Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1660
Peer-review started: May 21, 2021
First decision: June 22, 2021
Revised: July 16, 2021
Accepted: December 2, 2021
Article in press: December 2, 2021
Published online: December 27, 2021
Processing time: 216 Days and 13.3 Hours
As a common gastrointestinal malignancy, colorectal cancer (CRC) poses a serious health threat globally. Robotic surgery is one of the future trends in surgical treatment of CRC. Robotic surgery has several technical advantages over laparoscopic surgery, including 3D visualization, elimination of the fulcrum effect, and better ergonomic positioning, which together lead to better surgical outcomes and faster recovery. However, analysis of independent factors of postoperative complications after robotic surgery is still insufficient.
To analyze the incidence and risk factors for postoperative complications after robotic surgery in patients with CRC.
In total, 1040 patients who had undergone robotic surgical resection for CRC between May 2015 and May 2020 were analyzed retrospectively. Postoperative complications were categorized according to the Clavien-Dindo (C-D) classification, and possible risk factors were evaluated.
Among 1040 patients who had undergone robotic surgery for CRC, the overall, severe, local, and systemic complication rates were 12.2%, 2.4%, 8.8%, and 3.5%, respectively. Multivariate analysis revealed that multiple organ resection (P < 0.001) and level III American Society of Anesthesiologists (ASA) score (P = 0.006) were independent risk factors for overall complications. Multivariate analysis identified multiple organ resection (P < 0.001) and comorbidities (P = 0.029) as independent risk factors for severe complications (C-D grade III or higher). Regarding local complications, multiple organ resection (P = 0.002) and multiple bowel resection (P = 0.027) were independent risk factors. Multiple organ resection (P < 0.001) and level III ASA score (P = 0.007) were independent risk factors for systemic complications. Additionally, sigmoid colectomy had a lower incidence of overall complications (6.4%; P = 0.006) and local complications (4.7%; P = 0.028) than other types of colorectal surgery.
Multiple organ resection, level III ASA score, comorbidities, and multiple bowel resection were risk factors for postoperative complications, with multiple organ resection being the most likely.
Core Tip: This retrospective study of 1040 cases was performed to analyze the incidence and risk factors for postoperative complications after robotic colorectal cancer surgery. The postoperative complications were defined into four types: Overall, severe, local, and systemic complications, and their rates were 12.2%, 2.4%, 8.8%, and 3.5%, respectively. Their independent risk factors were as follows: (1) Overall complications: Multiple organ resection and a level III American Society of Anesthesiologists (ASA) score; (2) Severe complications: Multiple organ resection and comorbidities; (3) Local complications: Multiple organ resection and multiple bowel resection; and (4) Systemic complications: Multiple organ resection and a level III ASA score.
- Citation: Huang ZX, Zhou Z, Shi HR, Li TY, Ye SP. Postoperative complications after robotic resection of colorectal cancer: An analysis based on 5-year experience at a large-scale center. World J Gastrointest Surg 2021; 13(12): 1660-1672
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1660.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1660
As a common malignant tumor of the digestive tract, colorectal cancer (CRC) poses a serious health threat globally. According to the global tumor epidemiology statistics[1,2] released in 2020 by the International Agency for Research on Cancer of the World Health Organization, approximately 1931600 new cases of CRC and 935200 deaths occurred worldwide in 2020. The incidence and mortality of CRC are ranked third and second among all malignant tumors, respectively[3,4]. Overall, compared with the trend of stabilization or decline in developed countries, the incidence and mortality of CRC in developing countries have been rising slowly in recent years[5,6]. China accounts for 31% of the total number of patients with CRC globally, and 83% of patients in China are at an advanced stage when first diagnosed[1,7].
Surgical resection is the cornerstone of radical intent treatment[3]. Ensuring surgical operation quality is crucial because it is directly related to the patient’s survival and quality of life. With the emergence and development of laparoscopy and robotics, minimally invasive surgery (MIS) for CRC can substitute for conventional open surgery with similar or better perioperative and oncologic outcomes[8-10]. However, during laparoscopic surgery, surgeons are faced with challenging conditions, such as a narrow pelvic cavity, anatomical complexity, and restricted surgical view[11]. The da Vinci surgical system, which has several technical advantages, including 3D visualization, elimination of the fulcrum effect, and better ergonomic positioning, overcomes these limitations and is very likely leading to better surgical outcomes and faster recovery than laparoscopic surgery[12,13]. However, because of the lack of high-quality randomized controlled studies, analysis of independent factors of post
Considering the limitations of previous studies and lack of large-scale studies, we analyzed retrospectively more than 1040 cases of short-term postoperative complications after robotic surgery for CRC to assess related risk factors.
In this retrospective clinical study, we gathered and analyzed the information of 1302 patients who underwent robotic surgery for CRC between May 2015 and May 2020 at the First Affiliated Hospital of Nanchang University, a large-scale center. The inclusion criteria were as follows: (1) Age older than 18 and younger than 80 years; (2) Primary colonic adenocarcinoma confirmed pathologically by endoscopic biopsy; (3) Path
Patients who met the diagnostic criteria of related diseases were all subjected to routine preoperative chest X-ray, abdominal ultrasound, tumor markers, abdominal computed tomography, colonoscopy, magnetic resonance imaging, and other examinations to improve the evaluation of the patient's staging and condition. All the patients’ medical records were extracted from the prospectively maintained database at the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Nanchang University. This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University.
For information regarding surgical principles and procedures, the Chinese expert consensus on robotic surgery for CRC[15] should be referenced. In all cases, the surgical approach was to remove the colon and mesocolon of adjacent organs within the range of resection, cut the tumor-bearing segment, and ligate the origin of the aorta to maximize lymph node dissection (LND) without damaging the visceral fascia layer. The surgeon attempted to secure 10 cm or more for the proximal and distal resection margins (over 5 cm distal margin for rectosigmoid lesions). For colon resection and rectal resection, we followed D3 LND (D3) + complete mesocolic excision principles[17-19] and total mesorectal excision (TME) principles[20-22], respectively.
