Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1550
Peer-review started: April 19, 2021
First decision: June 13, 2021
Revised: June 27, 2021
Accepted: November 30, 2021
Article in press: November 30, 2021
Published online: December 27, 2021
Processing time: 248 Days and 11.7 Hours
The incidence of hepatocellular carcinoma (HCC) remains high globally. Surgical treatment is the best treatment for improving the prognosis of patients with HCC. Neoadjuvant therapy plays a key role in preventing tumor progression and even downstaging HCC. The liver transplantation rate and resectability rate have increased for neoadjuvant therapy. Neoadjuvant therapy is effective in different stages of HCC. In this review, we summarized the definition, methods, effects, indications and contraindications of neoadjuvant therapy in HCC, which have significance for guiding treatment.
Core Tip: Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world. A considerable number of patients cannot receive radical therapy due to advanced HCC at the first diagnosis, leading to a poor prognosis. Neoadjuvant treatment enables more patients with HCC inside or outside the Milan criteria to receive surgical treatment, such as partial liver resection and liver transplantation. In this study, we reviewed the current status of neoadjuvant therapy in HCC.
- Citation: Xu L, Chen L, Zhang W. Neoadjuvant treatment strategies for hepatocellular carcinoma. World J Gastrointest Surg 2021; 13(12): 1550-1566
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1550.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1550
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide[1]. The incidence and mortality of HCC are still increasing in most parts of the world, including China[2]. Viral hepatitis B is the main risk factor for HCC in East Asia and Africa, while nonalcoholic fatty liver disease is becoming an important risk factor in developed countries[1,3,4]. For patients with HCC with surgical indications, surgery [liver resection (LR) and liver transplantation (LT)] is the best treatment for improving their prognosis, with a 5-year survival rate of 60%-80%[5]. However, many patients are beyond the indications for surgery due to advanced tumor stage or severe liver disease at the time of diagnosis, leading to a median overall survival between 3 and 26 mo[6,7].
Neoadjuvant therapy is a new concept of multidisciplinary treatment for malignancies to prevent tumor progression and even downstage solid tumors in recent years[8]. Neoadjuvant therapies for HCC include transcatheter embolization (TACE), radiotherapy, ablation therapy, chemotherapy, targeted therapy and immunotherapy[9]. LT is the optimal treatment for HCC and liver cirrhosis, but many patients with HCC outside the Milan criteria are not suitable candidates for LT[10]. With neoa
Over the past decade, the overall survival rate of patients who underwent LT has continued to rise. Due to the shortage of livers for transplantation (even patients with HCC within the Milan criteria need to wait for liver donors), the dropout rate during the waiting period remains high[16]. Increasing tumor burden during the waiting period is also detrimental to survival after transplantation. In addition, one of the major factors for the poor prognosis of patients with HCC is the low resectability rate, which is only approximately 20%[17]. How to slow the progression of tumors before surgical treatment and lower the tumor stage to surgical indications is the focus of oncologists and surgeons, and this is the significance of neoadjuvant therapy for HCC.
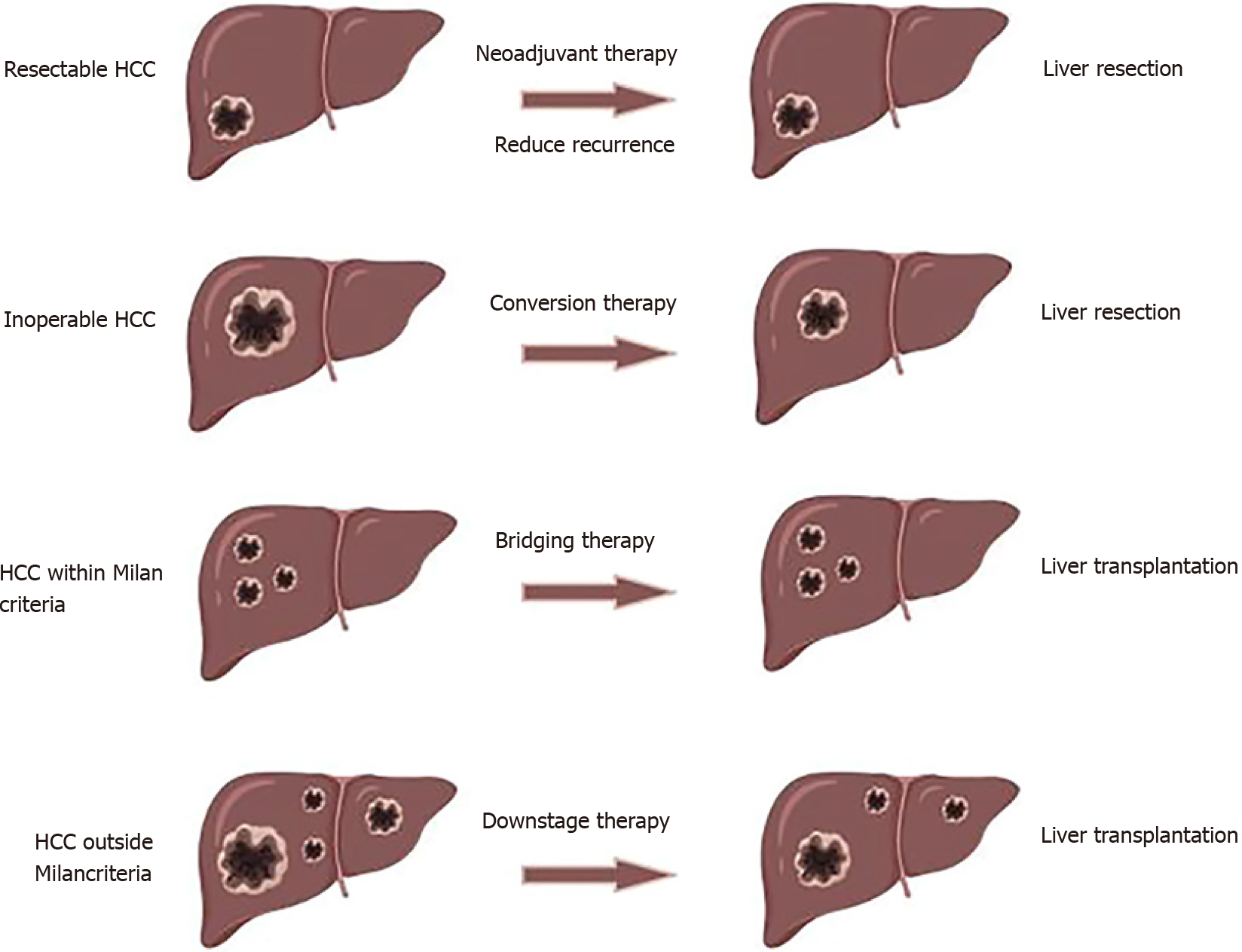
When defining neoadjuvant therapy, we have to distinguish between bridging, downstaging and conversion therapy and clarify the difference between neoadjuvant therapy and adjuvant therapy. Neoadjuvant therapy refers to local or systemic treatment applied before surgical treatment for malignant tumors, and there are four purposes of neoadjuvant therapy for HCC.
The first point is to prevent patients from dropping out due to tumor progression during the waiting period, ensuring that the patients meet the indications for LT. This is the so-called bridging therapy[18]. In an observational study, up to 8.2% of patients with T1 stage and 13.5% of patients with T2 stage who initially had operable HCC were not candidates for LT due to tumor progression while waiting for the 6th mo without intervention[19]. Alpha fetoprotein ≥ 500 ng/mL on the first diagnosis of T1 stage HCC and rapid tumor progression were risk factors for dropping out during the waiting period for LT[20], which suggests that the bridging effect of neoadjuvant therapy is critical. Bridging therapy can reduce the dropout rate to 0%-10% in candidates for LT with HCC meeting the Milan criteria[21]. One of the focuses of oncology surgery is whether patients with HCC within the Milan criteria should undergo direct radical resection if a long waiting period for a donor liver is required, but no clinical studies have yet confirmed this.
