Published online Nov 27, 2021. doi: 10.4240/wjgs.v13.i11.1372
Peer-review started: July 29, 2021
First decision: October 3, 2021
Revised: October 6, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: November 27, 2021
Processing time: 120 Days and 11 Hours
Although the “Step-up” strategy is the primary surgical treatment for infected pancreatic necrosis, it is not suitable for all such patients. The “One-step” strategy represents a novel treatment, but the safety, efficacy, and long-term follow-up have not yet been compared between these two approaches.
To compare the safety, efficacy, and long-term follow-up of two surgical approaches to provide a reference for infected pancreatic necrosis treatment.
This was a retrospective analysis of infectious pancreatic necrosis patients who underwent “One-step” or “Step-up” necrosectomy at Xuan Wu Hospital, Capital Medical University, from May 2014 to December 2020. The primary outcome was the composite endpoint of severe complications or death. Patients were followed up every 6 mo after discharge until death or June 30, 2021. Statistical analysis was performed using SPSS 21.0 and GraphPad Prism 8.0, and statistical significance was set at P < 0.05.
One-hundred-and-fifty-eight patients were enrolled, of whom 61 patients underwent “One-step” necrosectomy and 97 patients underwent “Step-up” necrosectomy. During the long-term follow-up period, 40 patients in the “One-step” group and 63 patients in the “Step-up” group survived. The time from disease onset to hospital admission (53.69 ± 38.14 vs 32.20 ± 20.75, P < 0.001) and to initial surgical treatment was longer in the “Step-up” than in the “One-step” group (54.38 ± 10.46 vs 76.58 ± 17.03, P < 0.001). Patients who underwent “Step-up” necrosectomy had a longer hospitalization duration (65.41 ± 28.14 vs 52.76 ± 24.71, P = 0.02), and more interventions (4.26 ± 1.71 vs 3.18 ± 1.39, P < 0.001). Postoperative inflammatory indicator levels were significantly lower than preoperative levels in each group. Although the incisional hernia incidence was higher in the “One-step” group, no significant difference was found in the composite outcomes of severe complications or death, new-onset organ failure, postoperative complications, inflammatory indicators, long-term complications, quality of life, and medical costs between the groups (P > 0.05).
Compared with the “Step-up” approach, the “One-step” approach is a safe and effective treatment method with better long-term quality of life and prognosis. It also provides an alternative surgical treatment strategy for patients with infected pancreatic necrosis.
Core Tip: This was a retrospective study comparing the safety, efficacy, and long-term follow-up between the “One-step” approach and “Step-up” approach for patients with infected pancreatic necrosis. The results indicated that the “One-step” approach is a safe and effective treatment method, with better long-term quality of life and prognosis, which provides a novel surgical treatment strategy for infected pancreatic necrosis patients.
- Citation: Zheng Z, Lu JD, Ding YX, Guo YL, Mei WT, Qu YX, Cao F, Li F. Comparison of safety, efficacy, and long-term follow-up between “one-step” and “step-up” approaches for infected pancreatic necrosis. World J Gastrointest Surg 2021; 13(11): 1372-1389
- URL: https://www.wjgnet.com/1948-9366/full/v13/i11/1372.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i11.1372
Acute pancreatitis (AP) is a common disease of the digestive system[1]. The associated mortality rate of severe AP is 15%–20%, while that of infectious necrotizing pancreatitis (IPN) is as high as 30%[2]. Generally, surgical intervention for IPN is often delayed[3]. In terms of surgical intervention, pancreatic debridement has changed from a large incision and a wide range of anatomical debridement to minimally invasive treatment over time. The “Step-up” surgical treatment strategy has become the mainstream IPN surgical treatment. In this strategy, at the early stage of the disease, percutaneous drainage (PCD) is first performed under imaging guidance, and surgical debridement is then performed when necessary using incremental and progressive treatment[4,5]. Compared with traditional open necrosectomy, the “Step-up” strategy can effectively reduce the incidence of complications and mortality in patients and has good long-term efficacy[6]. However, in clinical practice, the pancreatic necrotic tissue cannot be removed completely even after repeated puncture and drainage treatment in some IPN patients due to the lack of a safe and effective puncture and drainage paths; this prolongs the treatment cycle and may even make it impossible to complete the PCD treatment[7]. In addition, due to individual differences among IPN patients, the degree of necrosis and liquefaction of pancreatic tissue vary. Therefore, when some IPN patients with “dry” necrosis undergo “Step-up” treatment, the poor drainage effect of PCD often leads to insignificant relief of infection and poisoning symptoms, and there remains a need to remove necrotic tissue[8,9]. This not only increases the surgical trauma and medical burden on the patient but may also delay the patient’s optimal treatment time, which is not conducive to recovery[3]. Therefore, it remains unclear whether the “Step-up” strategy is suitable for all patients with IPN.
Based on this, we have developed and applied the “One-step” surgical approach to treat IPN. This approach involves direct minimally invasive debridement instead of PCD. A preliminary retrospective study from our center confirmed that the “One-step” approach has better surgical efficacy and safety, but there is a lack of clinical data comparing it with the “Step-up” approach[7].
Consequently, the present study compared the safety, efficacy, and long-term follow-up survival data in IPN patients treated with the “One-step” approach and those treated with the “Step-up” approach, aiming to present a new surgical approach to guide clinical treatment. We present the following article in accordance with the STROBE reporting checklist.
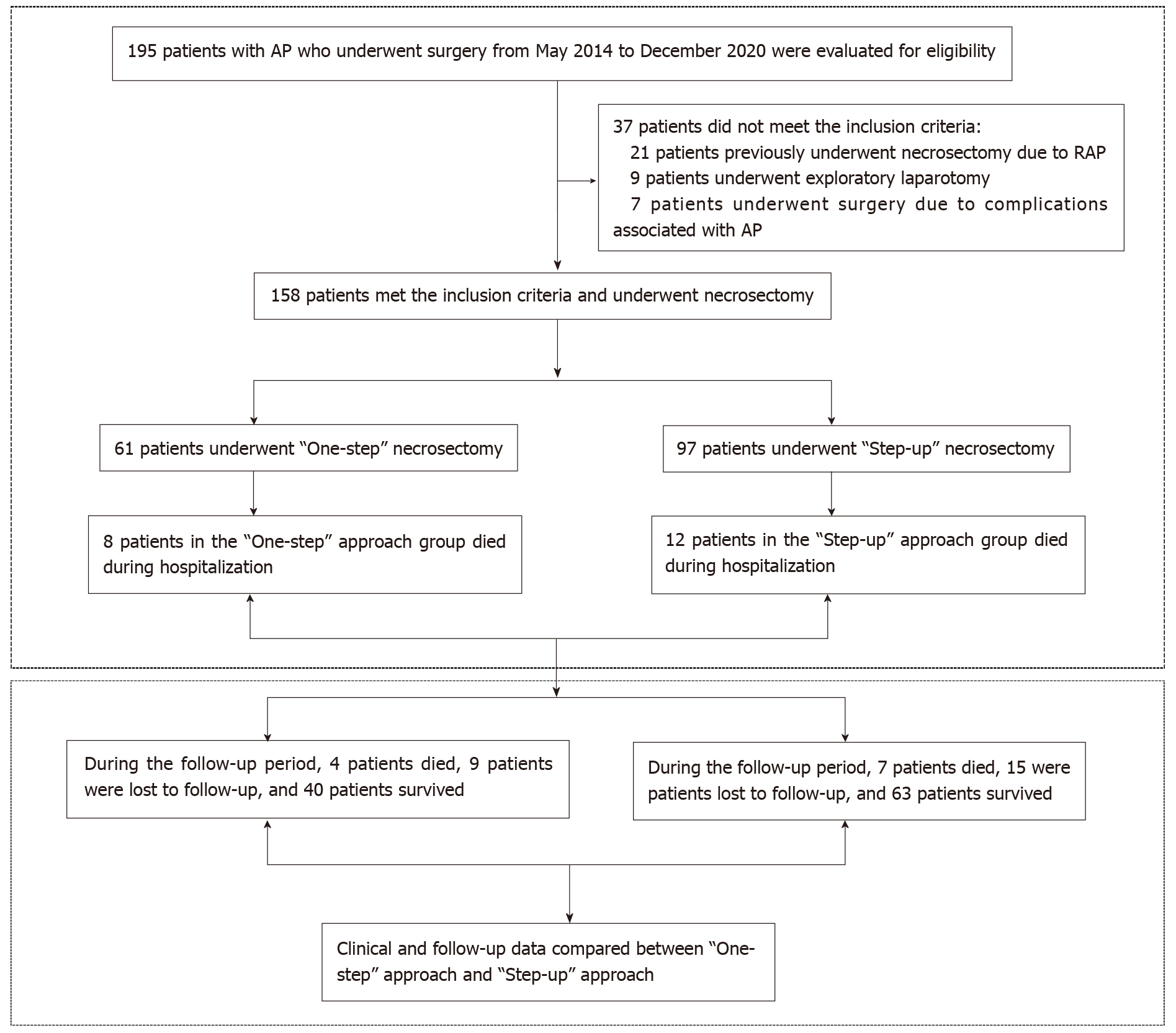
This study was a retrospective analysis of the clinical data of IPN patients who underwent “One-step” necrosectomy or “Step-up” necrosectomy at Xuan Wu Hospital, Capital Medical University, from May 2014 to December 2020. The study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University, No. 2020-158 and was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). The registration number was ChiCTR2100044348. Because this was a retrospective study that only analyzed existing clinical and follow-up data, the need to obtain informed patient consent was waived. All patient data were analyzed anonymously using an electronic data capture system. A detailed flowchart is illustrated in Figure 1.
