Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1736
Peer-review started: April 19, 2021
First decision: July 27, 2021
Revised: August 11, 2021
Accepted: November 18, 2021
Article in press: November 18, 2021
Published online: December 27, 2021
Processing time: 249 Days and 0.8 Hours
Anastomotic leak constitutes a major problem in abdominal surgery. Technical insufficiency, topical or systemic factors contribute to disrupted healing of the performed bowel anastomosis and result in anastomosis leakage, with detrimental effects on patient postoperative outcomes. Despite the investigation of several factors and the invention of protective materials, the ideal agent to prevent anastomotic leaks is yet to be determined.
To study the effect of platelet rich plasma (PRP) on the healing of bowel ana
A systematic literature search was performed in PubMed, EMBASE, and Scopus databases to identify studies investigating the effect of PRP application on bowel anastomosis.
Eighteen studies were eligible with a total population of 712 animals including rats (14 studies), rabbits (2 studies) and pigs (2 studies). No postoperative complications were reported following PRP application. Fourteen out of 18 studies reported a statistically significant higher anastomosis bursting pressure in PRP groups compared to control either in healthy animals or animal models with underlying condition or intervention, such as intraperitoneal chemotherapy or peritonitis. Similar results were reported by ten studies in terms of tissue hydroxyproline levels. One study reported significant increase in collagen deposition in PRP groups. PRP application resulted in significantly decreased inflammatory cell infiltration in the presence of peritonitis or intraperitoneal chemotherapy (6 studies).
The application of PRP is associated with improved bowel anastomosis outcomes, especially in animal models having an underlying condition affecting the normal healing process. PRP application seems to augment the normal healing process under these circumstances. However, further studies are needed to investigate the potential role of PRP on bowel anastomosis healing, especially in clinical settings.
Core Tip: The positive effect of platelet rich plasma (PRP) in bowel anastomoses has been shown by several studies. The application of PRP in bowel anastomoses in the presence of impaired wound healing conditions like ischemia, infection or chemotherapy significantly improved anastomosis burst pressure and tissue hydroxyproline, two of the most common used parameters to test anastomosis integrity. The current literature supports the effectiveness of PRP in animal models. Further studies are needed in order to determine the potential role of PRP in clinical practice.
- Citation: Geropoulos G, Psarras K, Giannis D, Martzivanou EC, Papaioannou M, Kakos CD, Pavlidis ET, Symeonidis N, Koliakos G, Pavlidis TE. Platelet rich plasma effectiveness in bowel anastomoses: A systematic review. World J Gastrointest Surg 2021; 13(12): 1736-1753
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1736.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1736
Bowel anastomosis related complications are frequently encountered in patients undergoing major abdominal surgery involving bowel excision. Anastomotic leak seems to be the most common complication and its rate is approximately 10% in operations involving bowel anastomosis[1-4]. However, in the presence of an underlying condition, such as malignancy or intraperitoneal hyperthermic chemotherapy, an anastomotic leak may occur in up to 25% of the cases[5-7]. Multiple factors have been previously investigated and have been proven to affect the integrity of bowel anastomosis. Advanced age, sepsis, hypoalbuminemia, low hematocrit, immunosuppression, diabetes mellitus, and reduced blood supply are systemic factors that may negatively affect anastomotic healing[8,9]. In addition, topical factors, including suturing technique, anastomotic tension, bowel infection, fecal contamination and peritonitis, could also result in delayed healing and increase the rate of anastomotic leak[10].
Several topical mechanical and pharmaceutic agent applied to bowel anastomosis have been reported in the literature, demonstrating variable effects in the healing process of anastomoses. The vast majority of these agents have been tested in experimental animal (mainly rat) models. However, very few agents were applied to the clinical practice[9].
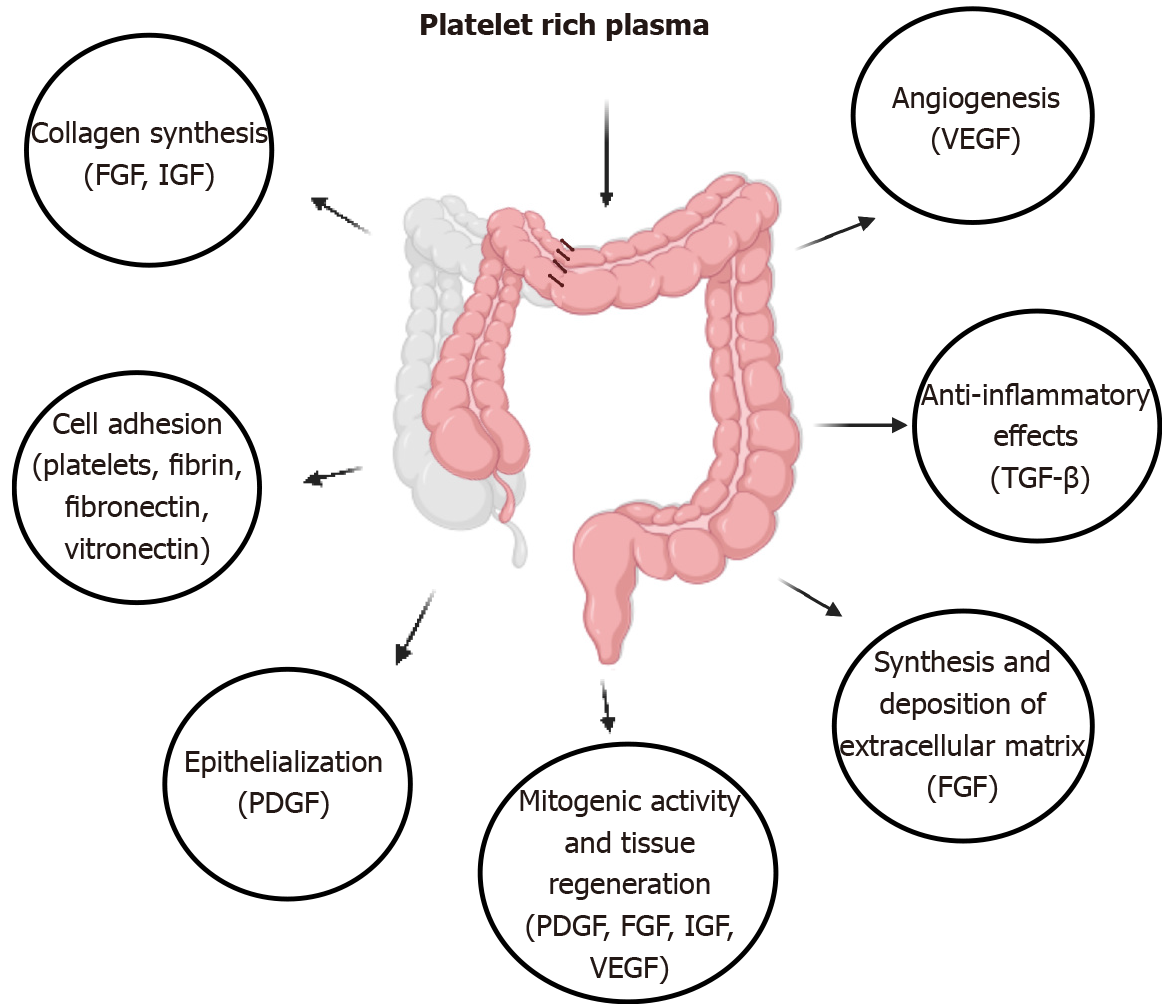
Platelet rich plasma (PRP) is widely used in maxillofacial reconstructive surgery, orthopedic surgery, plastic surgery, and diabetic skin ulcers with highly acceptable effects in terms of improved wound healing and tissue regeneration[11-13]. PRP is easily extracted from a small amount of peripheral blood and its production roughly requires a two-step centrifugation or even a one-step centrifugation technique[14]. The effects of PRP are mainly attributed to its endogenous concentration of growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor, and insulin-like growth factor (IGF)[15]. Furthermore, inflammatory biomolecules like interleukin (IL)-1β, IL-6 and IL-4 have been also reported in the PRP biochemical analysis[16].
The synergic effect of these factors modulates and/or augments angiogenesis, cell mitosis and extracellular matrix remodeling, which are processes involved in normal wound healing[17-19].
The aim of this study is to systematically review the current literature on the effects of PRP application on bowel anastomosis.