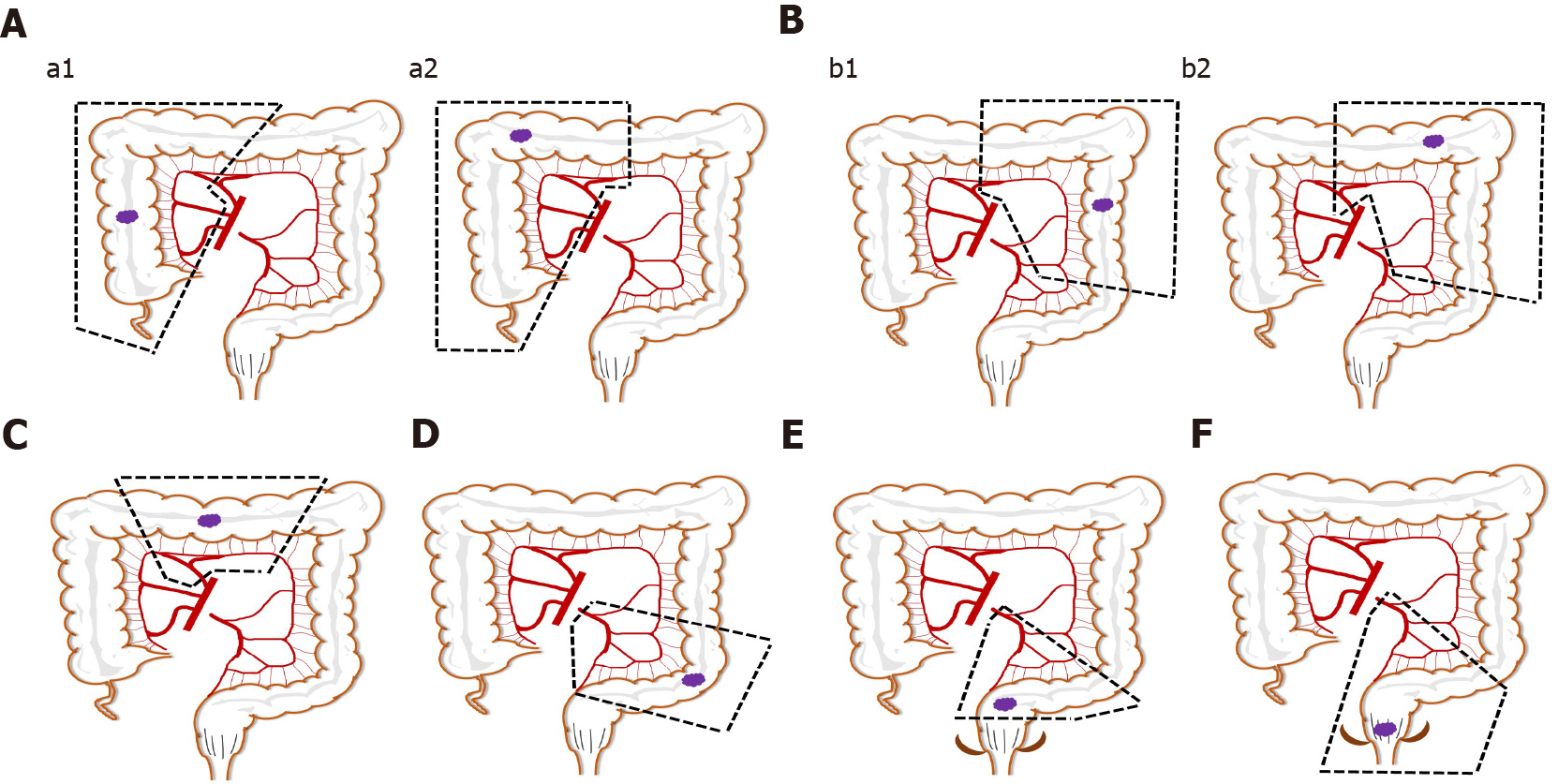
Different surgical methods were applied to tumors in different areas of invasion, and they have different characteristics (Figure 1). Right hemicolectomy or extended right hemicolectomy cases were included in group A (right colon resection). LND was performed along the superior mesenteric pedicle, including its front side, with high ligation of the ileocolic vessels, middle colic vessels (for hepatic flexure and proximal transverse colon lesion), or right branch of the middle colic vessels (for lesions proximal to hepatic flexure colon). Left hemicolectomy or extended left hemicolectomy cases were included in group B (left colon resection). LND was performed on the origin site of the middle colic vessels (left branch of the middle colic vessels for left hemicolectomy) and the origin site of the left colic artery for complete removal of the mesocolon. Full splenic flexure mobilization was also required for all patients in these cases. For transverse colectomy (group C), LND was only performed on the origin site of the middle colic vessels, and the gastroepiploic vessels were only meticulously dissected, instead of routinely ligated. Sigmoid colectomy (group D) cases required LND only around the inferior mesenteric artery (IMA). The surgical treatment of rectal cancer mainly included low anterior resection (LAR) of rectal cancer (group E) and abdominoperineal resection (group F). Although the scope of resection is different, the scope of LND involves the origin site of IMA. All of the above procedures only involve resection of one bowel segment of the primary tumor, hereinafter referred to as single bowel resection. When at least two primary tumor lesions invaded different parts of the intestine, multiple bowel resection (group G), simultaneous resections of multiple bowel segments of primary tumors, or even (sub-) total colectomy was applied. Multiple organ resection was performed in cases with peripheral organ tumor invasion or organ diseases requiring surgery.
