Published online Nov 27, 2021. doi: 10.4240/wjgs.v13.i11.1423
Peer-review started: May 3, 2021
First decision: May 27, 2021
Revised: May 31, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: November 27, 2021
Processing time: 207 Days and 14.7 Hours
Pancreatic ductal adenocarcinoma (PDAC) is a serious disease with a poor prognosis. Only a minority of patients undergo surgery due to the advanced stage of the disease, and patients with early-stage disease, who are expected to have a better prognosis, often experience recurrence. Thus, it is important to identify the risk factors for early recurrence and to develop an adequate treatment plan.
To evaluate the predictive factors associated with the early recurrence of early-stage PDAC.
This study enrolled 407 patients with stage I PDAC undergoing upfront surgical resection between January 2000 and April 2016. Early recurrence was defined as a diagnosis of recurrence within 6 mo of surgery. The optimal cutoff values were determined by receiver operating characteristic (ROC) analyses. Univariate and multivariate analyses were performed to identify the risk factors for early recurrence.
Of the 407 patients, 98 patients (24.1%) experienced early disease recurrence: 26 (26.5%) local and 72 (73.5%) distant sites. In total, 253 (62.2%) patients received adjuvant chemotherapy. On ROC curve analysis, the optimal cutoff values for early recurrence were 70 U/mL and 2.85 cm for carbohydrate antigen 19-9 (CA 19-9) levels and tumor size, respectively. Of the 181 patients with CA 19-9 level > 70 U/mL, 59 (32.6%) had early recurrence, compared to 39 (17.4%) of 226 patients with CA 19-9 level ≤ 70 U/mL (P < 0.001). Multivariate analysis revealed that CA 19-9 level > 70 U/mL (P = 0.006), tumor size > 2.85 cm (P = 0.004), poor differentiation (P = 0.008), and non-adjuvant chemotherapy (P = 0.025) were significant risk factors for early recurrence in early-stage PDAC.
Elevated CA 19-9 level (cutoff value > 70 U/mL) can be a reliable predictive factor for early recurrence in early-stage PDAC. As adjuvant chemotherapy can prevent early recurrence, it should be recommended for patients susceptible to early recurrence.
Core Tip: Pancreatic ductal adenocarcinoma (PDAC) is a serious disease with a poor prognosis. Only a minority of patients undergo surgery due to the advanced stage of the disease, and recurrence, an important prognostic factor, often occurs even after surgical resection. We identified the factors associated with the early recurrence of early-stage PDAC evaluating 407 patients with stage I PDAC undergoing upfront surgical resection. Early recurrence was defined as disease recurrence within 6 mo of surgery. Preoperative carbohydrate antigen 19-9 level > 70 U/mL determined by receiver operating characteristic analyses was a significant risk factor for early recurrence in early-stage PDAC.
- Citation: Hong S, Song KB, Hwang DW, Lee JH, Lee W, Jun E, Kwon J, Park Y, Park SY, Kim N, Shin D, Kim H, Sung M, Ryu Y, Kim SC. Preoperative serum carbohydrate antigen 19-9 levels predict early recurrence after the resection of early-stage pancreatic ductal adenocarcinoma. World J Gastrointest Surg 2021; 13(11): 1423-1435
- URL: https://www.wjgnet.com/1948-9366/full/v13/i11/1423.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i11.1423
Pancreatic ductal adenocarcinoma (PDAC) is a serious disease with a poor prognosis, with a 5-year survival rate of only 6%-10%[1,2]. While surgical resection offers the only possibility of cure[3], only a minority of patients are diagnosed with resectable disease because of local advancement or metastases at initial presentation[4]. Furthermore, even if patients undergo surgical treatment, about 70% experience disease recurrence[5-7]. Thus, efforts have been made to improve prognosis by early detection of the disease. However, even if patients are diagnosed and undergo surgery in the early stages, recurrence often occurs, and early recurrence is an important factor associated with a poor prognosis[8-10]. Therefore, it is necessary to identify the factors associated with the early recurrence of early-stage PDAC.
Various factors associated with PDAC prognosis have been reported including tumor size, preoperative carbohydrate antigen 19-9 (CA 19-9) concentration, histological grade, resection margin status, lymph node metastasis, and vascular invasion[11,12]. Among them, CA 19-9 levels, histological grade, and microvascular invasion are also associated with early recurrence[9,13-15]. Especially, serum CA 19-9 level, the only parameter that can be evaluated before surgery, has been regarded as a means of diagnosing malignant pancreatic neoplasms with high sensitivity and specificity[16,17]. Previous studies have also shown that CA 19-9 levels are a predictive factor for poor prognosis[18-22]. Elevated serum CA 19-9 levels are suggestive of pancreatic cancer recurrence, and serum CA 19-9 measurement is usually performed during surveillance, along with imaging tests, to detect cancer progression. Although imaging tests are performed to confirm cancer recurrence, CA 19-9 measurement is easier and more reproducible in terms of surveillance.
