Published online Nov 27, 2021. doi: 10.4240/wjgs.v13.i11.1436
Peer-review started: July 18, 2021
First decision: August 15, 2021
Revised: August 29, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 27, 2021
Processing time: 131 Days and 13.5 Hours
Clostridium difficile infection (CDI) occurs due to a dysbiosis in the colon. The appendix is considered a ‘safe house’ for gut microbiota and may help repopulate gut flora of patients with CDI.
To study the impact of prior appendectomy on the severity and outcomes of CDI.
We retrospectively reviewed data of 1580 patients with CDI, admitted to our hospital between 2008 to 2018. Patients were grouped based on the presence or absence of the appendix. The primary aim was to (1) assess all-cause mortality and (2) the severity of CDI. Severity was defined as per the Infectious Diseases Society of America criteria. Logistic regression, and propensity score analysis using inverse probability of treatment weights (IPTW) was performed.
Of the 1580 patients, 12.5% had a history of appendectomy. There was no statistical difference in mortality between patients with a prior appendectomy or without (13.7% vs 14%, P = 0.877). However, a history of appendectomy affected the severity of CDI [odds ratio (OR) = 1.32, 95% confidence interval: 1.01-1.75]. On IPTW, this association remained significant (OR = 1.59, P < 0.05). On multivariable analysis of secondary outcomes, prior appendectomy was also associated with toxic megacolon (OR = 5.37, P < 0.05) and colectomy (OR = 2.77, P < 0.05).
Prior appendectomy may affect the severity of CDI, development of toxic megacolon and the eventual need for colectomy. Since treatment of CDI is governed by its severity, stronger antibiotic regimens or earlier use of fecal microbiota transplant may be a viable option for patients with prior appendectomy.
Core Tip: Clostridium difficile (C. difficile) infection occurs due to a dysbiosis of the gut. The appendix is known to host immune tissue and favorable gut microbiota, which may indirectly influence the disease course and outcomes in C. difficile infection. We found that prior appendectomy may affect the severity of C. difficile infection, and it may also increase the risk of developing toxic megacolon or requiring colectomy in these patients. Thus, earlier implementation of advanced therapeutic options may be necessary in patients without an appendix who develop C. difficile infection.
- Citation: Shaikh DH, Patel H, Munshi R, Sun H, Mehershahi S, Baiomi A, Alemam A, Pirzada U, Nawaz I, Naher K, Hanumanthu S, Nayudu S. Patients with Clostridium difficile infection and prior appendectomy may be prone to worse outcomes. World J Gastrointest Surg 2021; 13(11): 1436-1447
- URL: https://www.wjgnet.com/1948-9366/full/v13/i11/1436.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i11.1436
Clostridium difficile (C. difficile) is a gram positive, spore forming bacterium that spreads via the fecal-oral route, and causes an opportunistic Infection when a disruption in the normal intestinal flora occurs. C. difficile infection (CDI) is typically acquired in the healthcare setting, such as during hospitalizations, however community spread is also established. Recent prevalence studies demonstrate a decline in health care-associated CDI (by 24% from 2011 through 2017) as a result of better prevention practices and antibiotic stewardship programs, whereas the national burden of community-associated CDI has remained unchanged[1,2]. The clinical spectrum of CDI ranges from a mild diarrheal illness to a fulminant colitis, leading to shock and possible death. It is diagnosed via stool studies (presence of C. difficile toxins or toxigenic strain of C. difficile in stool), or the presence of typical colonoscopy findings of pseudomembranous colitis.
Treatment of CDI is governed by its severity on presentation, and in order to define the severity, several scoring systems are available. The components of the majority of these scales include patient comorbidities, clinical manifestations, laboratory tests, and imaging studies[3]. The most widely used of these scores was published in the 2010 Society for Healthcare Epidemiology of America and Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines (Table 1). It categorizes CDI into mild, severe, and severe, complicated[4]. The term “fulminant” is sometimes used to describe severe, complicated CDI[5]. This classification was derived from expert opinion and includes factors that predict unfavorable outcomes in CDI, such as serum creatinine > 1.5 mg/dL, leukocyte count > 15 × 109/L, ileus, toxic megacolon and shock.
| Severity | IDSA criteria |
| Mild | WBC < 15 × 109/L and serum creatinine < 1.5 times premorbid level |
| Severe | WBC ≥ 15 × 109/L, or serum creatinine ≥ 1.5 times premorbid level |
| Fulminant (severe, complicated) | Hypotension, shock, ileus, or megacolon |
In recent times, reports have emerged that recognize a history of appendectomy as an influence on the severity of CDI[6-8]. The vermiform appendix has long been considered a vestigial organ, however recent studies have elicited an abundance of favorable gut microbiota within the appendix, with the highest concentration in the innermost region, safely sheltered from colonic fecal stream[9,10]. The appendix is also said to harbor gut associated lymphoid tissue comprising of plasma B cells that produce high levels of secretory immunoglobulin A[11]. These antibodies may play a protective role in CDI, as reported by several small studies[12,13]. Larger epidemiological studies have also reinforced the appendix’s role as an immunomodulatory organ. A history of appendectomy is believed to prevent the development of ulcerative colitis (UC)[14,15], and reduce the risk of colectomy in these patients[16].
We postulate that the appendix may be able to repopulate the large intestine with commensal organisms, or provide passive immunity should the colon fall prey to C. difficile. Accordingly, its absence may be detrimental to recovery from CDI. Previous reports have corroborated a similar claim. Im et al[8], demonstrated the protective role of the appendix in CDI recurrences in 254 patients. Clanton et al[17] reported a higher rate of appendectomy in patients with fulminant CDI that resulted in a colectomy.