In our center, there are two types of robotic surgery for CRC: Totally robotic surgery and robot-assisted surgery. Totally robotic surgery uses robotic arms to complete the process of naked intestine, anastomosis, cutting, reinforcement, and removal in the abdominal cavity under the field of endoscopy. Robot-assisted surgery is used to pull out the intestine segment from an additional auxiliary incision after dissection and nakedness by robotic arms in the abdominal cavity, and to complete the process of anastomosis, cutting and reinforcing under direct vision. Surgical procedures for totally robotic CRC resection or robotic-assisted resection have been previously described in detail[6,23]. All robotic surgery procedures were performed by surgeons experienced in laparoscopic surgery for CRC.
The patients’ general demographics data were as follows: Age, sex, body mass index (BMI), history of abdominal surgery, smoking and drinking history, comorbidity (e.g., diabetes, cardiopathy, hypertension, and other basic diseases). The surgical parameters of the patients were as follows: ASA-class, operation time, intraoperative evaluated blood loss, types of colorectal surgery (e.g., right resection, left resection, sigmoid colectomy, rectal resection and multiple bowel resection), types of robotic surgery (e.g., totally robotic or robotic-assisted), number of retrieved lymph nodes, multiple organ resection (cases with peripheral organs tumor invasion or organ diseases requiring surgery), operation number per year. The pathology parameters were as follows: Diameter of the neoplasm, histological type, pathological tumor, node and metastasis (TNM) stage, number of metastatic lymph nodes, lymphovascular invasion, resection margin. The postoperative complications were recorded using the Clavien-Dindo (C-D) classification and divided into local and systemic complications[24,25].
The primary outcomes of the study were postoperative complications. When complications were associated with surgical techniques near the field of operation, such as wounds or anastomosis, they were considered local complications. Complications were classified as systemic when they were not associated with the field of operation, such as pulmonary or hepatic complications. We reviewed morbidity and mortality that occurred during hospitalization after surgery.
All statistical analyses were performed using SPSS, ver.26.0 (IBM Corp., Armonk, NY, United States). Categorical variables were presented as counts and percentages. Normally distributed continuous variables were expressed as mean ± SD. Variables with P values less than 0.05 in univariate analysis were included in the multivariate analysis. Multivariate analysis was conducted using the logistic regression model to identify independent risk factors for postoperative complications. P values less than 0.05 were considered statistically significant.
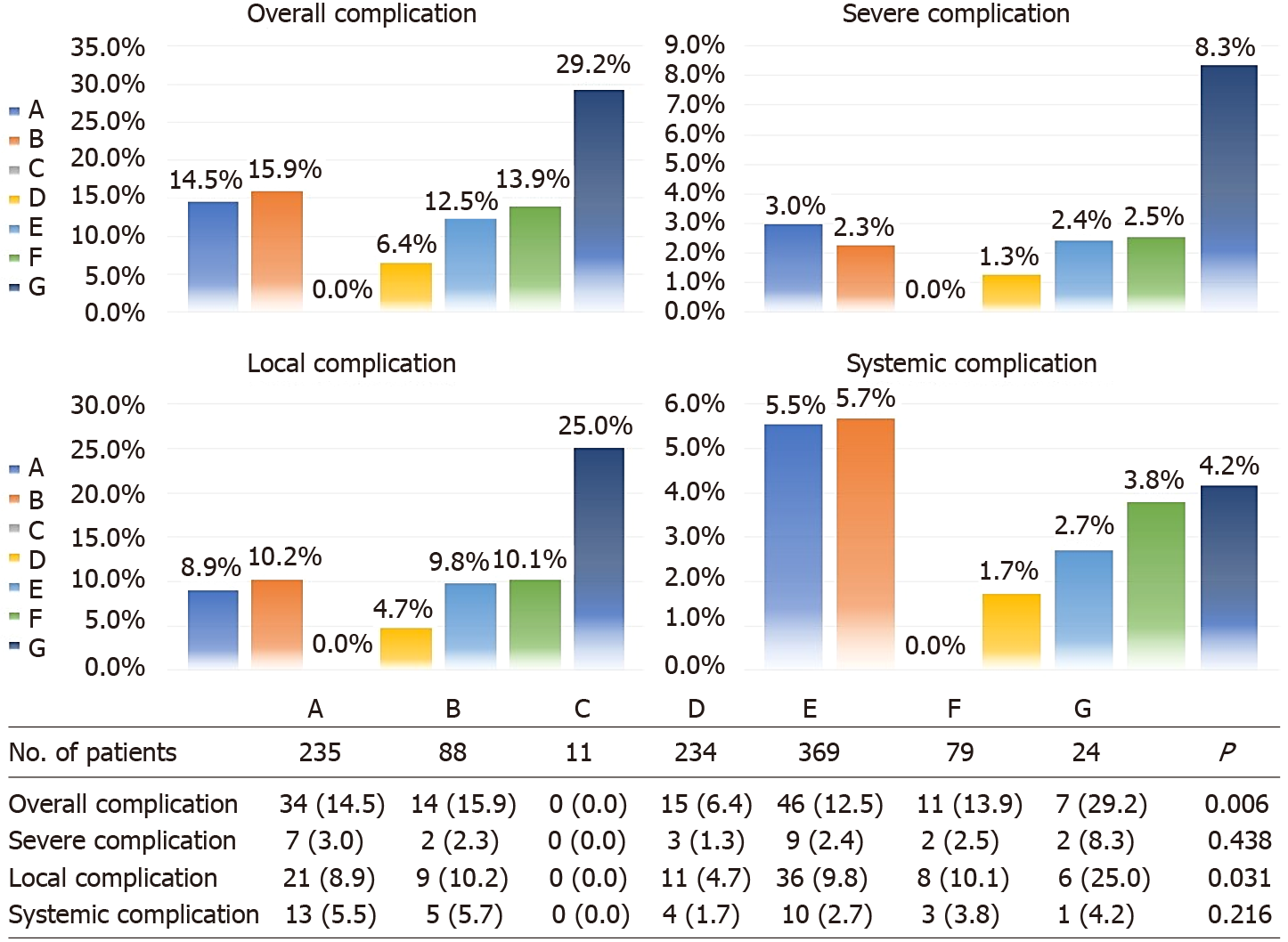
Table 1 shows the patient demographics, baseline pathologic characteristics and perioperative outcomes. Of the 1040 patients, 133 had a history of abdominal surgery, and 239 had other comorbidities, such as diabetes, hypertension, and heart disease. Regarding operative parameters, approximately 12.4% of surgical patients were rated as class III by anesthesiologists using the ASA classification standard. The mean operation time and evaluated blood loss were 173.6 ± 51.1 min and 108.4 ± 87.3 mL, respectively. In total, 235 right colon resections, 88 left colon resections, 11 transverse colectomies, 234 sigmoid colectomies, 369 LARs, and 79 abdominoperineal resections were performed. Multiple bowel resection was applied to 24 cases (2.3%) with multiple cancer foci inside the intestinal tube. The number of totally robotic (507 cases) and robotic-assisted (533 cases) surgeries performed was similar. Thirty-six cases (3.5%) involved multiple organ resection, including seven cases with partial small bowel enterectomy, six cases of oophorocystectomy, four cases of cholecystectomy, three cases of cystectomy, three cases of gastrectomy, three cases of hysterectomy, two cases of pneumonectomy, two cases of adnexectomy, two cases of splenectomy, two cases of nephrectomy, one case of partial hepatectomy, and one case of appendectomy.
