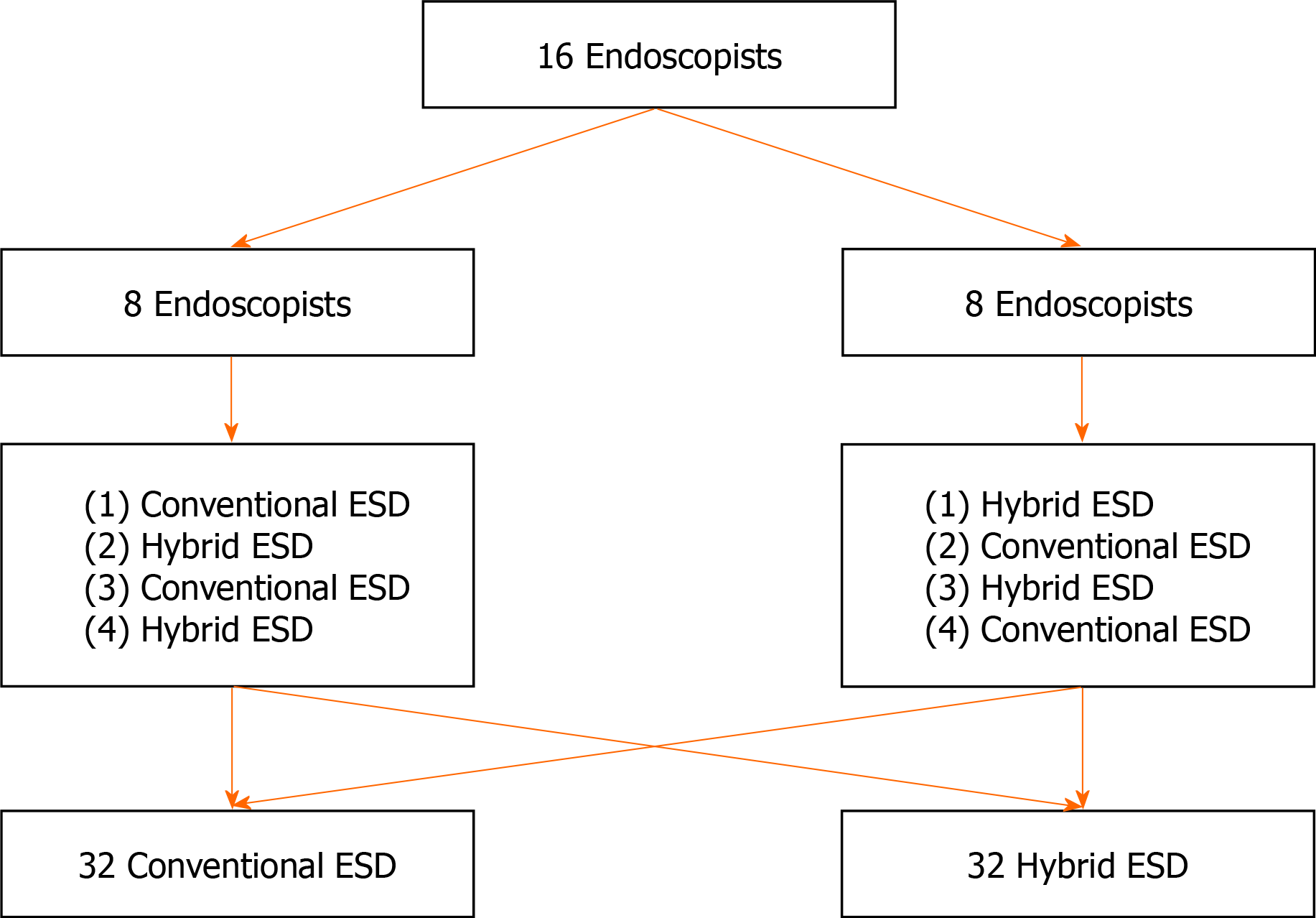
Sixteen endoscopists participated in this basic study in August 2020 at Kyushu University, performing 32 procedures each for hybrid ESD and conventional ESD. Mock lesions (10-15 mm, diameter) were created in the porcine stomach. The primary outcome was total procedure time and secondary outcomes were en bloc or complete resection, perforation, procedure time/speed for both, mucosal incision, and submucosal dissection. Factors associated with difficulty in ESD including longer procedure time, incomplete resection, and perforation, were also investigated. Categorical and continuous data were analyzed using the chi-square test or Fisher’s exact test and the Mann-Whitney U test, respectively.