To improve the prognosis of pancreatic cancer, the risk factors for early recurrence should be evaluated, and active treatment, such as surgical treatment followed by chemotherapy, should be performed. Furthermore, as patients with early-stage disease, who are expected to have a better prognosis, often experience early recurrence, it is important to identify the risk factors for early recurrence and develop an adequate treatment plan. Pre- and post-operative CA 19-9 levels have been used to predict disease progression; however, few studies have demonstrated the effectiveness of CA 19-9 as a marker for early recurrence. This study evaluated the risk factors for early recurrence in patients with American Joint Committee on Cancer (AJCC) 8th edition stage I PDAC after upfront surgery. We set the optimal cutoff CA 19-9 level and evaluated the power of CA 19-9 as a detector of early recurrence of early-stage PDAC. We also evaluated the importance of adjuvant chemotherapy as a therapeutic modality for early-stage patients to reduce the chance of early recurrence.
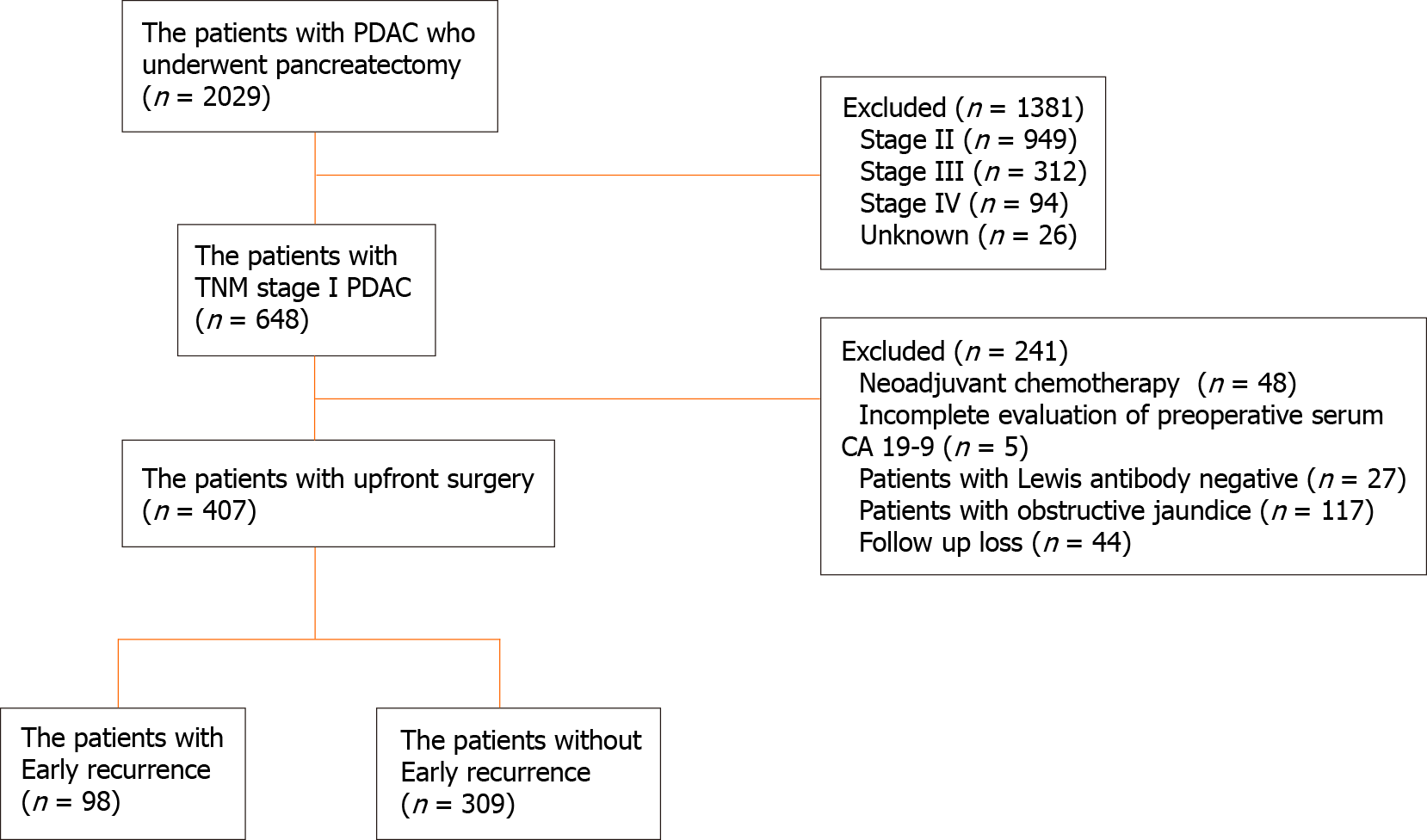
Between January 2000 and April 2016, 2029 consecutive patients underwent surgical resection for PDAC at Asan Medical Center (Seoul, South Korea). PDAC was histologically confirmed in all patients, and patients with other pancreatic tumors such as intraductal papillary mucinous adenocarcinoma, adenosquamous carcinoma, mucinous carcinoma, acinar cell carcinoma, and malignant endocrine carcinoma were excluded. Of these, 648 patients had tumor-node-metastasis (TNM) stage IA and IB disease based on permanent pathologic reports. Forty-eight patients who received neoadjuvant chemotherapy, forty-four who were lost to follow-up, and five with incomplete data on preoperative serum CA 19-9 levels were excluded. Patients whose CA 19-9 levels were measured when they had jaundice (preoperative total bilirubin levels ≥ 2 mg/dL) were excluded to avoid the effect of obstructive jaundice on CA 19-9 values. Patients with preoperative CA 19-9 level < 2 U/mL were considered as Lewis antibody-negative patients; thus, they were considered to be unable to express CA 19-9 and were excluded from this study. Finally, 407 patients who underwent upfront surgical resection for stage I PDAC were enrolled in this study (Figure 1). Data regarding age, sex, body mass index, type of operation, pathology, recurrence, and preoperative serum CA 19-9 levels were obtained retrospectively from medical records. All patients underwent either abdominal computed tomography (CT), magnetic resonance imaging, or both preoperatively for the evaluation of tumor lesion and resectability. The pathologic stage was determined according to the TNM Classification of Malignant Tumors, 8th edition, from the AJCC.
