Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1523
Peer-review started: April 25, 2021
First decision: June 13, 2021
Revised: June 20, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: December 27, 2021
Processing time: 242 Days and 23.8 Hours
Liver cancer is one of the most common cancers in the world. Of all types of liver cancer, hepatocellular carcinoma (HCC) is known to be the most frequent primary liver malignancy and has seriously compromised the health status of the general population. Locoregional thermal ablation techniques such as radiofrequency and microwave ablation, have attracted attention in clinical practice as an alternative strategy for HCC treatment. However, their aggressive thermal effect may cause undesirable complications such as hepatic decompensation, hemorrhage, bile duct injury, extrahepatic organ injuries, and skin burn. In recent years, photodynamic therapy (PDT), a gentle locoregional treatment, has attracted attention in ablation therapy for patients with superficial or luminal tumors as an alternative treatment strategy. However, some inherent defects and extrinsic factors of PDT have limited its use in clinical practice for deep-seated HCC. In this contribution, the aim is to summarize the current status and challenges of PDT in HCC treatment and provide potential strategies to overcome these deficiencies in further clinical translational practice.
Core tip: The application of photodynamic therapy (PDT) in hepatocellular carcinoma (HCC) therapy is limited due to its low penetration depth of light irradiation, the reduced generation of reactive oxygen species by conventional photosensitizers in the aggregated state, and the nontargeted accumulation in cancer cells. Once these problems are resolved, PDT will be a promising alternative treatment strategy for HCC.
- Citation: Zhu F, Wang BR, Zhu ZF, Wang SQ, Chai CX, Shang D, Li M. Photodynamic therapy: A next alternative treatment strategy for hepatocellular carcinoma? World J Gastrointest Surg 2021; 13(12): 1523-1535
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1523.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1523
Liver cancer is one of the most common causes of cancer-related death worldwide[1]. Of all types of liver cancer, hepatocellular carcinoma (HCC) is known to be the most frequent liver malignancy[2,3]. The main risk factors for HCC are chronic hepatitis B virus or hepatitis C virus infection, alcohol consumption and the resulting cirrhosis, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, dietary intake of aflatoxin B1, etc[4,5]. The incidence and mortality of HCC are rapidly rising in the USA and several European regions and slightly declining in traditionally high-risk regions such as East Asia and Africa[4]. Population-based studies have revealed that the incidence rate continues to approximate the death rate, indicating that most patients who develop HCC die of it[6]. HCC has seriously compromised the health status of the general population. In general, there are several treatment options for the management of HCC, but each treatment has its limitations and side effects[7]. In recent years, photodynamic therapy (PDT) has been a palliative treatment option that could improve quality of life and median survival with minimal invasion for cancer patients[8] and some studies have investigated its applications in ablation therapy for HCC. The aim of this frontier article was to summarize the current status and challenges of PDT for HCC as an alternative locoregional ablation and to propose potential strategies to overcome the deficiencies in clinical translational practice.
In general, several treatment options have emerged for the management of HCC. These options include surgical treatment with curative intents such as hepatic resection[9] or liver transplantation[10], systemic therapy (e.g., sorafenib, lenvatinib, regorafenib and apatinib)[11,12], immunotherapy (e.g., atezolizumab plus bevaci
PDT is a palliative treatment option that can improve quality of life and median survival with minimal invasion for patients, and has caused extensive concern for tumor therapy in recent years since Paramecium spp. killing was described through the interaction between acridine and infrared radiation by Oscar Raab in 1900[31]. Due to its low economic cost, few side effects, less invasiveness than surgery, short treatment time, precise targeting, and repeated treatment at the same site, PDT has been extended to the treatment of a variety of tumors, such as brain tumors[32], head and neck tumors[33,34], skin tumors[35], breast cancer[36], esophageal cancer[37], gastrointestinal tumors[38], lung cancer[39], extrahepatic cholangiocarcinoma[40-43], and bladder cancer[44].
PDT kills cancer cells by reactive oxygen species (ROS) generated from light-activated photosensitizers (PSs), resulting in the destruction of tumor cells and blood vessels and the stimulation of the host immune system[45-47]. Specifically, after activation by light irradiation, PSs accumulating in malignant tissues are electronically excited and transfer an electron to molecular oxygen or other electron acceptors to yield superoxide anions and radicals (i.e., type I reaction, in a hypoxic microenvironment) or transfer their electronic energy to ground-state molecular oxygen to yield singlet oxygen (i.e., type II reaction in a hyperoxic microenvironment)[48], which leads to antitumor effects and stimulates immune effects[49]. Moreover, activating the innate immune system increases the priming of tumor-specific T lymphocytes that can recognize and destroy distant tumor cells and lead to the development of immune memory that can combat the recurrence of cancer at a later point in time[50].
Among the three essential elements, PSs play a crucial role in ensuring the successful implementation of PDT. However, several inherent limitations of conventional PSs, such as high demand for oxygen in the microenvironment, inefficient generation of ROS and no organelle targeting, limit therapeutic outcomes in PDT[51]. In other words, several extrinsic factors impact the effectiveness of PDT. For instance, conventional PSs hardly have active accumulation in tumor lesions and tumor cell uptake[52], resulting in inefficient anticancer effects and phototoxicity of other normal tissues.
Although the clinical practice of PDT for deep-seated solid tumors has been limited by the penetration of laser irradiation and the defects of PSs, many studies have shown that PDT has better potential to improve HCC treatment than other traditional therapies owing to its noninvasiveness and localized therapeutic effect in the presence of specialized laser irradiation[8]. For example, experimental studies have shown that PDT can effectively kill hepatoma cells and shrink tumor tissues[53-55], and clinical investigations have also revealed that PDT can prolong the survival rate in patients with inoperable cancers to significantly improve their quality of life[56,57]. Specifically, this work summarizes the previous literature on PDT for HCC in Tables 1 and 2, to provide some insight for future research on PDT for HCC.