This systematic review was performed according to the PRISMA guidance[20] after approval of the study protocol by all authors. A comprehensive literature search (last search date as of October 1, 2020) was performed by two researchers (Kakos CD and Martzivanou EC) in PubMed (Medline), EMBASE, and Scopus. The search term included several combinations of “platelet rich plasma”, “PRP”, “colon” and “anastomosis” keywords (Supplementary Table 1). A manual search was also performed using the snowball methodology to identify any relevant studies in the list of references of the included articles[21].
Our systematic review included retrospective animal studies that investigated the effect of PRP on bowel anastomosis. There was no restriction regarding the animal models that were used and these included healthy animals as well as animals with peritonitis or undergoing intraperitoneal chemotherapy. Studies were excluded based on the following criteria: (1) Non-available full texts; (2) Non-peer reviewed publications, including theses, conference papers, and book chapters; (3) Non-original studies, such as systematic reviews and narrative reviews; (4) Studies with non-extractable data; and (5) Studies with overlapping or duplicated data.
A data extraction template was created and modified based on an initial pilot testing. Three investigators (Kakos CD, Martzivanou EC and Geropoulos G) independently identified and extracted the variables of interest. Extracted variables included study details (author, year, country, study type), animal type, underlying animal condition, study subgroups, origin of PRP, preparation method of PRP, dose of PRP, PRP application technique, type of anastomosis, interval between PRP application and animal sacrifice, postoperative complications, postoperative outcomes (bursting pressure, hydroxyproline levels, adhesions) and histopathology results (inflammatory cell infiltration, necrosis, angiogenesis, edema, collagen deposition, fibrosis, fibroblast count, anastomotic epithelialization, granulation). Any discrepancies between the results of extraction were discussed and resolved, while a fourth investigator (Giannis D) was consulted if needed.
The risk of bias of included studies was evaluated with the Systematic Review Centre for Laboratory animal Experimentation risk of bias tool (SYRCLE's RoB tool)[22]. The quality assessment tool is based on the Cochrane Risk Of Bias tool, but it is adjusted to estimate the risk of bias in animal/preclinical studies. Each question is answered as “yes” (low risk of bias), “no” (high risk of bias), or “unknown” (unknown/unclear risk of bias). Two authors (Martzivanou EC and Geropoulos G) independently assessed the 10 components of the SYRCLE's RoB tool. Any conflicts were resolved by discussion with a third investigator (Giannis D).
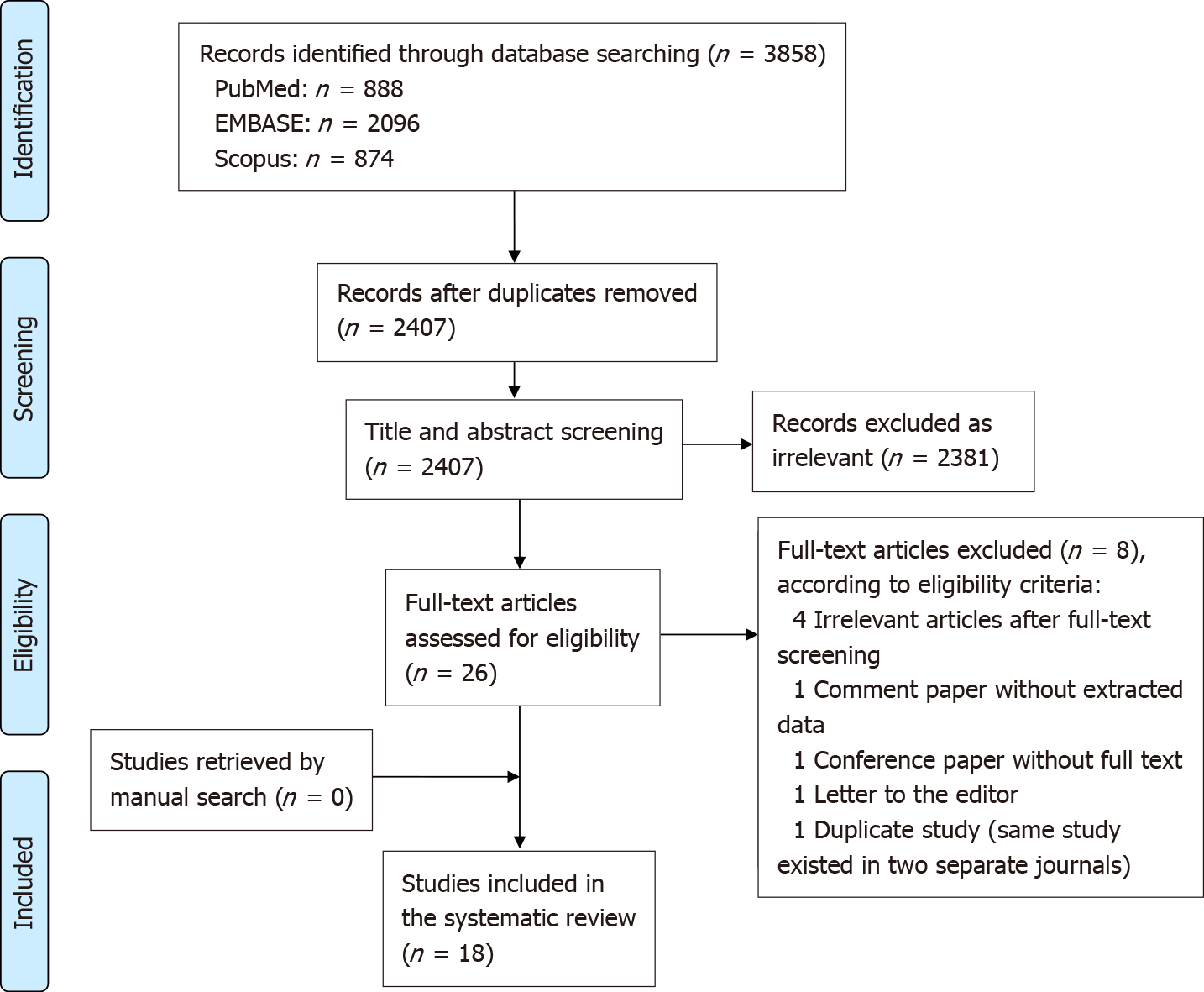
Among the 3858 studies that were identified, 2407 were screened after removal of duplicates, through the use of Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia) and manual screening of titles and abstracts[23]. According to the predefined inclusion and exclusion criteria, 26 studies were selected for full text screening. Eventually, eight studies were excluded (four studies not describing the effect of PRP on bowel anastomosis, one duplicate study published in two different journals[24,25], one comment paper, one conference paper and one letter to the editor without extractable data. After manual literature search in the references of the eligible studies, which did not provide any additional eligible studies, 18 studies were finally included in this systematic review (Figure 1). Two out of the 18 included studies investigated the effect of platelet rich fibrin (PRF), which is similar to PRP in terms of high platelet concentration. However, PRF is rich in fibrin, which is thought to trap platelets and ease the application around the anastomotic surface[16,26] (Table 1).