All serum CA 19-9 values were measured using an electrochemiluminescence immunoassay kit in the institution’s laboratory. The recommended upper normal limit for CA 19-9 is 37 U/mL. CA 19-9 levels were examined within 1 mo before the surgery. When patients developed jaundice due to tumor invasion of the biliary tract, interventions were performed, including endoscopic nasobiliary drainage, endoscopic retrograde biliary drainage, or percutaneous transhepatic biliary drainage.
Distal pancreatectomy was the standard procedure for tumors of the pancreatic neck, body, or tail. Pancreaticoduodenectomy (pylorus-preserving or pylorus-resecting) was performed for tumors located in the pancreas head or uncinate. Total pancreatectomy was performed in patients in whom intra-operative frozen biopsy showed positive resection margin, remnant pancreas was atrophied, pancreatitis was very severe involving the whole pancreas, and pancreatic duct was dilated throughout the pancreas. The surgeries were performed using either an open approach or laparoscopically. The pathologic characteristics included tumor size, resection margin status, lymph node metastasis, differentiation, lymphovascular invasion, and perineural invasion status. The resection margins were evaluated by a pathologist as either R0 (no cancer cells observed microscopically at the resection margin) or R1 (cancer cells observed microscopically at the resection margin or a free margin of < 1 mm).
The patients were followed up with abdominal CT and blood tests, including tests for tumor markers, CA 19-9, and carcinoembryonic antigen levels, every 3 mo for the first 2 years after surgery and every 3-6 mo thereafter. When the CA 19-9 level was elevated or abdominal CT suggested tumor recurrence, additional positron emission tomography (PET) was performed. Tumor recurrence was defined based on radiological or biopsy-proven evidence. Radiological recurrence was determined by radiologists and defined as progressive soft-tissue growth or hypermetabolic lesions at specific sites, as determined by CT or PET. Biopsy was not routinely required for the diagnosis of tumor recurrence.
Overall survival (OS) was defined as the time from surgery to the date of death from any cause or the last follow-up visit. Disease-free survival (DFS) was defined as the time from surgery to the first documented detection of recurrence on CT or PET during regular follow-up or death, whichever occurred first. Early recurrence was defined as disease relapse within 6 mo of surgery.
Continuous variables are expressed as medians and interquartile ranges. OS and DFS were estimated using the Kaplan–Meier method, and the values were compared using log-rank tests. Receiver operating characteristic (ROC) curves were constructed to estimate the optimal cutoff values for preoperative CA 19-9 levels and tumor size as predictors of postoperative early recurrence, with the Youden index used as a summary measure of the ROC curve. The χ2 or Fisher’s exact test was performed for categorical variables. Univariate and multivariate analyses were performed using a logistic regression model to determine the predictive variables associated with early recurrence. P < 0.05 was considered statistically significant. The statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, United States).
This study included 407 patients. Of them, 225 (55.3%) were male and 182 (44.7%) were female, with a median age of 62 years (30-88). The median follow-up time was 31 mo (1-227). A total of 254 patients (62.4%) underwent pancreatectomy for tumors located at the head or uncinate, and 151 (37.1%) underwent pancreatectomy for tumors located at the pancreatic neck, body, or tail. Permanent biopsy result revealed that the tumor involved both head and body in two cases (0.5%). The median tumor size was 2.5 cm (0.3-4), and the median number of harvested lymph nodes was 14. A total of 253 patients (62.2%) received adjuvant chemotherapy. The median OS durations in the early and non-early recurrence groups were 11 and 42 mo, respectively (P < 0.001). The demographic and pathologic findings are summarized in Table 1.
| Characteristics | Patients, n = 407 (%) |
| Age in yr, median (range) | 62 (30-88) |
| Sex, n (%) | |
| Male | 225 (55.3) |
| Female | 182 (44.7) |
| BMI in kg/m2, median (range) | 23.2 (15.3-31.6) |
| Pre-op CA 19-9 in U/mL, n (%) | |
| Normal | 167 (41) |
| Abnormal | 240 (59) |
| Tumor location, n (%) | |
| Head/uncinate | 254 (62.4) |
| Neck/body/tail | 151 (37.1) |
| Head/body | 2 (0.5) |
| Tumor size, median, cm (range) | 2.5 (0.3-4.0) |
| Total number of harvested lymph nodes, median (range) | 14 (1-74) |
| Differentiation, n (%) | |
| Well | 60 (14.9) |
| Poor | 288 (71.6) |
| Unknown | 54 (13.4) |
| Moderate | 5 (1.2) |
| Stage, n (%) | |
| IA | 109 (26.8) |
| IB | 298 (73.2) |
| Adjuvant chemotherapy, n (%) | |
| No | 154 (37.8) |
| Yes | 253 (62.2) |
| Recurrence within 6 mo, n (%) | |
| No | 309 (75.9) |
| Yes | 98 (24.1) |
The median follow-up duration was 31 mo. A total of 304 patients (75.4%) showed disease recurrence, with a median time to recurrence of 10 mo. In this study, 99 (32.6%) and 205 (67.4%) patients had local and distant recurrences, respectively. Among the patients with distant recurrence, the most common recurrence site was the liver, followed by peritoneal seeding and the lungs. A total of 98 patients (24.1%) had early recurrence, and 309 (75.9%) had either non-early or no recurrence. Among patients with early recurrence, 26 (26.5%) had local recurrence and 72 (73.5%) had distant recurrence. The most common recurrence site was the liver (37.8%).