The median total procedure time of hybrid ESD was significantly shorter than that of conventional ESD (median: 8.3 min vs 16.2 min, P < 0.001). Time, speed, and the amount of hyaluronic acid during submucosal dissection were more favorable in hybrid ESD than conventional ESD (time, 5.2 min vs 10.4 min, P < 0.001; speed, 43.7 mm2/min vs 23.8 mm2/min, P < 0.00; injection volume, 1.5 mL vs 3.0 mL, P < 0.001), although no significant differences in those factors were observed between both groups during mucosal incision. There was also no significant difference between both groups in the en bloc/complete resection rate and perforation rate (complete resection, 93.8% vs 87.5%, P = 0.67; perforation, 0% vs 3.1%, P = 1). Selection of conventional ESD as the treatment method was significantly associated with difficulties during ESD (odds ratio = 10.2; highest among factors).
Hybrid ESD with SOUTEN improves the treatment outcomes of gastric lesions. It also has the potential to reduce medical costs since SOUTEN is a single multifunctional device that is inexpensive.
Core Tip: Hybrid endoscopic submucosal dissection (ESD) is an intermediate technique between endoscopic mucosal resection (EMR) and ESD, which has been developed to overcome the curative barrier of EMR and the technical barrier of ESD in treating gastrointestinal neoplasms. We conducted an ex vivo porcine model basic study to determine the superiority of hybrid ESD with SOUTEN over conventional ESD in treating gastric lesions. The 32 outcomes of each hybrid ESD and conventional ESD were compared. Hybrid ESD had a significantly shorter total procedure time, favorable curability, and low complication rates, compared to conventional ESD. Hybrid ESD with SOUTEN could reduce the costs of ESD.
- Citation: Esaki M, Ihara E, Hashimoto N, Abe S, Aratono C, Shiga N, Sumida Y, Fujii H, Haraguchi K, Takahashi S, Iwasa T, Nakano K, Wada M, Somada S, Nishioka K, Minoda Y, Ogino H, Ogawa Y. Efficacy of hybrid endoscopic submucosal dissection with SOUTEN in gastric lesions: An ex vivo porcine model basic study. World J Gastrointest Surg 2021; 13(6): 563-573
- URL: https://www.wjgnet.com/1948-9366/full/v13/i6/563.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i6.563
Therapeutic endoscopic resection (ER) has been widely accepted as a local treatment for early gastric neoplasms, with negligible risk of lymph node metastasis[1]. ER is less invasive than surgery and preserves the patient’s quality of life after treatment[2,3]. Endoscopic mucosal resection (EMR) was the initial ER method employed; however, it had limitations, such as poor histological assessment and high risk of local recurrence due to piecemeal resection of large or ulcerative lesions[4]. Subsequently, an advanced ER method, endoscopic submucosal dissection (ESD), was developed and this allowed en bloc resection even for large or ulcerative lesions[5]. Previous comparison studies and meta-analysis that evaluated EMR and ESD, preferred ESD over EMR for en bloc resection, complete resection, and curative resection. Although less local recurrence was reported with ESD, the procedure was more complicated and took a longer time owing to its technical complexity[4,6]. According to the largest cohort study of gastric endoscopic resection in Japan, ESD was indeed performed in more than 99% of patients with gastric neoplasms treated with ER in spite of its technical complexity, because ESD has a higher cure rate compared to EMR[7].
In order to overcome both the curative barrier of piecemeal resection in EMR and the technical barrier of technical complexity in ESD, a more advanced technique, termed hybrid ESD, was recently developed and was initially meant for the resection of colorectal neoplasms[8]. Hybrid ESD is characterized by a combination of the ESD procedure with snaring resection, where partial submucosal dissection is followed by snaring resection in a planned manner. A meta-analysis reported that hybrid ESD had a shorter procedure time, fewer adverse events, but similar recurrence rates compared to conventional ESD in ER of colorectal neoplasms, although it was associated with a lower rate of en bloc resection[9]. Furthermore, a recently developed single multifunctional device (SOUTEN; Kaneka Medics, Tokyo, Japan) has enabled us to conduct hybrid ESD at lower cost with a single device than with conventional ESD[10,11]. Recently, we conducted a retrospective study, and showed that hybrid ESD with SOUTEN has shorter total procedure time than conventional ESD in ER for gastric neoplasms, and this finding was consistent with those of other previously reported studies on colonic neoplasms[8,12]. However, the real superiority of hybrid ESD with SOUTEN over conventional ESD remains to be determined since the previously conducted clinical studies had a limitation that SOUTEN was used only in hybrid the ESD group and not in the conventional ESD group where other common endoscopic knives, such as Dual knife, Flush knife and Splash-M knife were used, due to the ethical issues. Therefore, we conducted this ex vivo porcine model basic study to determine whether, and if so how, hybrid ESD with SOUTEN was actually superior to conventional ESD.