Based on the appendix’s ‘safe house’ theory, we set out to determine if there exists an association between prior appendectomy and outcomes (severity, recurrence, mortality) of CDI.
This was a single-center, retrospective cohort study, spanning a period of 10 years from April 2008 to November 2018. The study protocol was approved by the Institution Review Board at BronxCare Health System and was performed as per the Declaration of Helsinki (IRB # 12131804). Data for hospitalized patients with positive C. difficile stool test was retrieved using the electronic medical record (EMR). A positive stool test was defined as (1) positive results of stool C. difficile toxin (Toxin A or Toxin B) and glutamate dehydrogenase (GDH) antigen, or (2) positive C. difficile nucleic acid amplification test in cases of discrepancy between C. difficile toxin and GDH antigen. Medical records for patients with CDI were reviewed, and all asymptomatic carriers were excluded.
Baseline socio-demographic characteristics included age, gender, body mass index (BMI) and ethnicity. Data was extracted from admission records except for ethnicity, which was self-reported. EMR was used to obtain patients’ comorbidities. The blood chemistry and cell counts were obtained from the first set of the laboratory parameters acquired after the diagnosis of CDI. Past surgical history was scanned for information on prior appendectomy. Available abdominal imaging [ultrasound or computed tomography (CT) scan] prior to the onset of CDI was also reviewed to assess for the presence or absence of the appendix. When information was not available on history or imaging, the patient was considered to have an intact appendix. Records were also reviewed to assess prior use (within the preceding three months of CDI onset) of antibiotics, proton pump inhibitors (PPI), steroids and chemotherapy.
The primary outcomes for the current study were: (1) All-cause mortality; and (2) Severity of CDI. The charts of all patients, including their hospital course, were reviewed to document these findings including the recurrence rate, development of toxic megacolon, and the subsequent need for colectomy attributable to CDI. Mortality was defined as death within the same hospital admission as the CDI. Ileus was obtained from reported abdominal imaging (X-ray or CT scan) during the same hospital admission as the CDI. Additionally, data regarding the incidence of toxic megacolon was obtained from abdominal imaging (X-ray or CT scan) or the surgical operative note in cases where patients underwent colectomy during the same hospital admission as the CDI diagnosis. The severity of CDI was defined as per the IDSA guidelines (Table 1).
Recurrence was defined as a new episode of symptom onset, and positive assay result following a successfully treated prior episode of CDI in the previous 2-8 wk. Length of stay (LOS) was calculated from the EMR from the day of admission to discharge or death.
Patients were divided into two groups based on the history of appendectomy. The demographic information, comorbid medical conditions, and laboratory parameters were collected and stratified across both groups. Frequencies and percentages were reported for categorical variables. The mean and standard deviation were used to summarize continuous normal variables, while the median and interquartile range were used for continuous non-normal variables such as the LOS.
Univariate analysis was performed using Chi-square test independence for categorical variables. Continuous variables were compared using unpaired t-test and one-way ANOVA for two and more than two groups, respectively. For non-normal variables, Mann-Whitney and Kruskal-Wallis tests were used alternatively.
Multivariate analysis was performed using two methods in order to control and reduce the selection bias and other potential confounders in retrospective studies: (1) Multivariate logistic regression; and (2) Inverse probability of treatment weights (IPTW). Logistic regression was used to assess the association of prior appendectomy with primary and secondary outcomes after correcting for age, gender, BMI, comorbidities, and the prior use of antibiotics, steroids, and PPI. The binominal logistic regression was used for mortality and recurrence. Ordinal logistic regression and Poisson regression were used for CDI severity and LOS, respectively.
For IPTW, propensity scores were created by matching groups based on gender, age, prior PPI use, prior steroid use, and use of chemotherapy. Weights were calculated as the inverse of the propensity scores for patients with appendectomy. The control group weights were calculated by subtracting the propensity scores from unity and inverting the resulting score. Boosted logistic regression (using 20000 trees) was used to calculate propensity score. Average treatment effect (ATE) was used as an estimate during calculations. The balance was measured and assessed using the standardized effect size or standardized mean difference (SMD).
SPSS version 25 and R version 3.6.3 was used to perform the analysis.
CDI was diagnosed in 1580 hospitalized patients during the study period. The mean age of the patients was 57.1 ± 15.7 years at diagnosis. Females represented 51.2% of all patients. Data regarding race was missing for 40% of the patients. Of the remaining, African Americans constituted the majority (39%). Less than half of the patients presented with a mild CDI presentation (45%, n = 710), 36% (n = 566) were categorized into the severe CDI presentation and 19% (n = 304) presented with fulminant (severe, complicated) CDI. The all-cause mortality in the study population was 14% (n = 220). The recurrence rate was 14.4% (n = 228), with a mean and a median of 1.48 and 1.0 recurrences, respectively. The average LOS was 17.8 ± 31.89 d.