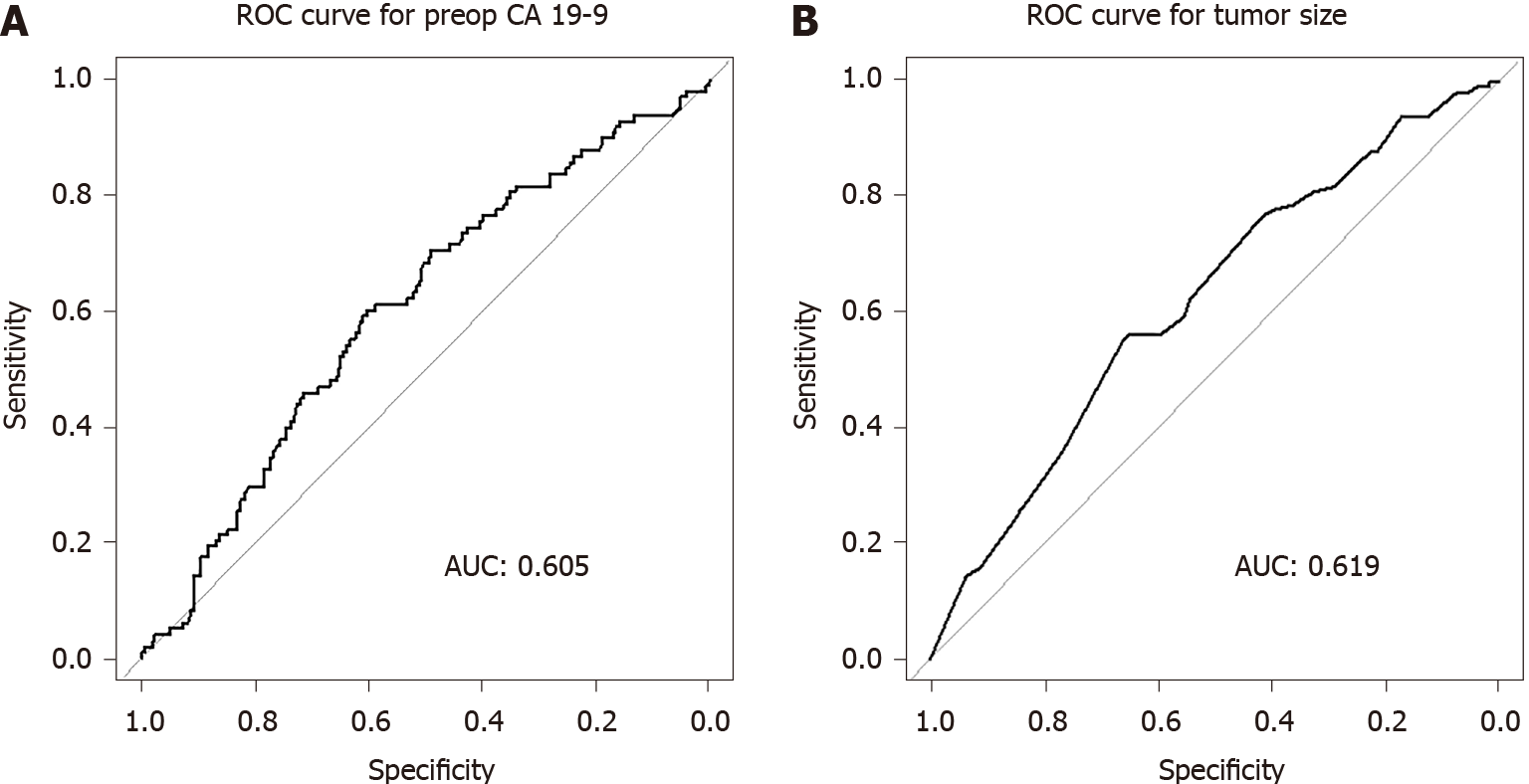
ROC curve analysis revealed 70 U/mL as the optimal cutoff preoperative CA 19-9 level for predicting early recurrence (area under the curve [AUC] 0.605; sensitivity 60.2%, specificity 60.5%; Figure 2A). In this study, 181 patients had preoperative serum CA 19-9 level ≥ 70 U/mL; among them, 59 patients (32.6%) had early recurrence. In contrast, 39 of the 226 patients (17.4%) with CA 19-9 level < 70 U/mL had early recurrence (P < 0.001). We had postoperative serum CA 19-9 values checked within 1 mo after the operation. Among the 181 patients with preoperative serum CA 19-9 values ≥ 70 U/mL, 171 patients (94%) had decreased serum CA 19-9 values after the operation, and of these, 49 patients (28.7%) experienced early disease recurrence. Nine patients had rather increased serum CA 19-9 value, and all of these patients experienced early recurrence. In one patient, we did not check the postoperative CA 19-9 value. ROC curve analysis also revealed 2.85 cm as the optimal cutoff tumor size for predicting early recurrence (AUC 0.619; sensitivity 56.1%, specificity 65.0%; Figure 2B).