In this experimental study, the treatment outcomes between hybrid ESD and conventional ESD, using ex vivo porcine gastric models, were compared. The study was conducted by 16 endoscopists, in August 2020, at Kyushu University. Each endoscopist performed a total of four ESDs; two hybrid ESDs and two conventional ESDs. The schedule of ESD for each endoscopist was shown in Figure 1. The approval of the Institutional Review Board was waived because this was a basic study. All endoscopists participating in this study provided informed written consent prior to study enrollment. This study was conducted in accordance with the guidelines of the Animal Research Reporting In Vivo Experiments and we adhered to the guidelines as much as possible, although this was an ex vivo animal model study using porcine stomach originally harvested for food. This study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry.
Domestic porcine, harvested for food, were slaughtered, before the ESD procedure, in Fukuoka Meat Wholesale Market Corp. Resected porcine stomach with the esophagus was transported to the endoscopic training room in Kyushu University (Figure 2A). The inside of the porcine stomach and esophagus was sufficiently rinsed in a water. An overtube (MD-48518, Sumitomo Bakelite, Tokyo, Japan) was inserted into the esophagus, and the pylorus of the stomach was tied. The stomach with the esophagus was set and fixed to a training kit for the endoscopic procedure (Figure 2B). Mock lesions, 10-15 mm in diameter, were made in the stomach by marking dots with an endo-knife.
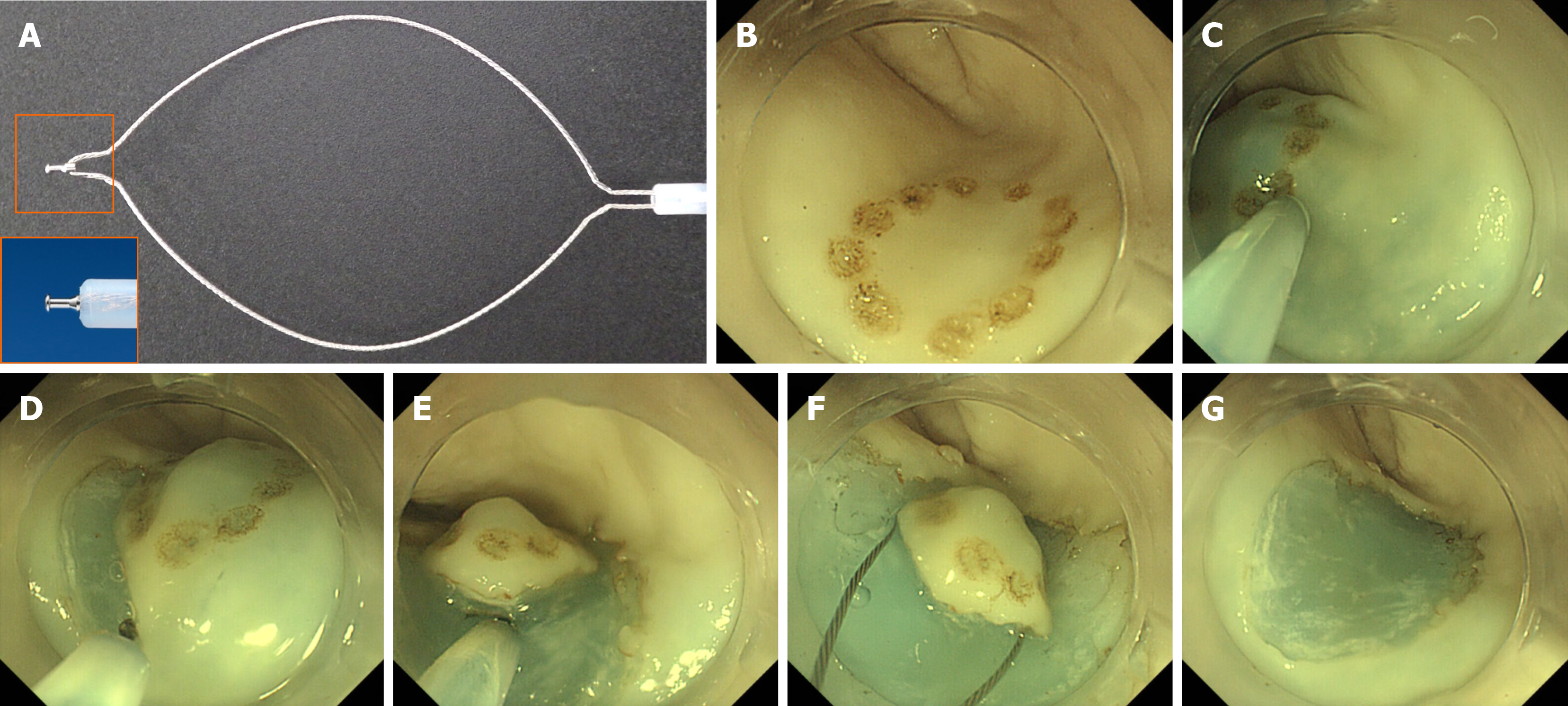
An upper gastrointestinal endoscope (GIF-Q260J, Olympus, Tokyo, Japan) with distal attachments (D-201-11804; Olympus) was used in both hybrid ESD and conventional ESD. ESG-100 (Olympus) was used as an electrical power unit. A multifunctional endoscopic device, SOUTEN was used for all endoscopic procedures in both groups (Figure 3A). An injection, using a needle (TOP Corp., Tokyo, Japan), was used to administer hyaluronic acid in the submucosal layer.