There was evidence of prior appendectomy in 12.5% (n = 198) of the patients. The appendectomy status in most of these patients (61%, n = 122) was documented on the CT scan of the abdomen. Comparing baseline characteristics between both groups (Table 2) revealed some differences. The percentage of females who had a prior appendectomy was significantly higher than the percentage who did not (59% vs 50%, P = 0.022). Information regarding BMI was available in less than half of the study population (n = 691), and there was no significant difference observed between the two groups. The age of the patients in both groups was also comparable, with no statistical significance. The prior use of antibiotics and PPI was more prevalent in individuals with prior appendectomy than individuals with no history of appendectomy, however only prior PPI use was statistically significant (P = 0.001). Prevalence of major comorbid conditions such as hypertension, diabetes, asthma/chronic obstructive pulmonary disease, coronary artery disease, and chronic kidney disease was comparable between the two groups. The prevalence of UC in patients with CDI was slightly higher amongst patients with a history of appendectomy (7.45%), compared to those with an intact appendix (4.54%), though this was not clinically significant (P = 0.203). We found no difference in the prevalence of Crohn's disease in our study population between patients with and without an appendix (2.02% vs 2.67%, P = 0.803). There were no differences in the laboratory parameters except for leukocytosis. Individuals with prior appendectomy demon
| Variable | All patients (%) | No prior appendectomy (%) | Prior appendectomy (%) | P value | n |
| 1580 | 1382 (87.5) | 198 (12.5) | |||
| Age | 57.1 ± 15.7 | 57.1 ± 15.6 | 56.8 ± 17.0 | 0.812 | 1580 |
| Gender | 0.022 | 1580 | |||
| Female | 809 (51.2) | 692 (50.1) | 117 (59.1) | ||
| Male | 771 (48.8) | 690 (49.9) | 81 (40.9) | ||
| Ethnicity | 0.439 | 1580 | |||
| African American | 611 (38.67) | 540 (39.1) | 71 (35.9) | ||
| Hispanic | 262 (16.6) | 228 (16.5) | 34 (17.2) | ||
| Caucasian | 58 (3.73) | 52 (3.8) | 6 (3.03) | ||
| Others | 10 (0.63) | 7 (0.51) | 3 (1.52) | ||
| Not available | 639 (40.4) | 555 (40.2) | 84 (42.4) | ||
| Comorbidities | 1580 | ||||
| Hypertension | 1210 (76.58) | 1022 (73.95) | 153 (77.27) | 0.815 | |
| Diabetes mellitus | 741 (46.89) | 618 (44.71) | 102 (51.51) | 0.384 | |
| Obstructive lung disease | 752 (47.56) | 633 (45.80) | 95 (47.97) | 0.933 | |
| Coronary artery disease | 480 (30.37) | 407 (29.45) | 57 (28.78) | 0.802 | |
| Chronic kidney disease | 477 (30.18) | 406 (29.37) | 56 (28.28) | 0.819 | |