| PSs | Animal model | Ref. |
| ICG | Patient-derived orthotopic xenograft mice | Hong et al[58] |
| ICG | Huh-7 tumor-bearing nude mice | Shirata et al[49] |
| m-THPC (Foscan®) | Rat model with Walker-256 hepatoma cells | Wang et al[59] |
| Endogenous PpIX from 5-ALA | Diethylnitrosamine-induced HCC in Fisher-344 rats | Otake et al[60] |
| HpD | 2-Acetylaminofluorene-induced HCC in Fisher-344 rats | Kita et al[61] |
| PSs | Delivery vehicle | Ligand | Matching receptor | Drug agent | Animal model | Ref. |
| Pu-18-N-butylimide-NMGA | Gold NPs | / | / | / | Huh-7 tumor-bearing nude mice | Kwon et al[62] |
| ZnPc | BSA-assembled NPs | / | / | Sorafenib | SMMC-7721 tumor-bearing nude mice | Yu et al[51] |
| ICG | Nanoliposomes | / | / | Sorafenib | Hep3B tumor-bearing nude mice | He et al[63] |
| Porphyrin | MOF | Folic acid | Folate receptor | / | Doxycycline-induced HCC in krasG12V zebrafish | Chen et al[64] |
| Ce6 | SPIONs | Cancer cell membrane | / | / | SMMC-7721 tumor-bearing nude mice | Li et al[65] |
| Porphyrin | PEGylated Zr-MOF | Galactose | ASGPR | DOX | Huh-7 tumor-bearing nude mice | Hu et al[66] |
| Mitoxantrone | PEGylated UCNP micelles | Anti-EpCAM antibody | EpCAM | / | BEL-7404 tumor-bearing nude mice | Han et al[46] |
| Ce6 | DNA hybrids | TLS11a aptamer | / | DOX | HepG2 tumor-bearing nude mice | Zhang et al[67] |
| Ce6 | Gold NPs | TLS11a aptamer | / | AQ4N | HepG2 tumor-bearing nude mice | Zhang et al[68] |
| IR780 | Phospholipid/Pluronic F68 NPs | Pullulan | ASGPR | Paclitaxel | MHCC-97H tumor-bearing nude mice | Wang et al[69] |
As described in Table 1, indocyanine green (ICG) is a clinical infrared imaging agent approved by the US Food and Drug Administration[70,71] and has been applied in optical imaging in liver surgery[72-74], fluorescence angiography[75], cancer theranostics[72], surgical navigation[76], vascular grafts[77] and so on. In addition, a large number of studies have shown that ICG is widely used as a PS in PDT, and is able to rapidly generate singlet oxygen upon exposure to a near-infrared (NIR) laser and thus destroy cancerous cells[78,79]. Hence, ICG has been considered a promising theranostic agent. In addition, HCC cells notably take up ICG molecules with high efficiency but it cannot be easily excreted to bile ducts owing to the abnormal structures of bile capillaries[80]; thus, the retained ICG in HCC can kill cancer cells via PDT. For example, Kim et al[58] tested the cytotoxicity of ICG after NIR light irra
However, traditional PSs have low selectivity for accumulation in neoplastic tissues with an affinity for healthy tissues, which results in phototoxicity during treatment[83,84]. Therefore, a long period of light protection is required for patients after PDT. Additionally, PSs are easily degraded and excreted in blood circulation and have a tendency to aggregate in aqueous milieu, resulting in low bioavailability and the loss of photodynamic activity[85]. Recently, nanocarrier systems have shown potential to overcome the defects mentioned above[86-88]. In tumorous tissues, the absence of vasculature supportive tissues intimates the formation of leaky vessels and pores (100 nm to 2 μm in diameter). Meanwhile, the poor lymphatic system offers a great opportunity to treat cancer, and this phenomenon is known as the enhanced permeability and retention (EPR) effect[89,90]. Nanoparticles (NPs) can essentially deliver PSs to tumor lesions, which contribute to their passive tumor-targeting abilities (via the EPR effect)[91-93]. For example, He’s group[94] reported a new type of NP, copper–cysteamine (Cu–Cy), as a novel PS for anti-HCC treatment. Cu–Cy NPs not only significantly reduced the activity of HepG2 cells at a low dose after a short time of ultraviolet radiation in vitro, but also inhibited tumor growth in vivo. To further enhance the anti-HCC effects, Xu and his colleagues[63] designed NIR fluorescence imaging-guided nanoliposomes co-encapsulated with ICG and sorafenib. As expected, this nanocarrier could overcome the drawbacks of free ICG solution, such as instability in aqueous solution, rapid clearance in blood circulation, and lack of targeting, which leads it to achieve the PDT effect with negative targeting. Moreover, sorafenib also decreased the expression of vascular endothelial growth factor (VEGF) that was upregulated by PDT, which is a critical signaling factor for tumor recurrence. As such, this nanocarrier could inhibit HCC with synergistic therapeutic effects in a Hep3B tumor-bearing xenograft nude mouse model in vivo.
The free NPs used by PDT are subjected to inactive uptake and lack cancer cell-targeting abilities; hence, they cannot be internalized into cancer cells via active targeting with high efficiency[95,96]. Due to this limitation of free NPs, the paradigm of HCC treatment by PDT is now markedly shifting from NPs conjugating PSs to the tumor-specific targeting approach, which could lead to significantly improved PDT efficacy due to enhanced cellular uptake and minimize the toxic effects of associated therapeutic molecules[97,98]. Active targeting strategies using, for instance, specific ligands such as vitamins, antibodies or peptides, aptamers, could be a solution to overcome this limitation and achieve tumor-specific targeting properties[93]. The ligands can specifically bind with matching receptors on the hepatoma cell membrane and trigger receptor-mediated endocytosis[99]. For example, Li et al[64] designed and synthesized nanoscale gadolinium–porphyrin metal-organic frameworks as a skeleton for folic acid (FA) conjugation (FA–NPMOFs) to enhance the delivery of porphyrin into HCC cells. FA–NPMOFs exhibited a strong affinity for HCC cells with positive folate receptors and were delivered to tumor tissues in a targeted manner. Then, the porphyrin that accumulated in the tumor tissues could possess dual-function of fluorescence imaging and PDT in HCC tumor-bearing zebrafish model. After exposure to light at a specific wavelength, the singlet oxygen generated from porphyrin exerts a prominent anti-HCC effect rather than damaging the normal tissues contributing to the active targeting between FA of FA–NPMOFs and FR on HCC cells.