| Ref. | Country | Animal model (race) | Sample size (n) | Number of groups (n) | Animal per group (n) | Day at animal sacrification | Underlying animal condition that PRP was tested | PRP amount in anastomosis (and factors mixed with PRP) | Control | Primary comparison |
| Daglioglu et al[9], 2018 | Turkey | Rat (Winstar-Albino) | 36 | 3 | 12 | Day 7 | Normal | 0.5 mL PRP | Simple end-end colon anastomosis | PRP vs fibrin glue |
| Ocak et al[34], 2019 | Turkey | Rat (Winstar-Albino) | 35 | 3 | 10 | Day 7 | Hyperthermic intraperitoneal chemotherapy (HIPEC) | 200 μL PRP (200 μL thrombin and 100 μL calcium solution) | Hyperthermic saline after anastomosis | PRP vs non PRP application in rats having HIPEC with cisplatin |
| Yol et al[10], 2008 | Turkey | Rat (Sprague Danwley) | 30 | 3 | 10 | Day 7 | Normal | 1 mL PRP (0.1 mL thrombin and 1 mL calcium solution) | Simple end-end colon anastomosis | PRP vs bioglue |
| Buk et al[35], 2020 | Turkey | Rat (Winstar-Albino) | 35 | 3 | 10 | Day 7 | HIPEC | 1 mL PRP (1 mL thrombin and 0.5 mL calcium solution) | Hyperthermic saline after anastomosis | PRP vs non PRP application in rats having HIPEC with oxaliplatin |
| Dzhumabekov et al[25], 2019 | Kazakhstan | Rabbit (Chinchillas) | 81 | 3 | 27 | Day 7 | Normal | 0.2 mL/m2 PRP | Normal saline injected in the muscular layer of end-end small bowel anastomosis | PRP injection in bowel muscular layers vs soaking of bowel edges in PRP before anastomosis |
| Aydin et al[17], 2020 | Turkey | Rat (Sprague Dawley) | 24 | 3 | 8 | Day 7 | Normal | 0.7 μL PRP absorbed by sutures | Simple end-end colon anastomosis | Higher vs lower platelet concentration PRP-impregnated vicryl sutures |
| Dauser et al[26], 2020 | Austria | Pig | 16 | 4 | 4 | Day 0, 4, 10 and 30 | Normal | PRF spray | Each group had one animal as a control: A simple anastomosis was performed with a circular stapler | PRF vs no PRF application tested in several postoperative days |
| Giusto et al[28], 2017 | Italy | Pig (Landace X Large White) | 8 | 2 | 4 | Day 8 | Normal | 1 mL PRP (50 μL calcium solution) | 2 out of 6 anastomoses performed in each animal used as a control anastomosis [no PRP or platelet rich in growth factors (PRGF) applied] | PRP vs PRGF |
| Zhou et al[29], 2014 | China | Rat (Sprague Dawley) | 30 | 3 | 10 | Day 7 | Open abdomen. A polypropylene mesh used for abdomen closing in the open abdomen group | 1 mL PRP | Simple end-end colon anastomosis | PRP vs non PRP application in a background of open abdomen |
| Göksu et al[30], 2020 | Turkey | Rat (Wistar Albino) | 24 | 3 | 8 | Day 7 | HIPEC | PRP alone (dose not mentioned) | Hyperthermic saline after anastomosis | PRP vs non PRP application in rats having HIPEC with 5-fluorouracil (5-FU) |
| Özçay et al[16], 2018 | Turkey | Rat (Sprague Dawley) | 40 | 4 | 10 | Day 7 | Mesenteric ischemia/reperfusion injury (IR injury) | PRF membrane applied around the anastomosis | Simple end-end colon anastomosis | PRF vs non PRF application following IR injury |
| Fresno et al[19], 2010 | Spain | Pig (White) | 35 | 7 | 3 or 10 | Day 1, 2, 3, 4 and 7 | Normal | 1 mL PRP (50 μL calcium solution) | 1 out of 2 anastomoses performed in each animal used as a control anastomosis (no PRP or PRGF applied) | PRP effect on several postoperative days |
| Daradka et al[27], 2019 | Jordan | Rabbit (mixed-breed) | 30 | 3 | 10 | Day 3 and 10 | Normal | Sutures submerged in 1 ml PRP solution | Simple end-end ileal anastomosis | PRP vs sodium citrate coated sutures |
| Yalı et al[36], 2020 | Turkey | Rat (Wistar-Albino) | 56 | 4 | 12 | Day 5 | Peritonitis | 1 mL PRP (1 mL calcium solution) | Simple end-end colon anastomosis | PRP in normal abdomen vs peritonitis |
| Pehlivanli et al[33], 2019 | Turkey | Rat (Wistar Albino) | 55 | 5 | 10 | Day 10 | Mesenteric ischemia | 1 mL PRP | Simple end-end colon anastomosis | PRP vs Zeolite vs thymoquinone |
| Sozutek et al[31], 2016 | Turkey | Rat (Wistar Albino) | 50 | 4 | 10 | Day 7 | Peritonitis | 1 mL PRP (1 mL thrombin and 50 μL calcium solution) | Simple end-end colon anastomosis | PRP in normal abdomen vs peritonitis |
| Yamaguchi et al[18], 2012 | Japan | Rat (Sprague-Dawley) | 77 | 4 | 12 | Day 5 | Normal | 180 μL PRP (180 units of bovine thrombin and 30 μL of calcium solution). | Simple end-end colon anastomosis | Platelet poor plasma vs low vs high platelet rich plasma |
| Gorur et al[32], 2020 | Turkey | Rat (Wistar Albino) | 50 | 4 | 10 | Day 7 | Intraperitoneal administration of 5-FU | 1 mL PRP (1 mL thrombin and 50 μL of calcium solution) | Simple end-end colon anastomosis | PRP vs non PRP application in rats having intraperitoneal administration of 5-FU |
The majority of the studies (12 studies) investigated the effect of PRP on colonic anastomosis, while six studies investigated the effect on small bowel anastomosis[16,18,19,25,27]. End-to-end anastomosis was performed to restore the bowel continuity in all included studies. Concurrent bowel resection was reported in three studies[16,28,29]. Suturing method was continuous in four studies[16,17,28,29] or simple interrupted in six studies[18,19,30-33]. Circular stapler was used in one study[26].
The origin of PRP was homologous or autologous. Autologous PRP was used in three rat studies[9,10,34], two rabbit studies[25,27] and three pig studies[19,26,28]. Daglioglu et al[9] and Özçay et al[16], in the autologous PRP group, extracted 2.5 mL and 1 mL from each rat, respectively, while Yol et al[10] did not report the amount of blood taken from each rat. In the pig and rabbit groups, a total of 60-100 mL and 8-10 was taken, respectively. All ten studies that investigated the effect of homologous PRP were conducted on rats[17,18,29-36] and the number of rat donors ranged from five to twelve rats, while the amount of blood drawn from each donor ranged between 5-10 mL. A two-step centrifugation technique was applied in 16 studies investigating PRP. Dauser et al[26] utilized a specific kit for the preparation of PRF, while Özçay et al[16] used an one step centrifugation technique to extract PRF.
Direct application of PRP on bowel anastomosis was mentioned in 13 studies[9,10,16,18,26,29-36], merging of the bowel edges with PRP enriched material in three studies[19,25,28], PRP injection adjacent to anastomosis in one study[25], and anastomosis performed with PRP coated sutures in two studies[17,27]. Lastly, two studies investigated PRP pharmacokinetics[18,27]. PDGF subunit A release to the media from PRP coated sutures was stable and showed no significant changes at 1, 2, 24 and 48 h post application. Similarly, the release of TGF-β1 was increased significantly in the first hour, but thereafter the release was stable without any major changes[27]. PDGF-BB and TGF-β1 showed statistically significant higher concentration in the high concentration PRP vs low concentration PRP and platelet poor plasma groups[18].
In total, eight deaths were reported and included one death in the PRP group[9] and seven deaths in the comparison groups. No postoperative complications related to PRP were reported among the included studies.
Common anastomosis related parameters measured among the included studies are the anastomotic bursting pressure, tissue hydroxyproline, collagen deposition and inflammatory cell infiltration. These results are summarized in Table 2. The comparison and the associated statistical significance of PRP, control and other agents are presented with the related P value. Other reported outcomes are descripted subsequently.