| Inflammatory bowel diseases | 1580 | ||||
| Ulcerative colitis | 117 (7.40) | 103 (7.45) | 9 (4.54) | 0.203 | |
| Crohn’s disease | 42 (2.66) | 37 (2.67) | 4 (2.02) | 0.803 | |
| Risk Factor of CDI | |||||
| Prior use of antibiotics | 768 (52.2) | 656 (51.3) | 112 (58.3) | 0.086 | 1470 |
| Prior use of PPI | 429 (29.0) | 353 (27.4) | 76 (39.4) | 0.001 | 1480 |
| Prior use of steroids | 128 (8.73) | 110 (8.63) | 18 (9.1) | 0.838 | 1467 |
| Prior chemotherapy | 50 (3.35) | 45 (3.46) | 5 (2.59) | 0.678 | 1492 |
| Known malignancy | 175 (11.4) | 147 (11.0) | 28 (14.1) | 0.232 | 1539 |
| Liver cirrhosis | 117 (8.42) | 98 (8.19) | 19 (9.84) | 0.531 | 1389 |
| HIV infection | 406 (34.5) | 362 (35.2) | 44 (29.3) | 0.186 | 1389 |
| C. difficile related complication | |||||
| Ileus on imaging | 52 (3.90) | 41 (3.59) | 11 (5.67) | 0.237 | 1335 |
| Admission to ICU | 615 (40.6) | 526 (39.8) | 89 (46.1) | 0.113 | 1514 |
| Intubation | 298 (19.9) | 258 (19.8) | 40 (20.7) | 0.846 | 1494 |
| Clinical variable | |||||
| Body mass index (kg/m2) | 27.69 ± 7.7 | 27.78 ± 7.79 | 26.70 ± 6.75 | 0.1890 | 690 |
| Mean arterial pressure (mmHg) | 92.4 ± 18.2 | 92.6 ± 18.3 | 90.5 ± 17.5 | 0.115 | 1467 |
| Pulse beats per minute | 91.7 ± 20.8 | 91.6 ± 21.0 | 92.3 ± 20.0 | 0.700 | 1464 |
| Laboratory parameters | |||||
| Hemoglobin g/dL | 11.2 ± 2.93 | 11.2 ± 2.98 | 11.2 ± 2.58 | 0.819 | 1533 |
| White blood cell (cells/mm3) | 11.6 ± 8.04 | 11.4 ± 8.06 | 13.1 ± 7.71 | 0.005 | 1533 |
| Albumin (mg/dL) | 3.37 ± 0.82 | 3.38 ± 0.81 | 3.31 ± 0.87 | 0.309 | 1502 |
| Blood urea nitrogen (mg/dL) | 26.9 ± 26.0 | 26.9 ± 25.8 | 26.7 ± 27.3 | 0.925 | 1531 |
| Serum creatinine (mg/dL) | 1.85 ± 2.26 | 1.85 ± 2.24 | 1.88 ± 2.43 | 0.830 | 1531 |
| Lactic acid (mg/dL) | 2.13 ± 2.27 | 2.16 ± 2.33 | 1.92 ± 1.82 | 0.127 | 1372 |
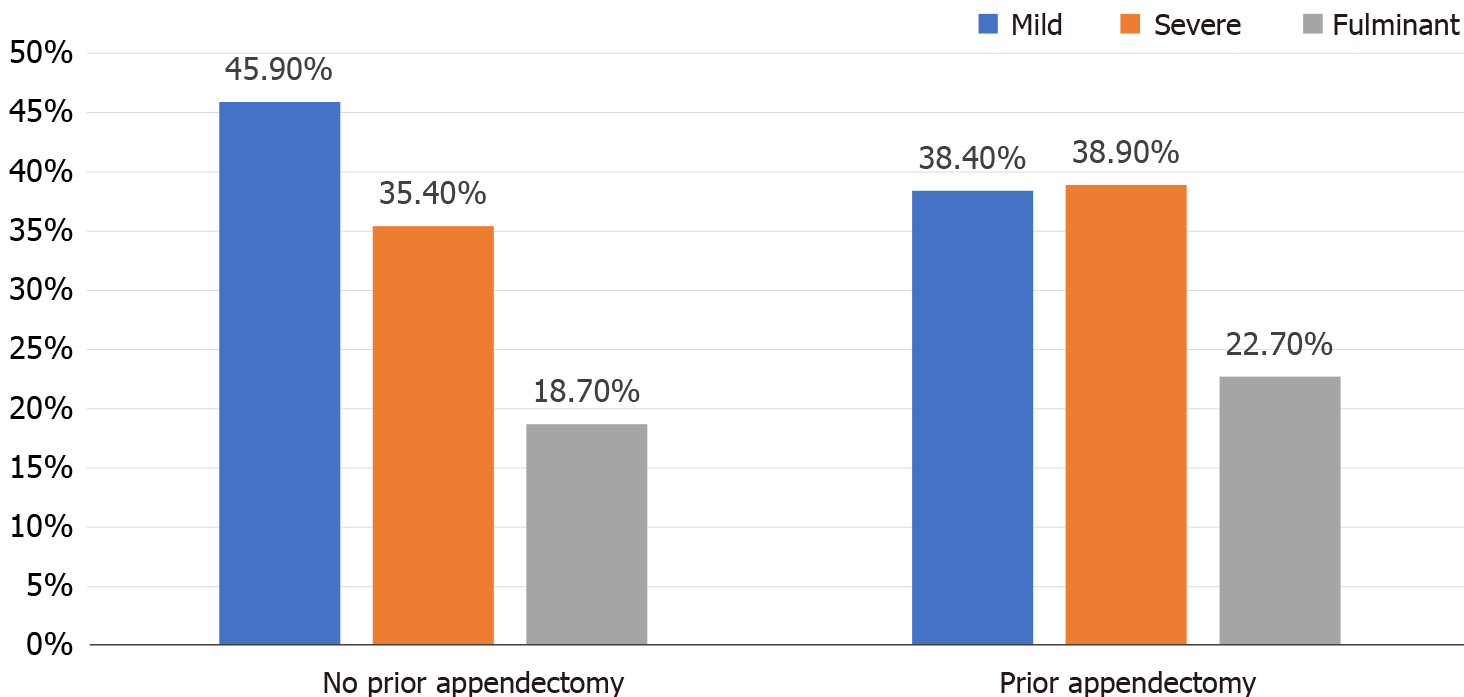
Prior appendectomy was associated with the severity of presentation, toxic megacolon attributable to C. difficile colitis, and colectomy (Table 3). The association with the latter two parameters was statistically significant at the 0.1 level, whereas, association of prior appendectomy with the severity of presentation was significant at P < 0.05 level. Patients with prior appendectomy were more likely to present with a higher grade of severity [odds ratio (OR) = 1.32, P < 0.05]. The rates of severe and fulminant CDI were higher in patients with prior appendectomy (39% and 23%, respectively) than patients with no prior appendectomy (35% and 19%, respectively). Mild presentation was more common in patients with no prior appendectomy. There was no significant difference in the recurrence rates of CDI.