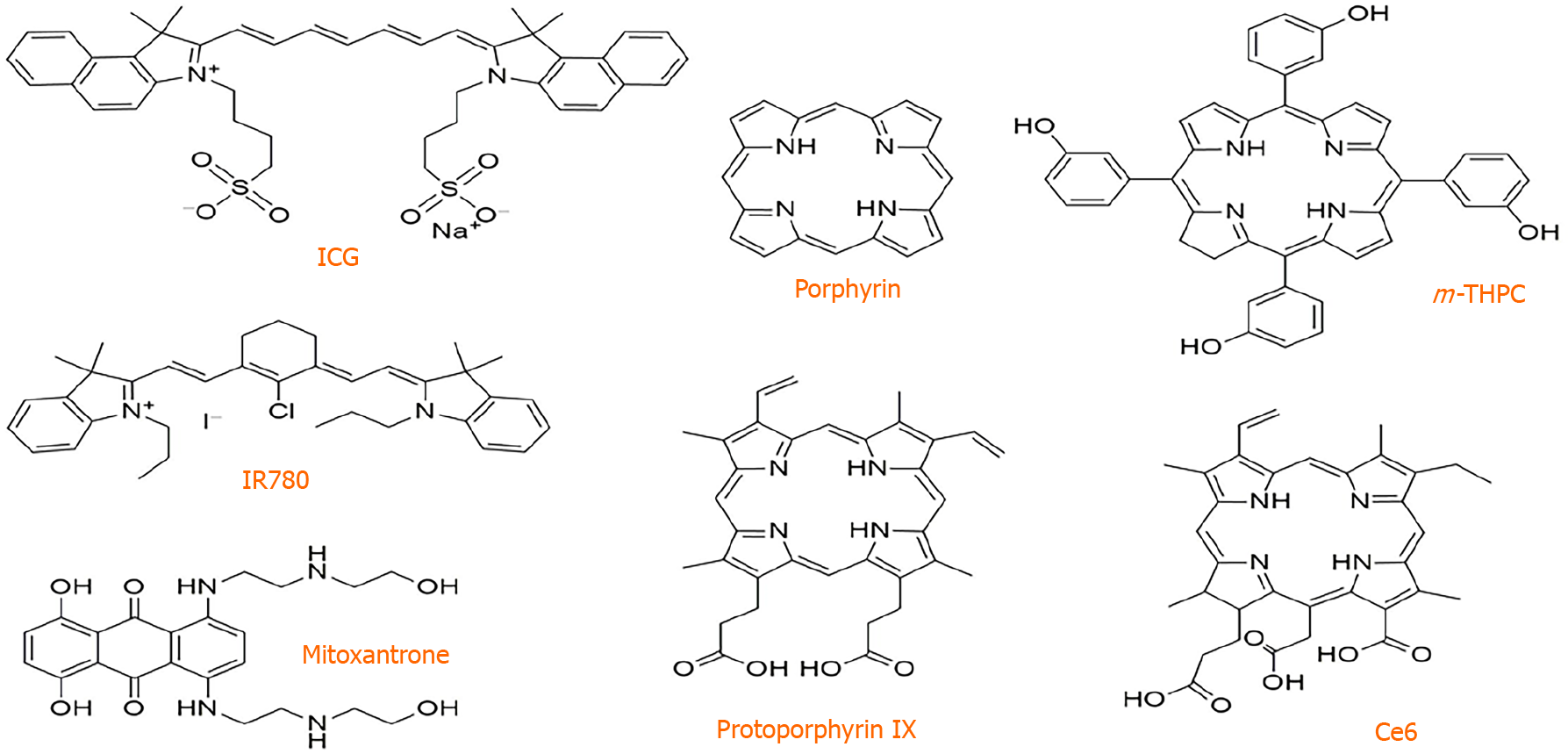
Another common problem of traditional PSs, such as the most widely used porphyrin derivatives and ICG, lies in their high hydrophobia and rigid planar structures as shown in Figure 1. Such a problem can collectively cause them to form aggregates in aqueous media through π–π stacking, resulting in an aggregation-caused quenching effect. This performance induces quenched fluorescence and a significant decrease in ROS generation that diminishes the imaging quality and PDT efficacy[100,101]. Conversely, aggregation-induced emission (AIE) molecules with a twisted configuration that suppresses strong intermolecular interactions represent a new class of PSs for image-guided PDT[102-104]. These PSs with AIE characteristics (denoted as AIE PSs) present weak emission in the molecular state but exhibit strong fluorescence emission and efficient photosensitization ability in the aggregated state[105-107]. Thus, formulating targeted AIE PS dots for image-guided PDT is expected to be a new treatment for tumors[40,105,106,108,109]. In previous work[40], our group designed and fabricated integrin ανβ3-targeted organic nanodots for image-guided PDT based on a red emissive AIE PS. The tetraphenylene derivative with typical AIE characteristic (TPETS)-encapsulated nanodots was prepared by nanoprecipitation method and further conjugated with thiolated cRGD through a click reaction to yield the targeted TPETS nanodots (T-TPETS nanodots), which could facilitate cellular uptake through active targeting by specific binding between cRGD and integrin ανβ3 and enhance ROS generation based on AIE PSs as the core of nanodots in the aggregate state. The data showed that the obtained nanodots showed bright red fluorescence and highly effective 1O2 generation in the aggregated state. The T-TPETS nanodots could accumulate in tumor tissue through the EPR effect and further expedite internalization by HCC cells via receptor-mediated endocytosis. Based on these multiple features, both in vitro and in vivo experiments demonstrated that the nanodots exhibited excellent HCC-targeted imaging performance, which promoted image-guided PDT for tumor ablation in a HepG2-bearing nude mouse model. After light irradiation, the nanodots inhibited the growth of tumor foci and significantly extended survival. Moreover, further analysis revealed that nanodot-mediated PDT could induce time- and concentration-dependent cell death. Specifically, the high PDT intensity resulted in direct cell necrosis, while the mitochondria-apoptosis pathway was triggered under low PDT intensity. These results suggest that the targeted NPs loaded with AIE PSs are promising image-guided PDT agents in HCC treatment.