| Variables | Total (n = 1040) |
| Patient demographics | |
| Age (yr) | 60.4 ± 12.4 |
| Sex (male/female) | 611/429 |
| BMI (kg/m2) | 22.5 ± 3.2 |
| With previous abdominal surgery, no. (%) | 133 (12.8) |
| Smoking and drinking history, no. (%) | 426 (41.0) |
| Comorbidity, no. (no/one or more) | 239 (23.0%) |
| Operative parameters | |
| ASA class, no. (I/II/III) | 593/518/129 |
| Operation time (min) | 173.6 ± 51.1 |
| Evaluated blood loss (mL) | 108.4 ± 87.3 |
| Types of colorectal surgery, no. (right-/left-/transverse-/sigmoid-/LAR/abdominoperineal-/multiple-) | 235/88/11/234/369/79/24 |
| Types of robotic surgery, no. (totally robotic/robotic-assisted) | 507/533 |
| No. lymph nodes retrieved | 17.8 ± 7.5 |
| Multiple organ resection, no. (%) | 36(3.5) |
| Operation number, no. (yr) | |
| 2015/5-2016/5 | 226 (21.7%) |
| 2016/5-2017/5 | 226 (21.7%) |
| 2017/5-2018/5 | 259 (24.9%) |
| 2018/5-2019/5 | 280 (26.9%) |
| 2019/5-2020/5 | 311 (29.9%) |
| Pathology results | |
| Neoplasm longest diameter, cm | 4.5 ± 2.3 |
| Histological type, no. (well or moderately/poorly or undifferentiated) | 947/93 |
| pT stage, no. (T1/T2/T3/T4) | 107/126/218/589 |
| pN stage, no. (0/1/2) | 659/252/129 |
| pTNM stage, no. (I/II/III) | 197/462/381 |
| With lymph node metastasis, no. (%) | 381 (36.6) |
| With lymphovascular invasion, no. (%) | 423 (40.7) |
| With positive resection margin, no. (%) | 8 (0.8) |
| In-hospital outcomes | |
| Time to 1st bowel movement, h | 25.4 ± 6.3 |
| Time to 1st first flatus, h | 58.6 ± 8.9 |
| Time to 1st liquid diet, h | 71.5 ± 9.3 |
| Overall complications, no. (%) | 127 (12.2) |
| Complications, no. (II/III/IV/V) | 20/82/15/6/4 |
| Severe complication, no. (C-D grade ≥ III, %) | 25 (2.4) |
| Local complications, no. (%) | 91 (8.8) |
| Systemic complication, no. (%) | 36 (3.5) |
| Mortality, no. (%) | 4 (0.4) |
| Postoperative hospital stay of all patients (d) | 7.4 ± 2.3 |
| Postoperative hospital stay of patients without complications (d) | 6.5 ± 1.1 |
| Postoperative hospital stay of patients with complications (d) | 14.1 ± 5.2 |
Regarding the in-hospital outcomes, the overall complication rate was 12.2%, the severe complication rate was 2.4%, and the mortality rate was 0.4%.
The local and systemic complications classified by C-D are shown in Table 2. The incidence of local complication was 8.8%, among which anastomotic leakage was the most common, followed by wound problems, intra-abdominal infection, and effusion. Three cases of anastomosis leakage and one case of intra-abdominal bleeding required reoperation under intravenous or inhalation anesthesia. The systemic complication rate was 3.5%, among which hematologic complications were the most common, with severe anemia (13 cases) accounting for the majority, followed by coagulation abnormalities (2 cases). Four patients died after surgery: Three from severe infection leading to shock and one from severe pneumonia resulting in respiratory failure.