| Outcome | Total | No prior appendectomy | Prior appendectomy | OR (95%CI) | P value |
| n | 1580 | 1382 | 198 | ||
| n IP-weighted | 1572 | 1354 | |||
| Mortality | |||||
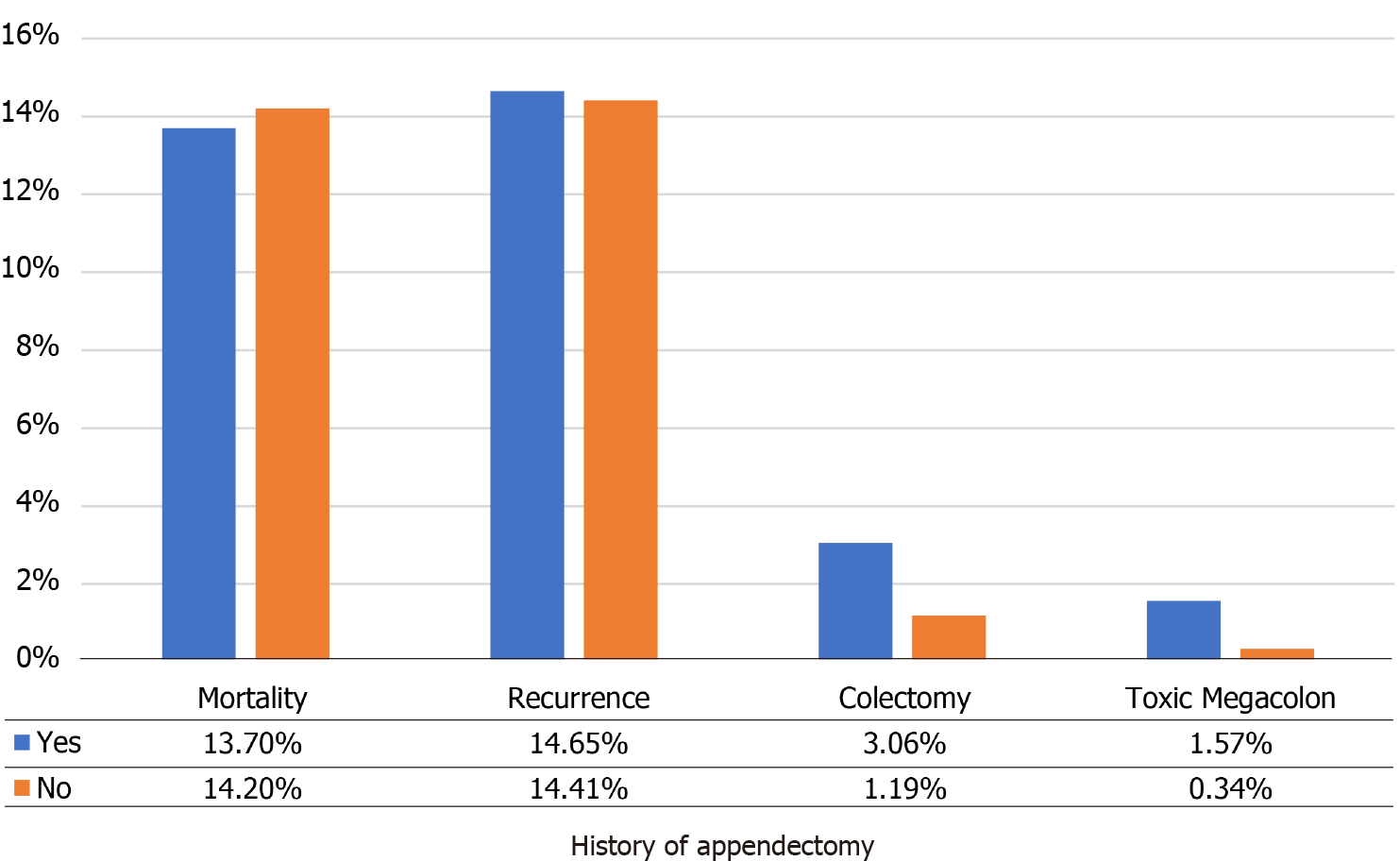
| n (%) | 220 (13.9) | 193 (14.2) | 27 (13.7) | 0.97 (0.61-1.47) | 0.877 |
| n IP-weighted (%) | 218 (14) | 207 (15.5) | 1.12 (0.65-1.92) | 0.685 | |
| Recurrence | |||||
| n (%) | 228 (14.4) | 199 (14.4) | 29 (14.6) | 1.02 (0.66-1.54) | 0.915 |
| n IP-weighted (%) | 228 (14.5) | 204 (15.1) | 1.05 (0.66-1.67) | 0.847 | |
| Toxic megacolon | |||||
| n (%) | 7 (0.4) | 4 (0.34) | 3 (1.57) | 4.75 (0.87-22.9) | 0.069 |
| n IP-weighted (%) | 5 (0.3) | 19 (1.5) | 4.32 (0.91-20.57) | 0.066 | |
| Colectomy | |||||
| n (%) | 22 (1.4) | 16 (1.19) | 6 (3.06) | 2.65 (0.93-6.59) | 0.067 |
| n IP-weighted (%) | 18 (1.2) | 24 (1.8) | 1.53 (0.55-4.27) | 0.413 | |
| Severity (IDSA) | |||||
| Before IPTW | 1.32 (1.01-1.75) | 0.043 | |||
| Mild, n (%) | 710 (44.9) | 634 (45.9) | 76 (38.4) | ||
| Severe, n (%) | 566 (35.8) | 489 (35.4) | 77 (38.9) | ||
| Fulminant, n (%) | 304 (19.2) | 259 (18.7) | 45 (22.7) | ||
| After IPTW | 1.59 (1.15-2.18) | 0.005 | |||
| Mild, n (%) | 724 (46.1) | 472 (34.8) | |||
| Severe, n (%) | 557 (35.4) | 526 (38.8) | |||
| Fulminant, n (%) | 291 (18.5) | 356 (26.3) | |||
| LOS among survivors | |||||
| Median (IQR) | 9 (5.00; 18.0) | 10 (6.50; 20.0) | 1.1 (0.94-1.28) | 0.233 | |
| Median (IQR)-IPTW | 10 (5.00; 20.0) | 12 (7.00; 23.00) | 0.9 (0.74-1.1) | 0.318 |
After the use of IPTW, the association between prior appendectomy and the IDSA severity did not change (OR = 1.59, P < 0.05), which indicates that a history of appendectomy in CDI is associated with 59% higher odds of presenting with a higher IDSA severity. The association between appendectomy and toxic megacolon did not change either. Prior appendectomy did not show a statistically significant association with recurrence, LOS, the need for colectomy, or mortality after the use of IPTW.