The second point is to shrink or reduce tumors outside the Milan criteria to meet the indications for LT[22]. This is the definition of downstage treatment. The expected 5-year survival rate of patients with HCC within the Milan criteria receiving LT was approximately 65%-80%, which was far higher than those outside the Milan criteria[23]. In all, 25%-70% of patients with HCC outside the Milan criteria achieve tumor downstaging after receiving neoadjuvant therapy; they received LT and achieved comparable prognosis to those who underwent initial LT[24] (Table 1). A meta-analysis also confirmed this conclusion[25]. Patients with T3 stage HCC who received neoadjuvant therapy before LT had significantly improved prognosis compared with patients who did not. However, patients with T1 and T2 stage HCC showed no difference[26]. Even patients who have failed downstaging can achieve better prognosis than those without neoadjuvant therapy (median overall survival: 10.3 mo vs 4.0 mo)[27]. Patients with ruptured advanced HCC may also be candidates for LT after successful downstaging, with a significantly improved prognosis compared with nonsurgical treatment[28]. This confirmed the efficacy and broad applicability of neoadjuvant therapy. Several clinical studies have shown similar outcomes for patients who received neoadjuvant therapy and those who did not[29-31], which was related to the patients enrolled in the studies. Although some studies have suggested that neoadjuvant therapy may increase the risk of recurrence after LT, the prognosis of patients with advanced HCC is encouraging enough[32].
| Year | Study design | Neoadjuvant group | Resectable or transplantable group | Ref. | |||||||
| Neoadjuvant therapy | Times of neoadjuvant therapy | Tumor condition | Success rate | Subsequent therapy | Prognosis | Tumor condition | Tumor treatment | Prognosis | |||
| 2017 | Retrospective study | DEB-TACE | 1.38 | Within Milan criteria 88% | 89.0% | OLT | 3-yr OS: 79%; 3-yr DFS: 79% | Within Milan criteria 77% | OLT | 3-yr OS: 73.0%; 3-yr DFS: 70.0% | [29] |
| 2015 | Retrospective study | TACE | NA | Over 10 cm | 28.4% | LR/OLT | 1-yr OS: 76.5% | HCC over 10 cm | BSC | 1-yr OS: 3.7% | [27] |
| 2019 | Retrospective study | TACE, RFA; TACE + RFA | NA | Within Milan criteria 56.7% | 25.2% | LT | Downstage: 5-yer DFS: 86%; No downstage: 5-yr DFS: 71.5% | Within Milan criteria 68.4% | LT | 5-yr DFS: 83.0% | [30] |
| 2013 | Retrospective study | TACE, RFA; HIFU, etc. | 1.6 ± 0.4 | Outside Milan criteria | NA | LT | 5-yr OS: 70.7% | Within Milan criteria | LT | 5-yr OS: 74.1% | [14] |
| 2015 | Retrospective study | TACE, RFA | NA | Outside UNOS T2 criteria | 65.3% | LT | 5-yr OS: 77.8%; 5-yr DFS: 90.8% | Within UNOS T2 criteria | LT | 5-yr OS: 81.0%; 5-yr DFS: 88.0% | [41] |
| 2019 | Retrospective study | TACE, RFA; SIRT, etc. | NA | Outside Milan criteria | 45.2% | LT | 5-yr OS: 76.0%; 5-yr DFS: 89.0% | Within Milan criteria | LT | 5-yr OS: 81.0%; 5-yr DFS: 98.3% | [42] |
| 2017 | Retrospective study | TACE, RFA; Sorafenib | NA | Outside Milan criteria | 26.7% | OLT | NA, comparable with those within Milan criteria | Within Milan criteria | OLT | NA | [43] |
| 2015 | Retrospective study | TACE, RFA | NA | Outside Milan criteria | 36.4% | LT | 5-yr RFS: 81.8% | Within Milan criteria | LT | 5-yr RFS: 94.6% | [44] |
| 2019 | Retrospective study | NA | NA | Outside Milan criteria | 68.4% | LT | 5-yr OS: 63.0% | Within Milan criteria | LT | 5-yr OS: 77.0% | [45] |
The third point is to increase the LR rate of HCC through neoadjuvant therapy and convert unresectable HCCs into resectable tumors[33]. Conversion therapy can be performed to increase future liver volume and reduce tumor stage[34]. In this case, more patients would have the opportunity to receive salvage LR. A meta-analysis suggested that the prognosis of patients with extensive HCC after hepatectomy was poorer than that of patients with non-extensive HCC, and tumor volume was related to the efficacy of LR[35]. Recent studies have shown that the prognosis of patients receiving hepatectomy after successful conversion is comparable to that of patients receiving initial resection (5-year overall survival: 24.9%-57.0% vs 42.0%-64.0%)[15,36,37]. Conversion therapy is necessary and beneficial in resectable or unresectable HCC.
Finally, approximately 40% of patients are eligible for radical treatment with an overall survival rate of 70%[38]. Metastasis and new lesions are common types of recurrence. Neoadjuvant therapy plays a certain role in preventing recurrence after radical treatment. Patients with operable HCC receiving neoadjuvant therapy (5-year disease-free survival: about 50%) tend to achieve superior prognosis compared with those receiving hepatectomy only (5-year disease-free survival: 0%-31%)[39]. The effect of reducing tumor recurrence is related to the tumor response of neoadjuvant therapy[40] (Figure 1). Prognostic comparison of patients with neoadjuvant therapy and those with initial resectable or transplantable hepatocellular carcinoma was summarized in Table 1[14,27,29,30,41-45].
Bridging treatment is necessary for patients with HCC within the Milan criteria during a long waiting period. Patients with HCC for tumor downstaging require a high degree of selection. A clinical study showed that neoadjuvant therapy was not beneficial for the prognosis of patients with Barcelona Clinic Liver Cancer (BCLC) stage 0/A HCC[46], increasing the recurrence risk after LT instead[38]. Moreover, a meta-analysis demonstrated that neoadjuvant therapy had no efficacy for the overall survival and disease-free survival of patients with HCC within the Milan criteria[27].
The indications for downstaging treatment involve physical condition, liver function and tumor stage as well as tumor biomarkers such as alpha fetoprotein and abnormal prothrombin are often considered one of the protocols[47]. There is no uniform and definite limit on the number and size of HCC in downstaging treatment. One retrospective study limited no other restrictions on the tumor conditions of patients with HCC, except no distant metastasis, and their results showed a success rate of 30% in downstaging treatment and comparable prognosis with patients within the Milan criteria after LT[48].
There are some guidelines for downstaging treatment in HCC. One of the most widely used recommendations is the University of California, San Francisco (UCSF) protocol. The indications for downstaging treatment according to the UCSF criteria were as follows: (1) Single HCC > 5 and ≤ 8 cm; (2) 2-3 lesions, each no more than 5 cm in diameter, with the sum of diameters ≤ 8 cm; and (3) 4-5 lesions, each ≤ 3 cm, with the sum of diameters ≤ 8 cm[29]. The success rate of downstaging treatment was approximately 24%-58% according to UCSF criteria[14,29,48,49]. The criteria adopted by the Bologna Liver Transplant Committee are: (1) Single HCC ≤ 8 cm; (2) Two lesions, each ≤ 5 cm; and (3) Multiple lesions within 5 nodules, with the sum of diameters ≤ 12 cm. The success rate was 68.3% on the basis of the Bologna criteria[32]. The Brazilian selection protocol is a relatively relaxed standard and is as follows: (1) No extrahepatic metastasis or major vascular invasion; and (2) Only TACE was applied as downstaging treatment[50]. Some studies have also used total tumor volume as a criterion for downstaging treatment in HCC[37]. Even if tumors develop definite progression during downstaging therapy, treatment should be continued as long as tumors are within the indication[51].