The following patients were included: (1) Patients suspected of having or diagnosed with IPN based on abdominal computed tomography (CT) and laboratory examinations, such as the “bubble” sign on CT or bacteria or fungi detected by culture of fine-needle aspiration samples; (2) Patients with IPN, mainly those with acute necrotic collection with infection and walled-off necrosis with infection; (3) Patients of either sex who were aged 18-80 years; (4) Patients who had undergone “One-step” or “Step-up” necrosectomy; (5) Patients who underwent video-assisted minimally invasive debridement; (6) Patients who had not previously undergone necrosectomy or surgery for pancreatic-related complications; and (7) Patients with complete clinical and follow-up data.
The exclusion criteria were as follows: (1) A previous history of pancreatic necrotic tissue drainage or debridement; (2) Previous exploratory laparotomy for acute abdominal disease or pancreatitis. (3) Acute exacerbation of chronic pancreatitis or recurrent AP (RAP); (4) Inability to tolerate video-assisted minimally invasive debridement and anesthesia due to physical conditions; (5) AP with abdominal compartment syndrome or abdominal organ perforation; and (6) Incomplete clinical data or data that could not be statistically analyzed.
“One-step” minimally invasive necrosectomy: “One-step” minimally invasive necrosectomy can be performed via the omentum sac, retroperitoneal, or combined approach. The surgical procedure was described in detail previously[7] and included incision via the omental sac and/or retroperitoneal approach, after which video-assisted pancreatic necrotic tissue debridement was performed.
“Step-up” minimally invasive necrosectomy: “Step-up” minimally invasive pancreatic necrosectomy used PCD as the initial treatment option for IPN. The surgical approach was the same as for “One-step” treatment. If there was residual infection in the abdominal cavity after minimally invasive surgery, PCD treatment was preferred. The detailed surgical procedures have been described previously[4].
Primary outcomes: The primary outcome of this study was the composite endpoint of severe complications or death. Severe complications were defined as Clavien-Dindo grade IIIa or higher[10].
Secondary outcomes: The secondary outcomes included the time from disease onset to hospital admission; time from disease onset to initial surgical treatment; new-onset organ failure; number of organs in failure; postoperative short-term complications (such as pancreatic fistula, intra-abdominal bleeding, enterocutaneous fistula, or viscera perforation); length of stay in the intensive care unit (ICU); total length of hospital stay; number of operations; operation time; number of interventions; number of drainage tubes used; overall survival rate; changes in perioperative inflammatory indicators, including white blood cell (WBC), interleukin-6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT); long-term complications (such as endocrine insufficiency, exocrine insufficiency, chronic pancreatitis, incisional hernia, recurrence pancreatitis, pancreatic pseudocyst, pancreatic portal hypertension, and pancreatic cancer); scores on the SF-36 and EQ-5D rating scales; Izbicki pain score; and perioperative medical costs. The specific definitions of the primary and secondary outcomes are described in Table 1. Clinical data were extracted from internet-based case records of Xuanwu Hospital, Capital Medical University, by three local doctors (DYX, GYL, and MWT). Follow-up was performed by three other local doctors (ZZ, LJD, and QYX).
| Endpoints | Definition |
| Primary endpoints | |
| Composite endpoint consisting of mortality and severe complications (Clavien-Dindo ≥ IIIa) | There are five grades of Clavien-Dindo Classification: Grade I, any complication that deviates from the natural course after surgery; Grade II, medications other than those permitted for Grade I complications are required; Grade III, surgical, endoscopic, and radiotherapy are required, including Grade IIIa (no general anesthesia is required) and IIIb (need for general anesthesia); Grade IV, life-threatening complication, including Grade IVa (single organ dysfunction) and IVb (multi-organ dysfunction) that require intermittent monitoring or ICU treatment; Grade V, death |
| Secondary endpoints | |
| New-onset organ failure | New-onset failure of one or more organs in the 24 h prior to the first intervention |
| Pulmonary failure | Partial pressure of oxygen (PO2) < 60 mmHg with or without partial pressure of carbon dioxide (PCO2) > 50 mmHg, or need for mechanical ventilation |
| Circulatory failure | Blood pressure < 90/60 mmHg, or need for inotropic catecholamine to maintain blood pressure |
| Renal failure | The level of creatinine (Cr) > 177 μmoL/L, or need for hemofiltration or hemodialysis |
| Postoperative intra-abdominal bleeding | Need for operation, radiological, or endoscopic intervention |
| Pancreatic fistula | Drainage fluid amylase level more than 3 times that of serum amylase |
| Enterocutaneous fistula | Intestinal contents, including intestinal fluids, food residues, and feces, break through the intestinal wall (small bowel or large bowel) and leak into the abdominal cavity or outside the body. It can also be confirmed by radiology or surgery |
| Viscera perforation | Need for operation, radiological, or endoscopic intervention |
| Endocrine insufficiency | Oral hypoglycemic drugs or insulin therapy for at least 6 mo, with no need to take these drugs before the onset of AP |
| Pancreatic enzyme | Clinical symptoms were improved by oral pancreatic enzyme use for more than 6 mo, with no need to take this drug before the onset of AP |
| Recurrent pancreatitis | A history of two or more episodes with and interval of at least 3 mo |
| Chronic pancreatitis | Patients experience abdominal pain, weight loss, diabetes, and fatty diarrhea. The condition is also confirmed by radiological and laboratory examinations. The symptoms did not occur before the onset of AP |
| Incisional hernia | Six months after discharge, the full-thickness abdominal wall is discontinuous and abdominal contents bulge, with or without obstruction |
| Pancreatic portal hypertension | AP causes splenic vein thrombosis, which causes obstruction of splenic venous return |
The patients were followed up every 6 mo after surgery. After discharge, the patients were followed up by means of outpatient visits, inpatient visits, telephone contact, or mail. During the follow-up period, patients were required to undergo physical examinations, abdominal CT scans, and laboratory tests. In addition, researchers monitored related clinical symptoms, such as abdominal pain, bloating, weight loss, and diarrhea. Physical examinations were conducted mainly to check for postoperative incisional hernia. Abdominal CT scans primarily focused on morphological changes of the pancreas and blood vessels in the abdominal cavity. Laboratory tests included routine blood tests, blood biochemistry tests, and fecal elastase-1 examination to clarify whether patients had long-term complications. In addition, enrolled patients also needed to receive the SF-36, EQ-5D, and Izbicki pain scores to further evaluate the quality of life of patients after surgery. The survival time was estimated from the date of operation to the date of death or until June 30, 2021. The follow-up period ended on June 30, 2021.