| Ref. | Anastomotic burst pressure (mm/hg) | Tissue hydroxyproline (μg/mg) | Collagen deposition | Inflammatory cells deposition | ||||||
| PRP | Control | Other agent | P value | PRP | Control | Other agent | P value | |||
| Daglioglu et al[9], 2018 | 146 ± 44.55 mm/hg | 119 ± 35.65 mm/hg | 149.1 ± 72.29 mm/hg (Fibrin glue) | vs Control (0.026); vs Fibrin glue (0.896) | 120.1 ± 51.5 μg/mg | 96.2 ± 29.22 μg/mg | 118.71 ± 42.18 μg/mg | vs Control (0.023); vs Fibrin glue (0,745) | No significant difference between groups | No significant difference between groups |
| Ocak et al[34], 2019 | 146 ± 21.85 mm/hg | 180 ± 9.14 mm/hg | 115.8 ± 18.19 mm/hg (HIPEC with cisplatin group) | vs Control (< 0.001); vs HIPEC with cisplatin (0.01) | 256.59 ± 84.03 ng/mg | 314.69 ± 47.56 ng/mg | 148.02 ± 26.57 ng/mg (HIPEC with cisplatin) | vs Control (0.335); vs Hyperthermic saline group (< 0.001) | - | Inflammatory cell infiltration is significant decreased with PRP application in HIPEC and cisplatin model |
| Yol et al[10], 2008 | 270 ± 29.8 mm/hg | 195 ± 15.3 mm/hg | 214 ± 16.46 mm/hg (bioglue) | vs Control (< 0.001); vs Bioglue (< 0.001) | 18.2 ± 4.95 μg/mg | 10.96 ± 5.94 μg/mg | 11.08 ± 5.08 μg/mg | vs Control (0.016); vs Bioglue (0.026) | Rich collagen production was observed in the PRP group. No comparison between groups | Less inflammatory cell infiltration in the PRP group |
| Buk et al[35], 2020 | 125.7 ± 15.64 mm/hg | 180 ± 9.14 mm/hg | 94.90 ± 9.9 mm/hg (HIPEC with oxiliplatin) | vs Control (< 0.001); vs HIPEC with oxiliplatin group (< 0.0011) | 280.92 ± 45.85 ng/mg | 314.69 ± 75.57 ng/mg | 92 ± 26.97 ng/mg (HIPEC with oxiliplatin) | vs Control (< 0.001); vs HIPEC with oxiliplatin) (< 0.0011) | - | Inflammatory cell infiltration is significant decreased with PRP application in oxiplatin model |
| Dzhumabekov et al[25], 2019 | 1.76 ± 0.28 (PRP soakinggroup)1 | 1.54 ± 0.231 | 1.81 ± 0.171 (PRP injecting group) | vs Control (0.05); vs PRP injecting group (0.69) | - | - | - | - | No significant differences between groups | Inflammatory cell infiltration significantly lower in the PRP soaking or injection group |
| Aydin et al[17], 2020 | 121 ± 57 mm/hg | 124 ± 61 mm/hg | 180 ± 49 mm/hg (low concentration PRP) | vs Control (> 0.05); vs low concentration PRP (< 0.0011) | 0.39 ± 0.10 μg/mg | 0.25 ± 0.17 μg/mg | 0.56 ± 0.37 μg/mg (low concentration PRP) | vs Control (< 0.001); vs low concentration PRP (< 0.051) | - | No significant difference between groups |
| Dauser et al[26], 2020 | Median = 210 mm/hg (day 10) | Median = 60 mm/hg (day 10) | - | The study reports no statistically significant changes between groups due to small sample size | - | - | - | - | Matrix treated animals showed less immature collagen deposition (type III) compared to the control group (day 10). However no significant differences were observed | No significant changes in the M2 or non-M2 macrophage density in the mucosal, mural and serosal layers. No significant changes in inflammatory cell infiltration |
| Giusto et al[28], 2017 | 117.5 mm/hg (range: 80-190) | 154 mm/hg (range: 50-180) | 165 mm/hg (range: 100-190) (PRGF); And 175 mm/hg (range: 160-190) (intact bowel) | vs Control or PRGF (> 0.05); vs Intact bowel (0.00071) | - | - | - | - | No significant difference between groups | No significant difference between groups |
| Zhou et al[29], 2014 | 177 ± 6.95 mm/hg | 184.8 ± 6.6 mm/hg | 158 ± 5.08 mm/hg (open abdomen group without PRP application) | vs Control (0.398); vs non-PRP application in open abdomen (0.041) | 399.7 ± 9.46 μg/mg | 403.6 ± 8.55 μg/mg | 353.5 ± 6.75 μg/mg (open abdomen group without PRP application) | vs Control (0.74); vs non-PRP application in open abdomen (0.001) | Significantly higher in the PRP and control group | No significant differences between groups |
| Göksu et al[30], 2020 | 143 ± 17.35 mm/hg | 150 ± 20.49 mm/hg | 119.38 ± 17.65 mm/hg (5-FU HIPEC without PRP application) | vs Control (0.718); vs non-PRP 5-FU HIPEC (0.047) | 253.64 ± 5.35 μg/mg | 259.6 ± 7.95 μg/mg | 244.04 ± 7.28 μg/mg (5-FU HIPEC without PRP application) | vs Control (0.224); vs non-PRP 5-FU HIPEC (0.03) | - | Decreased lymphocytes in the PRP compared to the other groups. No statistically significant changes in neutrophil infiltration |
| Özçay et al[16], 2018 | 198.1 ± 36.5 mm/hg | 205.1 ± 41.1 mm/hg | 106.1 ± 33.9 mm/hg (IR injury without PRF) | vs Control (> 0.05); vs non PRF in IR injury (< 0.01) | - | - | - | - | Moderate to severe collagen deposition in all groups but no significant changes between groups | Moderate to severe cellular infiltration but no significant changes between groups |
| Fresno et al[19], 2010 | 1.34 ± 0.07 kgf 1(day 3); 1.14 ± 0.11 kgf 1(day 7) | 1.21 ± 0.08 kgf 1(day 3); 1.08 ± 0.08 kgf 1(day 7) | 1.8 ± 0.08 kgf 1(normal tissue) | vs Normal tissue (< 0.05); vs Control day 3 or 7 (> 0.05) | - | - | - | - | No significant difference between groups | - |
| Daradka et al[27], 2019 | 60.2 ± 5.5 mm/hg | 54.5 ± 7.5 mm/hg | 55.6 ± 10.2 mm/hg (sodium citrate coated sutures) | vs Control (0.211) | 0.76 ± 0.1 μg/mg | 0.47 ± 0.13 μg/mg | 0.52 ± 0.07 μg/mg (sodium citrate- coated sutures) | vs Control (< 0.05) on day 10; vs Control (> 0.05) on day 3 | Statistically significant higher collagen deposition compared to uncoated suture groups on day 10 | Statistically significant less inflammatory infiltration compared to PRP uncoated suture groups |
| Yalı et al[36], 2020 | 129.66 ± 26.6 mmH20 | 143.25 ± 37.47 mmH20 | 154.9 ± 27.64 mmH20 (colon anastomosis in peritonitis) and 173.5 ± 29.49 mmH20 (colon anastomosis and PRP application in peritonitis) | vs Control (> 0.05); vs Colon anastomosis and PRP application in peritonitis (< 0.05) | - | - | - | - | Statistically significant higher collage storage values in PRP treated group compared to control and peritonitis model | Statistically significant differences between groups in terms of inflammatory reaction |
| Pehlivanli et al[33], 2019 | 225 (range: 180-250)2 | 200 (range: 90-230)2 | 235 (range: 220-250)2 thymoquinone; 132.5 (range: 85-150)2 Zeolite | vs Control (> 0.05); vs Zeolite (< 0.05); vs Thymoquinone (> 0.05) | 613.22 (range: 158.55-801.82)2 | 371.27 (range: 164.51-785.45)2 | 473.03 (range: 215.33-963.43)2 thymoquinone; 459.15 (range: 182.44-738.21)2 Zeolite | vs Control (> 0.05); vs Zeolite (> 0.05); vs Thymoquinone (> 0.05) | - | No significant difference in terms of inflammation at the anastomotic line in between groups |
| Sozutek et al[31], 2016 | 209 ± 14.4 mm/hg | 179.5 ± 10.3 mm/hg | 129.3 ± 14.2 mm/hg (colon anastomosis in peritonitis); 167.5 ± 7.5 mm/hg (colon anastomosis and PRP application in peritonitis) | vs Control (0.01); vs Colon anastomosis in peritonitis (0.01); vs Colon anastomosis and PRP application in peritonitis (0.01) | 17.4± 1.21 μg/mg | 10.8± 0.67 μg/mg | 8.98 ± 1.04 μg/mg (colon anastomosis in peritonitis); 10.6 ± 0.52 μg/mg (colon anastomosis and PRP application in peritonitis) | vs Control (0.023); vs Colon anastomosis in peritonitis (0.01); vs Colon anastomosis and PRP application in peritonitis (0.012) | Application of PRP in peritonitis group did no increase collagen deposition significantly | Macrophages significantly increased in PRP vs control group and lymphocytes were significantly decreased in PRP + peritonitis compared to peritonitis group |
| Yamaguchi et al[18], 2012 | 148 ± 25 mm/hg (H-PRP) | 171 ± 20 mm/hg | 174 ± 23 mm/hg (PPP); 189 ± 17 mm/hg (L-PRP) | vs Control (< 0.05); vs L-PRP (< 0.05); vs PPP (< 0.05) | 407 ± 143 μg/mg | 515 ± 130 μg/mg | 495 ± 123 μg/mg (PPP); 629 ± 120 μg/mg (L-PRP) | vs Control (< 0.05); vs L-PRP (< 0.05); vs PPP (< 0.05) | In L-PRP more collagen deposition in the serosa layer compared to other groups. H-PRP showed the lesser collagen deposition compared to other groups | - |
| Gorur et al[32], 2020 | 246.7± 25.1 mm/hg | 232.6± 19.5 mm/hg | 127.5± 17.7 mm/hg (colon anastomosis and 5-FU intraperitoneal); 202.9 ± 28.8 mm/hg (colon anastomosis + PRP and 5-FU intraperitoneal) | vs Control (> 0.05); vs Colon anastomosis and 5-FU intraperitoneal (< 0.05); Colon anastomosis + PRP vs non PRP and 5-FU intraperitoneal (< 0.05) | 1939.5 ± 586 μg/mg | 2994.6 ± 2132.4 μg/mg | 591 ± 84.4 μg/mg (colon anastomosis and 5-FU intraperitoneal); 1171 ± 301.7 μg/mg (colon anastomosis + PRP and 5-FU intraperitoneal) | vs Control (0.212); vs Colon anastomosis and 5-FU intraperitoneal (< 0.05); Colon anastomosis + PRP vs non PRP and 5-FU intraperitoneal (< 0.05) | Increased but no statistically significant collagen deposition in colon anastomosis + PRP vs non PRP on a background of intraperitoneal 5-FU administration | No significant differences between groups |
Intrabdominal adhesions were assessed in five studies[16,25-28]. Soaking of the bowel edges in PRP resulted in increased formation of intrabdominal adhesions compared to the injection of PRP along the anastomosis line and compared to the control group[25]. Compared to the platelet rich in growth factors (PRGF) and control groups, the use of PRP resulted in a non-significantly increased formation of intrabdominal adhesions[28]. In another technique, suture soaking in PRP material was associated with significantly lower adhesion scores in the anastomotic sites in a rabbit animal model[27]. Dauser et al[26], reported that the application of PRF was not associated with significant changes in adhesion formation, compared to the control group. In contrast, Özçay et al[16] reported that the application of PRF resulted in significantly decreased formation of intra-abdominal adhesions in the ischemia/reperfusion injury animal model compared to the non PRF groups[16].