The details of the ESD procedure are described elsewhere[5,13-16]. Marking dots, to identify mock lesions, were made via coagulation using a distal needle-tip of SOUTEN; for marking, the electric power source was set at 40 W in the forced coagulation 1 mode (Figure 3B). Hyaluronic acid was injected into the submucosa around the mock lesion (Figure 3C). After confirming that the lesion had been lifted, a circumferential mucosal incision was made using the distal needle-tip of SOUTEN (Figure 3D). During mucosal incision, the electric power source was set at 60 W in the pulse cut first mode or 40 W in the pure cut mode.
Subsequently, the submucosal layer was dissected using the distal needle-tip of the SOUTEN (Figure 3E); for submucosal dissection, the electric power source was set at 60 W in the forced coagulation 2 mode or 40 W in the pure cut mode.
In the procedure of conventional ESD, submucosal dissection was continued until the lesion was retrieved. In contrast, in the procedure of the hybrid ESD, snaring was performed in a planned manner after adequate submucosal dissection; based on the operator’s judgment when the lesion could be resected en bloc with snaring (Figure 3F and G). In both procedures, submucosal injection was added when needed. Regarding snaring for the target lesion, the electric power source was set at 60 W in forced coagulation 2 followed by 40 W in the pure cut mode. The resected specimen was flattened on a plastic plate, and its length (mm) was measured along its long and short axes with a ruler.
The primary outcome was the total of ESD procedure time beginning from the commencement of mucosal incision to the complete retrieval of the lesion. Secondary outcomes were procedure time and speed of each step, including mucosal incision, submucosal dissection, en bloc resection and complete resection, intraoperative perforation, and the volume of solution injected during the total procedure, mucosal incision and submucosal dissection. The factors associated with difficulty in ESD, including long procedure time, incomplete resection, and perforation, were also investigated. Location of the lesion (upper/middle third in the stomach or lower), position of the lesion (lesser curvature of the stomach wall or others), resected specimen size (≥ 250 mm2 or < 250 mm2), operator skill (expert or trainee), and the ESD method used (hybrid ESD or conventional ESD) were included among the investigated factors.
Total procedure time was defined as the time from the start of mucosal incision to the completion of lesion retrieval. En bloc resection was defined as non-piecemeal resection of the lesion. Complete resection was defined as en bloc resection with all marking dots in the resected specimen. Intraoperative perforation was defined as the occurrence of an immediately recognizable hole in the stomach wall. The circumferential length (mm) and resected area (mm2) were calculated using the long and short axes of resected specimens. The speed of mucosal incision was defined as “the circumferential length of the resected specimen/incision time” (mm/min). The speed of submucosal dissection was defined as “the area of resected specimen/dissection time” (mm2/min). Difficulty of ESD was defined as a procedure with a long procedure time of ≥ 20 min, incomplete resection, or perforation. Location and position of the lesion was classified according to the current Japanese classification of gastric carcinoma; location (upper, middle, and lower thirds of the stomach); position (lesser curvature, greater curvature, anterior wall, and posterior wall)[17]. With regard to the operator skill, an expert was defined as an operator with an experience of ≥ 50 ESD cases[18,19], while a trainee was defined as an operator with an experience of < 50 ESD cases.
Categorical data are described as frequencies with percentages and analysis of the two groups was done using the chi-square test or Fisher’s exact test. Continuous data are described as median with interquartile range and analyzed of both groups was performed using the Mann-Whitney U test. The factors associated with difficulty during ESD were investigated by univariate and multivariate logistic regression analyses. Values of P < 0.05 were considered significant. All statistical analyses were performed using the JMP Pro version 15.0 software (SAS, Cary, NC, 2019).