Table 2 shows the risk factors associated with early recurrence after curative surgical resection for TNM stage I PDAC. In the univariate analysis, preoperative serum CA 19-9 level (P < 0.001), tumor size (P < 0.001), and differentiation (P = 0.005) were significant. In the multivariate analysis, a CA 19-9 level ≥ 70 U/mL (odds ratio [OR] 1.987; P = 0.006), tumor size ≥ 2.85 cm (OR 2.039; P = 0.004), poor differentiation (OR 3.493 for poorly differentiated vs well differentiated; P = 0.008), and non-adjuvant chemotherapy (OR 1.745; P = 0.025) were significantly associated with early recurrence after surgical resection.
| Factors | Number of patients, n (%) | Univariate, P value | Odds ratio (95%CI) | Multivariate, P value |
| Age in yr | 0.211 | ` | ||
| < 65 | 234 (57.5) | |||
| ≥ 65 | 173 (42.5) | |||
| Sex | 0.261 | |||
| Male | 225 (55.3) | |||
| Female | 182 (44.7) | |||
| Tumor size in cm | < 0.001 | 0.004 | ||
| < 2.85 | 244 (60.0) | |||
| ≥ 2.85 | 163 (40.0) | 2.039 (1.251-3.323) | ||
| RM | 0.555 | 0.638 | ||
| Negative | 348 (85.5) | |||
| Positive | 59 (14.5) | 1.177 (0.583-2.287) | ||
| Tumor location | 0.394 | |||
| Head/uncinate | 254 (62.4) | |||
| Neck/body/tail | 151 (37.1) | |||
| Differentiation | 0.005 | 0.019 | ||
| Well | 60 (14.9) | |||
| Moderate | 288 (71.6) | 0.196 | 1.430 (0.652–3.133) | 0.372 |
| Poor | 54 (13.4) | 0.005 | 3.493 (1.377–8.858) | 0.008 |
| CA 19-9 in U/mL | < 0.001 | 0.006 | ||
| < 70 | 226 (55.5) | |||
| ≥ 70 | 181 (44.5) | 1.987 (1.217–3.243) | ||
| LVi | 0.126 | 0.372 | ||
| No | 263 (64.6) | |||
| Yes | 144 (35.4) | 1.270 (0.749–2.144) | ||
| PNi | 0.517 | 0.911 | ||
| No | 110 (27.0) | |||
| Yes | 297 (73.0) | 0.966 (0.535–1.780) | ||
| NLR | 0.768 | |||
| < 2 | 244 (60.0) | |||
| ≥ 2 | 163 (40.0) | |||
| Adj. CTx. | 0.059 | 0.025 | ||
| No | 154 (37.8) | |||
| Yes | 253 (62.2) | 0.573 (0.352–0.933) |
Table 3 shows the comparisons between the early and non-early recurrence groups. Of the 407 patients, 98 (24.1%) had early disease recurrence and 309 (75.9%) had non-early or no recurrence. The preoperative CA 19-9 level significantly differed between the groups (P = 0.004), with higher CA 19-9 levels prevalent among patients in the early recurrence group. Tumors in the early recurrence group were larger (P = 0.001) and showed a more poorly differentiated histology (P = 0.002) than those in the non-early recurrence group. Although the difference was not significant (P = 0.058), more patients in the non-early recurrence group received adjuvant chemotherapy. The recurrence pattern did not differ between the two groups.
| Factors | Early recurrence, n (%) | Non-early recurrence, n (%) | P value |
| N = 98 (24.1%) | N = 309 (75.9%) | ||
| Age in yr | 0.21 | ||
| <65 | 51 (52.0) | 183 (59.2) | |
| ≥ 65 | 47 (48.0) | 126 (40.8) | |
| Sex | 0.261 | ||
| Male | 59 (60.2) | 166 (53.7) | |
| Female | 39 (39.8) | 143 (46.3) | |
| Tumor size, median in cm | 0.001 | ||
| < 2.5 | 23 (23.5) | 129 (41.7) | |
| ≥ 2.5 | 75 (76.5) | 180 (58.3) | |
| RM | 0.555 | ||
| Negative | 82 (83.7) | 266 (86.1) | |
| Positive | 16 (16.3) | 43 (13.9) | |
| Tumor location | 0.712 | ||
| Head/uncinate | 63 (64.3) | 191 (62.2) | |
| Neck/body/tail | 35 (35.7) | 116 (37.8) | |
| Differentiation | 0.002 | ||
| Well | 9 (9.2) | 51 (16.5) | |
| Moderate | 65 (66.3) | 223 (72.2) | |
| Poor | 22 (22.4) | 32 (10.4) | |
| Preoperative CA 19-9 in U/mL | 0.004 | ||
| Normal | 28 (28.6) | 139 (45.0) | |
| Abnormal | 70 (71.4) | 170 (55.0) | |
| LVi | 0.125 | ||
| No | 57 (58.2) | 206 (66.7) | |
| Yes | 41 (41.8) | 103 (33.3) | |
| PNi | 0.516 | ||
| No | 24 (24.5) | 86 (27.8) | |
| Yes | 74 (75.5) | 223 (72.2) | |
| NLR | 0.768 | ||
| < 2 | 60 (61.2) | 184 (59.5) | |
| ≥ 2 | 38 (38.8) | 125 (40.5) | |
| Adj. CTx. | 0.058 | ||
| No | 45 (45.9) | 109 (35.3) | |
| Yes | 53 (54.1) | 200 (64.7) | |
| Recurrence pattern | 0.121 | ||
| Local | 26 (26.5) | 73 (35.4) | |
| Systemic | 72 (73.5) | 133 (64.6) |