Daglioglu et al[9], reported no statistically significant changes in proinflammatory cytokines IL-6, IL-10 and procalcitonin levels between the PRP and control groups. Higher circulating tumor necrosis factor-α and IL-1b levels in the PRP compared to the control group were observed by Pehlivanli et al[33].
Table 2 summarizes the collagen deposition and inflammatory cell infiltration in the PRP treated groups compared to the control or other agents that were also tested. Table 3 describes the results of the Verhofstad histopathology scale that was recorded by some of the included studies. The Verhofstad histopathology scale is used to analyze wound healing by assessing on a 0-3 scale the necrosis, polymorphonuclear leukocytes, macrophages, edema, mucosal epithelium and submucosal-muscular layer healing[37].
| Groups | Necrosis | Neutrophil | Lymphocyte | Macrophages | Oedema | Mucosal epithelium | Submucosal layer | Bridging | Total |
| Ocak et al[34] | |||||||||
| Control | 2.3 ± 0.82 | 2.5 ± 0.52 | 2.5 ± 0.52 | 2.5 ± 0.52 | 2.9 ± 0.31 | 2.6 ± 0.966 | 0.8 ± 0.63 | - | - |
| PRP | 2.6 ± 0.69 | 2.8 ± 0.42a | 2.7 ± 0.48a | 2.7 ± 0.48a | 2.9 ± 0.31 | 2.6 ± 0.516 | 1.4 ± 0.69 | - | - |
| Fibrin glue | 2 ± 0.66 | 2.1 ± 0.31a | 2.1 ± 0.31a | 2.1 ± 0.31a | 2 ± 0.47 | 2.6 ± 0.516 | 1.3 ± 0.67 | - | - |
| Buk et al[35] | |||||||||
| Control | 2.3 ± 0.82 | 1.9 ± 0.56a | 1.8 ± 0.42a | 2 ± 0.47 | 2.1 ± 0.56a | 2.6 ± 0.966 | 0.8 ± 0.63a | - | - |
| Oxaliplatin | 2.5 ± 0.52 | 2.9 ± 0.52a | 2.4 ± 0.51a | 2.5 ± 0.52 | 2.8 ± 0.42a | 2.6 ± 0.516 | 2 ± 0.94a | - | - |
| Oxaliplatin + PRP | 2.7 ± 0.58 | 2 ± 0.47a | 1.8 ± 0.42a | 2.3 ± 0.67 | 2.1 ± 0.56a | 2.5 ± 0.54 | 1.6 ± 0.96a | - | - |
| Aydin et al[17] | |||||||||
| Control | 1 ± 1 | 2 ± 1 | - | 2 ± 0 | 2 ± 1 | 3 ± 1 | 3 ± 0 | - | - |
| L-PRP | 0 ± 2 | 2 ± 1 | - | 2 ± 0 | 1 ± 0a | 2.5 ± 1a | 3 ± 2 | - | - |
| H-PRP | 1.5 ± 2 | 2 ± 2 | - | 2 ± 1 | 0 ± 0a | 1 ± 2a | 3 ± 0 | - | - |
| Göksu et al[30] | |||||||||
| Control | 2.38 ± 0.51 | 2.38 ± 0.518 | 2.38 ± 0.51a | - | 2.75 ± 0.46a | 2.63 ± 0.51 | 1.75 ± 0.46 | - | - |
| 5-FU | 2.63 ± 0.51 | 2.50 ± 0.463 | 2.63 ± 0.51a | - | 2.75 ± 0.46a | 2.50 ± 0.53 | 1.25 ± 0.46 | - | - |
| 5-FU + PRP | 2.13 ± 0.35 | 2.13 ± 0.518 | 2 ± 1a | - | 2 ± 0.53a | 2.63 ± 0.51 | 1.25 ± 0.46 | - | - |
| Sozutek et al[31] | |||||||||
| Control | 0.3 ± 0.48 | 1.3 ± 0.94 | 1 ± 0.47 | 1 ± 0a | 0.4 ± 0.51 | 0.3 ± 0.48 | - | 0.6 ± 0.51 | 4.9 ± 1.28 |
| Control + PRP | 0.2 ± 0.42 | 0.7 ± 0.67 | 1 ± 0.47 | 1.6 ± 0.51a | 0.3 ± 0.48 | 0.4 ± 0.48 | - | 0.2 ± 0.42 | 4.3 ± 1.33 |
| Septic | 1.1 ± 0.64 | 1.5 ± 0.53 | 1.6 ± 0.51a | 1.1 ± 0.83 | 1.2 ± 0.71a | 1 ± 0.53 | - | 1.2 ± 0.71 | 9.8 ± 1.12a |
| Septic + PRP | 0.7± 0.48 | 1.2 ± 0.42 | 1.3 ± 0.48a | 1.5 ± 0.52 | 0.4 ± 0.51a | 0.5 ± 0.51 | - | 0.8 ± 0.42 | 6.1 ± 1.37a |
| Gorur et al[32] | |||||||||
| Control | 0.3 ± 0.67a | 1.3 ± 0.9 | 1 ± 0.47 | 1 ± 0 | 1.1 ± 0.31a | 0.3 ± 0.57a | - | 1.2 ± 0.78a | - |
| 5-FU | 1 ± 1.05a | 1.5 ± 0.53 | 1.4 ± 0.32 | 1.1 ± 0.83 | 2 ± 0.73a | 1.1 ± 0.42a | - | 1.5 ± 0.52a | - |
| Control + PRP | 0a | 0.7 ± 0.67 | 1 ± 0.67 | 1.8 ± 0.31 | 1 ± 0.47a | 0.3 ± 0.57a | - | 0.9 ± 0.87a | - |
| 5-FU + PRP | 0.1 ± 0.3 | 1.2 ± 0.42 | 1.5 ± 0.58 | 1.6 ± 0.53 | 1.7 ± 0.48 | 0.6 ± 0.57 | - | 1.1 ± 0.31 | - |
Dauser et al[26] reported no significant difference in the PRF compared to the control group in terms of foreign body reactivity, mucosal regeneration and inflammatory cell infiltrates. Anastomotic thickness, mean mucin percentage, and microvascular density (at day 30 postoperatively) were also non-significantly increased in the PRF treated anastomosis. The application of PRF was associated with bacterial colonization and infiltration of neutrophils at day 4 in all animals. Both Özçay et al[16] and Dauser et al[26] did not observe residual PRF material on day 10 and day 30 postoperatively. In contrast, PRP material was visualized in the anastomosis microscopic examination as an eosinophilic material[19]. Epithelialization, cellular infiltration, fibroblast proliferation, and neovascularization did not present a significant increase in the PRF group in the ischemia/reperfusion injury animal model[16]. Staining for the endothelium specific Factor VIII did not present significant changes in the PRP compared to control groups[19].