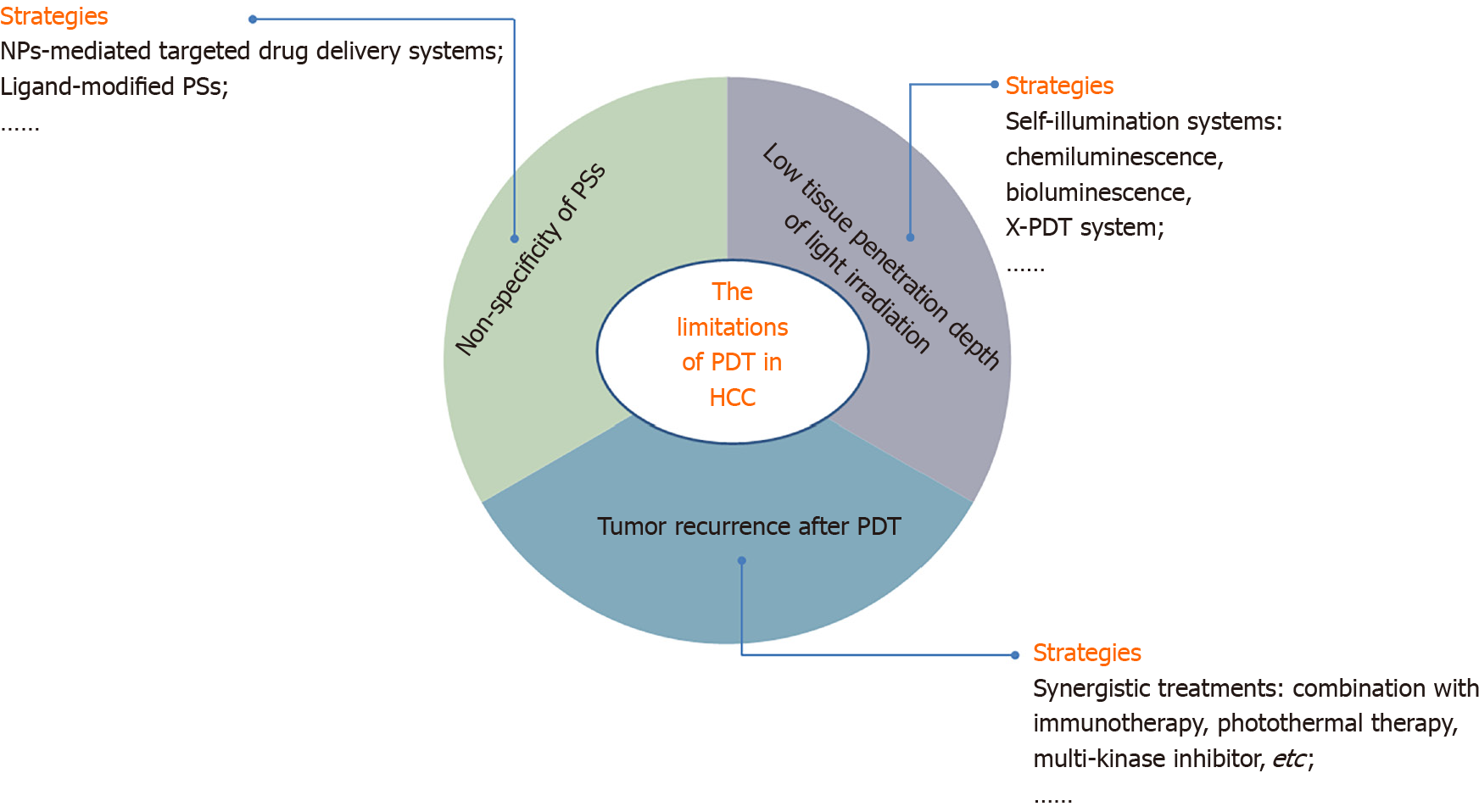
In recent years, numerous clinical trials have been registered of PDT for many types of tumors, but there are scarcely any trials on HCC. Therefore, some critical problems need to be conquered before further clinical practice of PDT for HCC can be realized (Figure 2). First, one major drawback of the currently available PDT is its low tissue penetration depth of light irradiation caused by the short-wavelength absorption of most PSs, which limits their clinical application[46]. The use of a self-illuminating system as a light source provides an intriguing solution to the light penetration issues of conventional PDT[110]. Some self-illuminating systems, including chemiluminescence[111] and bioluminescence[112], are promising candidates as internal light sources for PDT. These self-illuminators are small in size (ranging from the atomic/molecular to the nanometer scale) and thus can be delivered to any pathological tissue[113]. In addition, X-PDT exploits a nanoscale scintillator to down-convert external X-ray photons to visible light photons, and then the latter in turn activates nearby PSs to trigger PDT. Therefore, X-rays afford superior tissue penetration and can overcome this limitation of PDT[114,115]. Recently, Liu and her colleagues[116] developed a novel X-PDT system, taking advantage of an AIE PS with bright fluorescence and highly efficient 1O2 generation in the aggregated state. Based on the high penetration of X-ray irradiation, this system could use ionizing irradiation to trigger localized PDT, indicating that effective ·OH and SO generation was induced via radiosensitization-mediated energy transfer from X-rays to the AIE PS and then realized marked killing of cancer cells. This pioneering exploration revealed the great potential of AIE PSs in novel X-PDT systems to overcome the drawback of light irradiation penetration.
Second, another critical limiting factor of conventional cancer PDT is the lack of specificity of PSs. Moreover, most PSs accumulate in normal and cancer tissues indiscriminately. This performance leads to both significantly important side effects and decreased therapeutic efficacy[117,118]. Due to these obstacles, many studies have focused on the development of strategies to deliver effective therapeutic concentrations of PSs and anti-cancer agents specifically to the tumor, thereby increasing their therapeutic efficacy while reducing toxicity[99,118]. Therefore, targeted delivery of phototherapeutics, such as NP-mediated targeted drug delivery systems, is promising to minimize drug toxicity to healthy tissues through both target-specific drug delivery and by precisely controlling phototherapy-initiating external light sources[99,119,120].
Finally, the hypoxic microenvironment induced by PDT could secondarily accelerate the upregulation of angiogenic factors, such as hypoxia-inducible factor 1 and VEGF, and if the tumor cells are not killed completely under low light intensity, revascularization in tumor foci can be promoted, triggering the activation of signaling pathways for tumor recurrence[121,122]. Therefore, multiple combination regimens in the treatment of HCC, including immunotherapy, PDT/photothermal therapy, multikinase inhibitors and anti-VEGF agents, have attracted focus in recent years[123]. Combination therapies will hopefully increase objective responses and overall survival, contributing to the synergistic treatment of PDT and other anti-HCC therapies[124]. The multitude of available complementary and additive treatment modalities should encourage clinicians to implement a multidisciplinary treatment approach to improve the outcome in HCC patients[125].
The application of PDT in HCC has been limited due to its low tissue penetration depth of light irradiation, reduced generation of ROS, nontargeted accumulation in cancer cells, and tumor recurrence after PDT. There are several potential strategies to overcome these limitations, such as creating self-illuminating systems, NP-mediated targeted drug delivery systems, and synergistic treatments. Once these problems are resolved, PDT will be a promising alternative treatment strategy for HCC.