There are also contraindications of downstaging treatment for LT. First, the contraindications of the treatment itself cannot be ignored[52]. Second, extrahepatic metastasis and major vascular invasion are also contraindications to downstaging treatment[53]. Finally, downstaging treatment is not recommended for tumors exceeding the criteria. Clinical research has suggested that overall survival is significantly shortened in patients with HCC exceeding the UCSF criteria receiving LT after downstaging treatment[54].
Most patients receiving conversion therapy suffered from HCC that was more advanced than those receiving downstaging therapy. There were more restrictions for patients receiving conversion therapy. The neoplastic features of unresectable HCC include: (1) Insufficient future remnant liver (FLR) volume after hepatectomy; (2) Extensive multiple intrahepatic tumors; (3) Extrahepatic metastasis; and (4) Tumor thrombus in the main portal vein, hepatic vein and inferior vena cava[15]. First, insufficient residual liver volume after hepatectomy is a contraindication to hepatectomy but not an absolute contraindication. Portal vein embolization (PVE) can be performed to increase the volume of unembolized liver and improve liver function[55]. PVE should be an alternative when the standardized liver volume ratio is no more than 20% in normal liver, 30% in injured liver and 40% in cirrhosis or fibrosis[56]. Second, multiple tumors, major vascular invasion and distant metastasis are not contraindicated in neoadjuvant therapy for patients with normal liver function. A small proportion of patients with advanced HCC after conversion therapy can receive radical therapy, while others also benefit from neoadjuvant therapy[15,57]. Finally, only patients with Child-Pugh grade A and selected patients with Child-Pugh grade B can be candidates for hepatectomy after conversion therapy[58]. A Model of End-Stage Liver Disease score greater than 10 after conversion therapy should be considered a contraindication for hepatectomy[28]. Patients who cannot undergo hepatectomy due to decompensation of liver function are not eligible for conversion therapy.
Radiological assessment is the main method to evaluate the efficacy of HCC. World Health Organization (WHO) criteria were first performed to evaluate the efficacy of solid tumors based on tumor size[59]. However, WHO criteria lack specific requirements for tumor size measurement and imaging modality was also not clearly specified, leading to incorrect assessment of tumor burden[60]. Response Evaluation Criteria in Solid Tumors (RECIST) criteria made up for many deficiencies in WHO criteria, defining target lesions and non-target lesions, clarifying the method of tumor size measurement and specifying the tumor imaging modality[61]. RECIST 1.1 criteria supplemented the clear definition of lymph nodes and other state lesions on the basis of RECIST criteria, as well as a discussion for fluorodeoxyglucose-positron emission tomography to assess new lesions[62]. The effects of treatment other than tumor reduction were not included in WHO and RECIST/RECIST 1.1 criteria. Given the need to assess efficacy accurately, experts established European Association for the Study of the Liver (EASL) criteria in 2001. The highlight is the measurement of arterially enhanced tumors, taking into account tumor necrosis. EASL criteria also led to a stricter requirement of tumor response. The modified RECIST criteria simplified the complex steps of EASL criteria, integrates the main advantages of RECIST criteria and puts forward a new suggestion of target lesions, non-target lesions and new lesions[63]. The overall tumor response in modified RECIST criteria is comparable with that in EASL criteria[64]. Due to the delayed treatment of immune checkpoint inhibitors, immune RECIST criteria was also applied in HCC patients receiving immunotherapy[65].
The modified RECIST criteria were performed to evaluate the efficacy of patients receiving neoadjuvant treatment by computed tomography or magnetic resonance imaging in most HCC cases[63]. Efficacy evaluation only considers viable tumors. It takes a period of at least 3 mo of observation for successful downstaging to LT[66]. If the tumor progresses beyond the Milan criteria during this period, LT cannot be performed. If the tumor progresses within downstaging protocols, patients should continue to take downstaging treatment[67], but the Brazilian selection protocol requires no observation period[51]. Most protocols require patients undergoing downstaging treatment to undergo abdominal computed tomography or magnetic resonance imaging every 3 mo.
TACE combines local embolic ischemia and the cytotoxic effects of chemotherapy, and it has become the recommended first-line treatment for intermediate-stage HCC with preserved liver function[5,68]. Recent research has demonstrated that TACE is the most common first treatment for HCC in China, Korea, North America and Europe. The most common method of TACE is hepatic arterial emulsion with lipiodol plus chemotherapy drugs and embolization with gelatin. TACE can reduce the dropout rate to 3%-13% in patients with early-stage HCC being considered for LT, especially those patients whose waiting time is expected to exceed 6 mo[69,70]. The successful downstaging rate ranged from 23.7% to 63.0% in patients with advanced HCC[71,72]. Patients receiving TACE as downstaging treatment could achieve improved survival (5-year overall survival rate: 77.6%), but TACE cannot improve the long-term prognosis of patients with HCC receiving bridging treatment[73,74]. Clinical studies have shown that the tumor response of pre-transplantation TACE was related to the recurrence rate after transplantation[75].
Drug-eluting beads are non-absorbable embolic microspheres releasing drugs continuously. Compared with conventional TACE, some previous studies indicated that drug-eluting bead TACE (DEB-TACE) not only seemed to be more capable of inducing tumor necrosis but also reduced the systemic blood concentration[76-78]. Other studies have suggested that DEB-TACE led to no advantage in tumor response and survival time compared with conventional TACE[79-82]. There is not enough evidence to support that DEB-TACE is superior to conventional TACE in terms of treatment effect and complications in HCC patients[83]. Approximately 73%-78% of patients within the UCSF criteria achieved successful downstaging, and 40% of them received LT after DEB-TACE[82,84]. The disease control rate was 75%-94%[85-87].
Several studies have demonstrated that appropriate pre-transplant TACE does not increase the risk of LT[88], but others have suggested that the incidence of hepatic artery thrombosis and re-transplantation was significantly higher in patients who received pre-transplant TACE than in those who did not[89]. Tsochatzis et al[90] found that the high recurrence rate after LT is associated with the absence of pre-transplant TACE as neoadjuvant therapy (odds ratio 5.395, 95%CI: 1.289-22.577).
Trans-arterial radioembolization refers to the injection of radioactive substances through the hepatic artery, such as microspheres containing yttrium-90 (Y-90), iodine-131 and iodized oil[91]. HCC is sensitive to radiotherapy[92]. Radioembolization (RE) can achieve different degrees of regression in 25%-50% of HCC patients[93-96]; the success rate of bridging treatment with Y-90 RE can be up to 100%[97,98]. Approximately 20% of patients with an initially unresectable HCC received radical surgery after Y-90 RE[99]. Clinicians have found that Y-90 RE can even be a neoadjuvant treatment for BCLC C stage patients with portal vein tumor thrombosis[100]. However, others also indicated that Y-90 RE can prevent the progression of target lesions but not the generation of new lesions[101]. Complications of radiotherapy embolization mainly stem from the inability to predict precise dosimetry during RE. Table 2 summarized the outcomes of pre-transplant TACE and trans-arterial radioembolization in downstage treatment for hepatocellular carcinoma[86,89,90,95,96,102-105].