Οn postoperative days 1, 2, 3, and 7, Dzhumabekov et al[25] studied the fiber-crypt index, intraepithelial lymphocyte count, epithelial-stromal coefficient and mitosis count (mitosis observed outside lymphoid follicles). Higher mitosis rate in the mucosal crypt area was observed in the PRP injection group compared to PRP soaking and control groups on postoperative days 3 and 7. Epithelial-stromal coefficient decreased in the control group. Intraepithelial lymphocyte infiltration did not present any significant difference between groups[25].
Aydin et al[17] reported that PRP coated sutures with either high or low platelet concentration resulted in significantly decreased formation of granulation tissue compared to the control group[17]. In contrast, Fresno et al[19] reported that on postoperative day 7 the PRP treated anastomosis developed increased, but not significantly different, mature granulation tissue and fibrosis. Yalı et al[36] findings were significant for higher vascularization, fibroblast organization and epithelial formation in the PRP treated peritonitis model. Pehlivanli et al[33] compared several agents and concluded that PRP application was associated with better re-epithelialization scores compared to Zeolite application and control groups.
Giusto et al[28] reported no statistically significant changes in neovascularization and fibroblast proliferation. Mucosa epithelialization was significantly increased in the PRGF group. Yol et al[10] found that the fibroblast count was significantly increased in the PRP group compared to the control group, but the results were comparable between the PRP and the bioglue groups. In addition, Daglioglu et al[9] showed that fibroblast density and neovascularization were not significantly different between the fibrin glue or PRP application and control groups. Buk et al[35] reported that submucosal bridging was significantly increased in the control and PRP/oxaliplatin groups compared to the oxaliplatin group alone. Lastly, Zhou et al[29], utilizing an open abdomen animal model, reported that fibroblast ingrowth was significantly higher in the PRP group compared to the control and open abdomen group. The vascular ingrowth of the PRP was significantly increased compared to the open abdomen, but was comparable to the control group[29].
Regarding selection bias, 17 of the 18 included studies (94%) did not report whether the allocation sequence was adequately applied and concealed. Only one study reported the use of a random number generator. Concerning the baseline characteristics, 10 of the 18 studies (56%) described comparable groups at the baseline.
Regarding performance bias, one study reported that the researchers were blinded, while other studies did not report data regarding the housing parameters or researcher's blinding. Therefore, the risk of bias is considered unclear.
Regarding detection bias and specifically the animals’ selection method for the assessment of outcomes, all studies were scored as having unclear risk of bias due to missing relevant information. However, outcome assessment methods were similar between the groups in all studies and the risk of bias regarding the blinding of the outcome assessors is characterized as low.
Regarding attrition bias, 5 of the 18 included studies (28%) did not describe the handling method for incomplete data (unclear bias). Two studies were scored as high risk of bias, including one study where the authors excluded unequal number of animals that died in different groups and another study where the authors did not provide sufficient information about the death of two animals in one of the groups.
Regarding reporting bias, in 17 of the 18 studies (94%) adequately described the outcomes and the reporting bias risk was low. In one study, the tissue hydroxyproline levels were not reported, despite being included as an expected outcome in the materials and method section (high risk).
Regarding other sources of bias, in one study a preparation kit was used and one of the co-authors had a relevant conflict of interest with the manufacturer. Two studies (11%) did not provide information regarding any funding that may have affected their work.
Regarding the approval from an ethical committee, all included studies reported approval from their local ethical committee. The quality assessment results are summarized in Supplementary Table 2.
The application of PRP in bowel anastomosis is associated with improved outcomes in terms of anastomoses bursting pressure and tissue hydroxyproline, which are the two most common parameters used for the evaluation of anastomosis integrity[17].
Anastomotic bursting pressure is an indirect indicator of anastomosis healing. It reflects the balance between collagen synthesis and degradation[18,38]. Although 50% (9/18) of the included studies reported no statistically significant changes in the anastomosis bursting pressure in PRP-treated compared to control groups, five studies reported that the application of PRP in the presence of an underlying medical or surgical condition, improved the anastomosis bursting pressure. Furthermore, the application of PRP in the open abdomen, ischemic /reperfusion injury, peritonitis, intraperitoneal 5-fluorouracil (5-FU) infusion, and hyperthermic intraperitoneal chemotherapy with 5-FU animal models was associated with statistically significant improved anastomosis bursting pressure[16,29,30,32,36]. Among, the other four studies, Dauser et al[26] investigated the application of PRF, which presents some component differences compared to PRP. Giusto et al[28] reported significantly lower bursting pressure in the PRP compared to the control group, although the application of PRGF significantly increased the anastomotic bursting pressure compared to PRP or control group. Lastly, Daradka et al[27] used PRP coated sutures and Pehlivanli et al[33] studied the application of several topical factors in the anastomosis. Both studies report no significant changes in anastomotic bursting pressure in PRP compared to control groups.
Hydroxyproline level is a widely accepted marker of tissue collagen synthesis, including the anastomotic area[39]. Increased collagen synthesis and collagen maturation are thought to be induced by hydroxyproline molecules[10]. Low levels of tissue hydroxyproline exert a negative impact in wound healing[40,41]. According to the included studies, tissue hydroxyproline is measured on or close to the 7th postoperative day. Despite not being reported by six studies, tissue hydroxyproline levels were consistent with the anastomotic bursting pressure in all except three studies. In two studies the anastomotic bursting pressure was significantly increased in the PRP-treated group while anastomotic tissue hydroxyproline levels did not show any significant changes[17,34]. Gorur et al[32] reported that PRP application was associated with increased tissue hydroxyproline levels in the intraperitoneal 5-FU infusion animal model.
Similarly, anastomotic wound inflammatory cellular infiltration in control compared to PRP groups did not show any statistically significant changes among the included studies. However, it was reported that in the presence of an underlying detrimental condition like intraperitoneal chemotherapy or infection, PRP application significantly decreased the inflammatory cellular infiltration in bowel anastomosis[30-32,34-36]. Theoretically, enhanced anastomosis strength associated with PRP application could partially be attributed to decreased inflammatory cell-mediated collagen degradation[42].
The intestinal wound healing process can be roughly divided into three phases: inflammation, proliferation, and maturation. Following a surgical intervention, platelets are among the first cells that reach the traumatized tissue area, while their main functions include the formation of a protective clot and the release of growth factors[43]. The role of growth factors in wound healing has been extensively investigated in previous studies. PDGF secretion was shown to improve epithelialization, secretion of several other tissue growth factors, and tissue regeneration[44]. Synthesis and deposition of several extracellular matrix factors as well as increased in vitro keratinocyte motility have been associated with FGF[45]. VEGF family proteins play a significant role in early angiogenesis. In vitro studies have demonstrated that the VEGF family proteins facilitate the angiogenic properties of stem cells and improve the wound healing process[46,47]. IGF acts as a mitogenic growth factor for fibroblasts[48]. PRP, which is a carrier of growth factors, is expected to improve the anastomosis wound healing and reduce the incidence of postoperative anastomosis-related complications (Figure 2).
The first week following a bowel anastomosis seems to be the critical period for the development of anastomosis leaks. Most of the anastomosis leakages are reported 5-10 d postoperatively, when the strength of anastomosis is considered to be at its lowest level[49]. A possible explanation involves the collagen remodeling during wound healing process. Experimental studies have shown that collagen degradation starts on the third postoperative day and peaks on the seventh day following surgical trauma. Permanent collagen deposition in the anastomotic area is believed to take place a few days postoperatively. In view of initial collagen degradation during wound healing over the first few days, the anastomosis integrity is mainly supported by fibrin deposition and anastomotic technique (suturing method)[9,29,35,50,51]. Based on these experimental studies, all included studies investigated the effects of PRP application around postoperative day 7, when the anastomotic strength is considered to be at its lowest level.