The severity of C. difficile (Table 4) was associated with mortality. Mortality was highest in patients with a fulminant presentation (46%, n = 146 of 314), as compared to the severe or mild manifestation of CDI (P < 0.001). The severity of CDI was as per the IDSA criteria, and patients with toxic megacolon and colectomy were considered in the fulminant C. difficile colitis category. The median LOS increased with increasing IDSA severity. Patients with mild CDI had a median LOS of 8 d, while patients with severe and fulminant CDI had median LOS of 10 and 19 d, respectively (P < 0.001). The mean BMI was similar between mild and severe presentations of CDI, whereas, patients with fulminant CDI were noted to have a slight increase in mean BMI, though not statistically significant (Table 5).
| Severity (IDSA) | |||||
| Outcomes | Mild (n = 710) | Severe (n = 566) | Fulminant (n = 304) | P value | |
| Mortality (%) | 29 (4.1) | 44 (7.7) | 147 (48.3) | < 0.001 | |
| Recurrence (%) | 98 (13.8) | 81 (14.3) | 49 (16.1) | 0.063 | |
| Toxic megacolon (%) | 0 | 0 | 7 (2.3) | < 0.001 | |
| Colectomy (%) | 0 | 0 | 22 (7.2%) | < 0.001 | |
| Length of stay (median) | 8 (4.00; 14.00) | 10.0 (6.00; 18.0) | 19 (10.0; 30.0) | < 0.001 | |
| Severity (IDSA) | Body mass index (kg/m2) | P = 0.412 |
| Mild | 27.66 ± 7.39 | |
| Severe | 27.34 ± 7.63 | |
| Fulminant | 28.4 ± 8.59 |
Multivariate logistic regression analysis (Table 6) showed that older age was associated with higher mortality in CDI patients (OR = 1.02, P < 0.001). Age did not show a statistically significant association with recurrence, toxic megacolon, or the need for colectomy. None of the included factors showed a statistically significant association with recurrence of CDI. Interestingly, prior appendectomy status was associated with higher odds of toxic megacolon (OR = 5.37, P < 0.05) and higher odds of requiring a colectomy (OR = 2.77, P < 0.05).
| Mortality | Recurrence | Toxic megacolon | Need for colectomy | |||||
| Variable | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI | P value |
| Age | 1.02 (1.01-1.03) | < 0.001 | 1.00 (0.99-1.01) | 0.626 | 0.99 (0.96-1.02) | 0.547 | ||
| Female gender | Ref | Ref | Ref | Ref | ||||
| Male gender | 1.32 (0.97-1.81) | 0.079 | 0.91 (0.67-1.22) | 0.518 | 0.86 (0.17-4.00) | 0.846 | 1.36 (0.55-3.46) | 0.501 |
| Prior appendectomy = no | Ref | Ref | Ref | Ref | ||||
| Prior appendectomy = yes | 1.03 (0.64-1.59) | 0.905 | 0.92 (0.58-1.42) | 0.727 | 5.37 (1.03-25.09) | 0.031 | 2.77 (0.95-7.17) | 0.044 |
| Prior antibiotics (no) | Ref | Ref | Ref | Ref | ||||
| Prior antibiotics (yes) | 1.04 (0.76-1.43) | 0.788 | 1.07 (0.79-1.44) | 0.668 | 0.13 (0.01-0.81) | 0.065 | 1.58 (0.62-4.31) | 0.348 |
| Prior steroids (no) | Ref | Ref | Ref | Ref | ||||
| Prior steroids (yes) | 0.72 (0.37-1.30) | 0.309 | 1.08 (0.63-1.77) | 0.774 | 1.99 (0.10-12.38) | 0.533 | 0.41 (0.02-2.07) | 0.391 |
| Prior PPI (no) | Ref | Ref | Ref | Ref | ||||
| Prior PPI (yes) | 0.89 (0.62-1.27) | 0.530 | 1.05 (0.74-1.46) | 0.790 | 1.08 (0.15-5.38) | 0.933 | 1.83 (0.71-4.60) | 0.196 |
| Prior chemotherapy (no) | Ref | Ref | Ref | Ref | ||||
| Prior chemotherapy (yes) | 0.92 (0.34-2.07) | 0.851 | 0.76 (0.26-1.79) | 0.570 | - | 4.26 (0.65-16.24) | 0.063 | |
Appendectomy remains the standard of care for the treatment of acute uncomplicated appendicitis. More than 300000 appendectomies are performed annually in the United States, making it one of the most commonly performed procedures by general surgeons. Our study demonstrated a 12.5% prevalence of prior appendectomy in our patient population, which is similar to the general population (12%-23%), based on epidemiological studies. More females had prior appendectomy compared to males (59% vs 50%, P = 0.019), a claim which is also consistent with population studies that demonstrate a higher lifetime risk of appendectomy in females compared to males[18].
CDI is the leading cause of hospital-acquired diarrhea in the United States and accounts for significant morbidity and mortality, burdening the healthcare system an additional 1 to 3 billion dollars in costs annually[7]. The all-cause mortality, attributable to CDI in our study population was 14%, whereas epidemiological studies estimate a mortality rate directly related to CDI at 5%, and a mortality associated with CDI complications between 15%-25%[19].
Our results show that patients with a prior appendectomy had a more severe course of CDI (Figure 1), and outcomes of toxic megacolon and colectomy were also higher (Figure 2). Even though patients with appendectomy did have more severe and fulminant course of CDI, our research did not demonstrate a higher rate of mortality in these patients. Of the two markers of severity, namely serum creatinine and white cell count, there was no significant difference seen in the serum creatinine level between the two groups. Thus the higher severity was mostly attributable to a higher leukocyte count in CDI patients with a prior appendectomy. Furthermore, a history of appendectomy was positively associated with the development of toxic megacolon and the need for colectomy at the P < 0.1 level on bivariate analysis. We postulate that significance was not met at the P < 0.05 level due to a small overall number of patients with toxic megacolon (n = 7) and those that underwent colectomy (n = 22), in our study population. However, the trajectory of data suggests a possible association that could have yielded significance at the P < 0.05 level, if the study power was increased. Our conjecture was confirmed on multivariate regression analysis, where prior appendectomy in CDI patients was identified as an independent predictor of both toxic megacolon (P = 0.031) and colectomy (P = 0.044).