Statistical analysis was performed using SPSS (version 21.0; IBM Corp., Armonk, NY, United States) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, United States). Continuous variables with normal distributions were described as mean ± standard deviation, and an independent sample t-test was used for comparisons. Continuous variables with non-normal distributions were presented as medians (interquartile ranges), and the Mann-Whitney U test was used for comparisons. The chi-square test or Fisher’s exact test was used to compare categorical variables and assess adverse events. The Kaplan-Meier method was used for survival analysis. The log-rank test was used to compare the complication rates between the two surgical approaches. P values < 0.05, were considered statistically significant.
Overall, 195 patients with AP who underwent surgery between May 2014 and December 2020 were retrospectively analyzed. Of these, 37 patients did not meet the inclusion criteria and were excluded. Among them, 21 patients had previously undergone pancreatic necrosectomy due to RAP, 9 patients had undergone exploratory laparotomy, and 7 patients had undergone surgery because of AP-related complications. Finally, 158 patients met the inclusion criteria, of which 61 underwent “One-step” necrosectomy and 97 underwent “Step-up” necrosectomy. Eight and 12 patients died during hospitalization in the “One-step” necrosectomy and “Step-up” necrosectomy groups, respectively. After discharge, all patients underwent regular follow-ups. The research flow chart is shown in Figure 1.
The baseline characteristics were similar between the two groups. Gallstones were the most common etiology in our study. However, since the majority of IPN patients were referred to our center and most of the patients who underwent the “Step-up” approach had already undergone PCD treatment at other hospitals, the time from onset of disease to admission was significantly longer in the “Step-up” than in the “One-step” group (53.69 ± 38.14 vs 32.20 ± 20.75, P < 0.001). In the “One-step” group, the time from onset of disease to receiving initial surgical treatment was shorter than in the “Step-up” group (54.38 ± 10.46 vs 76.58 ± 17.03, P < 0.001). There were no significant differences in the ASA score, APACHE II score, CT severity index, pancreatic necrosis extent, and AP severity (Table 2).
| Groups | “One-step” approach (n = 61) | “Step-up” approach (n = 97) | P value | |
| Baseline characteristics | ||||
| Sex | 0.61 | |||
| Female | 19 | 34 | ||
| Male | 42 | 63 | ||
| Age (yr) | 52.16 ± 11.51 | 50.57 ± 13.71 | 0.45 | |
| BMI (kg/m2) | 26.20 ± 3.78 | 25.91 ± 3.91 | 0.73 | |
| Cause of AP | 0.36 | |||
| Gallstone | 31 | 48 | ||
| Alcohol | 3 | 2 | ||
| Hyperlipidaemia | 15 | 34 | ||
| Others | 12 | 13 | ||
| Concomitant disease | 0.15 | |||
| Cardiovascular disease | 29 | 37 | ||
| Pulmonary disease | 8 | 13 | ||
| Chronic renal insufficiency | 4 | 21 | ||
| Diabetes | 13 | 16 | ||
| Others | 7 | 10 | ||
| ASA score | 0.42 | |||
| I: Healthy status | 30 | 58 | ||
| II: Mild systemic status | 30 | 38 | ||
| III: Severe systemic status | 1 | 1 | ||
| APACHE-II score | 13.9 ± 5.1 | 14.2 ± 5.4 | 0.71 | |
| CT severity index | 6.65 ± 2.55 | 7.19 ± 2.18 | 0.18 | |
| Severity of AP | 0.79 | |||
| Moderately AP | 27 | 45 | ||
| Severely AP | 34 | 52 | ||
| Extent of pancreatic necrosis | 0.07 | |||
| < 30% | 35 | 41 | ||
| 30%-50% | 11 | 33 | ||
| > 50% | 15 | 23 | ||
| Preoperative inflammatory indicators | ||||
| White blood cells (109/L) | 11.04 ± 6.31 | 11.33 ± 6.03 | 0.77 | |
| Neutrophil percentage (%) | 80.86 ± 8.3 | 79.57 ± 8.9 | 0.36 | |
| C-reactive protein (mg/L) | 115.3 ± 81.8 | 118.5 ± 85.7 | 0.81 | |
| Procalcitonin (ng/mL) | 1.18 ± 1.0 | 1.08 ± 2.2 | 0.73 | |
| Interleukin 6 (pg/mL) | 113 ± 91.8 | 116 ± 95.1 | 0.93 | |
| Organ failure | 0.49 | |||
| Single | 10 | 14 | ||
| Multiple | 5 | 14 | ||
| None | 46 | 69 | ||
| Time from onset of disease to admission (days) | 32.20 ± 20.75 | 53.69 ± 38.14 | < 0.001 | |
| Time from onset of disease to initial surgical treatment (days) | 54.38 ± 10.46 | 76.58 ± 17.03 | < 0.001 | |
| Tertiary referral (%) | 56 (91.8%) | 96 (98.9%) | 0.06 | |
All patients underwent minimally invasive surgical treatment. In the “Step-up” group, 32 patients (32.9%) were cured after PCD treatment alone, without pancreatic necrosectomy. The remainder of the patients in the “Step-up” group (65 patients, 67.1%) underwent necrosectomy after PCD due to continuous progression of the disease. Among them, trans-lesser sac pancreatic necrosectomy was the most common surgical approach used in both groups at our center. In addition, severe complications or death composite outcomes (18% vs 20.6%, P = 0.69) were comparable between the two groups. Although new-onset organ failure involving pulmonary and cardiovascular diseases was common, there were no statistically significant differences between the groups (P = 0.73). No significant difference was found in postoperative complications, blood loss, number of operations, and operation time between the groups. Compared with the “One-step” group, patients who underwent “Step-up” necrosectomy had longer total length of hospitalization and underwent more interventions (total length of hospitalization: 65.41 ± 28.14 vs 52.76 ± 24.71, P = 0.02; number of interventions: 4.26 ± 1.71 vs 3.18 ± 1.39, P < 0.001). However, the postoperative ICU stay and total length of ICU stay were not significantly different between the groups. Additionally, there were fewer drainage tubes used in the “One-step” group than in the “Step-up” group (3.9 ± 1.0 vs 4.43 ± 1.77, P = 0.04) (Table 3).