Anastomotic leak is associated with increased morbidity and mortality rate[10,52]. Dysregulation of circulating platelets, the main component of PRP, has been associated with anastomotic leak. Both thrombocytosis and thrombocytopenia have been described as factors associated with anastomotic leak. However, these results should be interpreted with caution as the dysregulation of circulating platelets could be attributed to malnutrition (thrombocytopenia) or sepsis (thrombocytosis), which are well established risk factors associated with anastomotic leak[53,54]. Patients developing anastomotic leaks tend to have prolonged intensive care unit and hospital stay and significantly increased medical care costs[29]. Intra-abdominal infections, fistulas between adjacent organs, and poor abdominal wound healing are some of the long-term complications of anastomotic leak that may result in significant consequences on patient’s quality of life[55].
The optimal method and/or agent to prevent these detrimental complications has not been identified yet. The prevention of bowel anastomosis leak involves the modification of risk factors that predispose to impaired wound healing. To this end, immunomodulators, hormones, growth factors, antibiotics and proteinase inhibitors have been previously applied topically or administered systematically and have been associated with improved bowel anastomotic healing[56]. The underlying mechanisms ,that promote enhanced anastomosis integrity, include increased blood supply, reduced inflammatory cell infiltration, and rapid collagen deposition[56]. Despite the presence of numerous studies on agents that could promote wound healing, the ideal agent is yet to be determined. PRP contains a variety of growth factors, immunomodulators, as well as other constituents that promote tissue healing and is a promising candidate in terms of clinical applications.
Preparation of PRP is a simple process with very low cost compared to other materials used for anastomosis reinforcement[57]. Currently, most studies report that a sample of 5-20 mL of peripheral blood is required to extract 2-5 mL of PRP. The amount of peripheral blood required for PRP preparation depends on the technique or commercial kit that are used during the isolation process[14,58]. Furthermore, its autologous nature increases biocompatibility[9,32]. However, some technical issues and concerns were raised among the included studies. The majority of the growth factors are presynthesized within the platelets and are secreted within one hour after platelet activation. As a result PRP associated growth factors are released immediately after PRP application to the anastomotic area[19,59]. Interestingly, platelets could also synthesize and secrete growth factors during their lifespan in the area of bowel anastomosis for up to 7 d. This growth factor release is supplemental to the initial growth factor secretion taking place immediately after PRP application[10,19,60]. Nevertheless, the platelet concentration of PRP applied to the anastomotic area may also affect the healing outcomes. To that extend, Aydin et al[17] showed that low platelet concentration results in superior outcomes in terms of anastomotic bursting pressure and collagen concentration at the anastomotic site compared to high platelet concentration PRP.
Our study has several strengths, including the total number of included studies, the large number of animal models, as well the variety of conditions that the PRP was tested on. However, we have to recognize that our findings are not free of limitations and should be interpreted cautiously. Animal models, studies heterogeneity and small samples are among the major limitations of our study. Only two studies investigated the effects of PRP on pigs, which have intestines that are structurally closer to human bowel. As a result, the clinical application and generalizability of our findings in large animal models are questionable. Furthermore, high heterogeneity was observed in the histopathological scales used for the assessment of anastomotic cellular infiltration. Although pathologists were reported to be blinded regarding the origin of the samples, the lack of a uniform scale, such as the Verhofstad scale, pose difficulties in terms of results interpretation and measurement bias.
The application of PRP in bowel anastomosis is a feasible approach and it seems to improve the integrity of bowel anastomosis. PRP application compared to control groups did not show any significant changes in the majority of the included studies. However, in the presence of an underlying condition that impairs intestinal wound healing, including peritonitis or chemotherapy, the application of PRP could potentially improve the healing process. Its preparation does not require significant expertise and can be easily extracted from patient’s own blood. Taking into consideration its cost effectiveness, PRP could be considered in the clinical practice for bowel anastomosis reinforcement material. Apparently, further research is needed to confirm the safety and effectiveness of PRP on human bowel anastomoses.
Several applications of platelet rich plasma (PRP) have been reported in the literature. Some examples include maxillofacial, orthopedic and plastic surgery where PRP is considered to improve the wound healing process. PRP is easily extracted from patient’s blood and includes a variety of growth factor that is thought to improve the wound healing process.
Preclinical studies shows that the PRP has a positive impact in the healing process of bowel anastomosis.
The aim of this study is to define the role of PRP in general surgery, especially in procedures involving bowel anastomosis. Therefore, a systematic review of the literature was performed.
A systematic literature search was performed in PubMed, EMBASE, and Scopus databases. Animal studies that investigated the effect of PRP on bowel anastomosis were included in our analysis.
Among the 2407 studies screened, 18 animal studies were finally included in our analysis. An end-to-end bowel anastomosis was performed in all included studies. PRP origin was autologous in 8 studies and homologous in 10 studies. In 13 out of 18 studies PRP was applied topically to the bowel anastomosis. No postoperative complications attributed to PRP application were reported. Common anastomosis related parameters measured among the included studies were the anastomotic bursting pressure, tissue hydroxyproline, collagen deposition and inflammatory cell infiltration. The individual study results in the aforementioned parameters are presented in tables.
The application of PRP in bowel anastomosis is feasible and seems to be free of any major complications. PRP application compared to control groups did not show any significant changes in the majority of the included studies. However, in the presence of an underlying condition that impairs intestinal wound healing, including peritonitis or chemotherapy, the application of PRP could potentially improve the healing process.
Although the results of this study support the use of PRP in bowel anastomosis, further research is needed to confirm the safety and effectiveness of PRP on human bowel anastomoses.
This review is part of a PhD thesis research project, taking place at the Graduate School of Medicine, Aristotle University, Thessaloniki, Greece.
| 1. | Midura EF, Hanseman D, Davis BR, Atkinson SJ, Abbott DE, Shah SA, Paquette IM. Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum. 2015;58:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Murray AC, Chiuzan C, Kiran RP. Risk of anastomotic leak after laparoscopic vs open colectomy. Surg Endosc. 2016;30:5275-5282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 351] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Hompes D, D'Hoore A, Van Cutsem E, Fieuws S, Ceelen W, Peeters M, Van der Speeten K, Bertrand C, Legendre H, Kerger J. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol. 2012;19:2186-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Riss S, Chandrakumaran K, Dayal S, Cecil TD, Mohamed F, Moran BJ. Risk of definitive stoma after surgery for peritoneal malignancy in 958 patients: comparative study between complete cytoreductive surgery and maximal tumor debulking. Eur J Surg Oncol. 2015;41:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Whealon MD, Gahagan JV, Sujatha-Bhaskar S, O'Leary MP, Selleck M, Dumitra S, Lee B, Senthil M, Pigazzi A. Is Fecal Diversion Needed in Pelvic Anastomoses During Hyperthermic Intraperitoneal Chemotherapy (HIPEC)? Ann Surg Oncol. 2017;24:2122-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 9. | Daglioglu YK, Duzgun O, Sarici IS, Ulutas KT. Comparison of platelet rich plasma vs fibrin glue on colonic anastomoses in rats. Acta Cir Bras. 2018;33:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Yol S, Tekin A, Yilmaz H, Küçükkartallar T, Esen H, Caglayan O, Tatkan Y. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008;146:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Saleem M, Pisani F, Zahid FM, Georgakopoulos I, Pustina-Krasniqi T, Xhajanka E, Almasri M. Adjunctive Platelet-Rich Plasma (PRP) in Infrabony Regenerative Treatment: A Systematic Review and RCT's Meta-Analysis. Stem Cells Int. 2018;2018:9594235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Alio JL, Rodriguez AE, De Arriba P, Gisbert S, Abdelghany AA. Treatment with platelet-rich plasma of surgically related dormant corneal ulcers. Eur J Ophthalmol. 2018;28:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis : A prospective randomized controlled study. Orthopade. 2019;48:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 14. | Hamid MSA. Cost Effectiveness of a Platelet-rich Plasma Preparation Technique for Clinical Use. Wounds. 2018;30:186-190. [PubMed] |