| 1. | Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 871] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 2. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 373] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 3. | Choo SP, Tan WL, Goh BKP, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern vs Western populations. Cancer. 2016;122:3430-3446. [RCA] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 4. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 853] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 5. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1286] [Article Influence: 183.7] [Reference Citation Analysis (3)] |
| 6. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 703] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 7. | Dendy MS, Ludwig JM, Stein SM, Kim HS. Locoregional Therapy, Immunotherapy and the Combination in Hepatocellular Carcinoma: Future Directions. Liver Cancer. 2019;8:326-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Chen G, Xu M, Zhao S, Sun J, Yu Q, Liu J. Pompon-like RuNPs-Based Theranostic Nanocarrier System with Stable Photoacoustic Imaging Characteristic for Accurate Tumor Detection and Efficient Phototherapy Guidance. ACS Appl Mater Interfaces. 2017;9:33645-33659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Cucchetti A, Zhong J, Berhane S, Toyoda H, Shi K, Tada T, Chong CCN, Xiang BD, Li LQ, Lai PBS, Ercolani G, Mazzaferro V, Kudo M, Cescon M, Pinna AD, Kumada T, Johnson PJ. The chances of hepatic resection curing hepatocellular carcinoma. J Hepatol. 2020;72:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 357] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 11. | Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol. 2017;67:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 12. | Zhang XH, Cao MQ, Li XX, Zhang T. Apatinib as an alternative therapy for advanced hepatocellular carcinoma. World J Hepatol. 2020;12:766-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Chino F, Stephens SJ, Choi SS, Marin D, Kim CY, Morse MA, Godfrey DJ, Czito BG, Willett CG, Palta M. The role of external beam radiotherapy in the treatment of hepatocellular cancer. Cancer. 2018;124:3476-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Iñarrairaegui M, Melero I, Sangro B. Immunotherapy of Hepatocellular Carcinoma: Facts and Hopes. Clin Cancer Res. 2018;24:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, Lin X, Chen X, Li E, Wang L, Chen C, Zou J. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 16. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5337] [Article Influence: 889.5] [Reference Citation Analysis (29)] |
| 17. | Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 482] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 18. | Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 464] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 19. | Liu X, Wang Z, Chen Z, Liu L, Ma L, Dong L, Zhang Z, Zhang S, Yang L, Shi J, Fan J, Wang X, Gao Q. Efficacy and Safety of Transcatheter Arterial Chemoembolization and Transcatheter Arterial Chemotherapy Infusion in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Oncol Res. 2018;26:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Chen KL, Gao J. Factors influencing the short-term and long-term survival of hepatocellular carcinoma patients with portal vein tumor thrombosis who underwent chemoembolization. World J Gastroenterol. 2021;27:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016;379:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 22. | Verslype C, Rosmorduc O, Rougier P; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii41-vii48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 23. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 24. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 662] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 25. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 26. | Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 27. | Ryu T, Takami Y, Wada Y, Hara T, Sasaki S, Saitsu H. Actual 10-Year Survival After Surgical Microwave Ablation for Hepatocellular Carcinoma: A Single-Center Experience in Japan. Ann Surg Oncol. 2019;26:4126-4133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Yao XS, Yan D, Jiang XX, Li X, Zeng HY, Li H. Short-term outcomes of radiofrequency ablation for hepatocellular carcinoma using cone-beam computed tomography for planning and image guidance. World J Clin Cases. 2021;9:1580-1591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | National Health Commision of the People's Republic of China. Standardization for diagnosis and treatment of primary hepatic carcinom (2019 edition). Zhonghua Shiyong Waike Zazhi. 2020;40:121-138. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Maeda M, Saeki I, Sakaida I, Aikata H, Araki Y, Ogawa C, Kariyama K, Nouso K, Kitamoto M, Kobashi H, Sato S, Shibata H, Joko K, Takaki S, Takabatake H, Tsutsui A, Takaguchi K, Tomonari T, Nakamura S, Nagahara T, Hiraoka A, Matono T, Koda M, Mandai M, Mannami T, Mitsuda A, Moriya T, Yabushita K, Tani J, Yagi T, Yamasaki T. Complications after Radiofrequency Ablation for Hepatocellular Carcinoma: A Multicenter Study Involving 9,411 Japanese Patients. Liver Cancer. 2020;9:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Oniszczuk A, Wojtunik-Kulesza KA, Oniszczuk T, Kasprzak K. The potential of photodynamic therapy (PDT)-Experimental investigations and clinical use. Biomed Pharmacother. 2016;83:912-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 32. | Tsai YC, Vijayaraghavan P, Chiang WH, Chen HH, Liu TI, Shen MY, Omoto A, Kamimura M, Soga K, Chiu HC. Targeted Delivery of Functionalized Upconversion Nanoparticles for Externally Triggered Photothermal/Photodynamic Therapies of Brain Glioblastoma. Theranostics. 2018;8:1435-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 33. | van Driel PBAA, Boonstra MC, Slooter MD, Heukers R, Stammes MA, Snoeks TJA, de Bruijn HS, van Diest PJ, Vahrmeijer AL, van Bergen En Henegouwen PMP, van de Velde CJH, Löwik CWGM, Robinson DJ, Oliveira S. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J Control Release. 2016;229:93-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 34. | Schwake M, Nemes A, Dondrop J, Schroeteler J, Schipmann S, Senner V, Stummer W, Ewelt C. In-Vitro Use of 5-ALA for Photodynamic Therapy in Pediatric Brain Tumors. Neurosurgery. 2018;83:1328-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJ, Gilaberte Y, Calzavara-Pinton P, Sidoroff A, Wulf HC, Szeimies RM. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Wang X, Hu J, Wang P, Zhang S, Liu Y, Xiong W, Liu Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sodium. Theranostics. 2015;5:772-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Kuzyniak W, Schmidt J, Glac W, Berkholz J, Steinemann G, Hoffmann B, Ermilov EA, Gürek AG, Ahsen V, Nitzsche B, Höpfner M. Novel zinc phthalocyanine as a promising photosensitizer for photodynamic treatment of esophageal cancer. Int J Oncol. 