| Local complication | Total n (%) | Grade ≥ III | Systemic complication | Total n (%) | Grade ≥ III |
| Wound problem | 14 (1.3) | 0 (0.0) | Pulmonary | 9 (0.9) | 2 (0.2) |
| Anastomosis leakage | 43 (4.1) | 9 (0.9) | Hepatic | 0 (0.0) | 0 (0.0) |
| Intra-abdominal infection and effusion | 12 (1.2) | 3 (0.3) | Cardiovascular | 2 (0.2) | 1 (0.1) |
| Intra-abdominal bleeding | 2 (0.2) | 1 (0.1) | Urinary | 2 (0.2) | 0 (0.0) |
| Anastomosis bleeding | 3 (0.3) | 0 (0.0) | Central nervous | 2 (0.2) | 2 (0.2) |
| Ileus/motility disorder | 9 (0.9) | 2 (0.2) | Hematologic | 15 (1.4) | 0 (0.0) |
| Infection of presacral space | 4 (0.4) | 0 (0.0) | Infection | 6 (0.6) | 5 (0.5) |
| Others | 4 (0.4) | 0 (0.0) | Endocrine | 0 (0.0) | 0 (0.0) |
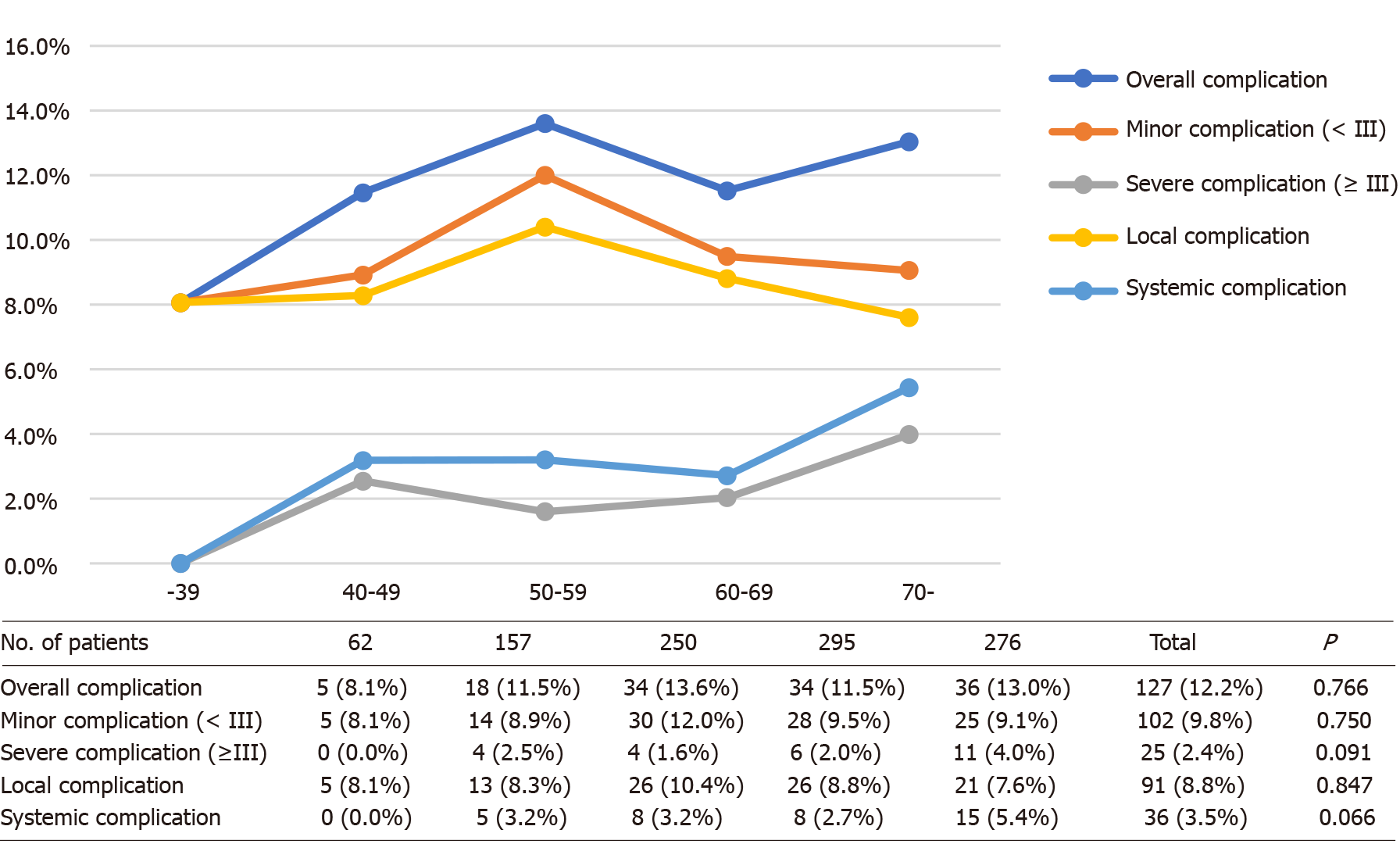
Overall complication rates among the five different age groups were similar (P = 0.766), as well as when broken down for minor (P = 0.750), severe (P = 0.091), local (P = 0.847), and systemic (P = 0.066) complications (Figure 2). Considering the trend of the broken line in Figure 2, the severe and systemic complication rates generally increased with age, and significant differences were found between the group aged older than 70 years and the other age groups (Supplementary Table 1). Postoperative complication rates in subgroups of CRC surgery approaches are outlined in Figure 3. The differences in the complication rates were significant among the seven types of colorectal surgery, including the overall (P = 0.006 < 0.10) and local (P = 0.031 < 0.10) complication rates. These differences may be caused by sigmoid colectomy (P = 0.002 for overall complications and P = 0.013 for local complications) or multiple bowel resection (P = 0.020 for overall complications and P = 0.013 for local complications) (Supplementary Table 2). Therefore, in multivariate analysis, we divided the types of colorectal surgery into three categories for comparison — multiple bowel resection, sigmoid colectomy and the other surgery types.
Univariate analyses for overall and severe complications are demonstrated in Supplementary Table 3. Multivariate analysis revealed that multiple organ resection (P < 0.001) and a level III ASA score (P = 0.006) were independent risk factors for overall complications, and multiple organ resection (P < 0.001) and comorbidities (P = 0.029) were independent risk factors for severe complications (C-D grade III or higher) (Supplementary Table 4).
Univariate analyses for local and systemic complications are outlined in Supplementary Table 5. For local complications, multiple organ resection (P = 0.002) and multiple bowel resection (P = 0.027) were identified as independent risk factors. Multiple organ resection (P < 0.001) and a level III ASA score (P = 0.007) were identified as independent risk factors for systemic complications. Additionally, sigmoid colectomy was identified as an independent protective factor for overall (P = 0.006) and local (P = 0.028) complications (Supplementary Table 6).