| Group | “One-step” approach (n = 61) | “Step-up” approach (n = 97) | P value | |
| Outcomes | ||||
| Primary composite outcomes | ||||
| Severe complications or death, n (%)1 | 11 (18) | 20 (20.6) | 0.69 | |
| Secondary outcomes | ||||
| New-onset organ failure | 10 | 18 | 0.73 | |
| Pulmonary | 6 | 12 | ||
| Cardiovascular | 8 | 10 | ||
| Renal | 1 | 3 | ||
| Single-organ failure | 5 | 12 | 0.41 | |
| Multiple-organ failure | 5 | 6 | 0.63 | |
| Death | 8 | 12 | 0.89 | |
| Postoperative intraabdominal bleeding | 2 | 5 | 0.58 | |
| Pancreatic fistula | 16 | 28 | 0.72 | |
| Enterocutaneous fistula or Viscera perforation | 3 | 5 | 0.95 | |
| Surgical approach | 0.77 | |||
| Trans-lesser sac | 40 | 39 | ||
| Retroperitoneal | 17 | 20 | ||
| Combined | 4 | 6 | ||
| Length of stay in ICU after operation (days) | 10.9 ± 22.7 | 14.4 ± 26.1 | 0.18 | |
| Total length of ICU stay (days) | 22.5 ± 30.1 | 25.7 ± 28.7 | 0.43 | |
| Total length of hospital stay (days) | 52.76 ± 24.71 | 65.41 ± 28.14 | 0.02 | |
| Number of operations | 2.89 ± 1.23 | 3.42 ± 1.69 | 0.65 | |
| Operation time (min) | 82.19 ± 29.34 | 87.01 ± 30.12 | 0.92 | |
| Number of interventions | 3.18 ± 1.39 | 4.26 ± 1.71 | 0.000 | |
| Blood loss during the operation (mL) | 49.5 ± 58.3 | 55.5 ± 72.1 | 0.51 | |
| Number of drainage tubes | 3.9 ± 1.0 | 4.43 ± 1.77 | 0.04 | |
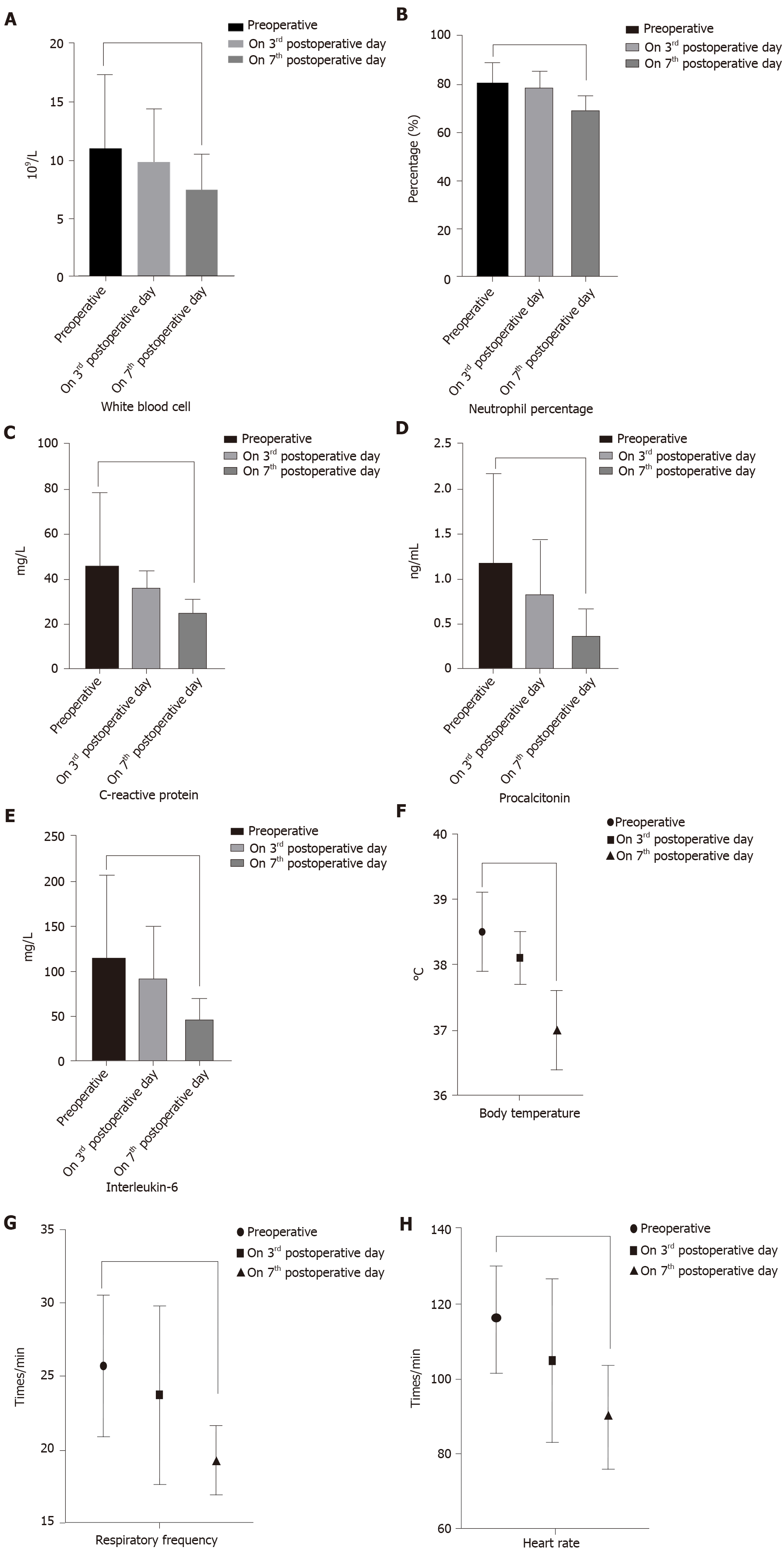
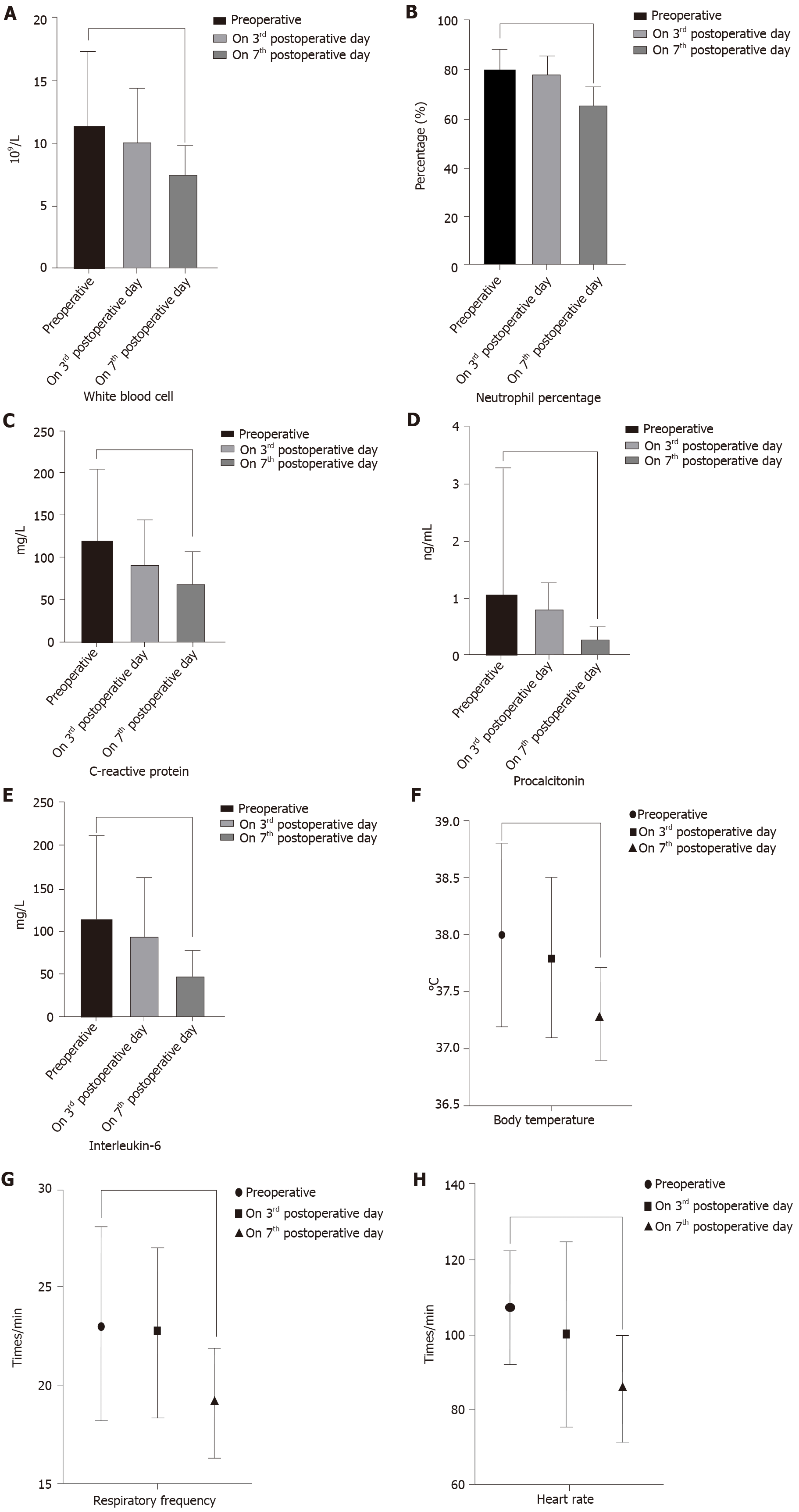
Interestingly, there were no significant differences in perioperative inflammatory indicators and vital signs between the two groups (Table 4). However, within each group, compared with before the operation, the inflammatory indicators were significantly decreased on the 3rd day after the operation. The vital signs and inflammatory indicators were significantly improved on the 7th day after surgery. In the “One-step” group, the postoperative level of WBC (11.04 ± 6.31 vs 7.51 ± 3.0, P = 0.003), NE (80.86 ± 8.3 vs 69.30 ± 6.1, P = 0.019), CRP (115.3 ± 81.8 vs 62.63 ± 25.6, P = 0.0002), PCT (1.18 ± 1.0 vs 0.37 ± 0.3, P < 0.001), and IL-6 (113 ± 91.8 vs 46.3 ± 22.6, P = 0.00097) were significantly lower than the preoperative levels. Postoperative vital signs were also better than preoperative values (Figure 2). In the “Step-up” group, the postoperative level of WBC (11.33 ± 6.03 vs 7.48 ± 2.4, P = 0.0012), NE (79.57 ± 8.9 vs 65.14 ± 7.8, P = 0.0367), CRP (118.5 ± 85.7 vs 68.05 ± 38.1, P = 0.0089), PCT (1.08 ± 2.2 vs 0.31 ± 0.2, P = 0.0004), IL-6 (116 ± 95.1 vs 49.7 ± 27.4, P = 0.00073) and vital signs were also better than the preoperative levels (Figure 3).
| Groups | “One-step” approach (n = 61) | “Step-up” approach (n = 97) | P value |
| Indicators | |||
| Preoperative inflammatory indicators | |||
| White blood cells (109/L) | 11.04 ± 6.31 | 11.33 ± 6.03 | 0.77 |