2017;50:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Rupinski M, Zagorowicz E, Regula J, Fijuth J, Kraszewska E, Polkowski M, Wronska E, Butruk E. Randomized comparison of three palliative regimens including brachytherapy, photodynamic therapy, and APC in patients with malignant dysphagia (CONSORT 1a) (Revised II). Am J Gastroenterol. 2011;106:1612-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Kimura M, Miyajima K, Kojika M, Kono T, Kato H. Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis. Int J Mol Sci. 2015;16:25466-25475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Li M, Gao Y, Yuan Y, Wu Y, Song Z, Tang BZ, Liu B, Zheng QC. One-Step Formulation of Targeted Aggregation-Induced Emission Dots for Image-Guided Photodynamic Therapy of Cholangiocarcinoma. ACS Nano. 2017;11:3922-3932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 41. | Park DH, Lee SS, Park SE, Lee JL, Choi JH, Choi HJ, Jang JW, Kim HJ, Eum JB, Seo DW, Lee SK, Kim MH, Lee JB. Randomised phase II trial of photodynamic therapy plus oral fluoropyrimidine, S-1, vs photodynamic therapy alone for unresectable hilar cholangiocarcinoma. Eur J Cancer. 2014;50:1259-1268. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Moole H, Tathireddy H, Dharmapuri S, Moole V, Boddireddy R, Yedama P, Uppu A, Bondalapati N, Duvvuri A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:1278-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 43. | Zhou T, Zhu J, Shang D, Chai C, Li Y, Sun H, Gao M, Li M. Mitochondria-anchoring and AIE-active photosensitizer for self-monitored cholangiocarcinoma therapy. Mater Chem Front. 2020;4:3201-3208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Chan KM, Gleadle J, Li J, Vasilev K, MacGregor M. Shedding Light on Bladder Cancer Diagnosis in Urine. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Hu D, Chen L, Qu Y, Peng J, Chu B, Shi K, Hao Y, Zhong L, Wang M, Qian Z. Oxygen-generating Hybrid Polymeric Nanoparticles with Encapsulated Doxorubicin and Chlorin e6 for Trimodal Imaging-Guided Combined Chemo-Photodynamic Therapy. Theranostics. 2018;8:1558-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 46. | Han Y, An Y, Jia G, Wang X, He C, Ding Y, Tang Q. Theranostic micelles based on upconversion nanoparticles for dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Nanoscale. 2018;10:6511-6523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Gao Y, Zheng QC, Xu S, Yuan Y, Cheng X, Jiang S, Kenry, Yu Q, Song Z, Liu B, Li M. Theranostic Nanodots with Aggregation-Induced Emission Characteristic for Targeted and Image-Guided Photodynamic Therapy of Hepatocellular Carcinoma. Theranostics. 2019;9:1264-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 48. | Dąbrowski JM, Arnaut LG. Photodynamic therapy (PDT) of cancer: from local to systemic treatment. Photochem Photobiol Sci. 2015;14:1765-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 49. | Shirata C, Kaneko J, Inagaki Y, Kokudo T, Sato M, Kiritani S, Akamatsu N, Arita J, Sakamoto Y, Hasegawa K, Kokudo N. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci Rep. 2017;7:13958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 50. | Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol. 2011;7:75-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 51. | Yu XN, Deng Y, Zhang GC, Liu J, Liu TT, Dong L, Zhu CF, Shen XZ, Li YH, Zhu JM. Sorafenib-Conjugated Zinc Phthalocyanine Based Nanocapsule for Trimodal Therapy in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. ACS Appl Mater Interfaces. 2020;12:17193-17206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Kaneko J, Kokudo T, Inagaki Y, Hasegawa K. Innovative treatment for hepatocellular carcinoma (HCC). Transl Gastroenterol Hepatol. 2018;3:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Egger NG, Schoenecker JA Jr, Gourley WK, Motamedi M, Anderson KE, Weinman SA. Photosensitization of experimental hepatocellular carcinoma with protoporphyrin synthesized from administered delta-aminolevulinic acid: studies with cultured cells and implanted tumors. J Hepatol. 1997;26:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Shao J, Xue J, Dai Y, Liu H, Chen N, Jia L, Huang J. Inhibition of human hepatocellular carcinoma HepG2 by phthalocyanine photosensitiser PHOTOCYANINE: ROS production, apoptosis, cell cycle arrest. Eur J Cancer. 2012;48:2086-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Mirzaei H, Djavid GE, Hadizadeh M, Jahanshiri-Moghadam M, Hajian P. The efficacy of Radachlorin-mediated photodynamic therapy in human hepatocellular carcinoma cells. J Photochem Photobiol B. 2015;142:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | van Duijnhoven FH, Rovers JP, Engelmann K, Krajina Z, Purkiss SF, Zoetmulder FA, Vogl TJ, Terpstra OT. Photodynamic therapy with 5,10,15,20-tetrakis(m-hydroxyphenyl) bacteriochlorin for colorectal liver metastases is safe and feasible: results from a phase I study. Ann Surg Oncol. 2005;12:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Gillams AR. Liver ablation therapy. Br J Radiol. 2004;77:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Hong F, Park JS, Kim SW, Park SJ, Kim SK. Near-infrared phototherapy for patient-derived orthotopic xenograft model of hepatocellular carcinoma in combination with indocyanine green. J Photochem Photobiol B. 2020;209:111938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Wang JD, Shen J, Zhou XP, Shi WB, Yan JH, Luo FH, Quan ZW. Optimal treatment opportunity for mTHPC-mediated photodynamic therapy of liver cancer. Lasers Med Sci. 2013;28:1541-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Otake M, Nishiwaki M, Kobayashi Y, Baba S, Kohno E, Kawasaki T, Fujise Y, Nakamura H. Selective accumulation of ALA-induced PpIX and photodynamic effect in chemically induced hepatocellular carcinoma. Br J Cancer. 2003;89:730-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Kita K, Itoshima T, Ito T, Ogawa H, Ukida M, Kitadai M, Hattori S, Mizutani S, Tanaka R, Andoh M. Photodynamic therapy of rat liver cancer: protection of the normal liver by indocyanine green. Gastroenterol Jpn. 1987;22:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Kwon JG, Song IS, Kim MS, Lee BH, Kim JH, Yoon I, Shim YK, Kim N, Han J, Youm JB. Pu-18-N-butylimide-NMGA-GNP conjugate is effective against hepatocellular carcinoma. Integr Med Res. 2013;2:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | He Q, He X, Deng B, Shi C, Lin L, Liu P, Yang Z, Yang S, Xu Z. Sorafenib and indocyanine green co-loaded in photothermally sensitive liposomes for diagnosis and treatment of advanced hepatocellular carcinoma. J Mater Chem B. 2018;6:5823-5834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Chen Y, Liu W, Shang Y, Cao P, Cui J, Li Z, Yin X, Li Y. Folic acid-nanoscale gadolinium-porphyrin metal-organic frameworks: fluorescence and magnetic resonance dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Int J Nanomedicine. 2019;14:57-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Li J, Wang X, Zheng D, Lin X, Wei Z, Zhang D, Li Z, Zhang Y, Wu M, Liu X. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater Sci. 2018;6:1834-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 66. | Hu J, Wu WR, Qin YF, Liu C, Wei P, Hu J, Seeberger PH, Yin J. Fabrication of Glyco-Metal-Organic Frameworks for Targeted Interventional Photodynamic/Chemotherapy for Hepatocellular Carcinoma through Percutaneous Transperitoneal Puncture. Adv Funct Mater. 2020;30. [DOI] [Full Text] |