For CRC, MIS is now increasingly accepted and applied. Many clinical trials have shown that short-term outcomes after robotic surgery for CRC are better than those after laparoscopic surgery[26-29]. Robotic surgery is considered more accurate and reliable, reducing trauma and improving the quality of life while ensuring radical resection of the tumor[30,31]. However, the Jayne et al[14]’s study, a multicenter randomized clinical trial, found that robotic surgery performed by surgeons with varying robotic experience did not provide clinically important benefits over conventional laparoscopic surgery in the short term. In our study, which only included patients with malignant disease who had undergone robotic surgery at a single institution, the quality of the surgical procedures was consistently high and the data were sufficiently reliable. Additionally, chief surgeons had completed an initial phase of more than 30 cases[32] before 2015 and could master operations proficiently. Comparing the above two studies, we found that some in-hospital outcomes were numerically superior in our study, such as the mean length of stay (7.3 d vs 8.0 d), overall complications (12.2% vs 33.1%), and incidence of anastomotic fistula (4.1% vs 12.2%). Regarding the huge gap between the two studies, potential reasons may be responsible, such as the limited case volume and inadequate surgical experience that may compromise the quality of surgery[33,34]. A retrospective study[35] of robot-assisted colorectal surgery with the largest sample size worldwide verified the safety and efficacy of robotic techniques and confirmed its clinical advantages, particularly in reducing anastomotic fistulas. The short-term outcomes of our study, a low incidence of anastomoses (approximately 4%), and a short recovery time, were similar to those of this retrospective study except for mortality (0.1%, 6/5389 cases vs 0.4%, 4/1040 cases) and morbidity (9%, 487/5389 cases vs 12.2%, 127/1040 cases). Analysis of its data found that the incidence of complications that are C-D III or above accounted for 2.4% (129/5389 cases vs 25/1040 cases, 2.4%) in all patients. Among patients with CRC suitable for curative resection, compared with conventional laparoscopic surgery, the robotic procedure performed at an experienced medical unit resulted in more favorable clinical outcomes[14,35].
Many researchers have begun to analyze the different significant factors associated with complications after colorectal surgery. Manilich et al[33] examined the records of 3552 patients who had undergone colorectal surgery and concluded that BMI, operative time, and chief surgeon were the three most important factors influencing the re-admission rates, rates of transfusions, and surgical site infection. Kirchhoff et al[36] found that, of the 20 general background factors analyzed, the following 5 were significant factors for complications following laparoscopic colorectal procedures as an initial report: The surgeon’s level of experience, patient age, patient sex, ASA class, and neoplasia. The real world data of 1145 consecutive cases in China[37] revealed that male sex, tumors located in the mid-low rectum, combined organ resection, and clinical T category (cT3-4) were independent risk factors for robotic surgical complications.
In the present study, 21 general background variables were analyzed by univariate analysis, among which 5 were identified as significant factors: Age, comorbidity, ASA class, type of colorectal surgery, and multiple organ resection. Finally, age was excluded from the multivariate analysis of risk factors for all complications. Generally, elderly patients are considered a high-risk population for major abdominal surgery because of reduced functional reserve and increased comorbidities[38,39]. Some studies[40-43] have confirmed that aging is an independent risk factor for postoperative complications. Additionally, systemic complications are related to the increase in preoperative adverse conditions and comorbidities. We found that only severe and systemic complication rates increased mildly with age. Additionally, postoperative complications in elderly patients (age ≥ 70) tend to be more severe than those in nonelderly patients. Therefore, during preoperative assessment and postoperative management, medical personnel must focus more on patients aged 70 years and older. The incidence and severity of postoperative complications among elderly patients who had undergone robotic surgery were similar to those who had undergone laparoscopic surgery[44-46].
In our study, multiple organ resection was considered to be a primary independent risk factor for overall, severe, local, and systemic complications after robotic surgery. Chang et al[37] reported that combined organ resection was confirmed as an independent risk factor for surgical complications and significantly increased the risk of anastomotic fistula. The conclusions of other studies[47,48] were similar. The complex procedure of intraperitoneal surgery not only poses a challenge to the surgeon but is also a potential risk factor for postoperative complications. Add
This study has several limitations. First, this retrospective study involved only one single center where experienced surgeons operated on patients. This would limit the promotion to the population of physicians with less experience in robotic resection. Second, this study excluded patients with neoadjuvant therapy, which would limit the universality of our research results. Additionally, selection bias might influence the results, and the follow-up period was relatively short. Thus, the factors identified in this study require confirmation in future research.
The present study demonstrated, in detail, the postoperative complications of robotic surgery treating patients with CRC and identified several independent and significant predictors of the complication rate after robotic CRC surgery. Among them, multiple organ resection was the greatest independent risk factor for complications. We recommend that complex surgical procedures are best performed by experienced surgeons. Additionally, patients' comorbidities should be improved preoperatively, and more attention should be given to follow-up to prevent postoperative complications related to different surgical types.
As a common malignant tumor of the digestive tract, colorectal cancer (CRC) poses a serious health threat globally. Robotic surgery for the treatment of CRC is one of the future trends in surgical treatment. With several technical advantages of 3D visualization, elimination of the fulcrum effect, and better ergonomic positioning, the da Vinci surgical system is better than laparoscope and these technical benefits lead to better surgical outcomes and faster recovery. However, it is impossible to accurately explain which factors will affect the complications of robotic surgery because of the lack of high-quality randomized controlled studies.
To provide new ideas and directions for reducing complications, through the analysis of incidence and risk factors for postoperative complications after robotic surgery in patients with CRC.
To analyze the incidence and risk factors for postoperative complications after robotic surgery in patients with CRC.