A total of 64 lesions were treated with ESD (32 Lesions each with hybrid ESD and conventional ESD). Baseline characteristics of enrolled lesions are shown in Table 1. The proportion not only of upper or middle third in location but also of lesser curvature in position was not significantly different between the two groups. The proportion of trainees to experts in each procedure of treatment was also not different between the two groups.
| Hybrid ESD (n = 32) | Conventional ESD (n = 32) | P value | |
| Location, n (%) | 1 | ||
| Upper or middle third | 15 (46.8) | 15 (46.9) | |
| Lower third | 17 (53.1) | 17 (53.1) | |
| Position, n (%) | 1 | ||
| Lessor curvature | 16 (50) | 16 (50) | |
| Others | 16 (50) | 16 (50) | |
| Operator skills | 1 | ||
| Trainee | 16 (50) | 16 (50) | |
| Expert | 16 (50) | 16 (50) |
Comparison of treatment outcomes between the two groups are shown in Table 2. Hybrid ESD had a significant shorter total procedure time than conventional ESD [8.3 (6.6-12.0) min vs 16.2 (9.5-20.1) min, P < 0.001]. Although there was no significant difference in the time for mucosal incision between hybrid ESD and conventional ESD [3.5 (2.5-5.5) min vs 4.2 (2.7-5.3) min, P = 0.82], hybrid ESD had a shorter time for submucosal dissection than conventional ESD [5.2 (3.7-6.6) min vs 10.4 (7.0-15.1) min, P < 0.001]. The en bloc resection rate was 100% in both groups. There was no significant difference in the complete resection rate and perforation rate between hybrid ESD and conventional ESD (93.8% vs 87.5%, P = 0.67; 0.0% vs 3.1%, P = 1.0, respectively). Hybrid ESD had a significant higher speed for submucosal dissection than conventional ESD [43.7 (34.6-64.6) mm2/min vs 23.8 (17.0-30.1) mm2/min, P < 0.001], although no difference in the mucosal incision speed was observed. The injection volumes in the total procedure and submucosal dissection were significantly less in hybrid ESD group than in conventional ESD group [total procedure; 8.3 (6.6-12.0) mL vs 16.2 (9.5-20.1) mL, P < 0.001, submucosal dissection; 1.5 (1.0-2.1) mL vs 3.0 (2.4-5.6) mL, P < 0.001), although no significant difference in mucosal incision was observed.
| Hybrid ESD (n = 32) | Conventional ESD (n = 32) | P value | |
| ESD procedure time, min | |||
| Median (IQR) | 8.3 (6.6-12.0) | 16.2 (9.5-20.1) | < 0.001a |
| En bloc resection, n (%) | 32 (100) | 32 (100) | |
| Complete resection, n (%) | 30 (93.8) | 28 (87.5) | 0.67 |
| Perforation, n (%) | 0 (0) | 1 (3.1) | 1 |
| Mucosal incision time, min | |||
| Median (IQR) | 3.5 (2.5-5.5) | 4.2 (2.7-5.3) | 0.82 |
| Submucosal dissection time, min | |||
| Median (IQR) | 5.2 (3.7-6.6) | 10.4 (7.0-15.1) | < 0.001a |
| Resected specimen size, mm2 | |||
| Median (IQR) | 254.3 (201-282.6) | 247.3 (201.0-254.3) | 0.44 |
| Circumferential length, mm | |||
| Median (IQR) | 56.5 (50.3-59.7) | 55.8 (50.3-56.5) | 0.49 |
| Mucosal incision speed, mm/min | |||
| Median (IQR) | 15.3 (10.8-20.1) | 13.0 (10.0-20.3) | 0.67 |
| Submucosal dissection speed, mm2/min | |||
| Median (IQR) | 43.7 (34.6-64.6) | 23.8 (17.0-30.1) | < 0.001a |
| Total injection volume, mL | |||
| Median (IQR) | 8.3 (6.6-12.0) | 16.2 (9.5-20.1) | < 0.001a |
| Injection volume during mucosal incision, mL | |||
| Median (IQR) | 5.0 (4.5-7.0) | 6.0 (4.8-7.6) | 0.40 |
| Injection volume during submucosal dissection, mL | |||
| Median (IQR) | 1.5 (1.0-2.1) | 3.0 (2.4-5.6) | < 0.001a |
Results of the univariate and multivariate analyses of the factors associated with difficulty during ESD are summarized in Table 3. In the multivariate analysis, lesions located in the upper or middle third of the stomach [odds ratio (OR): 9.9], operators that are still trainee (OR: 6.2), and conventional ESD in the treatment method (OR: 10.1) were independent factors associated with difficulty during ESD.