| Neutrophil percentage (%) | 80.86 ± 8.3 | 79.57 ± 8.9 | 0.36 |
| C-reactive protein (mg/L) | 115.3 ± 81.8 | 118.5 ± 85.7 | 0.81 |
| Procalcitonin (ng/mL) | 1.18 ± 1.0 | 1.08 ± 2.2 | 0.73 |
| Interleukin-6 (pg/mL) | 113 ± 91.8 | 116 ± 95.1 | 0.93 |
| Preoperative vital signs | |||
| Body temperature (℃) | 38.5 ± 0.6 | 38.0 ± 0.8 | 0.94 |
| Respiratory frequency (times/min) | 25.8 ± 4.8 | 23.1 ± 4.9 | 0.67 |
| Heart rate (times/min) | 116.1 ± 14.2 | 107 ± 15.1 | 0.33 |
| Inflammatory indicators on the 3rd postoperative day | |||
| White blood cells (109/L) | 9.91 ± 4.5 | 10.1 ± 4.4 | 0.54 |
| Neutrophil percentage (%) | 78.9 ± 6.8 | 78.1 ± 7.2 | 0.97 |
| C-reactive protein (mg/L) | 91.6 ± 40.6 | 91.1 ± 53.3 | 0.92 |
| Procalcitonin (ng/mL) | 0.84 ± 0.6 | 0.79 ± 0.5 | 0.71 |
| Interleukin-6 (pg/mL) | 91.2 ± 60.2 | 94.7 ± 68.4 | 0.31 |
| Vital signs on the 3rd postoperative day | |||
| Body temperature (℃) | 38.1 ± 0.4 | 37.8 ± 0.7 | 0.49 |
| Respiratory frequency (times/min) | 23.8 ± 6.1 | 22.7 ± 4.3 | 0.86 |
| Heart rate (times/min) | 105.1 ± 21.9 | 100 ± 24.9 | 0.68 |
| Inflammatory indicators on the 7th postoperative day | |||
| White blood cells (109/L) | 7.51 ± 3.0 | 7.48 ± 2.4 | 0.96 |
| Neutrophil percentage (%) | 69.30 ± 6.1 | 65.14 ± 7.8 | 0.43 |
| C-reactive protein (mg/L) | 62.63 ± 25.6 | 68.05 ± 38.1 | 0.54 |
| Procalcitonin (ng/mL) | 0.37 ± 0.3 | 0.31 ± 0.2 | 0.94 |
| Interleukin-6 (pg/mL) | 46.3 ± 22.6 | 49.7 ± 27.4 | 0.48 |
| Vital signs on the 7th postoperative day | |||
| Body temperature (℃) | 37.0 ± 0.6 | 37.3 ± 0.4 | 0.95 |
| Respiratory frequency (times/min) | 19.4 ± 2.3 | 19.1 ± 2.8 | 0.83 |
| Heart rate (times/min) | 90.1 ± 13.7 | 85.2 ± 14.1 | 0.61 |
In the follow-up period, 103 patients survived and received regular follow-ups after discharge. Of these, 40 patients were in the “One-step” group and 63 were in the “Step-up” group. As shown in Table 5, the incidence of incisional hernia was higher in the “One-step” group (20.5% vs 6.3%, P = 0.03). New-onset endocrine insufficiency was present in 12 patients (30%) in the “One-step” group and in 28 patients (45%) in the “Step-up” group, which was not statistically significantly different (P = 0.143). Fecal elastase-1 (FE-1) levels were used for detecting exocrine insufficiency, with mean values of 254.1 ± 107.8% and 257.9 ± 93.3% in the two groups, respectively (P = 0.85). Levels of FE-1 < 200 μg/g were present in 14 patients (35%) in the “One-step” group, and 21 patients (33.3%) in “Step-up” group (P = 0.86). There was no significant difference in exocrine insufficiency between the two groups. In addition, there was no difference in other long-term complications between the two groups, which further confirmed that the “One-step” approach could obtain therapeutic effects similar to those of the “Step-up” approach.