| Year | Neoadjuvant treatment | Entry criteria | Success downstage rate | Subsequent therapy | Adverse events | Incidence rate | Ref. |
| 2015 | Conventional TACE; | Patients within USCF criteria | NA | OLT | Hepatic artery thrombosis hepatic aneurysm | 1.5% | [89] |
| 2015 | DEB-TACE | BCLC 0/A/B stage | 26.7% | OLT | Grade 3/4 | 3.2% | [102] |
| 2017 | TACE | NA | OLT | Hepatic artery thrombosisRetransplant | 27%22.7% | [90] | |
| 2020 | DEB-TACE | AJCC stage ≤ T3a | 73.3% | OLT | Grade 3Grade 4 | 3.1%0.0% | [86] |
| 2006 | Y-90 RE | UNOS stage T3 | 66.0% | OLT | NA | NA | [103] |
| 2017 | Y-90 RE | BCLC A/B/C stage | 78.9% | OLT | NA | NA | [104] |
| 2011 | Y-90 RE | UNOS stage T2, T3, T4a | 50.0% | OLT | Hyperbilirubinemia (Grade3) | 13.0% | [105] |
| 2013 | Y-90 RE | UNOS stage T3, T4a | 33.0% | OLT | NA | NA | [95] |
| 2021 | Y-90 RE | UNOS stage T1, T2, T3, T4 | 43.0% | OLT | NA | NA | [96] |
Hepatic arterial infusion chemotherapy (HAIC) can deliver chemotherapeutics to the arterial branches of the HCC at higher concentrations[106]. Compared with traditional systemic chemotherapy, HAIC provides a higher local drug concentration and fewer side effects. The tumor response rate of HAIC is 7%-81%[107,108]. Hepatic artery infusion of FOLFOX (folinic acid, fluorouracil and oxaliplatin), cisplatin plus 5-fluorouracil and cisplatin are common chemotherapy regimens[109-111]. Patients can tolerate HAIC well, and no adverse events above grade 3 have been observed[112]. Recent studies have shown that HAIC is more effective and safer than sorafenib in the treatment of HCC[113]. Preoperative HAIC prolongs the long-term survival of patients[114]. For initially unresectable HCCs, approximately 12% of patients can receive hepatectomy after successful conversion with HAIC[115]. HAIC can prevent the progression of inferior vena cava tumor thrombi, and clinicians have suggested that LR should be performed in patients who initially have no inferior vena cava tumor thrombus and inferior vena cava tumor thrombus controlled by HAIC[116]. Moreover, preoperative HAIC cannot prolong the overall survival of patients with early-stage HCC, but it may be able to prevent intrahepatic distant recurrence[117].
PVE was originally used to prevent the spread of portal vein thrombi[118] and was found to increase the volume of the unembolized liver. Postoperative liver insufficiency or even liver failure after hepatectomy is closely related to FLR volume. PVE can lead to a significant increase in FLR volume in normal livers or those with chronic disease[119]. There would be functional and volumetric increases in unembolized liver after PVE[120]. The increase in liver volume after PVE is a predictor of postoperative safety. Palavecino et al[121] suggested that preoperative PVE was helpful to reduce complications after hepatectomy, and patients with PVE achieved comparable prognosis with those without PVE. However, there were also researchers suggesting that PVE accelerates the growth of tumors in the embolized liver lobe[122].
Repeatedly reversible PVE has achieved satisfactory results in animal experiments, and this new method of PVE requires more evidence[123]. Portal vein ligation can achieve effects similar to PVE, but it is performed less due to its high invasiveness and the risk of treatment-related complications[124]. FLR volume could be insufficient in some patients receiving PVE, and a meta-analysis showed that hepatic and PVE could be an ideal alternative for patients who failed to increase FLR volume with PVE[125].
Radiotherapy can be used for more advanced HCC as compared to TACE[126]. Hasan et al[127] suggested that radiotherapy is effective in downstaging and bridging therapy for pre-transplant HCC, especially in advanced HCC, which is outside the indications for TACE. Various methods of radiotherapy have been applied in HCC. Clinical studies have demonstrated that stereotactic ablative radiation therapy, selective internal radiation therapy and stereotactic radiotherapy can be effective in the pre-transplant period, with a successful downstaging rate of approximately 60%[128]. For patients with HCC with portal vein tumor thrombosis, radiotherapy before major hepatectomy can achieve a significantly better prognosis. Radiotherapy combined with TACE seemed to be a more effective treatment option, providing a better prognosis[129].
Radiofrequency ablation is a radical alternative to surgical resection for BCLC stage 0/A HCC and a palliative treatment for advanced HCC at the same time[5,130]. de Haas et al[131] suggested that preoperative radiotherapy had no adverse effects on patient prognosis while providing downstaging and bridging effects. Radiofrequency ablation before LT may indeed cause inflammation and adhesions, increasing the difficulty of operation, but clinical studies have shown that the perioperative mortality and morbidity of the local ablation group are comparable with that of the non-local ablation group[131]. The disease control rate of radiofrequency ablation combined with TACE was significantly higher than that of monotherapy, and the sequence of radiofrequency ablation and TACE appeared to lead no effect on prognosis[132].
Chemotherapy is effective for the treatment of HCC, but the incidence of adverse events is very high. Up to 44% of patients develop grade 3-4 adverse events[133]. Neoadjuvant therapy rarely uses chemotherapy alone. Localized concurrent chemoradiotherapy could lead to a downstaging rate of 26.5% in advanced HCC so that surgery can be performed[134]. Even in patients with portal vein tumor thrombosis, the operation rate can reach 26.5% after concurrent chemoradiotherapy[134]. The feasibility of chemotherapy combined with targeted drugs requires more clinical research in downstaging and bridging in pre-transplant HCC[135,136].
Sorafenib is a milestone in the systematic treatment of HCC. It was clinically observed that one patient who received sorafenib for downstaging achieved a good prognosis after LT[137]. Sorafenib is also effective in conversion therapy of advanced HCC and even ruptured HCC[138,139]. A decline of more than 20% from baseline in early alpha fetoprotein levels is a predictor of tumor response to sorafenib[140]. However, due to the relatively low response rate of sorafenib in HCC, the application of neoadjuvant therapy is limited[141]. To date, there have been few reports of successful conversion after receiving sorafenib[142-144]. More evidence is required to support the role of sorafenib in neoadjuvant therapy because of the small sample size of clinical studies on sorafenib in neoadjuvant therapy[145]. Compared with other targeted drugs, lenvatinib leads to a higher response rate of approximately 40.6%[146]. Targeted therapy should be an alternative in patients who cannot benefit from TACE. It can be more effective when lenvatinib is administered before TACE in patients with BCLC B stage HCC[147]. Regorafenib and other targeted drugs can also be potential neoadjuvant treatments[148]. Surgery-related complications of molecular targeted drugs must be noted, such as increased bleeding and hindered liver regeneration[149], but clinical research has suggested that the surgical blood loss and complications in the sorafenib group were comparable to those in the control group[150].
Immunotherapy is an emerging systemic treatment for solid tumors[151]. The combination of atezolizumab and bevacizumab showed a strong antitumor effect, with a relatively low rate of grade 3-4 adverse events (15.2%)[152]. Targeted drugs plus immune checkpoint inhibitors can achieve a tumor response rate of 30%, leading to a new emerging treatment[153-155]. Lenvatinib plus pembrolizumab can also be an important treatment option for neoadjuvant therapy. The combination of immunotherapy and other treatments, such as chemotherapy and radiotherapy, still requires more evidence to demonstrate efficacy[155,156].
To reduce the drop-out rate during the waiting period and downstaging more HCCs outside the Milan criteria, effective neoadjuvant therapy is critical in prolonging patient prognosis. Adverse events of neoadjuvant therapy are manageable under strict indications. The establishment of unified protocols of neoadjuvant therapy requires more clinical studies.