| No. of patients | No. of events | Univariate | Multivariate | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | |||
| Location | ||||||||
| L | 34 | 6 | 1 | Ref | 0.25 | 1 | Ref | 0.72 |
| U or M | 30 | 9 | 2.0 | 0.62-6.5 | 1.32 | 0.28-6.3 | ||
| Position | ||||||||
| G or A or P | 32 | 3 | 1 | Ref | 0.01a | 1 | Ref | 0.004a |
| Lessor | 32 | 12 | 5.8 | 1.4-23.2 | 9.9 | 2.0-71.4 | ||
| Size | ||||||||
| < 250 mm2 | 31 | 6 | 1 | Ref | 0.45 | 1 | Ref | 0.33 |
| ≥ 250 mm2 | 33 | 9 | 1.6 | 0.48-5.1 | 2.1 | 0.48-10.1 | ||
| Operator skill | ||||||||
| Expert | 32 | 4 | 1 | Ref | 0.05 | 1 | Ref | 0.015a |
| Trainee | 32 | 11 | 3.7 | 1.0-13.1 | 6.2 | 1.4-37.7 | ||
| Method | ||||||||
| Hybrid ESD | 32 | 3 | 1 | Ref | 0.01a | 1 | Ref | 0.002a |
| Conventional ESD | 32 | 12 | 5.8 | 1.4-23.2 | 10.1 | 2.2-67.5 | ||
This is the first study that used an ex vivo porcine model to demonstrate the superiority of hybrid ESD with SOUTEN, a single multifunctional device, over the conventional ESD in terms of treatment outcomes of gastric ESD. We found that hybrid ESD with SOUTEN had significantly shorter total procedure time compared to conventional ESD with favorable curability and low perforation rate; this finding was similar to the findings of previously conducted clinical studies.
Hybrid ESD is an intermediate technique between EMR and ESD; mucosal incision and partial submucosal dissection are performed as in ESD, followed by final snaring in a planned manner similar to that of EMR. During the procedure, the mucosa is incised outside of the markings and the submucosa is partially dissected, this enables subsequent snaring in order to achieve en bloc resection. Indeed, snaring replaces submucosal dissection and tumor resection can be done in less time; this reduces the total procedure time. Therefore, hybrid ESD is expected to achieve not only higher curability than EMR but also a shorter procedure time than ESD.
Initially, hybrid ESD was used unintentionally as a rescue treatment in cases of ESD with technical difficulties[2,20]. A previous study indicated that most lesions that were smaller than 2 cm could be removed in en bloc fashion by rescue hybrid ESD. However, rescue hybrid ESD did not contribute reducing the procedure time because of its unintentional applications in cases of ESD with technical difficulties. Subsequently, hybrid ESD with SOUTEN in a planned manner has been used to treat gastric neoplasms[2]. A retrospective study with propensity score matching analysis showed that hybrid ESD with SOUTEN had a shorter procedure time, favorable curability, and low complication rate compared to conventional ESD for ER of gastric neoplasms that are smaller than 20 mm. Currently, a prospective randomized controlled trial to confirm the superiority of hybrid ESD with SOUTEN over conventional ESD, with regard to procedure time, is ongoing (UMIN000041244). However, in these clinical studies, SOUTEN was used only in the hybrid ESD group while other common endoscopic knives, such as Dual knife, Flush knife, and Splash-M knife were used in the conventional ESD group. This was because SOUTEN could not be applied to conventional ESD due to ethical issue in clinical practice. That is why we conducted this basic study to determine whether and how hybrid ESD with SOUTEN was actually superior to conventional ESD with SOUTEN.
As for a primary outcome, the total procedure time of hybrid ESD was notably shorter than that of conventional ESD; the time and speed of submucosal dissection of hybrid ESD were shorter and higher than those of conventional ESD, respectively, while those of mucosal incision were not significantly different between the two groups. Indeed, the outcomes of mucosal incision were reasonably quite similar in both groups, due to the usage of the same device (SOUTEN) in this basic study. In contrast, the outcomes of submucosal dissection of hybrid ESD were also reasonably better than those of conventional ESD due to snaring of the submucosal layer during dissection. This contributed to reducing the total procedure time in hybrid ESD. Moreover, according to the multivariate analysis, selection of conventional ESD as a treatment method was one of the independent factors associated with difficulty during ESD, with the highest OR.