In total, 1040 patients who had undergone robotic surgical resection for CRC between May 2015 and May 2020 were analyzed retrospectively. Postoperative complications were classified as minor complications, severe complications, local complications, and systemic complications, and their possible risk factors were assessed. Variables that were statistically significant (P < 0.05) in univariate analysis were included in multivariate analysis. To identify independent risk factors for postoperative complications, the logistic regression model was used in multivariate analysis.
Among 1040 patients who had undergone robotic surgery for CRC, the overall, severe, local, and systemic complication rates were 12.2%, 2.4%, 8.8%, and 3.5%, respectively. Multivariate analysis revealed that multiple organ resection (P < 0.001) and a level III American Society of Anesthesiologists (ASA) score (P = 0.006) were independent risk factors for overall complications. Multivariate analysis identified multiple organ resection (P < 0.001) and comorbidities (P = 0.029) as independent risk factors for severe complications (Clavien-Dindo grade III or higher). Regarding local complications, multiple organ resection (P = 0.002) and multiple bowel resection (P = 0.027) were identified as independent risk factors. Multiple organ resection (P < 0.001) and a level III ASA score (P = 0.007) were identified as independent risk factors for systemic complications. Additionally, sigmoid colectomy had a lower incidence of overall complications (6.4%; P = 0.006) and local complications (4.7%; P = 0.028) than other types of colorectal surgery.
The present study demonstrated, in detail, the postoperative complications of robotic procedure to treating patients with CRC, and identified several factors that were independent and significant predictors of the complication rate after robotic CRC surgery. Among them, multiple organ resection was the greatest independent risk factor for complications.
The development of robotic surgery is unstoppable, and the application of robotic surgery to CRC will become more and more widespread. Therefore, research on the risk factors of complications is essential. It will not only provide the possibility to reduce complications in the future but also promote the development of robotic surgery.
| 1. | World Health Organization (WHO). World cancer report: cancer research for cancer prevention. Wild C, Weiderpass E, Stewart B, editor. Lyon: International Agency for Research on Cancer, 2020: 23-33. |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68641] [Article Influence: 13728.2] [Reference Citation Analysis (201)] |
| 3. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3426] [Article Influence: 489.4] [Reference Citation Analysis (4)] |
| 4. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3516] [Article Influence: 390.7] [Reference Citation Analysis (4)] |
| 5. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3363] [Article Influence: 560.5] [Reference Citation Analysis (2)] |
| 6. | Ye SP, Zhu WQ, Liu DN, Lei X, Jiang QG, Hu HM, Tang B, He PH, Gao GM, Tang HC, Shi J, Li TY. Robotic- vs laparoscopic-assisted proctectomy for locally advanced rectal cancer based on propensity score matching: Short-term outcomes at a colorectal center in China. World J Gastrointest Oncol. 2020;12:424-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | He Y, Liang D, Li D, Shan B, Zheng R, Zhang S, Wei W, He J. Incidence and mortality of laryngeal cancer in China, 2015. Chin J Cancer Res. 2020;32:10-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Colon Cancer Laparoscopic or Open Resection Study Group; Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1076] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 9. | Clinical Outcomes of Surgical Therapy Study Group; Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2541] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 10. | Ye SP, Qiu H, Liao SJ, Ai JH, Shi J. Mini-invasive vs open resection of colorectal cancer and liver metastases: A meta-analysis. World J Gastroenterol. 2019;25:2819-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Cheng CL, Rezac C. The role of robotics in colorectal surgery. BMJ. 2018;360:j5304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Hu Y, Strong VE. Robotic Surgery and Oncologic Outcomes. JAMA Oncol. 2020;6:1537-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Bednarski BK, Nickerson TP, You YN, Messick CA, Speer B, Gottumukkala V, Manandhar M, Weldon M, Dean EM, Qiao W, Wang X, Chang GJ. Randomized clinical trial of accelerated enhanced recovery after minimally invasive colorectal cancer surgery (RecoverMI trial). Br J Surg. 2019;106:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA. 2017;318:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1015] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 15. | Professional Committee of Robotic Surgery; Colorectal Cancer Committee of Chinese Medical Doctor Association. Robotic and Laparoscopic Surgery Committee of Chinese Research Hospital Association. [Chinese expert consensus on robotic surgery for colorectal cancer (2020 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 16. | American Joint Committee on Cancer. Colon and rectum. AJCC Cancer Staging Manual. 8th ed. Mahul BA, Stephen BE, Frederick LG, editor. New York, NY: Springer Nature, 2017: 251–264. |
| 17. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-64; discussion 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1150] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 18. | Melich G, Jeong DH, Hur H, Baik SH, Faria J, Kim NK, Min BS. Laparoscopic right hemicolectomy with complete mesocolic excision provides acceptable perioperative outcomes but is lengthy--analysis of learning curves for a novice minimally invasive surgeon. Can J Surg. 2014;57:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Kim MK, Lee IK, Kye BH, Kim JG. Procedural difficulty differences according to tumor location do not compromise the clinical outcome of laparoscopic complete mesocolic excision for colon cancer: a retrospective analysis. Oncotarget. 2017;8:64509-64519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1069] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 21. | Wibe A, Møller B, Norstein J, Carlsen E, Wiig JN, Heald RJ, Langmark F, Myrvold HE, Søreide O; Norwegian Rectal Cancer Group. A national strategic change in treatment policy for rectal cancer--implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum. 2002;45:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 435] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 345] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 23. | Liu D, Li J, He P, Tang C, Lei X, Jiang Q, Li T. Short-and long-term outcomes of totally robotic versus robotic-assisted right hemicolectomy for colon cancer: A retrospective study. Medicine. 2019;98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Duraes LC, Stocchi L, Steele SR, Kalady MF, Church JM, Gorgun E, Liska D, Kessler H, Lavryk OA, Delaney CP. The Relationship Between Clavien-Dindo Morbidity Classification and Oncologic Outcomes After Colorectal Cancer Resection. Ann Surg Oncol. 2018;25:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9228] [Article Influence: 542.8] [Reference Citation Analysis (1)] |