We thank the hepatic surgery of Tongji Hospital for the platform support.
| 1. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1580] [Article Influence: 175.6] [Reference Citation Analysis (0)] |
| 2. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1286] [Article Influence: 183.7] [Reference Citation Analysis (3)] |
| 3. | Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the Burden of Nonalcoholic Fatty Liver Disease in a United States Cohort of Veterans. Clin Gastroenterol Hepatol. 2016;14:301-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 628] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6431] [Article Influence: 803.9] [Reference Citation Analysis (10)] |
| 6. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1959] [Article Influence: 195.9] [Reference Citation Analysis (4)] |
| 7. | Cabibbo G, Maida M, Genco C, Parisi P, Peralta M, Antonucci M, Brancatelli G, Cammà C, Craxì A, Di Marco V. Natural history of untreatable hepatocellular carcinoma: A retrospective cohort study. World J Hepatol. 2012;4:256-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Fujiki M, Aucejo F, Choi M, Kim R. Neo-adjuvant therapy for hepatocellular carcinoma before liver transplantation: where do we stand? World J Gastroenterol. 2014;20:5308-5319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med. 2018;16:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Lei JY, Yan LN, Wang WT. Transplantation vs resection for hepatocellular carcinoma with compensated liver function after downstaging therapy. World J Gastroenterol. 2013;19:4400-4408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 12. | Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol. 2013;19:7515-7530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Finkenstedt A, Vikoler A, Portenkirchner M, Mülleder K, Maglione M, Margreiter C, Moser P, Vogel W, Bale R, Freund M, Luger A, Tilg H, Petersen J, Schneeberger S, Graziadei I, Zoller H, Glodny B. Excellent post-transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int. 2016;36:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Lei J, Wang W, Yan L. Downstaging advanced hepatocellular carcinoma to the Milan criteria may provide a comparable outcome to conventional Milan criteria. J Gastrointest Surg. 2013;17:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007;14:3301-3309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, Rodés J, Bruix J. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 17. | Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-9; discussion 799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 591] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 18. | Kollmann D, Selzner N, Selzner M. Bridging to liver transplantation in HCC patients. Langenbecks Arch Surg. 2017;402:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Huo TI, Huang YH, Su CW, Lin HC, Chiang JH, Chiou YY, Huo SC, Lee PC, Lee SD. Validation of the HCC-MELD for dropout probability in patients with small hepatocellular carcinoma undergoing locoregional therapy. Clin Transplant. 2008;22:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Mehta N, Sarkar M, Dodge JL, Fidelman N, Roberts JP, Yao FY. Intention to treat outcome of T1 hepatocellular carcinoma with the "wait and not ablate" approach until meeting T2 criteria for liver transplant listing. Liver Transpl. 2016;22:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | She WH, Cheung TT. Bridging and downstaging therapy in patients suffering from hepatocellular carcinoma waiting on the list of liver transplantation. Transl Gastroenterol Hepatol. 2016;1:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (1)] |
| 23. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5397] [Article Influence: 179.9] [Reference Citation Analysis (7)] |
| 24. | Inomata K, Yagi H, Hibi T, Shinoda M, Matsubara K, Abe Y, Kitago M, Obara H, Itano O, Kawachi S, Tanabe M, Wakabayashi G, Shimazu M, Kitagawa Y. Long-term outcomes of living donor liver transplantation after locoregional treatment for hepatocellular carcinoma: an experience from a single institute. Surg Today. 2021;51:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Ravaioli M, Cucchetti A, Cescon M, Piscaglia F, Ercolani G, Trevisani F, Pinna AD. Systematic review of outcome of downstaging hepatocellular cancer before liver transplantation in patients outside the Milan criteria (Br J Surg 2011; 98: 1201-1208). Br J Surg. 2011;98:1674; author reply 1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, Murad MH, Mohammed K. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology. 2018;67:381-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 27. | Song DS, Nam SW, Bae SH, Kim JD, Jang JW, Song MJ, Lee SW, Kim HY, Lee YJ, Chun HJ, You YK, Choi JY, Yoon SK. Outcome of transarterial chemoembolization-based multi-modal treatment in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2015;21:2395-2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Jeng KS, Huang CC, Lin CC, Lin CK, Teng CJ, Chen KH. Liver Transplantation After Downstagings of Ruptured Advanced Hepatocellular Carcinoma in Cirrhotic Liver: Is It Advisable? Transplant Proc. 2019;51:1468-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Dorcaratto D, Udupa V, Hogan NM, Brophy DP, McCann JW, Maguire D, Geoghegan J, Cantwell CP, Hoti E. Does neoadjuvant doxorubicin drug-eluting bead transarterial chemoembolization improve survival in patients undergoing liver transplant for hepatocellular carcinoma? Diagn Interv Radiol. 2017;23:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Huang X, Lu S. Impact of preoperative locoregional therapy on recurrence and patient survival following liver transplantation for hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol. 2017;52:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Li HL, Ji WB, Zhao R, Duan WD, Chen YW, Wang XQ, Yu Q, Luo Y, Dong JH. Poor prognosis for hepatocellular carcinoma with transarterial chemoembolization pre-transplantation: retrospective analysis. World J Gastroenterol. 2015;21:3599-3606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Toso C, Meeberg G, Andres A, Shore C, Saunders C, Bigam DL, Shapiro AMJ, Compagnon P, Berney T, Majno P, Kneteman N. Downstaging prior to liver transplantation for hepatocellular carcinoma: advisable but at the price of an increased risk of cancer recurrence - a retrospective study. Transpl Int. 2019;32:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Zhang ZF, Luo YJ, Lu Q, Dai SX, Sha WH. Conversion therapy and suitable timing for subsequent salvage surgery for initially unresectable hepatocellular carcinoma: What is new? World J Clin Cases. 2018;6:259-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Tang ZY, Zhou XD, Ma ZC, Wu ZQ, Fan J, Qin LX, Yu Y. Downstaging followed by resection plays a role in improving prognosis of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:495-498. [PubMed] |
| 35. | Wang L, Liu Z, Liu X, Zeng Y, Liu J. The hepatectomy efficacy of huge hepatocellular carcinoma and its risk factors: A meta analysis. Medicine (Baltimore). 2017;96:e9226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Labgaa I, Taffé P, Martin D, Clerc D, Schwartz M, Kokudo N, Denys A, Halkic N, Demartines N, Melloul E. Comparison of Partial Hepatectomy and Transarterial Chemoembolization in Intermediate-Stage Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer. 2020;9:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Akoad ME, Pomfret EA. Surgical resection and liver transplantation for hepatocellular carcinoma. Clin Liver Dis. 2015;19:381-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Xu M, Doyle MM, Banan B, Vachharajani N, Wang X, Saad N, Fowler K, Brunt EM, Lin Y, Chapman WC. Neoadjuvant Locoregional Therapy and Recurrent Hepatocellular Carcinoma after Liver Transplantation. J Am Coll Surg. 2017;225:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207-15; discussion 1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, Fidelman N, Kerlan RK Jr, Roberts JP. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 43. | Kim Y, Stahl CC, Makramalla A, Olowokure OO, Ristagno RL, Dhar VK, Schoech MR, Chadalavada S, Latif T, Kharofa J, Bari K, Shah SA. Downstaging therapy followed by liver transplantation for hepatocellular carcinoma beyond Milan criteria. Surgery. 2017;162:1250-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Na GH, Kim EY, Hong TH, You YK, Kim DG. Effects of loco regional treatments before living donor liver transplantation on overall survival and recurrence-free survival in South Korean patients with hepatocellular carcinoma. HPB (Oxford). 