| 67. | Zhang D, Zheng A, Li J, Wu M, Cai Z, Wu L, Wei Z, Yang H, Liu X, Liu J. Tumor Microenvironment Activable Self-Assembled DNA Hybrids for pH and Redox Dual-Responsive Chemotherapy/PDT Treatment of Hepatocellular Carcinoma. Adv Sci (Weinh). 2017;4:1600460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Zhang D, Zheng A, Li J, Wu M, Wu L, Wei Z, Liao N, Zhang X, Cai Z, Yang H, Liu G, Liu X, Liu J. Smart Cu(II)-aptamer complexes based gold nanoplatform for tumor micro-environment triggered programmable intracellular prodrug release, photodynamic treatment and aggregation induced photothermal therapy of hepatocellular carcinoma. Theranostics. 2017;7:164-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 69. | Wang D, Zhang S, Zhang T, Wan G, Chen B, Xiong Q, Zhang J, Zhang W, Wang Y. Pullulan-coated phospholipid and Pluronic F68 complex nanoparticles for carrying IR780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int J Nanomedicine. 2017;12:8649-8670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Liu C, Ruan C, Shi R, Jiang BP, Ji S, Shen XC. A near infrared-modulated thermosensitive hydrogel for stabilization of indocyanine green and combinatorial anticancer phototherapy. Biomater Sci. 2019;7:1705-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Xu F, Liu M, Li X, Xiong Z, Cao X, Shi X, Guo R. Loading of Indocyanine Green within Polydopamine-Coated Laponite Nanodisks for Targeted Cancer Photothermal and Photodynamic Therapy. Nanomaterials (Basel). 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 72. | Wang H, Li X, Tse BW, Yang H, Thorling CA, Liu Y, Touraud M, Chouane JB, Liu X, Roberts MS, Liang X. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics. 2018;8:1227-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 73. | Achterberg FB, Sibinga Mulder BG, Meijer RPJ, Bonsing BA, Hartgrink HH, Mieog JSD, Zlitni A, Park SM, Farina Sarasqueta A, Vahrmeijer AL, Swijnenburg RJ. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann Transl Med. 2020;8:1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Inagaki Y, Kokudo T, Kamiya M, Uno SN, Sato M, Kaneko J, Kokudo N, Urano Y, Hasegawa K. A novel liver-specific fluorescent anti-cancer drug delivery system using indocyanine green. Sci Rep. 2019;9:3044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise P, Vignali A, Rosati R. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020;34:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 76. | Qian Y, Cai S. A safe and effective surgical navigation technique in laparoscopic radical gastrectomy: Indocyanine green-mediated near-infrared fluorescent imaging. Cancer Commun (Lond). 2020;40:270-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Yamamoto M, Ninomiya H, Miyashita K, Tashiro M, Orihashi K, Inoue K, Sato T, Hanazaki K. Influence of residual coronary flow on bypass graft flow for graft assessment using near-infrared fluorescence angiography. Surg Today. 2020;50:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Wang M, Xiao Y, Li Y, Wu J, Li F, Ling D, Gao J. Reactive oxygen species and near-infrared light dual-responsive indocyanine green-loaded nanohybrids for overcoming tumour multidrug resistance. Eur J Pharm Sci. 2019;134:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 79. | Hu Y, Wang R, Zhou Y, Yu N, Chen Z, Gao D, Shi X, Shen M. Targeted dual-mode imaging and phototherapy of tumors using ICG-loaded multifunctional MWCNTs as a versatile platform. J Mater Chem B. 2018;6:6122-6132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Hu H, Chen J, Yang H, Huang X, Wu H, Wu Y, Li F, Yi Y, Xiao C, Li Y, Tang Y, Li Z, Zhang B, Yang X. Potentiating photodynamic therapy of ICG-loaded nanoparticles by depleting GSH with PEITC. Nanoscale. 2019;11:6384-6393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 81. | Yang X, Wang D, Shi Y, Zou J, Zhao Q, Zhang Q, Huang W, Shao J, Xie X, Dong X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl Mater Interfaces. 2018;10:12431-12440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 82. | Casas A. Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Lett. 2020;490:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 83. | Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O, Kotowski K, Kulbacka J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 1370] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 84. | Hou YJ, Yang XX, Liu RQ, Zhao D, Guo CX, Zhu AC, Wen MN, Liu Z, Qu GF, Meng HX. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int J Nanomedicine. 2020;15:6827-6838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 85. | R Mokoena D, P George B, Abrahamse H. Enhancing Breast Cancer Treatment Using a Combination of Cannabidiol and Gold Nanoparticles for Photodynamic Therapy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Duse L, Pinnapireddy SR, Strehlow B, Jedelská J, Bakowsky U. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. Eur J Pharm Biopharm. 2018;126:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143:3050-3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 88. | Chen D, Tao R, Tao K, Chen B, Choi SK, Tian Q, Xu Y, Zhou G, Sun K. Efficacy Dependence of Photodynamic Therapy Mediated by Upconversion Nanoparticles: Subcellular Positioning and Irradiation Productivity. Small. 2017;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Bort G, Lux F, Dufort S, Crémillieux Y, Verry C, Tillement O. EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles. Theranostics. 2020;10:1319-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 90. | Wang Y, Wang Z, Xu C, Tian H, Chen X. A disassembling strategy overcomes the EPR effect and renal clearance dilemma of the multifunctional theranostic nanoparticles for cancer therapy. Biomaterials. 2019;197:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 91. | Kang H, Rho S, Stiles WR, Hu S, Baek Y, Hwang DW, Kashiwagi S, Kim MS, Choi HS. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv Healthc Mater. 2020;9:e1901223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (4)] |