PDAC is one of the most lethal malignancies and is a leading cause of cancer-related deaths worldwide. Despite substantial improvements in the survival rates of patients with other major malignancies, the survival rates of patients with PDAC have remained relatively unchanged. PDAC is usually detected in the advanced stage, and restricted treatment options contribute to its poor overall prognosis. Approximately 70%-80% of patients with PDAC experience locoregional and/or distant recurrence after surgery[5-7]. Recent efforts have sought to improve the early diagnosis of PDAC[23-27]. Early detection and treatment of PDAC can help improve the dismal prognosis of this aggressive cancer. We evaluated the OS of 407 early-stage (stage I) PDAC patients who underwent upfront pancreatic surgery between January 2000 and April 2016. The median OS of those with early-stage disease was 34.5 mo, significantly longer than that of those with advanced-stage disease (18.5 mo; P < 0.001). However, patients with early-stage PDAC often experience early recurrence after curative resection, leading to a poor prognosis. The results of the present study suggested the presence of a heterogeneous microenvironment in terms of pre-existing occult metastasis in early-stage PDAC as 24.1% (n = 98) of patients with early recurrence showed a relatively poor prognosis compared to that in the non-early recurrence group (75.9%, n = 309) (median OS: 11 vs 42 mo; P < 0.001). Therefore, it is important to identify the clinicopathological factors and therapeutic modalities that are significantly associated with early recurrence in early-stage PDAC to improve the prognosis of this dismal disease.
Several studies have reported risk factors associated with OS and recurrence after surgical resection for PDAC, including tumor size, histological grade, resection margin status, lymph node metastasis, perineural invasion, venous invasion, and preoperative CA 19-9 levels[6,28-31]. The results of our study suggested that high preoperative serum CA 19-9 levels, large tumor size, poor differentiation, and non-adjuvant chemotherapy were independent predictors of early recurrence in early-stage PDAC.
Tumor size is an independent predictor of poor prognosis in patients with PDAC[32-34]. Based on previous studies, we further evaluated the effect of tumor size on recurrence and survival in patients with early-stage PDAC treated with curative resection. The median DFS and OS were 10 mo and 23 mo, respectively, in the larger tumor group (≥ 2.85 cm) and 21 mo and 38 mo in the smaller tumor group (< 2.85 cm), demonstrating that tumor size was an independent clinical predictor for early recurrence in early-stage PDAC. Since tumor size, as expected, affected disease prognosis and early recurrence even in early-stage disease, scheduled surveillance for detecting early recurrence is necessary in early-stage patients with large tumors.
Tumor histological grade is an important independent prognostic factor for PDAC. In general, poorly differentiation reflects aggressive malignant behavior accompanying a larger tumor size, a high rate of nodal metastases, microvascular invasion, and perineural invasion, causing poor OS[35-38]. The results of this study demonstrated that poor tumor differentiation was a significant factor for early recurrence in early PDAC compared to well differentiation (P = 0.008). Tumor grade is associated with not only survival but also recurrence. Although tumor grade is not used to evaluate tumor stage in PDAC according to the AJCC 8th edition guidelines, it should be considered critical for predicting disease prognosis and recurrence, especially in patients with early-stage PDAC.
CA 19-9, also referred to as Sialyl Lewis-A, is regularly expressed on cancer cells and can be detected by the monoclonal antibody 19-9[8]. Although it was originally isolated from a human colorectal cancer cell line[39], CA 19-9 is a good marker for the diagnosis of PDAC and the detection of recurrence during routine surveillance. It can be easily evaluated by a simple blood test, and numerous reports have suggested CA 19-9 as a meaningful tumor marker not only for diagnosis but also for prognosis prediction[18]. However, the specific role of CA 19-9 and the optimal serum CA 19-9 cutoff values for predicting early PDAC recurrence have remained controversial. We focused on early-stage PDAC patients who underwent primary pancreatectomy to evaluate the clinical impact of preoperative serum CA 19-9 levels on early recurrence. To the best of our knowledge, this is the first study to identify the independent relationship between serum CA 19-9 levels and early recurrence of early-stage PDAC in a large number of patients. In this study, we demonstrated that an elevated CA 19-9 level (cutoff value: > 70 U/mL) can be a reliable predictive marker for early recurrence in early-stage PDAC. This finding supports the notion that preoperative serum CA 19-9 levels could reflect biological aggressiveness and the presence of tumor micrometastases in early-stage PDAC.