| Groups | “One-step” approach (n = 40) | “Step-up” approach (n = 63) | P value |
| Long-term complications | |||
| New-onset endocrine insufficiency, n (%) | |||
| Number of patients | 12 (30) | 28 (45) | 0.143 |
| Oral medication | 9 (75) | 20 (71.4) | |
| Insulin | 5 (41.7) | 13 (46.4) | |
| Exocrine insufficiency, n (%) | |||
| Fecal elastase-1, mean value | 254.1 ± 107.8 | 257.9 ± 93.3 | 0.85 |
| Fecal elastase-1 < 200 μg/g, n (%) | 14 (35) | 21 (33.3) | 0.86 |
| Pancreatic enzyme, n (%) | 8 (20) | 11 (20.8) | 0.93 |
| Recurrent pancreatitis, n (%) | 7(17.5) | 13(20.6) | 0.69 |
| Chronic pancreatitis, n (%) | 4 (10) | 7 (11.1) | 0.86 |
| Incisional hernia, n (%) | 9 (20.5) | 4 (6.3) | 0.03 |
| Clinical symptoms, n (%) | |||
| Diarrhea | 16 (40) | 23 (36.5) | 0.72 |
| Bloating | 21(52.5) | 28(44.4) | 0.43 |
| Abdominal pain | 10 (25) | 13 (20.6) | 0.60 |
| Weight loss | 31 (77.5) | 47 (74.6) | 0.74 |
| Pancreatic pseudocyst, n (%) | 2 (5) | 5 (7.9) | 0.56 |
| Pancreatic portal hypertension, n (%) | 2 (5) | 3 (4.8) | 0.96 |
| Pancreatic cancer, n (%) | 0 | 0 | - |
Patients underwent quality of life evaluation every 6 mo after discharge during the follow-up period. There was no statistically significant difference in the SF-36 physical or mental health score, EQ-5D health status score, or Izbicki pain score between the groups (Table 6).
As shown in Table 7, the surgical costs of the “One-step” approach were lower than those of the “Step-up” approach, as the former approach did not require PCD (18582.37 ± 5918.45 vs 29641.63 ± 6795.11, P < 0.001). However, due to the similar baseline characteristics of AP severity and extent of pancreatic necrosis, the ICU costs (P = 0.87) and perioperative total medical costs (P = 0.34) were not different between the two groups. The follow-up costs per year, which included outpatient costs (P = 0.71), auxiliary examination costs (P = 0.58), and drug costs (P = 0.82), were also similar for the two surgical approaches.
| Groups | “One-step” approach (n = 61) | “Step-up” approach (n = 97) | P value |
| Medical costs | |||
| Surgical costs1 (RMB) | 18582.37 ± 5918.45 | 29641.63 ± 6795.11 | < 0.001 |
| ICU costs (RMB) | 276812.39 ± 183417.12 | 281133.73 ± 193252.47 | 0.87 |
| Perioperative medical total costs (RMB)2 | 529958.23 ± 171128.74 | 569768.07 ± 193184.68 | 0.34 |
| Follow-up costs per year | |||
| Outpatient costs (RMB) | 2040.79 ± 519.48 | 2169.08 ± 463.71 | 0.71 |
| Auxiliary examination costs3 (RMB) | 9751.96 ± 1012.37 | 9003.65 ± 1102.29 | 0.58 |
| Drug costs4 (RMB) | 2855.36 ± 318.12 | 2994.21 ± 372.95 | 0.82 |
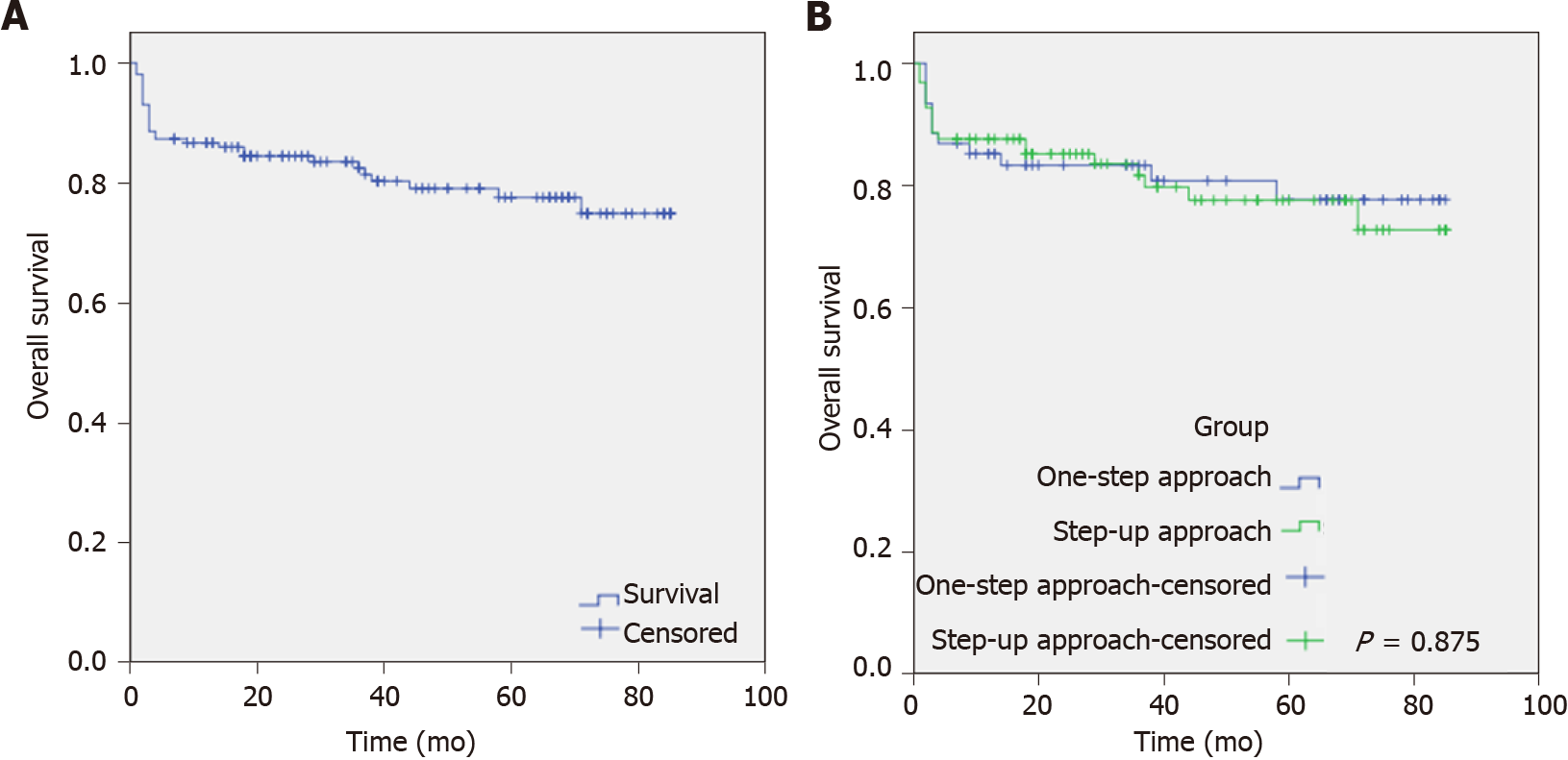
During the follow-up period, 11 patients died, 24 were lost to follow-up, and 103 patients survived. Of these, 4 patients who died, 9 patients who were lost to follow-up, and 40 patients who survived were in the “One-step” group, while 7 patients who died, 15 patients who were lost to follow-up, and 63 patients who survived were in the “Step-up” group. As illustrated in Figure 4, the mean follow-up time was 69.17 ± 2.53 mo (95%CI: 64.02-74.16). The total loss to follow-up rate was 15.2%. The overall survival (OS) in the “One-step” and “Step-up” groups was 10% (4/40) and 11.1% (7/63), respectively, with no significant difference (P = 0.875).