Prior antibiotic use, defined as use of antibiotics within 3 months prior to the onset of CDI, was more likely in patients with appendectomy than those without, however this was not statistically significant. Similarly, PPI use was also more likely in patients with a history of appendectomy, and this was met with statistical significance. Antibiotic use has a known association with the development of CDI[20], and several meta-analysis have also reported PPI use as a risk factor for CDI, even in the absence of antibiotics[21,22]. However, to date, both antibiotics and PPI have not been shown to affect the severity of CDI at presentation.
Our study did not demonstrate an increased risk of CDI recurrence in patients with prior appendectomy compared to those without, contrary to previous reports[8]. Although PPI use has also been associated with an increased risk of recurrent CDI[23], this was not observed in our study cohort.
Other risk factors for acquiring CDI, such as a history of steroid use, chemotherapy, cirrhosis and HIV infection were evenly distributed between the appendectomy and non-appendectomy group.
In 2015, an estimated 1.3% of US adults (3 million) reported being diagnosed with inflammatory bowel diseases (IBD), namely Crohn’s disease or UC[24]. The appendix may also serve a role in IBD. Several large epidemiological cohort studies have demonstrated the preventive effect of appendectomy on the development of UC, a finding that has been confirmed in murine colitis models[14], though this has not been replicated in CDI populations. A recent systematic review showed a significant inverse association between an appendectomy and the development of UC with an overall OR of 0.39 (95% confidence interval: 0.29-0.52)[15]. While it is known that CDI can complicate underlying IBD, given the immunosuppressive nature of the disease[25], a higher prevalence of UC was seen in our patients with CDI and appendectomy keeping in line with prior studies.
The study data was further validated as it rightfully portrayed the highest mortality and increased LOS in those with the most fulminant presentation of CDI. Our data also demonstrated that older age had a higher risk of mortality. Age is a well-known risk factor for CDI, especially greater than 65 years, and it also correlates with increasing severity of infection[26].
Based on our results and analysis, we postulate that a history of appendectomy may lead to worse outcomes in CDI, likely secondary to an attenuated response to the dysbiosis of the gut, leading to an increased inflammatory reaction. Since disease severity is used to guide therapy, perhaps it’s prudent to screen patients with new onset CDI for factors associated with impaired immune response, such as an absent appendix. It would be worthwhile to investigate stronger antibiotic regimens or earlier institution of fecal microbiota transplantation in such patients.
Contrary to our hypothesis, deterministic ecological models of the colon microbiome have not demonstrated a protective role of the appendix in CDI. These models studied the effect of the appendicular migration rate of commensal microbiota, and the boost to antibody production exerted by the appendix[27]. Further, a handful of small retrospective studies did not show a positive correlation between a history of appendectomy and CDI. Khanna et al[28], reported no difference in outcomes such as severity, treatment failure or recurrence in patients who had undergone an appendectomy before the development of CDI as compared to patients without an appendectomy. Ward et al[29], studied the presence and severity of CDI in relation to the presence or absence of an appendix, which did not demonstrate a statistically significant association. More recently, two further analyses demonstrated that C. difficile recurrence rate is not affected by a prior appendectomy[30], nor is there any statistical difference in the severity or complications of CDI in the presence or absence of the appendix[31]. It is worth noting that all of the above mentioned negative studies had a smaller patient population compared to ours, with most under 500 patients. Yet interestingly, the prevalence of patients with appendectomy in these studies was similar to ours and the general population at large.
The limitations of our study include its inherent retrospective design. Our case-control methodology does not allow for us to determine causality between appen
Prior appendectomy may affect the severity of CDI, development of toxic megacolon and the eventual need for colectomy. Since treatment of CDI is governed by its severity, stronger antibiotic regimens or earlier use of fecal microbiota transplant may be a viable option for patients with prior appendectomy.
Clostridium difficile (C. difficile) is the leading cause of hospital-acquired diarrhea in the United States and accounts for significant morbidity, mortality and healthcare costs.
The vermiform appendix hosts immune tissue and favorable gut microbiota, which may indirectly influence the disease course and outcomes in C. difficile infection (CDI).
We aimed to study the association between prior appendectomy and outcomes (severity, recurrence, mortality) of CDI.
Retrospective review of 1580 patients with CDI, assessing mortality and severity based on the presence or absence of the appendix, using logistic regression and propensity score analysis.
There was no statistical difference in mortality between C. difficile patients with a prior appendectomy or without (13.7% vs 14%, P = 0.877). However, a history of appendectomy affected the severity of CDI [odds ratio (OR) = 1.32, 95% confidence interval: 1.01-1.75] and was also associated with the development of toxic megacolon (OR = 5.37, P < 0.05), and colectomy (OR = 2.77, P < 0.05).
A history of appendectomy may lead to worse outcomes in CDI, likely secondary to an attenuated response to the dysbiosis of the gut, leading to an increased inflammatory reaction.