2016;18:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Ravaioli M, Odaldi F, Cucchetti A, Trevisani F, Piscaglia F, De Pace V, Bertuzzo VR, Neri F, Golfieri R, Cappelli A, D'Errico A, Cescon M, Del Gaudio M, Fallani G, Siniscalchi A, Morelli MC, Ciccarese F, Di Marco M, Farinati F, Giannini EG, Pinna AD. Long term results of down-staging and liver transplantation for patients with hepatocellular carcinoma beyond the conventional criteria. Sci Rep. 2019;9:3781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Yeh ML, Huang CI, Huang CF, Hsieh MY, Huang JF, Dai CY, Lin ZY, Chen SC, Yu ML, Chuang WL. Neoadjuvant transcatheter arterial chemoembolization does not provide survival benefit compared to curative therapy alone in single hepatocellular carcinoma. Kaohsiung J Med Sci. 2015;31:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Grąt M, Kornasiewicz O, Lewandowski Z, Hołówko W, Grąt K, Kobryń K, Patkowski W, Zieniewicz K, Krawczyk M. Combination of morphologic criteria and α-fetoprotein in selection of patients with hepatocellular carcinoma for liver transplantation minimizes the problem of posttransplant tumor recurrence. World J Surg. 2014;38:2698-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Chapman WC, Garcia-Aroz S, Vachharajani N, Fowler K, Saad N, Lin Y, Wellen J, Tan B, Khan AS, Doyle MB. Liver Transplantation for Advanced Hepatocellular Carcinoma after Downstaging Without Up-Front Stage Restrictions. J Am Coll Surg. 2017;224:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Mehta N, Guy J, Frenette CT, Dodge JL, Osorio RW, Minteer WB, Roberts JP, Yao FY. Excellent Outcomes of Liver Transplantation Following Down-Staging of Hepatocellular Carcinoma to Within Milan Criteria: A Multicenter Study. Clin Gastroenterol Hepatol. 2018;16:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 50. | Massarollo PC, Coppini AZ, Salzedas-Netto AA, Coelho FF, Minami T, Gonzalez AM. Favorable Long-term Outcome in Patients Submitted to Liver Transplantation After Downstaging of Hepatocellular Carcinoma According to a Brazilian Selection Protocol. Transplant Proc. 2016;48:2338-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Dirican A, Karakas S. What Should Be the Rules for Downstaging for Hepatocellular Carcinoma? J Gastrointest Cancer. 2020;51:1148-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Kumar Y, Sharma P, Bhatt N, Hooda K. Transarterial Therapies for Hepatocellular Carcinoma: a Comprehensive Review with Current Updates and Future Directions. Asian Pac J Cancer Prev. 2016;17:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 798] [Article Influence: 57.0] [Reference Citation Analysis (2)] |
| 54. | Onaca N, Davis GL, Goldstein RM, Jennings LW, Klintmalm GB. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Aoki T, Kubota K. Preoperative portal vein embolization for hepatocellular carcinoma: Consensus and controversy. World J Hepatol. 2016;8:439-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-80; discussion 680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 338] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 57. | Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, Aikata H, Nagata Y, Chayama K, Ohdan H. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg. 2017;44:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 591] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 59. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 60. | Warr D, McKinney S, Tannock I. Influence of measurement error on assessment of response to anticancer chemotherapy: proposal for new criteria of tumor response. J Clin Oncol. 1984;2:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13141] [Article Influence: 505.4] [Reference Citation Analysis (8)] |
| 62. | Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 1467] [Article Influence: 146.7] [Reference Citation Analysis (0)] |
| 63. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3446] [Article Influence: 215.4] [Reference Citation Analysis (43)] |
| 64. | Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 65. | Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143-e152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1852] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 66. | Ersan V, Barut B, Yilmaz S. The Timing of Liver Transplantation Following Downstaging: Wait of Not to Wait? J Gastrointest Cancer. 2020;51:1152-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Sharr WW, Chan SC, Lo CM. Section 3. Current status of downstaging of hepatocellular carcinoma before liver transplantation. Transplantation. 2014;97 Suppl 8:S10-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 69. | Alba E, Valls C, Dominguez J, Martinez L, Escalante E, Lladó L, Serrano T. Transcatheter arterial chemoembolization in patients with hepatocellular carcinoma on the waiting list for orthotopic liver transplantation. AJR Am J Roentgenol. 2008;190:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Aloia TA, Adam R, Samuel D, Azoulay D, Castaing D. A decision analysis model identifies the interval of efficacy for transarterial chemoembolization (TACE) in cirrhotic patients with hepatocellular carcinoma awaiting liver transplantation. J Gastrointest Surg. 2007;11:1328-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, Cooper A, Esquivel C, Nguyen MH. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | Shi XJ, Jin X, Wang MQ, Wei LX, Ye HY, Liang YR, Luo Y, Dong JH. Effect of resection following downstaging of unresectable hepatocelluar carcinoma by transcatheter arterial chemoembolization. Chin Med J (Engl). 2012;125:197-202. [PubMed] |
| 73. | Yao FY, Kinkhabwala M, LaBerge JM, Bass NM, Brown R Jr, Kerlan R, Venook A, Ascher NL, Emond JC, Roberts JP. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Bharat A, Brown DB, Crippin JS, Gould JE, Lowell JA, Shenoy S, Desai NM, Chapman WC. Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survival. J Am Coll Surg. 2006;203:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Jin X, Shi XJ, Wang MQ, Wei LX, Ye HY, Liang YR, Luo Y, Dong JH. Experience of the treatment following downstaging of larger hepatocellular carcinomas by transcathetheter hepatic arterial chemoembolization in 58 patients. Zhonghua Yi Xue Za Zhi. 2011;91:950-955. [PubMed] |
| 76. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 439] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 77. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1218] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 78. | Yu CY, Ou HY, Weng CC, Huang TL, Chen TY, Leung-Chit L, Hsu HW, Chen CL, Cheng YF. Drug-Eluting Bead Transarterial Chemoembolization as Bridge Therapy for Hepatocellular Carcinoma Before Living-Donor Liver Transplantation. Transplant Proc. 2016;48:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F; PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 496] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 80. | Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. 2016;48:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 81. | Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, Cascella T, Marchianò A, Camerini T, Bhoori S, Brunero F, Barone M, Mazzaferro V. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 82. | Frenette CT, Osorio RC, Stark J, Fok B, Boktour MR, Guy J, Rhee J, Osorio RW. Conventional TACE and drug-eluting bead TACE as locoregional therapy before orthotopic liver transplantation: comparison of explant pathologic response. Transplantation. 2014;98:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 84. | Orlacchio A, Chegai F, Merolla S, Francioso S, Giudice CD, Angelico M, Tisone G, Simonetti G. Downstaging disease in patients with hepatocellular carcinoma outside up-to-seven criteria: Strategies using degradable starch microspheres transcatheter arterial chemo-embolization. World J Hepatol. 2015;7:1694-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Domaratius C, Settmacher U, Malessa C, Teichgräber U. Transarterial chemoembolization with drug-eluting beads in patients with hepatocellular carcinoma: response analysis with mRECIST. Diagn Interv Radiol. 2021;27:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Ou HY, Wu YN, Yu CY, Chen CL, Hsu HW, Weng CC, Leung-Chit Tsang L, Huang TL, Tong YS, Lim WX, Cheng YF. Transarterial Chemoembolization Using 100-μm Drug-Eluting Microspheres in Patients with Hepatocellular Carcinoma: A Prospective Study and Midterm Follow-up. J Vasc Interv Radiol. 