Adjuvant chemotherapy was introduced following the assessment of its benefits, in which 5-fluorouracil (5-FU) and gemcitabine (GEM)-based regimens showed a survival effect[40,41]. The CONKO-005 trial also demonstrated that adjuvant chemotherapy with GEM and capecitabine doubled the 5-year OS rate to approximately 30%-50% compared to mono-regimen chemotherapy[42]. Adjuvant chemotherapy improved not only OS but also DFS[43,44]. In our institution, adjuvant chemotherapy is recommended to basically all patients regardless of the disease stage. However, the final decision is made based on the oncologists’ decision and patients’ postoperative general condition. In our study, patients who were in poor general condition, with postoperative complication, old, or reluctant to chemotherapy did not undergo adjuvant chemotherapy. Otherwise, 5-FU or GEM-based regimens were generally administered. We found that the number of patients who received adjuvant chemotherapy was higher in the late- and non-recurrence groups than in the early recurrence group (n = 200 vs 53), with adjuvant chemotherapy being an independent predictor of early recurrence (OR 0.573 [0.352–0.933]; P = 0.025) in early-stage PDAC. As few studies have assessed the effect of chemotherapy in early-stage disease, this result is meaningful in that we focused on early-stage patients. Adjuvant chemotherapy could be an effective treatment modality for reducing recurrence rates even in early-stage patients.
In conclusion, early recurrence often occurs even in stage I PDAC patients after upfront surgery, suggesting the need for the evaluation of predictive factors for early recurrence. In particular, CA 19-9 levels can be easily checked preoperatively and elevated CA 19-9 level (cutoff value > 70 U/mL) can be a reliable predictive factor. Furthermore, adjuvant chemotherapy should be considered for patients who are susceptible to early recurrence to achieve a better prognosis, even in patients with early-stage PDAC.
One of the reasons that pancreatic ductal adenocarcinoma (PDAC) has a poor prognosis is that the disease is diagnosed at advanced stage. Various factors associated with PDAC prognosis have been evaluated and effort have been made to improve prognosis by early detection of the disease.
Serum carbohydrate antigen 19-9 (CA 19-9) has been used as a means of diagnosing malignant pancreatic neoplasm and detection of disease recurrence. However, the effectiveness of CA 19-9 as a marker for early recurrence of disease has not been well studied yet.
This study aimed to set the optimal cutoff preoperative CA 19-9 level and evaluate the effectiveness of CA 19-9 as a detector of early recurrence of early-stage PDAC.
A total of 407 patients with stage I PDAC undergoing upfront surgical resection between January 2000 and April 2016 were evaluated. The optimal cutoff values were determined by receiver operating characteristic and the risk factors for early recurrence were identified using a logistic regression model.
Ninety-eight patients (24.1%) experienced early disease recurrence. The optimal cutoff value of preoperative CA 19-9 for early recurrence was determined as 70 U/mL. Patients with high CA 19-9 level showed the tendency to have early recurrence more frequently. Tumor size > 2.85 cm, poor differentiation, and non-adjuvant chemotherapy were also demonstrated to be significant risk factors for early recurrence in early-stage PDAC.
Elevated CA 19-9 level can be regarded as a reliable parameter predicting early disease recurrence. Adjuvant chemotherapy should be recommended for patients susceptible to early recurrence.
Preoperative CA 19-9 can be a guidance for patients to undergo effective treatment modality to reduce early recurrence, thus leading to a better prognosis.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13305] [Article Influence: 1663.1] [Reference Citation Analysis (4)] |
| 2. | Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Benson AB 3rd, Binder E, Cardin DB, Cha C, Chiorean EG, Chung V, Czito B, Dillhoff M, Dotan E, Ferrone CR, Hardacre J, Hawkins WG, Herman J, Ko AH, Komanduri S, Koong A, LoConte N, Lowy AM, Moravek C, Nakakura EK, O'Reilly EM, Obando J, Reddy S, Scaife C, Thayer S, Weekes CD, Wolff RA, Wolpin BM, Burns J, Darlow S. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 752] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 3. | Yamada S, Fujii T, Yabusaki N, Murotani K, Iwata N, Kanda M, Tanaka C, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Kodera Y. Clinical Implication of Inflammation-Based Prognostic Score in Pancreatic Cancer: Glasgow Prognostic Score Is the Most Reliable Parameter. Medicine (Baltimore). 2016;95:e3582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, Imrie CW, Carter R, McKay CJ, McMillan DC. A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2318-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 398] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Tummers WS, Groen JV, Sibinga Mulder BG, Farina-Sarasqueta A, Morreau J, Putter H, van de Velde CJ, Vahrmeijer AL, Bonsing BA, Mieog JS, Swijnenburg RJ. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br J Surg. 2019;106:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2184] [Article Influence: 145.6] [Reference Citation Analysis (6)] |
| 8. | Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 372] [Reference Citation Analysis (3)] |
| 9. | Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Sasaki K, Furukawa H, Matsunaga K, Maeda A. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:977-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Optimal duration of the early and late recurrence of pancreatic cancer after pancreatectomy based on the difference in the prognosis. Pancreatology. 2014;14:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 11. | Izumo W, Higuchi R, Furukawa T, Yazawa T, Uemura S, Shiihara M, Yamamoto M. Evaluation of preoperative prognostic factors in patients with resectable pancreatic ductal adenocarcinoma. Scand J Gastroenterol. 2019;54:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Fang LP, Xu XY, Ji Y, Huang PW. The Prognostic Value of Preoperative Neutrophil-to-Lymphocyte Ratio in Resected Patients with Pancreatic Adenocarcinoma. World J Surg. 2018;42:3736-3745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Nishio K, Kimura K, Amano R, Yamazoe S, Ohrira G, Nakata B, Hirakawa K, Ohira M. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J Surg Oncol. 2017;15:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Tsuchiya N, Matsuyama R, Murakami T, Yabushita Y, Sawada YU, Kumamoto T, Endo I. Risk Factors Associated With Early Recurrence of Borderline Resectable Pancreatic Ductal Adenocarcinoma After Neoadjuvant Chemoradiation Therapy and Curative Resection. Anticancer Res. 2019;39:4431-4440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kurahara H, Maemura K, Mataki Y, Sakoda M, Iino S, Kawasaki Y, Arigami T, Mori S, Kijima Y, Ueno S, Shinchi H, Natsugoe S. A Therapeutic Strategy for Resectable Pancreatic Cancer Based on Risk Factors of Early Recurrence. Pancreas. 2018;47:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 16. | Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 631] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 18. | Azizian A, Rühlmann F, Krause T, Bernhardt M, Jo P, König A, Kleiß M, Leha A, Ghadimi M, Gaedcke J. CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020;10:1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 19. | Bergquist JR, Puig CA, Shubert CR, Groeschl RT, Habermann EB, Kendrick ML, Nagorney DM, Smoot RL, Farnell MB, Truty MJ. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J Am Coll Surg. 2016;223:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Dong Q, Yang XH, Zhang Y, Jing W, Zheng LQ, Liu YP, Qu XJ. Elevated serum CA19-9 Level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World J Surg Oncol. 2014;12:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Takagi C, Kikuchi Y, Shirakawa H, Hoshimoto S, Tomikawa M, Ozawa I, Hishinuma S, Ogata Y. Predictive Factors for Elevated Postoperative Carbohydrate Antigen 19-9 Levels in Patients With Resected Pancreatic Cancer. Anticancer Res. 2019;39:3177-3183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Asaoka T, Miyamoto A, Maeda S, Tsujie M, Hama N, Yamamoto K, Miyake M, Haraguchi N, Nishikawa K, Hirao M, Ikeda M, Sekimoto M, Nakamori S. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016;16:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, Almario CV, Halloran C, Lennon AM, Costello E. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (1)] |
| 24. | Zhou B, Xu JW, Cheng YG, Gao JY, Hu SY, Wang L, Zhan HX. Early detection of pancreatic cancer: Where are we now and where are we going? Int J Cancer. 2017;141:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 25. | Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2108] [Cited by in RCA: 2283] [Article Influence: 207.5] [Reference Citation Analysis (0)] |
| 26. | O'Brien DP, Sandanayake NS, Jenkinson C, Gentry-Maharaj A, Apostolidou S, Fourkala EO, Camuzeaux S, Blyuss O, Gunu R, Dawnay A, Zaikin A, Smith RC, Jacobs IJ, Menon U, Costello E, Pereira SP, Timms JF. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res. 2015;21:622-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 27. | Zhou M, Diao Z, Yue X, Chen Y, Zhao H, Cheng L, Sun J. Construction and analysis of dysregulated lncRNA-associated ceRNA network identified novel lncRNA biomarkers for early diagnosis of human pancreatic cancer. Oncotarget. 2016;7:56383-56394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Lüttges J, Schemm S, Vogel I, Hedderich J, Kremer B, Klöppel G. The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J Pathol. 2000;191:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Takahashi H, Ohigashi H, Ishikawa O, Gotoh K, Yamada T, Nagata S, Tomita Y, Eguchi H, Doki Y, Yano M. Perineural invasion and lymph node involvement as indicators of surgical outcome and pattern of recurrence in the setting of preoperative gemcitabine-based chemoradiation therapy for resectable pancreatic cancer. Ann Surg. 2012;255:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 30. | Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol. 2014;20:10802-10812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Ansari D, Bauden M, Bergström S, Rylance R, Marko-Varga G, Andersson R. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg. 2017;104:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Marchegiani G, Andrianello S, Malleo G, De Gregorio L, Scarpa A, Mino-Kenudson M, Maggino L, Ferrone CR, Lillemoe KD, Bassi C, Castillo CF, Salvia R. Does Size Matter in Pancreatic Cancer? Ann Surg. 2017;266:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Shimada K, Sakamoto Y, Sano T, Kosuge T, Hiraoka N. Reappraisal of the clinical significance of tumor size in patients with pancreatic ductal carcinoma. Pancreas. 2006;33:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Fortner JG, Klimstra DS, Senie RT, Maclean BJ. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann Surg. 1996;223:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 35. | Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Reber H, Tomlinson JS. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17:2312-2320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, Büchler MW, Werner J. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 37. | Crippa S, Partelli S, Zamboni G, Barugola G, Capelli P, Inama M, Bassi C, Pederzoli P, Falconi M. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery. 2012;152:S112-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316-3322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 871] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 40. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1028] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 41. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1425] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 42. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 43. | Chikhladze S, Lederer AK, Kousoulas L, Reinmuth M, Sick O, Fichtner-Feigl S, Wittel UA. Adjuvant chemotherapy after surgery for pancreatic ductal adenocarcinoma: retrospective real-life data. World J Surg Oncol. 2019;17:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Parikh AA, Maiga A, Bentrem D, Squires MH 3rd, Kooby DA, Maithel SK, Weber SM, Cho CS, Katz M, Martin RC, Scoggins CR, Sutton J, Ahmad SA, Abbott DE, Carr J, Kim HJ, Yakoub D, Idrees K, Merchant N. Adjuvant Therapy in Pancreas Cancer: Does It Influence Patterns of Recurrence? J Am Coll Surg. 2016;222:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ling Q, Ostwal V, Xie Q S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu RR