| 26. | Sheng S, Zhao T, Wang X. Comparison of robot-assisted surgery, laparoscopic-assisted surgery, and open surgery for the treatment of colorectal cancer: A network meta-analysis. Medicine (Baltimore). 2018;97:e11817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Ng KT, Tsia AKV, Chong VYL. Robotic Versus Conventional Laparoscopic Surgery for Colorectal Cancer: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. World J Surg. 2019;43:1146-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis of eight studies. J Gastrointest Surg. 2015;19:516-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Gómez Ruiz M, Lainez Escribano M, Cagigas Fernández C, Cristobal Poch L, Santarrufina Martínez S. Robotic surgery for colorectal cancer. Ann Gastroenterol Surg. 2020;4:646-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Kim HJ, Choi GS, Park JS, Park SY, Yang CS, Lee HJ. The impact of robotic surgery on quality of life, urinary and sexual function following total mesorectal excision for rectal cancer: a propensity score-matched analysis with laparoscopic surgery. Colorectal Dis. 2018;20:O103-O113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Park EJ, Baik SH. Robotic Surgery for Colon and Rectal Cancer. Curr Oncol Rep. 2016;18:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Barrie J, Jayne DG, Wright J, Murray CJ, Collinson FJ, Pavitt SH. Attaining surgical competency and its implications in surgical clinical trial design: a systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol. 2014;21:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Manilich E, Vogel JD, Kiran RP, Church JM, Seyidova-Khoshknabi D, Remzi FH. Key factors associated with postoperative complications in patients undergoing colorectal surgery. Dis Colon Rectum. 2013;56:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Asari SA, Cho MS, Kim NK. Safe anastomosis in laparoscopic and robotic low anterior resection for rectal cancer: a narrative review and outcomes study from an expert tertiary center. Eur J Surg Oncol. 2015;41:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Xu J, Tang B, Li T, Jia B, Yao H, Zhao R, Yuan W, Zhong M, Chi P, Zhou Y, Yang X, Cheng L, He Y, Li Y, Tong W, Sun X, Jiang Z, Wang K, Li X, Wang X, Wei Y, Chen Z, Zhang X, Ye Y, Han F, Tao K, Kong D, Wang Z, Zhang C, He G, Feng Q. Robotic colorectal cancer surgery in China: a nationwide retrospective observational study. Surg Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Kirchhoff P, Dincler S, Buchmann P. A multivariate analysis of potential risk factors for intra- and postoperative complications in 1316 elective laparoscopic colorectal procedures. Ann Surg. 2008;248:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Chang W, Wei Y, Ren L, Jian M, Chen Y, Chen J, Liu T, Huang W, Peng S, Xu J. Short-term and long-term outcomes of robotic rectal surgery-from the real word data of 1145 consecutive cases in China. Surg Endosc. 2020;34:4079-4088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 39. | Richardson JD, Cocanour CS, Kern JA, Garrison RN, Kirton OC, Cofer JB, Spain DA, Thomason MH. Perioperative risk assessment in elderly and high-risk patients. J Am Coll Surg. 2004;199:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Hida K, Yamaguchi T, Hata H, Kuroyanagi H, Nagayama S, Tada H, Teramukai S, Fukushima M, Koizumi K, Sakai Y. Risk factors for complications after laparoscopic surgery in colorectal cancer patients: experience of 401 cases at a single institution. World J Surg. 2009;33:1733-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Zhang X, Wang Z, Chen J, Wang P, Luo S, Xu X, Mai W, Li G, Wang G, Wu X, Ren J. Incidence and risk factors of surgical site infection following colorectal surgery in China: a national cross-sectional study. BMC Infect Dis. 2020;20:837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Chan DKH, Ang JJ, Tan JKH, Chia DKA. Age is an independent risk factor for increased morbidity in elective colorectal cancer surgery despite an ERAS protocol. Langenbecks Arch Surg. 2020;405:673-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Wang D, Zhang J, Bai Z, Yang Y, Wang T, Jin L, Wang J, Wu G, Kou T, Zhang Z. Associations of Postoperative Complications Assessed by Clavien-Dindo Classification and Comprehensive Complication Index with Long-Term Overall Survival in Elderly Patients after Radical CRC Resection. Clin Interv Aging. 2020;15:1939-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Devoto L, Celentano V, Cohen R, Khan J, Chand M. Colorectal cancer surgery in the very elderly patient: a systematic review of laparoscopic versus open colorectal resection. Int J Colorectal Dis. 2017;32:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | de'Angelis N, Abdalla S, Bianchi G, Memeo R, Charpy C, Petrucciani N, Sobhani I, Brunetti F. Robotic Versus Laparoscopic Colorectal Cancer Surgery in Elderly Patients: A Propensity Score Match Analysis. J Laparoendosc Adv Surg Tech A. 2018;28:1334-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Germain A, Perrenot C, Scherrer ML, Ayav C, Brunaud L, Ayav A, Bresler L. Long-term outcome of robotic-assisted laparoscopic rectopexy for full-thickness rectal prolapse in elderly patients. Colorectal Dis. 2014;16:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Lee KG, Lee HJ, Yang JY, Oh SY, Bard S, Suh YS, Kong SH, Yang HK. Risk factors associated with complication following gastrectomy for gastric cancer: retrospective analysis of prospectively collected data based on the Clavien-Dindo system. J Gastrointest Surg. 2014;18:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Li ZY, Zhao YL, Qian F, Tang B, Chen J, Zhang F, Li PA, Luo ZY, Shi Y, Yu PW. Incidence and risk factors of postoperative complications after robotic gastrectomy for gastric cancer: an analysis of 817 cases based on 10-year experience in a large-scale center. Surg Endosc. 2021;35:7034-7041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A, Stamos MJ. Ureteral injuries in colorectal surgery: an analysis of trends, outcomes, and risk factors over a 10-year period in the United States. Dis Colon Rectum. 2014;57:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Caycedo-Marulanda A, Cianci P, Hashida H S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