Previous studies that compared the efficacy of EMR and that of ESD have shown that EMR has a lower rate of en bloc resection than ESD especially for the lesions larger than 10 mm[4,21]. Although there were concerns about the curability of hybrid ESD for lesion that were 10-15 mm in size, hybrid ESD achieved 100% en bloc resection and 93.8% complete resection, which were similar to what was obtainable with conventional ESD. This result was consistent with that of previous clinical studies on hybrid ESD and the treatment of gastric lesions[12,20]. Circumferential mucosal incision, followed by partial mucosal dissection before snaring in a planned manner improves curability using hybrid ESD.
One limitation of hybrid ESD is that it usually requires two endoscopic devices: One for mucosal incision/submucosal dissection and the other for snaring. Since SOUTEN is an inexpensive single multifunctional device originally designed for hybrid ESD, it could be used to perform all steps associated with ESD except submucosal injection. Similar to other commonly used endoscopic knives, SOUTEN has a knife with a distal tip at the top of the snare that is suitable for mucosal incision and submucosal dissection[13,14]. Therefore, a single SOUTEN device enables us to conduct hybrid ESD at a lower cost not only compared to hybrid ESD with two endoscopic devices, but also to conventional ESD with a single, commonly used endoscopic knife. Therefore, hybrid ESD with SOUTEN will contribute to reduction in the total medical cost for the local treatment of gastric neoplasms.
There are several limitations to this study. First, this was an ex vivo porcine model basic study that used an isolated stomach without any blood flow. An in vivo porcine model basic study and clinical study in human should be conducted in future. Second, the subjects were not randomized in this study. There remains a confounding bias although multivariate logistic regression analyses were performed to reduce the bias. As a result, selection of conventional ESD as the treatment method was revealed to be associated with difficulties during the procedures. Third, lesions were limited to those that were 10-15 mm in size without ulceration. Furthermore, the depth of the resected specimens was not investigated. The findings of this study are not applicable to large lesions, or those lesions with ulcers and/or deep invasion. Fourth, both procedures of hybrid ESD and conventional ESD were performed with SOUTEN. It remains to be determined whether the curative efficacy for early gastric neoplasms using hybrid ESD with SOUTEN is equivalent to conventional ESD using conventional endoscopic knives in clinical practice. We are expecting the result of a prospective randomized controlled trial currently conducted to confirm the superiority of hybrid ESD with SOUTEN over conventional ESD with conventional endoscopic needle-knives for gastric neoplasms in clinical practice.
Hybrid ESD with SOUTEN, a single multifunctional device, has a significantly shorter total procedure time, with favorable curability, and a low complication rate compared to conventional ESD. Furthermore, hybrid ESD with SOUTEN could reduce the costs of ESD since it uses a single multifunctional device, and is less expensive compared to other commonly used endoscopic knives. Based on this basic study, randomized controlled trials in humans on the use of SOUTEN in hybrid ESD for gastric neoplasms are warranted.
Hybrid endoscopic submucosal dissection (ESD) has been developed as an advanced technique combining conventional ESD and endoscopic mucosal resection procedures. A multifunctional device called SOUTEN has been invented for hybrid ESD procedures.
It is unclear whether hybrid ESD using SOUTEN is superior to conventional ESD for gastric lesions.
This study aimed to determine whether and how hybrid ESD is superior to conventional ESD in the endoscopic treatment of gastric lesions in an ex vivo porcine model basic study.
A total of 32 ESD on mock lesions in an ex vivo porcine stomach model were performed using SOUTEN; 16 hybrid ESD and 16 conventional ESD. Technical outcomes were compared between the two groups.
Hybrid ESD achieved significantly shorter procedure time than conventional ESD, with favorable curability, and a low complication rate. The selection of conventional ESD as the treatment method was significantly associated with the difficulties during ESD on multivariate analysis.
Hybrid ESD with SOUTEN improves the technical outcomes of ESD for gastric lesions.
A randomized controlled trial in humans is expected to be conducted to confirm that hybrid ESD using SOUTEN is superior to the conventional ESD in the future.