The PANTER study, conducted by the Dutch Pancreatitis Working Group in 2010, was regarded as a milestone event in IPN treatment and has attracted extensive attention, making the minimally invasive “Step-up” strategy become an important method for the current treatment of IPN[4,11]. However, as this strategy does not apply to all IPN patients, we here compared it to our “One-step” approach. We showed that, compared with the “Step-up” approach, the “One-step” approach has the advantages of effectively reducing the total length of hospital stay, number of interventions, number of drainage tubes used, and surgical costs. While it does not increase the incidence of severe complications, organ failure, or mortality, it significantly improves the perioperative inflammatory indicators and stabilizes the vital signs of patients, achieving a short-term efficacy and safety similar to the “Step-up” approach. In addition, our long-term follow-up showed that pancreatic endocrine or exocrine insufficiency incidence, quality of life, and long-term prognosis were not significantly different in patients who underwent the “One-step” approach and those who underwent the “Step-up” approach. Moreover, the medical costs to patients were not increased. The results of this study demonstrated that there was a similar effect between the two approaches in the long-term follow-up. However, the disadvantage of the “One-step” approach is that the long-term probability of incisional hernia is higher than that of the “Step-up” approach. The main reason for this is that the “One-step” approach usually involves a small incision to assist in pancreatic necrosectomy. The long-term indwelling drainage tube in the local abdominal wall and repeated inflammatory stimulation lead to a decrease in abdominal wall tension, which may cause incisional hernia.
In addition, since the vast majority of patients enrolled in our center were referred from other hospitals, the referral rate was over 90%. Among them, some patients who underwent “Step-up” treatment were transferred to our center for further treatment after receiving PCD, which failed, at another hospital. Consequently, the time from disease onset to hospital admission of the patients and then to the initial surgical treatment was significantly longer for these patients than for those treated with the “One-step” approach. This also partly delayed the optimal timing of treatment, increased the number of interventions, and increased the total length of hospital stay for patients in the “Step-up” group.
The present study found that compared to preoperatively, the inflammatory indicators and vital signs in the “One-step” group were significantly improved on the 7th day after the operation. Among them, the level of WBC decreased from 11.04 ± 6.31 to 7.51 ± 3.0, the level of NE decreased from 80.86 ± 8.3 to 69.30 ± 6.1, the level of CRP decreased from 115.3 ± 81.8 to 62.63 ± 25.6, the level of PCT decreased from 1.18 ± 1.0 to 0.37 ± 0.3 and the level of IL-6 decreased from 113 ± 91.8 to 46.3 ± 22.6; these differences were statistically significant (P < 0.05). However, there were no significant differences in perioperative inflammatory indicators and vital signs between the “One-step” approach and the “Step-up” approach. This demonstrates that the “One-step” approach has similar surgical efficacy as the “Step-up” approach.
In terms of surgical safety, the 61 patients who underwent “One-step” treatment in this study were all treated successfully. The mortality rate of the patients was 13.1% (8/61). The incidence of new-onset organ failure was 16.4% (10/61), of which 5 patients had multiple organ failure (mainly respiratory and circulatory failure). The incidence of postoperative complications, such as intrabdominal bleeding (3.3%, 2/61), pancreatic fistula (26.2%, 16/61), and enterocutaneous fistula or viscera perforation (4.9%, 3/61), with the “One-step” approach were similar to those with the “Step-up” approach. Some studies have reported that the “One-step” approach was first used by endoscopists for the treatment of pancreatic pseudocysts, and its therapeutic effect and surgical safety were better than that of the “Step-up” approach[12,13]. Currently, only a few centers have reported using the “One-step” approach, including the trans-lesser sac approach, retroperitoneal approach, or combined approach for treating IPN[7,14]. Among these approaches, the trans-lesser sac is the most commonly used surgical approach in our center[7]. The advantage of “One-step” necrosectomy through the trans-lesser sac is that it can reduce interference with the patient’s abdominal cavity and reduce the incidence of operation-related complications. It can help avoid PCD treatment, shorten the overall hospitalization time to some extent, and promote the patient’s recovery. In addition, under laparoscopy, the surgical view is wide, and the surgeon can better perform accurate anatomical positioning, which facilitates thorough removal of pancreatic necrotic tissue, reduces iatrogenic injury, and reduces surgical risks, while ensuring the patient’s safety to the greatest extent and facilitating the therapeutic effect.
During the follow-up period, we analyzed the clinical data of 103 surviving IPN patients to explore long-term complications, quality of life, medical costs, and OS rate. The OS rate in the “One-step” group was 10%, similar to that of the “Step-up” group. Except for incisional hernia, other long-term complications, including pancreatic endocrine or exocrine insufficiency, chronic pancreatitis, recurrent pancreatitis, pancreatic pseudocyst, and pancreatic portal hypertension, were not significantly different between the two surgical groups. However, although the “One-step” approach can achieve a similar long-term effect with the “Step-up” approach, postoperative pancreatic endocrine/exocrine insufficiency and patient’s quality of life still require attention from clinicians[15].
According to the research results, approximately 1/3rd of patients had pancreatic endocrine insufficiency, and about 40% of patients had diabetes or pre-diabetes after AP, which was mainly caused by irreversible pancreatic damage[16,17]. Winter Gasparoto et al[18] followed-up AP patients for an average of 2.9 years and found that 43.7% of the patients developed pre-diabetes and 31.3% developed diabetes after AP[18]. A large study in Taiwan that followed 2966 patients with AP and 11864 healthy controls over a long period found that the incidence of diabetes in the first 3 mo after the onset of AP was 60.8/1000 per year compared to 8.0/1000 per year in the control group[19]. The risk of developing diabetes in the first 3 mo after AP onset was 5.9 times higher than that in the control group. The incidence of diabetes 3 mo after the onset of AP was 22.5/1000 per year compared to 6.7/1000 per year in the control group. The risk of diabetes 3 mo after the onset of AP was 2.54 times higher than that in the control group[20]. Additionally, Gupta et al[20] found that 40% of AP patients developed diabetes after an average of 31.3 mo of follow-up[20]. The above results are consistent with the results of the present study, indicating that the proportion of AP patients with long-term pancreatic endocrine insufficiency gradually increases with prolonged follow-up time and patient age. This suggests that clinicians should pay attention to postoperative glucose monitoring and regulation, as well as long-term follow-up of patients’ pancreatic secretion function.
In addition, another study reported that the probability of pancreatic exocrine insufficiency (PEI) within 1 year in patients with AP is 61%-85% and that PEI will last for 6-18 mo in some patients[16,21]. However, pancreatic function improves with the passage of postoperative time in some patients, and the incidence of PEI also decreases[22]. However, some patients still require long-term oral trypsin to improve their clinical symptoms, which is consistent with the present study results. As a common complication after pancreatic surgery, PEI can lead to decreased quality of life and malnutrition in patients, which can be life-threatening in severe cases. Pancreatic surgeons should pay appropriate attention to this issue. Additionally, because some patients with pancreatic endocrine or exocrine insufficiency require long-term oral medications or need to undergo necessary imaging and laboratory examinations regularly, the medical expenses of these patients are significantly increased, and their long-term quality of life is affected to varying degrees. However, there was no significant difference between the two surgical approaches in terms of long-term complications and medical costs. Consequently, the present study confirms that the “One-step” surgical approach has similar long-term efficacy and prognosis as the “Step-up” approach.
In summary, we believe that the “One-step” approach is indicated when the lesion area is mainly involved in the peripancreatic or peripancreatic involving the posterior colonic space. The patients are usually generally stable and can tolerate minimally invasive surgery. The advantages of this procedure are: (1) The lesions on the pancreatic head and pancreatic body and tail can be treated simultaneously; (2) Laparoscopy has a broad field of vision, strong ability to remove necrotic tissue, and can be used to control intraoperative bleeding by a variety of means; (3) This procedure has little interference with laparoscopy and is helpful to the rapid recovery of digestive tract function; (4) The anatomical position is clear, and the localization is more accurate; and (5) This procedure avoids PCD treatment and helps to shorten the length of hospital stay. The disadvantage is that there is the possibility of stomach and colon injury. For non-low drainage, negative pressure suction should be carried out in the early postoperative period to maintain the drainage effect. Meanwhile, there is a risk of long-term incisional hernia. In addition to the condition mentioned above, the “Step-up” approach can be applicable for IPN patients in other conditions.