2020;31:1784-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Zhou GH, Han J, Sun JH, Zhang YL, Zhou TY, Nie CH, Zhu TY, Chen SQ, Wang BQ, Yu ZN, Wang HL, Chen LM, Wang WL, Zheng SS. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres® beads in Chinese hepatocellular carcinoma patients. BMC Cancer. 2018;18:644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 88. | RETRACTION of "Immune memory responses to HBV vaccine 13-18 years after primary vaccination" by L. Hou, W. Li, X. Wei, Y. Zhou, Y. Zhuo, H. Wu, B. Shen. Genet. Mol. Res. 14 (3): 8466-8472 (2015). Genet Mol Res. 2016;15:150159121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Ince V, Ersan V, Karakas S, Kutluturk K, Karadag N, Kutlu R, Yilmaz S. Does Preoperative Transarterial Chemoembolization for Hepatocellular Carcinoma Increase the Incidence of Hepatic Artery Thrombosis After Living-Donor Liver Transplant? Exp Clin Transplant. 2017;15:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Tsochatzis E, Garcovich M, Marelli L, Papastergiou V, Fatourou E, Rodriguez-Peralvarez ML, Germani G, Davies N, Yu D, Luong TV, Dhillon AP, Thorburn D, Patch D, O'Beirne J, Meyer T, Burroughs AK. Transarterial embolization as neo-adjuvant therapy pretransplantation in patients with hepatocellular carcinoma. Liver Int. 2013;33:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Brown DB, Nikolic B, Covey AM, Nutting CW, Saad WE, Salem R, Sofocleous CT, Sze DY; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012;23:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 92. | Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010;25:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, Ryu RK, Sato KT, Gates V, Abecassis MM, Omary RA, Baker TB, Salem R. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 797] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 96. | Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, Panizo A, Gil B, Inarrairaegui M, Herrero I, Quiroga J, Prieto J. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 97. | Tohme S, Sukato D, Chen HW, Amesur N, Zajko AB, Humar A, Geller DA, Marsh JW, Tsung A. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol. 2013;24:1632-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 98. | Gabr A, Kulik L, Mouli S, Riaz A, Ali R, Desai K, Mora RA, Ganger D, Maddur H, Flamm S, Boike J, Moore C, Thornburg B, Alasadi A, Baker T, Borja-Cacho D, Katariya N, Ladner DP, Caicedo JC, Lewandowski RJ, Salem R. Liver Transplantation Following Yttrium-90 Radioembolization: 15-Year Experience in 207-Patient Cohort. Hepatology. 2021;73:998-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 99. | Tabone M, Calvo A, Russolillo N, Langella S, Carbonatto P, Lo Tesoriere R, Richetta E, Pellerito R, Ferrero A. Downstaging unresectable hepatocellular carcinoma by radioembolization using 90-yttrium resin microspheres: a single center experience. J Gastrointest Oncol. 2020;11:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Levi Sandri GB, Ettorre GM, Colasanti M, De Werra E, Mascianà G, Ferraro D, Tortorelli G, Sciuto R, Lucatelli P, Pizzi G, Visco-Comandini U, Vennarecci G. Hepatocellular carcinoma with macrovascular invasion treated with yttrium-90 radioembolization prior to transplantation. Hepatobiliary Surg Nutr. 2017;6:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Iñarrairaegui M, Martinez-Cuesta A, Rodríguez M, Bilbao JI, Arbizu J, Benito A, Alegre F, D'Avola D, Herrero JI, Quiroga J, Prieto J, Sangro B. Analysis of prognostic factors after yttrium-90 radioembolization of advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Spreafico C, Cascella T, Facciorusso A, Sposito C, Rodolfo L, Morosi C, Civelli EM, Vaiani M, Bhoori S, Pellegrinelli A, Marchianò A, Mazzaferro V. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Intervent Radiol. 2015;38:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, Hunter RD, Nemcek AA Jr, Abecassis MM, Haines KG 3rd, Salem R. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 104. | Ettorre GM, Levi Sandri GB, Laurenzi A, Colasanti M, Meniconi RL, Lionetti R, Santoro R, Lepiane P, Sciuto R, Pizzi G, Cianni R, Golfieri R, D'Offizi G, Pellicelli AM, Antonini M, Vennarecci G. Yttrium-90 Radioembolization for Hepatocellular Carcinoma Prior to Liver Transplantation. World J Surg. 2017;41:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Ibrahim SM, Kulik L, Baker T, Ryu RK, Mulcahy MF, Abecassis M, Salem R, Lewandowski RJ. Treating and downstaging hepatocellular carcinoma in the caudate lobe with yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2012;35:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Bartkowski R, Berger MR, Aguiar JL, Henne TH, Dörsam J, Geelhaar GH, Schlag P, Herfarth C. Experiments on the efficacy and toxicity of locoregional chemotherapy of liver tumors with 5-fluoro-2'-deoxyuridine (FUDR) and 5-fluorouracil (5-FU) in an animal model. J Cancer Res Clin Oncol. 1986;111:42-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer. 2012;1:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 108. | Obi S, Sato S, Kawai T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer. 2015;4:188-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 109. | Kojima H, Hatano E, Taura K, Seo S, Yasuchika K, Uemoto S. Hepatic Resection for Hepatocellular Carcinoma with Tumor Thrombus in the Major Portal Vein. Dig Surg. 2015;32:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, Guo RP, Shi M. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 111. | Kondo M, Morimoto M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, Aikata H, Hatanaka T, Takizawa D, Matsunaga K, Okuse C, Suzuki M, Taguri M, Ishibashi T, Numata K, Maeda S, Tanaka K. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19:954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 112. | Huang SX, Wu YL, Tang CW, Feng WM, Xu YQ, Bao Y, Zheng YY. Prophylactic hepatic artery infusion chemotherapy improved survival after curative resection in patients with hepatocellular carcinoma. Hepatogastroenterology. 2015;62:122-125. [PubMed] |
| 113. | Zhuang BW, Li W, Xie XH, Hu HT, Lu MD, Xie XY. Sorafenib versus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: a systematic review and meta-analysis. Jpn J Clin Oncol. 2019;49:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Lee BH, Lee DS, Cho CW, Yun SS. Role and limitation of neoadjuvant hepatic arterial infusion chemotherapy in advanced hepatocelluar carcinoma patients with Child-Pugh class A. World J Surg Oncol. 2019;17:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Meric F, Patt YZ, Curley SA, Chase J, Roh MS, Vauthey JN, Ellis LM. Surgery after downstaging of unresectable hepatic tumors with intra-arterial chemotherapy. Ann Surg Oncol. 2000;7:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Kasai Y, Hatano E, Seo S, Taura K, Yasuchika K, Okajima H, Kaido T, Uemoto S. Proposal of selection criteria for operative resection of hepatocellular carcinoma with inferior vena cava tumor thrombus incorporating hepatic arterial infusion chemotherapy. Surgery. 2017;162:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Oyama A, Nouso K, Yoshimura K, Morimoto Y, Nakamura S, Onishi H, Takaki A, Iwadou S, Kariyama K, Kuwaki K, Yabushita K, Sakaguchi K, Toshimori J, Kobashi H, Moriya A, Ando M, Okada H; Okayama Hepatocellular Carcinoma Study Group. Randomized controlled study to examine the efficacy of hepatic arterial infusion chemotherapy with cisplatin before radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2021;51:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 312] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 119. | Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 431] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 120. | Narula N, Aloia TA. Portal vein embolization in extended liver resection. Langenbecks Arch Surg. 2017;402:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 121. | Palavecino M, Chun YS, Madoff DC, Zorzi D, Kishi Y, Kaseb AO, Curley SA, Abdalla EK, Vauthey JN. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: Perioperative outcome and survival. Surgery. 2009;145:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 122. | Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, Aikou T, Komokata T, Nakamura N, Sakata R. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 123. | Tranchart H, Koffi GM, Gaillard M, Lainas P, Poüs C, Gonin P, Nguyen TH, Dubart-Kupperschmitt A, Dagher I. Liver regeneration following repeated reversible portal vein embolization in an experimental model. Br J Surg. 2016;103:1209-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Isfordink CJ, Samim M, Braat MNGJA, Almalki AM, Hagendoorn J, Borel Rinkes IHM, Molenaar IQ. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol. 2017;26:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 125. | Esposito F, Lim C, Lahat E, Shwaartz C, Eshkenazy R, Salloum C, Azoulay D. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB (Oxford). 2019;21:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 126. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4370] [Article Influence: 546.3] [Reference Citation Analysis (6)] |