Clinicians should be aware of the association between CDI and a history of appendectomy, and may consider screening all patients with C. difficile for a history of appendectomy. Further investigation into stronger antibiotic regimens or earlier institution of fecal microbiota transplantation for patients with prior appendectomy should be conducted if larger prospective studies can confirm and validate our results.
| 1. | Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, Wilson LE, Holzbauer SM, Phipps EC, Dumyati GK, Beldavs ZG, Kainer MA, Karlsson M, Gerding DN, McDonald LC; Emerging Infections Program Clostridioides difficile Infection Working Group. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med. 2020;382:1320-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 647] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 3. | Hamo Z, Azrad M, Nitzan O, Sagie A, Tkhawkho L, Binyamin D, Peretz A. Role of Single Procalcitonin Test on Admission as a Biomarker for Predicting the Severity of Clostridium difficile Infection. Front Microbiol. 2017;8:2532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 2212] [Article Influence: 201.1] [Reference Citation Analysis (0)] |
| 5. | Debast SB, Bauer MP, Kuijper EJ; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20 Suppl 2:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 799] [Article Influence: 66.6] [Reference Citation Analysis (12)] |
| 6. | Merchant R, Mower WR, Ourian A, Abrahamian FM, Moran GJ, Krishnadasan A, Talan DA. Association Between Appendectomy and Clostridium difficile Infection. J Clin Med Res. 2012;4:17-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Na X, Kelly C. The vermiform appendix and recurrent Clostridium difficile infection: a curious connection. Clin Gastroenterol Hepatol. 2011;9:1017-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Im GY, Modayil RJ, Lin CT, Geier SJ, Katz DS, Feuerman M, Grendell JH. The appendix may protect against Clostridium difficile recurrence. Clin Gastroenterol Hepatol. 2011;9:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Killinger B, Labrie V. The Appendix in Parkinson's Disease: From Vestigial Remnant to Vital Organ? J Parkinsons Dis. 2019;9:S345-S358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Kooij IA, Sahami S, Meijer SL, Buskens CJ, Te Velde AA. The immunology of the vermiform appendix: a review of the literature. Clin Exp Immunol. 2016;186:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Male DK. Immunology. Milton Keynes, MK, United States: Elsevier/Saunders, 2013. |
| 12. | Johal SS, Lambert CP, Hammond J, James PD, Borriello SP, Mahida YR. Colonic IgA producing cells and macrophages are reduced in recurrent and non-recurrent Clostridium difficile associated diarrhoea. J Clin Pathol. 2004;57:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Mulligan ME, Miller SD, McFarland LV, Fung HC, Kwok RY. Elevated levels of serum immunoglobulins in asymptomatic carriers of Clostridium difficile. Clin Infect Dis. 1993;16 Suppl 4:S239-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Cheluvappa R, Thomas DG, Selvendran S. The Role of Specific Chemokines in the Amelioration of Colitis by Appendicitis and Appendectomy. Biomolecules. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Koutroubakis IE, Vlachonikolis IG. Appendectomy and the development of ulcerative colitis: results of a metaanalysis of published case-control studies. Am J Gastroenterol. 2000;95:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Myrelid P, Landerholm K, Nordenvall C, Pinkney TD, Andersson RE. Appendectomy and the Risk of Colectomy in Ulcerative Colitis: A National Cohort Study. Am J Gastroenterol. 2017;112:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Clanton J, Subichin M, Drolshagen K, Daley T, Firstenberg MS. Fulminant Clostridium difficile infection: An association with prior appendectomy? World J Gastrointest Surg. 2013;5:233-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1335] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 19. | Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A, Goldman S, Wultańska D, Garlicki A, Biesiada G. Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis. 2019;38:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 503] [Article Influence: 71.9] [Reference Citation Analysis (1)] |
| 20. | Alekhova TA. [Isolation of a plasmid from actinomycin C-producing Streptomyces chrysomallus and its characteristics]. Mol Gen Mikrobiol Virusol. 1985;26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 377] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 21. | Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, Chiriac SA, Ciobica A, Boiculese L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol. 2017;23:6500-6515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (5)] |
| 22. | Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047-56; quiz 2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 429] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 23. | Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Hernandez AV, Donskey CJ, Fraser TG. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 493] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 25. | Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 26. | Leffler DA, Lamont JT. Clostridium difficile Infection. N Engl J Med. 2015;373:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Joshi T, Elderd BD, Abbott KC. No appendix necessary: Fecal transplants and antibiotics can resolve Clostridium difficile infection. J Theor Biol. 2018;442:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Khanna S, Baddour LM, Dibaise JK, Pardi DS. Appendectomy is not associated with adverse outcomes in clostridium difficile infection: a population-based study. Am J Gastroenterol. 2013;108:626-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Ward WH, Puttler KM, Lucha PA. Clostridium difficile colitis: is severity increased with previous appendectomy? Am Surg. 2013;79:E258-E259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Franko J, Ferrel B, Pierson P, Raman S, Frankova D, Rearigh LM, Afroze A, Guevara Hernandez MA, Terrero-Salcedo D, Kermode D, Gorvet M. Influence of prior appendectomy and cholecystectomy on Clostridioides difficile infection recurrence and mortality. Am J Surg. 2020;220:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Essrani R, Saturno D, Mehershahi S, Essrani RK, Hossain MR, Ravi SJK, Berger A, Mehmood A. The Impact of Appendectomy in Clostridium difficile Infection and Length of Hospital Stay. Cureus. 2020;12:e10342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy; American Association for the Study of Liver Diseases.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu L S-Editor: Gao CC L-Editor: A P-Editor: Wu RR