| 15. | Estcourt LJ, Malouf R, Hopewell S, Trivella M, Doree C, Stanworth SJ, Murphy MF. Pathogen-reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev. 2017;7:CD009072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Özçay N, Özdemir H, Besim H. Role of platelet-rich fibrin on intestinal anastomosis wound healing in a rat. Biomed Mater. 2018;13:045006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Aydin MA, Guler EM, Demiroz AS, Aydin MF, Saglam G. Comparison of Platelet-Rich Plasma-Impregnated Suture Material with Low and High Platelet Concentration to Improve Colonic Anastomotic Wound Healing in Rats. Gastroenterol Res Pract. 2020;2020:7386285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Fresno L, Fondevila D, Bambo O, Chacaltana A, García F, Andaluz A. Effects of platelet-rich plasma on intestinal wound healing in pigs. Vet J. 2010;185:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13819] [Article Influence: 812.9] [Reference Citation Analysis (3)] |
| 21. | Wohlin C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering (EASE ’14); 2014 May. New York, NY, USA: Association for Computing Machinery, 2014: 1-10. [DOI] [Full Text] |
| 22. | Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2511] [Cited by in RCA: 2889] [Article Influence: 240.8] [Reference Citation Analysis (2)] |
| 23. | Covidence. Covidence systematic review software, Veritas Health Innovation, Melbourne AA. [cited 10 March 2021]. In: Covidence [Internet]. Available from: www.covidence.org. |
| 24. | Dzhumabekov BN, Dzhumabekov AT, Ismailov DK, Baitileuov TA, Fakhradyiev IR. Platelet-rich autoplasma effect on intestinal anastomosis regeneration in rabbits. Arch Balk Med Union. 2019;54:621-629. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Dzhumabekov BN, Dzhumabekov AT, Ismailov DK, Baitileuov TA, Fakhradyiev IR. Platelet-rich autoplazma effect on intestinal anastomosis regeneration in rabbits. Libr Oncol. 2019;47:55-63. [DOI] [Full Text] |
| 26. | Dauser B, Heitland W, Bader FG, Brunner W, Nir Y, Zbar AP. Histologic changes in early colonic anastomotic healing using autologous platelet-rich fibrin matrix. Eur Surg - Acta Chir Austriaca. 2020;52:155-164. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Daradka M, Alardah MM, Ismail ZB. Effects of autologous platelet-rich plasma coated sutures on intestinal anastomotic healing in rabbits. Heliyon. 2019;5:e02713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Giusto G, Vercelli C, Iussich S, Tursi M, Perona G, Gandini M. Comparison of the effects of platelet-rich or growth factor-rich plasma on intestinal anastomosis healing in pigs. BMC Vet Res. 2017;13:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Zhou B, Ren J, Ding C, Wu Y, Chen J, Wang G, Gu G, Li J. Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. Injury. 2014;45:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Göksu M, Alakuş H, Ertan S, Akgün S. Effect of platelet-rich plasma on colon anastomosis in rats in which hyperthermic intra-peritoneal chemotherapy was performed using 5-fluorouracil. ANZ J Surg. 2020;90:2290-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Sozutek A, Colak T, Cetinkunar S, Reyhan E, Irkorucu O, Polat G, Cennet A. The Effect of Platelet-Rich-Plasma on the Healing of Left Colonic Anastomosis in a Rat Model of Intra-Abdominal Sepsis. J Invest Surg. 2016;29:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Gorur M, Sozutek A, Irkorucu O, Karakaya B. The influence of platelet-rich plasma (PRP) on colonic anastomosis healing impaired by intraperitoneal 5-flourouracil application. An experimental study. Acta Cir Bras. 2020;35:e202000504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Pehlivanli F, Karaca G, Aydin O, Altunkaya C, Şahiner İt, Özden H, Hafize U, Pekicici Mr. Effects of thymoquinone, zeolite and platelet rich plasma on the healing of ischemic colonic anastomosis. Kırıkkale Üniversitesi Tıp Fakültesi Derg. 2019;21:65-72. [DOI] [Full Text] |
| 34. | Ocak S, Buk OF, Genc B, Avcı B, Uzuner HO, Gundogdu SB. The effects of platelet-rich-plasma gel application to the colonic anastomosis in hyperthermic intraperitoneal chemotherapy: An experimental rat model. Int Wound J. 2019;16:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Buk OF, Ocak S, Genc B, Avcı B, Uzuner HO. Is platelet-rich plasma improves the anastomotic healing in hyperthermic intraperitoneal chemotherapy with oxaliplatin: an experimental rat study. Ann Surg Treat Res. 2020;98:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Yalı AC, Karagöz Eren S, Ertan T, Topuz Ö. Application of Platelet Rich Plasma in Experimental Colonic Anastomosis for Improved Strength. Indian J Surg. 2020;83:500-504. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Verhofstad MH, Lange WP, van der Laak JA, Verhofstad AA, Hendriks T. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum. 2001;44:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Kerem M, Bedirli A, Karahacioglu E, Pasaoglu H, Sahin O, Bayraktar N, Yilmaz TU, Sakrak O, Goksel F, Oguz M. Effects of soluble fiber on matrix metalloproteinase-2 activity and healing of colon anastomosis in rats given radiotherapy. Clin Nutr. 2006;25:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Munireddy S, Kavalukas SL, Barbul A. Intra-abdominal healing: gastrointestinal tract and adhesions. Surg Clin North Am. 2010;90:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Srivastava AK, Khare P, Nagar HK, Raghuwanshi N, Srivastava R. Hydroxyproline: A Potential Biochemical Marker and Its Role in the Pathogenesis of Different Diseases. Curr Protein Pept Sci. 2016;17:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 41. | Bucknall TE. The effect of local infection upon wound healing: an experimental study. Br J Surg. 1980;67:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Newton PM, Watson JA, Wolowacz RG, Wood EJ. Macrophages restrain contraction of an in vitro wound healing model. Inflammation. 2004;28:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 462] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 44. | Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996;32:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Lee SH, Lee JH, Cho KH. Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice. Ann Dermatol. 2011;23:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Huang SP, Hsu CC, Chang SC, Wang CH, Deng SC, Dai NT, Chen TM, Chan JY, Chen SG, Huang SM. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg. 2012;69:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Song SH, Lee MO, Lee JS, Jeong HC, Kim HG, Kim WS, Hur M, Cha HJ. Genetic modification of human adipose-derived stem cells for promoting wound healing. J Dermatol Sci. 2012;66:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 493] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 49. | Li YW, Lian P, Huang B, Zheng HT, Wang MH, Gu WL, Li XX, Xu Y, Cai SJ. Very Early Colorectal Anastomotic Leakage within 5 Post-operative Days: a More Severe Subtype Needs Relaparatomy. Sci Rep. 2017;7:39936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg. 2007;245:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 465] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 51. | Schiff A, Roy S, Pignot M, Ghosh SK, Fegelman EJ. Diagnosis and Management of Intraoperative Colorectal Anastomotic Leaks: A Global Retrospective Patient Chart Review Study. Surg Res Pract. 2017;2017:3852731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2017;32:549-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 53. | Yeap E, Teoh WMK, Nguyen TC, Suhardja TS. Preoperative anaemia and thrombocytopenia are associated with venous thromboembolism complications after colorectal resection. ANZ J Surg. 2021;91:E32-E37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Nikolian VC, Kamdar NS, Regenbogen SE, Morris AM, Byrn JC, Suwanabol PA, Campbell DA Jr, Hendren S. Anastomotic leak after colorectal resection: A population-based study of risk factors and hospital variation. Surgery. 2017;161:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 55. | Vertrees A, Wakefield M, Pickett C, Greer L, Wilson A, Gillern S, Nelson J, Aydelotte J, Stojadinovic A, Shriver C. Outcomes of primary repair and primary anastomosis in war-related colon injuries. J Trauma. 2009;66:1286-91; discussion 1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Oines MN, Krarup PM, Jorgensen LN, Agren MS. Pharmacological interventions for improved colonic anastomotic healing: a meta-analysis. World J Gastroenterol. 2014;20:12637-12648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Casella G, Soricelli E, Genco A, Ferrazza G, Basso N, Redler A. Use of platelet-rich plasma to reinforce the staple line during laparoscopic sleeve gastrectomy: feasibility study and preliminary outcome. J Laparoendosc Adv Surg Tech A. 2015;25:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Samadi P, Sheykhhasan M, Khoshinani HM. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast Surg. 2019;43:803-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 59. | Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1259] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 60. | Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1070] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yasukawa K S-Editor: Gao CC L-Editor: A P-Editor: Gao CC