| 92. | Kim Y, Uthaman S, Pillarisetti S, Noh K, Huh KM, Park IK. Bioactivatable reactive oxygen species-sensitive nanoparticulate system for chemo-photodynamic therapy. Acta Biomater. 2020;108:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Gries M, Thomas N, Daouk J, Rocchi P, Choulier L, Jubréaux J, Pierson J, Reinhard A, Jouan-Hureaux V, Chateau A, Acherar S, Frochot C, Lux F, Tillement O, Barberi-Heyob M. Multiscale Selectivity and in vivo Biodistribution of NRP-1-Targeted Theranostic AGuIX Nanoparticles for PDT of Glioblastoma. Int J Nanomedicine. 2020;15:8739-8758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | Huang X, Wan F, Ma L, Phan JB, Lim RX, Li C, Chen J, Deng J, Li Y, Chen W, He M. Investigation of copper-cysteamine nanoparticles as a new photosensitizer for anti-hepatocellular carcinoma. Cancer Biol Ther. 2019;20:812-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 95. | García Calavia P, Chambrier I, Cook MJ, Haines AH, Field RA, Russell DA. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J Colloid Interface Sci. 2018;512:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 96. | Xia F, Niu J, Hong Y, Li C, Cao W, Wang L, Hou W, Liu Y, Cui D. Matrix metallopeptidase 2 targeted delivery of gold nanostars decorated with IR-780 iodide for dual-modal imaging and enhanced photothermal/photodynamic therapy. Acta Biomater. 2019;89:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 97. | Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 585] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 98. | Song Y, Shi Q, Zhu C, Luo Y, Lu Q, Li H, Ye R, Du D, Lin Y. Mitochondrial-targeted multifunctional mesoporous Au@Pt nanoparticles for dual-mode photodynamic and photothermal therapy of cancers. Nanoscale. 2017;9:15813-15824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Li M, Zhang W, Wang B, Gao Y, Song Z, Zheng QC. Ligand-based targeted therapy: a novel strategy for hepatocellular carcinoma. Int J Nanomedicine. 2016;11:5645-5669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 100. | Shi L, Hu F, Duan Y, Wu W, Dong J, Meng X, Zhu X, Liu B. Hybrid Nanospheres to Overcome Hypoxia and Intrinsic Oxidative Resistance for Enhanced Photodynamic Therapy. ACS Nano. 2020;14:2183-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 101. | Sun B, Chen Y, Yu H, Wang C, Zhang X, Zhao H, Chen Q, He Z, Luo C, Sun J. Photodynamic PEG-coated ROS-sensitive prodrug nanoassemblies for core-shell synergistic chemo-photodynamic therapy. Acta Biomater. 2019;92:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 102. | Dai J, Wu X, Ding S, Lou X, Xia F, Wang S, Hong Y. Aggregation-Induced Emission Photosensitizers: From Molecular Design to Photodynamic Therapy. J Med Chem. 2020;63:1996-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 103. | Hu JJ, Jiang W, Yuan L, Duan C, Yuan Q, Long Z, Lou X, Xia F. Recent advances in stimuli‐responsive theranostic systems with aggregation‐induced emission characteristics. Aggregate. 2021;2:48-65. [DOI] [Full Text] |
| 104. | Qi J, Ou H, Liu Q, Ding DJA. Gathering brings strength: How organic aggregates boost disease phototheranostics. Aggregate. 2021;2:95-113. [DOI] [Full Text] |
| 105. | Min X, Fang T, Li L, Li C, Zhang ZP, Zhang XE, Li F. AIE nanodots scaffolded by mini-ferritin protein for cellular imaging and photodynamic therapy. Nanoscale. 2020;12:2340-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Yang Y, Wang L, Cao H, Li Q, Li Y, Han M, Wang H, Li J. Photodynamic Therapy with Liposomes Encapsulating Photosensitizers with Aggregation-Induced Emission. Nano Lett. 2019;19:1821-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 107. | Kang M, Zhang Z, Song N, Li M, Sun P, Chen X, Wang D, Tang BZ. Aggregation‐enhanced theranostics: AIE sparkles in biomedical field. Aggregate. 2020;1:80-106. [DOI] [Full Text] |
| 108. | Zhang P, Kuang H, Xu Y, Shi L, Cao W, Zhu K, Xu L, Ma J. Rational Design of a High-Performance Quinoxalinone-Based AIE Photosensitizer for Image-Guided Photodynamic Therapy. ACS Appl Mater Interfaces. 2020;12:42551-42557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 109. | Zhang L, Li Y, Che W, Zhu D, Li G, Xie Z, Song N, Liu S, Tang BZ, Liu X, Su Z, Bryce MR. AIE Multinuclear Ir(III) Complexes for Biocompatible Organic Nanoparticles with Highly Enhanced Photodynamic Performance. Adv Sci (Weinh). 2019;6:1802050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 110. | Zhang Y, Hao Y, Chen S, Xu M. Photodynamic Therapy of Cancers With Internal Light Sources: Chemiluminescence, Bioluminescence, and Cerenkov Radiation. Front Chem. 2020;8:770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Jeon J, You DG, Um W, Lee J, Kim CH, Shin S, Kwon S, Park JH. Chemiluminescence resonance energy transfer-based nanoparticles for quantum yield-enhanced cancer phototheranostics. Sci Adv. 2020;6:eaaz8400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 112. | Yang Y, Hou W, Liu S, Sun K, Li M, Wu C. Biodegradable Polymer Nanoparticles for Photodynamic Therapy by Bioluminescence Resonance Energy Transfer. Biomacromolecules. 2018;19:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 113. | Yang K, Wang C, Wei X, Ding S, Liu C, Tian F, Li F. Self-Illuminating Photodynamic Therapy with Enhanced Therapeutic Effect by Optimization of the Chemiluminescence Resonance Energy Transfer Step to the Photosensitizer. Bioconjug Chem. 2020;31:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Sun W, Zhou Z, Pratx G, Chen X, Chen H. Nanoscintillator-Mediated X-Ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications. Theranostics. 2020;10:1296-1318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 115. | Cline B, Delahunty I, Xie J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 116. | Liu J, Hu F, Wu M, Tian L, Gong F, Zhong X, Chen M, Liu Z, Liu B. Bioorthogonal Coordination Polymer Nanoparticles with Aggregation-Induced Emission for Deep Tumor-Penetrating Radio- and Radiodynamic Therapy. Adv Mater. 2021;33:e2007888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 117. | Fernandes SRG, Fernandes R, Sarmento B, Pereira PMR, Tomé JPC. Photoimmunoconjugates: novel synthetic strategies to target and treat cancer by photodynamic therapy. Org Biomol Chem. 2019;17:2579-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 118. | Fathi M, Abdolahinia ED, Barar J, Omidi Y. Smart stimuli-responsive biopolymeric nanomedicines for targeted therapy of solid tumors. Nanomedicine (Lond). 2020;15:2171-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 119. | Zhu G, Chen X. Aptamer-based targeted therapy. Adv Drug Deliv Rev. 2018;134:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 120. | Zhang X, Ng HLH, Lu A, Lin C, Zhou L, Lin G, Zhang Y, Yang Z, Zhang H. Drug delivery system targeting advanced hepatocellular carcinoma: Current and future. Nanomedicine. 2016;12:853-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 121. | Weijer R, Broekgaarden M, van Golen RF, Bulle E, Nieuwenhuis E, Jongejan A, Moerland PD, van Kampen AH, van Gulik TM, Heger M. Low-power photodynamic therapy induces survival signaling in perihilar cholangiocarcinoma cells. BMC Cancer. 2015;15:1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 122. | Jiang S, Gao Y, Yu QH, Li M, Cheng X, Hu SB, Song ZF, Zheng QC. P-21-activated kinase 1 contributes to tumor angiogenesis upon photodynamic therapy via the HIF-1α/VEGF pathway. Biochem Biophys Res Commun. 2020;526:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Bangaru S, Marrero JA, Singal AG. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 124. | Sangiovanni A, Colombo M. Treatment of hepatocellular carcinoma: beyond international guidelines. Liver Int. 2016;36 Suppl 1:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 125. | Lurje I, Czigany Z, Bednarsch J, Roderburg C, Isfort P, Neumann UP, Lurje G. Treatment Strategies for Hepatocellular Carcinoma ⁻ a Multidisciplinary Approach. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar SKY, Tajiri K S-Editor: Wang LL L-Editor: Kerr C P-Editor: Wu RR