The authors thank all members of the Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University for assisting with data collection. The authors thank Junji Kishimoto (Center for Clinical and Translational Research at Kyushu University Hospital) for reviewing the statistical methods of this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moradi L, Xie HP S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 277] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Ahmed O, Kim YJ, Patel MV, Tullius TG Jr, Navuluri R, Funaki B, Van Ha T. A Single-Institutional Comparative Analysis of Advanced Versus Standard Snare Removal of Inferior Vena Cava Filters. J Vasc Interv Radiol 2020; 31: 53-60. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Tae CH, Shim KN, Kim BW, Kim JH, Hong SJ, Baik GH, Song HJ, Kim YS, Jang SH, Jung HK. Comparison of subjective quality of life after endoscopic submucosal resection or surgery for early gastric cancer. Sci Rep. 2020;10:6680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, Wada T, Kubota E, Yamada T, Mori Y, Fujita F, Nakao H, Ohara H, Inukai M, Kasugai K, Joh T. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1162] [Article Influence: 46.5] [Reference Citation Analysis (5)] |
| 6. | Tao M, Zhou X, Hu M, Pan J. Endoscopic submucosal dissection vs endoscopic mucosal resection for patients with early gastric cancer: a meta-analysis. BMJ Open. 2019;9:e025803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Suzuki H, Takizawa K, Hirasawa T, Takeuchi Y, Ishido K, Hoteya S, Yano T, Tanaka S, Endo M, Nakagawa M, Toyonaga T, Doyama H, Hirasawa K, Matsuda M, Yamamoto H, Fujishiro M, Hashimoto S, Maeda Y, Oyama T, Takenaka R, Yamamoto Y, Naito Y, Michida T, Kobayashi N, Kawahara Y, Hirano M, Jin M, Hori S, Niwa Y, Hikichi T, Shimazu T, Ono H, Tanabe S, Kondo H, Iishi H, Ninomiya M; Ichiro Oda for J-WEB/EGC group. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: 'Real-world evidence' in Japan. Dig Endosc. 2019;31:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 8. | Bae JH, Yang DH, Lee S, Soh JS, Lee HS, Lee HJ, Park SH, Kim KJ, Ye BD, Myung SJ, Yang SK, Byeon JS. Optimized hybrid endoscopic submucosal dissection for colorectal tumors: a randomized controlled trial. Gastrointest Endosc. 2016;83:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | McCarty TR, Bazarbashi AN, Thompson CC, Aihara H. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Arimoto J, Ohata K, Chiba H, Tachikawa J, Okada N, Kuwabara H, Nakaoka M, Ashikari K, Ishii R, Minato Y, Takita M, Sakai E, Muramoto T, Matsuhashi N, Goto T, Nakajima A. Evaluation of colorectal endoscopic submucosal dissection using a multifunctional snare: a prospective clinical feasibility study (with videos). Gastrointest Endosc. 2021;93:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Ohata K, Muramoto T, Minato Y, Chiba H, Sakai E, Matsuhashi N. Usefulness of a multifunctional snare designed for colorectal hybrid endoscopic submucosal dissection (with video). Endosc Int Open. 2018;6:E249-E253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Esaki M, Suzuki S, Horii T, Ichijima R, Yamakawa S, Shibuya H, Kusano C, Ikehara H, Gotoda T. Reduction in the procedure time of hybrid endoscopic submucosal dissection for early gastric neoplasms: a multi-center retrospective propensity score-matched analysis. Therap Adv Gastroenterol. 2020;13:1756284820939420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Toyonaga T, Man-I M, Fujita T, Nishino E, Ono W, Morita Y, Sanuki T, Masuda A, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther. 2010;32:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Esaki M, Suzuki S, Hayashi Y, Yokoyama A, Abe S, Hosokawa T, Ogino H, Akiho H, Ihara E, Ogawa Y. Splash M-knife vs Flush Knife BT in the technical outcomes of endoscopic submucosal dissection for early gastric cancer: a propensity score matching analysis. BMC Gastroenterol. 2018;18:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Sakaguchi Y, Tsuji Y, Fujishiro M, Kataoka Y, Saito I, Shichijo S, Minatsuki C, Asada-Hirayama I, Yamaguchi D, Niimi K, Ono S, Kodashima S, Yamamichi N, Koike K. Evaluation of endoscopic submucosal dissection using a new endosurgical knife DN-D2718B: a first clinical feasibility study. Endosc Int Open. 2017;5:E670-E674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Fujishiro M, Sugita N. Animal feasibility study of an innovated splash-needle for endoscopic submucosal dissection in the upper gastrointestinal tract. Dig Endosc. 2013;25:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2943] [Article Influence: 196.2] [Reference Citation Analysis (0)] |
| 18. | Hong KH, Shin SJ, Kim JH. Learning curve for endoscopic submucosal dissection of gastric neoplasms. Eur J Gastroenterol Hepatol. 2014;26:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, Bae JM. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc. 2005;62:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (4)] |
| 20. | Goto O, Fujishiro M, Kodashima S, Kakushima N, Ono S, Yahagi N, Omata M. Feasibility of electrocautery snaring as the final step of endoscopic submucosal dissection for stomach epithelial neoplasms. Dig Liver Dis. 2009;41:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 295] [Article Influence: 19.7] [Reference Citation Analysis (0)] |