However, the present study also has some limitations. First, this was a retrospective, single-center study involving a small sample size; as such, there may be a certain bias in the analysis of clinical data. Secondly, the research and exploration of the “One-step” approach mainly focused on patients without organ failure or who had passed the delayed operative waiting period through conservative treatment. Thus, this surgical approach has not been applied in the treatment of patients with IPN complicated with organ failure. Hence, we need to confirm the current findings further using large-sample, randomized controlled studies.
Compared with the “Step-up” approach, the “One-step” approach is a safe and effective treatment method, with better long-term quality of life and prognoses. These findings provide a new concept that allows diversification of surgical treatment strategies for IPN patients and also indicates that an individualized treatment strategy for each patient is more appropriate than a single set treatment strategy for a complex and variable disease such as IPN. Thus, this approach can be used as a new surgical treatment option for IPN in the future.
Although the “Step-up” strategy is the primary surgical treatment for infected pancreatic necrosis, it is not suitable for all infected pancreatic necrosis patients.
The “One-step” strategy represents a novel treatment; however, there is a lack of safety, efficacy, and long-term follow-up clinical data compared with the “Step-up” approach.
This study aimed to compare the safety, efficacy, and long-term follow-up of two surgical approaches.
Patients were retrospectively assessed, with a composite endpoint of severe complications or death as the primary outcome. t-test, chi-square test or Fisher’s exact test, and Kaplan-Meier curves were used for further analysis.
The time from disease onset to hospital admission (53.69 ± 38.14 vs 32.20 ± 20.75, P < 0.001) and to initial surgical treatment was longer in the “Step-up” than in the “One-step” group (54.38 ± 10.46 vs 76.58 ± 17.03, P < 0.001). Patients who underwent “Step-up” necrosectomy had a longer hospitalization duration (65.41 ± 28.14 vs 52.76 ± 24.71, P = 0.02), and more interventions (4.26 ± 1.71 vs 3.18 ± 1.39, P < 0.001). Postoperative inflammatory indicator levels were significantly lower than preoperative levels in each group. Although the incisional hernia incidence was higher in the “One-step” group, there were no significant differences in other observation indicators.
The “One-step” approach is a safe and effective treatment method with better long-term quality of life and prognosis.
The “One-step” approach provides an alternative surgical treatment strategy for patients with infected pancreatic necrosis.
| 1. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 624] [Article Influence: 124.8] [Reference Citation Analysis (1)] |
| 2. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 589] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 3. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 470] [Article Influence: 78.3] [Reference Citation Analysis (3)] |
| 4. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1080] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 5. | Li A, Cao F, Li J, Fang Y, Wang X, Liu DG, Li F. Step-up mini-invasive surgery for infected pancreatic necrosis: Results from prospective cohort study. Pancreatology. 2016;16:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck CH, Fockens P, van Goor H, van Grevenstein WM, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven CJ, Laméris JS, van Leeuwen MS, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling GP, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Superiority of Step-up Approach vs Open Necrosectomy in Long-term Follow-up of Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 7. | Cao F, Duan N, Gao C, Li A, Li F. One-Step verse Step-Up Laparoscopic-Assisted Necrosectomy for Infected Pancreatic Necrosis. Dig Surg. 2020;37:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Hollemans RA, Bollen TL, van Brunschot S, Bakker OJ, Ahmed Ali U, van Goor H, Boermeester MA, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Predicting Success of Catheter Drainage in Infected Necrotizing Pancreatitis. Ann Surg. 2016;263:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Burek J, Jaworska K, Witkowski G, Durlik M. A case of acute pancreatitis - does step-up protocol always indicated? Pol Merkur Lekarski. 2020;48:100-102. [PubMed] |
| 10. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26200] [Article Influence: 1190.9] [Reference Citation Analysis (2)] |
| 11. | Besselink MG, van Santvoort HC, Nieuwenhuijs VB, Boermeester MA, Bollen TL, Buskens E, Dejong CH, van Eijck CH, van Goor H, Hofker SS, Lameris JS, van Leeuwen MS, Ploeg RJ, van Ramshorst B, Schaapherder AF, Cuesta MA, Consten EC, Gouma DJ, van der Harst E, Hesselink EJ, Houdijk LP, Karsten TM, van Laarhoven CJ, Pierie JP, Rosman C, Bilgen EJ, Timmer R, van der Tweel I, de Wit RJ, Witteman BJ, Gooszen HG; Dutch Acute Pancreatitis Study Group. Minimally invasive 'step-up approach' vs maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [ISRCTN13975868]. BMC Surg. 2006;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Yoo J, Yan L, Hasan R, Somalya S, Nieto J, Siddiqui AA. Feasibility, safety, and outcomes of a single-step endoscopic ultrasonography-guided drainage of pancreatic fluid collections without fluoroscopy using a novel electrocautery-enhanced lumen-apposing, self-expanding metal stent. Endosc Ultrasound. 2017;6:131-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Mangiavillano B, Arcidiacono PG, Masci E, Mariani A, Petrone MC, Carrara S, Testoni S, Testoni PA. Single-step vs two-step endo-ultrasonography-guided drainage of pancreatic pseudocyst. J Dig Dis. 2012;13:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zhao G, Hu M, Liu R, Xu Y. Retroperitoneoscopic Anatomical Necrosectomy: A Modified Single-Stage Video-Assisted Retroperitoneal Approach for Treatment of Infected Necrotizing Pancreatitis. Surg Innov. 2015;22:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 689] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 16. | Hollemans RA, Hallensleben NDL, Mager DJ, Kelder JC, Besselink MG, Bruno MJ, Verdonk RC, van Santvoort HC; Dutch Pancreatitis Study Group. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology. 2018;18:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 18. | Winter Gasparoto RC, Racy Mde C, De Campos T. Long-term outcomes after acute necrotizing pancreatitis: what happens to the pancreas and to the patient? JOP. 2015;16:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of Diabetes Mellitus after First-Attack Acute Pancreatitis: A National Population-Based Study. Am J Gastroenterol. 2015;110:1698-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Gupta R, Wig JD, Bhasin DK, Singh P, Suri S, Kang M, Rana SS, Rana S. Severe acute pancreatitis: the life after. J Gastrointest Surg. 2009;13:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Working Party of the Australasian Pancreatic Club, Smith RC, Smith SF, Wilson J, Pearce C, Wray N, Vo R, Chen J, Ooi CY, Oliver M, Katz T, Turner R, Nikfarjam M, Rayner C, Horowitz M, Holtmann G, Talley N, Windsor J, Pirola R, Neale R. Summary and recommendations from the Australasian guidelines for the management of pancreatic exocrine insufficiency. Pancreatology. 2016;16:164-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Italian Association for the Study of the Pancreas (AISP); Pezzilli R, Zerbi A, Campra D, Capurso G, Golfieri R, Arcidiacono PG, Billi P, Butturini G, Calculli L, Cannizzaro R, Carrara S, Crippa S, De Gaudio R, De Rai P, Frulloni L, Mazza E, Mutignani M, Pagano N, Rabitti P, Balzano G. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis. 2015;47:532-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution-NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dambrauskas Z, Gadour E S-Editor: Fan JR L-Editor: A P-Editor: Fan JR