| 127. | Hasan S, Abel S, Uemura T, Verma V, Koay EJ, Herman J, Thai N, Kirichenko A. Liver transplant mortality and morbidity following preoperative radiotherapy for hepatocellular carcinoma. HPB (Oxford). 2020;22:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Lemaire M, Lucidi V, Bouazza F, Katsanos G, Vanderlinden B, Levillain H, Delatte P, Garcia CA, Vouche M, Galdon MG, Demetter P, Deleporte A, Hendlisz A, Flamen P, Donckier V. Selective internal radiation therapy (SIRT) before partial hepatectomy or radiofrequency destruction for treatment of hepatocellular carcinoma in cirrhotic patients: a feasibility and safety pilot study. HPB (Oxford). 2018;20:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 129. | Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 130. | Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, Yoshida S, Miyauchi Y, Kuriyama S. Combined use of percutaneous ethanol injection and radiofrequency ablation for the effective treatment of hepatocelluar carcinoma. Int J Oncol. 2002;21:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 131. | de Haas RJ, Lim C, Ricci C, Lahat E, Fuentes L, Salloum C, Azoulay D. Local Ablation Does Not Worsen Perioperative Outcomes After Liver Transplant for Hepatocellular Carcinoma. AJR Am J Roentgenol. 2019;213:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 132. | El Dorry AK, Shaker MK, El-Fouly NF, Hussien A, El-Folly RF, El Fouly AH, Abd El Tawab K. Effectiveness of combined therapy radiofrequency ablation/transarterial chemoembolization versus transarterial chemoembolization/radiofrequency ablation on management of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2021;33:1573-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 133. | Zaanan A, Williet N, Hebbar M, Dabakuyo TS, Fartoux L, Mansourbakht T, Dubreuil O, Rosmorduc O, Cattan S, Bonnetain F, Boige V, Taïeb J. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013;58:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 134. | Chong JU, Choi GH, Han DH, Kim KS, Seong J, Han KH, Choi JS. Downstaging with Localized Concurrent Chemoradiotherapy Can Identify Optimal Surgical Candidates in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Ann Surg Oncol. 2018;25:3308-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 135. | Williet N, Dubreuil O, Boussaha T, Trouilloud I, Landi B, Housset M, Botti M, Rougier P, Belghiti J, Taieb J. Neoadjuvant sorafenib combined with gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma. World J Gastroenterol. 2011;17:2255-2258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 136. | Dinh VY, Bhatia S, Narayanan G, Yrizarry J, Savaraj N, O'Brien C, Martin P, Feun L. Pilot Study of Intrahepatic Artery Chemotherapy in Combination with Sorafenib in Hepatocellular Carcinoma. Anticancer Res. 2016;36:3555-3563. [PubMed] |
| 137. | Borentain P, Gregoire E, Louis G, Gerolami R. Successful liver transplantation for hepatocellular carcinoma following down-staging using sorafenib single therapy. Liver Int. 2016;36:1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 138. | Bertacco A, Vitale A, Mescoli C, Cillo U. Sorafenib treatment has the potential to downstage advanced hepatocellular carcinoma before liver resection. Per Med. 2020;17:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 139. | Zheng SZ, Liu DJ, Sun P, Yu GS, Xu YT, Gong W, Liu J. Feasibility and safety of sorafenib treatment in hepatocellular carcinoma patients with spontaneous rupture. World J Gastroenterol. 2014;20:16275-16281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 140. | Lee S, Kim BK, Kim SU, Park JY, Kim do Y, Ahn SH, Han KH. Early α-fetoprotein response predicts survival in patients with advanced hepatocellular carcinoma treated with sorafenib. J Hepatocell Carcinoma. 2015;2:39-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 141. | Lee S, Yoon SH, Park JY, Kim DY, Ahn SH, Han KH, Choi HJ. Sorafenib versus cytotoxic chemotherapy for patients with advanced hepatocellular carcinoma: a retrospective, single-institution study. Invest New Drugs. 2012;30:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 142. | Takeyama H, Beppu T, Higashi T, Kaida T, Arima K, Taki K, Imai K, Nitta H, Hayashi H, Nakagawa S, Okabe H, Hashimoto D, Chikamoto A, Ishiko T, Tanaka M, Sasaki Y, Baba H. Impact of surgical treatment after sorafenib therapy for advanced hepatocellular carcinoma. Surg Today. 2018;48:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 143. | Nakamura K, Beppu T, Hayashi H, Okabe H, Imai K, Nitta H, Chikamoto A, Ishiko T, Sasaki M, Baba H. Recurrence-free survival of a hepatocellular carcinoma patient with tumor thrombosis of the inferior vena cava after treatment with sorafenib and hepatic resection. Int Surg. 2015;100:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 144. | Kermiche-Rahali S, Di Fiore A, Drieux F, Di Fiore F, François A, Scotté M. Complete pathological regression of hepatocellular carcinoma with portal vein thrombosis treated with sorafenib. World J Surg Oncol. 2013;11:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 145. | Golse N, Radenne S, Rode A, Ducerf C, Mabrut JY, Merle P. Liver Transplantation After Neoadjuvant Sorafenib Therapy: Preliminary Experience and Literature Review. Exp Clin Transplant. 2018;16:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 146. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4122] [Article Influence: 515.3] [Reference Citation Analysis (5)] |
| 147. | Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, Takita M, Hagiwara S, Minami Y, Ida H, Takenaka M, Sakurai T, Watanabe T, Morita M, Ogawa C, Wada Y, Ikeda M, Ishii H, Izumi N, Nishida N. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 148. | Goio E, Ielasi L, Benevento F, Renzulli M, Tovoli F. Long-lasting remission in a metastatic hepatocellular carcinoma patient after combined regorafenib therapy and surgery. Hepat Oncol. 2020;7:HEP24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 149. | Kudo M. Current status of molecularly targeted therapy for hepatocellular carcinoma: clinical practice. Int J Clin Oncol. 2010;15:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Barbier L, Fuks D, Pessaux P, Muscari F, Le Treut YP, Faivre S, Belghiti J. Safety of liver resection for hepatocellular carcinoma after sorafenib therapy: a multicenter case-matched study. Ann Surg Oncol. 2013;20:3603-3609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 151. | Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 1016] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 152. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5337] [Article Influence: 889.5] [Reference Citation Analysis (29)] |
| 153. | Cheng AL, Qin S, Ikeda M, Galle P, Ducreux M, Zhu A, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, Li D, Verret W, Xu Z, Hernandez S, Liu J, Huang C, Mulla S, Lim HY, Finn R. IMbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) 1 bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30:ix186-ix187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 154. | Llovet J, Finn RS, Ikeda M, Sung M, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Mody K, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Zhu AX. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): Updated results. Ann Oncol. 2019;30:v253-v324. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 155. | Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined Stereotactic Body Radiotherapy and Checkpoint Inhibition in Unresectable Hepatocellular Carcinoma: A Potential Synergistic Treatment Strategy. Front Oncol. 2019;9:1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 156. | DE Pasquale MD, de Ville de Goyet J, Monti L, Grimaldi C, Crocoli A, Castellano A. Bevacizumab Combined with Chemotherapy in Children Affected by Hepatocellular Carcinoma: a Single-center Experience. Anticancer Res. 2017;37:1489-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boninsegna E, Gupta P S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL