Published online Jul 27, 2020. doi: 10.4240/wjgs.v12.i7.326
Peer-review started: December 30, 2019
First decision: April 12, 2020
Revised: May 10, 2020
Accepted: May 15, 2020
Article in press: May 15, 2020
Published online: July 27, 2020
Processing time: 204 Days and 17.8 Hours
Postoperative acute kidney injury (AKI) is a complex pathological process involved intrarenal and systemic inflammation caused by renal hypoperfusion, nephrotoxic drugs and urinary obstruction. Neutrophil-to-lymphocyte ratio (NLR) is a marker of inflammation reflecting the progress of many diseases. However, whether NLR at admission can predict the occurrence of AKI after surgery in the intensive care unit (ICU) remains unknown.
To clarify the relationship between NLR and the occurrence of AKI in patients with gastrointestinal and hepatobiliary surgery in the ICU.
A retrospective analysis of 282 patients receiving surgical ICU care after gastrointestinal and hepatobiliary surgery in our hospital from December 2014 to December 2018 was performed.
Postoperative AKI occurred in 84 patients (29.79%) in this cohort. NLR by the multivariate analysis was an independent risk factor for occurrence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in the ICU. In this cohort, receiver operating characteristic curves of AKI occurrence showed that the optimal cut-off value of NLR was 8.380. NLR was found to be significantly correlated with the white blood cell count, neutrophil count, lymphocyte count, arterial lactate and dialysis (P < 0.05). Additionally, NLR value at admission was higher in AKI patients compared with the non-AKI patients and increased with the severity of AKI. Patients with NLR ≥ 8.380 exhibited significantly higher incidences of postoperative AKI and severe AKI than patients with NLR < 8.380 (AKI: 38.12% vs 14.85%, P < 0.001; severe AKI: 14.36% vs 1.98%, P = 0.001).
NLR at admission is a predictor of AKI occurrence in patients with gastrointestinal and hepatobiliary surgery in ICU. NLR should be included in the routine assessment of AKI occurrence.
Core tip: This was a retrospective study to clarify the relationship between neutrophil-to-lymphocyte ratio (NLR) and the occurrence of acute kidney injury (AKI) in patients with gastrointestinal and hepatobiliary surgery in the surgical intensive care unit (ICU). We found that patients with NLR ≥ 8.380 exhibited significantly higher incidences of postoperative AKI and severe AKI. NLR at admission is a predictor of AKI in patients with gastrointestinal and hepatobiliary surgery in ICU. We recommend that NLR should be included in the routine assessment of AKI occurrence.
- Citation: Bi JB, Zhang J, Ren YF, Du ZQ, Wu Z, Lv Y, Wu RQ. Neutrophil-to-lymphocyte ratio predicts acute kidney injury occurrence after gastrointestinal and hepatobiliary surgery. World J Gastrointest Surg 2020; 12(7): 326-335
- URL: https://www.wjgnet.com/1948-9366/full/v12/i7/326.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i7.326
Acute kidney injury (AKI) is one of the most common complications after gastrointestinal and hepatobiliary surgery. Numerous studies have reported high incidences of postoperative AKI, ranging from 22% to 66% in intensive care unit (ICU)[1-4]. Postoperative AKI and its stages are independent risk factors for the prognosis of surgical patients[5]. In addition, a large number of studies have reported that AKI significantly increases the risk of chronic renal insufficiency and end-stage kidney disease[6,7]. Patients with severe AKI often require renal replacement therapy, and once developed into end-stage kidney disease, patients would require long-term hemodialysis, which decreases quality of life[8]. Early detection of AKI is critical to the treatment of perioperative patients. Serum creatinine is a classic indicator of AKI, but changes in serum creatinine levels often occur at a later stage. Some new tests are either too expensive or too difficult to implement, making them difficult for clinical use[9]. Therefore, indicators that can predict the occurrence of AKI after surgery are urgently needed.
The neutrophil-to-lymphocyte ratio (NLR) is a marker of inflammation that can be calculated directly from a patient's complete blood count. Extensive studies have shown that NLR can predict the outcome of cardiac surgery, sepsis, and cancer[10-13]. A recent study showed that sepsis patients with NLR > 17.11 were more likely to develop AKI[10]. The risk factors and early diagnosis of postoperative AKI have always been urgent problems in clinic. Nevertheless, whether NLR at admission can predict the occurrence of AKI after surgery in patients receiving ICU care remains unknown. We hypothesize that NLR is an independent risk factor for AKI after surgery, and patients with high NLR are more likely to develop postoperative AKI. The main purpose of this article was to clarify the relationship between NLR and the occurrence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in the ICU.
This study retrospectively analyzed the electronic medical records of 282 patients after gastrointestinal and hepatobiliary surgery in the ICU of the First Affiliated Hospital of Xi’an Jiaotong University from December 2014 to December 2018. The inclusion criteria were: Patients admitted to the ICU after gastrointestinal or hepatobiliary surgery; aged over 18 years; hospitalized in the ICU for at least 24 hours. The exclusion criteria were: Patients admitted with known acute or chronic kidney disease; patients with liver transplantation; patients without complete clinical data. This study complied with the provisions of Declaration of Helsinki[14]. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University.
The clinical outcomes in this study included occurrence of AKI and severe AKI, length of ICU stay, ICU re-admission, ICU mortality and 28-day overall mortality. The definition of AKI complied with Kidney Disease: Improving Global Outcomes criteria[15] as follows: The serum creatinine level increased by ≥ 0.3 mg/dL (≥ 26 mmol/L) within 48 h, or serum creatinine levels increased by 1.5 times of the baseline within 7 d after surgery, or urine volume was less than 0.5mL/kg per h and lasted more than 6 h. The staging of AKI is defined as follows: Stage 1, serum creatinine level increased by ≥ 0.3 mg/dL (≥ 26 mmol/L) within 48 h, or serum creatinine levels increased by 1.5-1.9 times of the baseline within 7 d after surgery; Stage 2, serum creatinine serum creatinine levels increased by 2.0-2.9 times of the baseline within 7 d after surgery; Stage 3, serum creatinine level increased by ≥ 4.0 mg/dL (≥ 354 mmol/L) within 48 h, or serum creatinine levels increased by more than 3.0 times of the baseline within 7 d after surgery, or patients require renal replacement therapy. Severe AKI is defined as AKI of stage 2 and 3.
The distribution of the continuous variables was checked for normality using the Kolmogorov-Smirnov test. Normally distributed variables were expressed as mean ± SD, and differences between the two groups were analyzed by the t test. Nonnormally distributed variables were expressed as medians (interquartile range) and differences between the two groups were analyzed by the Mann-Whitney. Categorical variables were expressed as absolute numbers and percent frequencies and differences between the two groups were analyzed by χ2 or Fisher’s exact test. Univariate and multivariate analyses were performed using logistic regression models. Variables with P < 0.05 in the univariate analysis were incorporated into the multivariate analysis. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off value (with the highest sum of specificity plus sensitivity). PASW 18.0 software (SPSS Inc., Chicago, Illinois, United States) was used for statistical analysis. A two-tailed P < 0.05 was considered statistically significant.
A total of 84 patients (29.79%) developed postoperative AKI in this cohort. To identify risk factors for AKI in patients with gastrointestinal and hepatobiliary surgery in ICU, univariate and multivariate analyses were performed (Table 1). The results of univariate analysis exhibited that the following factors were significantly associated with occurrence of AKI in patients with gastrointestinal and hepatobiliary surgery in ICU, including sex (HR: 1.761, 95%CI: 1.008-3.075, P = 0.047), drinking (HR: 0.547, 95%CI: 0.306-0.977, P = 0.042), coexisting condition of ischemic heart disease (HR: 0.387, 95%CI: 0.166-0.901, P = 0.028), sequential organ failure assessment score (SOFA score) at ICU admission (HR: 1.092, 95%CI: 1.030-1.157, P = 0.003), acute physiology and chronic health evaluation (APACHE II) at ICU admission (HR: 1.038, 95%CI: 1.005-1.073, P = 0.023), serum creatinine (HR: 1.005, 95%CI: 1.003-1.007, P < 0.001), serum K concentration (HR: 1.676, 95%CI: 1.190-2.362, P = 0.003) and NLR (HR: 1.052, 95%CI: 1.026-1.078, P < 0.001). Further multivariate analysis revealed that NLR (HR: 1.290, 95%CI: 1.212-1.373, P < 0.001) was an independent risk factor for occurrence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in ICU.
| Parameters | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age ( < 65 yr/≥ 65 yr) | 1.010 (0.996-1.024) | 0.146 | - | - |
| Sex (male/femal) | 1.761 (1.008-3.075) | 0.047 | 0.684 (0.325-1.438) | 0.316 |
| Smoking (yes/no) | 0.599 (0.351-1.021) | 0.060 | - | - |
| Drinking (yes/no) | 0.547 (0.306-0.977) | 0.042 | 0.916 (0.401-2.097) | 0.836 |
| MAP (mmHg) | 1.001 (0.988-1.014) | 0.923 | ||
| Hypertension (yes/no) | 1.218 (0.668-2.223) | 0.519 | - | - |
| Diabetes mellitus (yes/no) | 0.828 (0.393-1.744) | 0.619 | - | - |
| Cirrhosis (yes/no) | 0.563 (0.239-1.324) | 0.188 | - | - |
| Ischemic heart disease (yes/no) | 0.387 (0.166-0.901) | 0.028 | 1.111 (0.385-3.212) | 0.845 |
| Stroke (yes/no) | 2.500 (0.834-7.493) | 0.102 | - | - |
| Malignant diseases (yes/no) | 0.949 (0.514-1.751) | 0.867 | - | - |
| SOFA score at ICU admission | 1.092 (1.030-1.157) | 0.003 | 1.010 (0.937-1.088) | 0.801 |
| APACHE II at ICU admission | 1.038 (1.005-1.073) | 0.023 | 1.006 (0.954-1.060) | 0.836 |
| PLT (109/L) | 0.999 (0.997-1.002) | 0.487 | - | - |
| WBC (109/L) | 1.026 (0.999-1.055) | 0.062 | - | - |
| TB | 1.001 (0.999-1.003) | 0.216 | ||
| Creatinine | 1.005 (1.003-1.007) | < 0.001 | 1.000 (0.998-1.002) | 0.989 |
| K | 1.676 (1.190-2.362) | 0.003 | 0.918 (0.594-1.416) | 0.698 |
| Na | 1.012 (0.979-1.046) | 0.473 | - | - |
| INR | 1.269 (0.980-1.641) | 0.070 | - | - |
| PaO2/FiO2 | 0.994 (0.883-1.119) | 0.920 | - | - |
| Arterial lactate1 | 0.936 (0.876-1.000) | 0.051 | ||
| Procalcitonin2 | 1.003 (0.096-1.011) | 0.348 | - | - |
| Blood transfusion (yes/no) | 0.898 (0.555-1.452) | 0.660 | - | - |
| NLR | 1.052 (1.026-1.078) | < 0.001 | 1.290 (1.212-1.373) | < 0.001 |
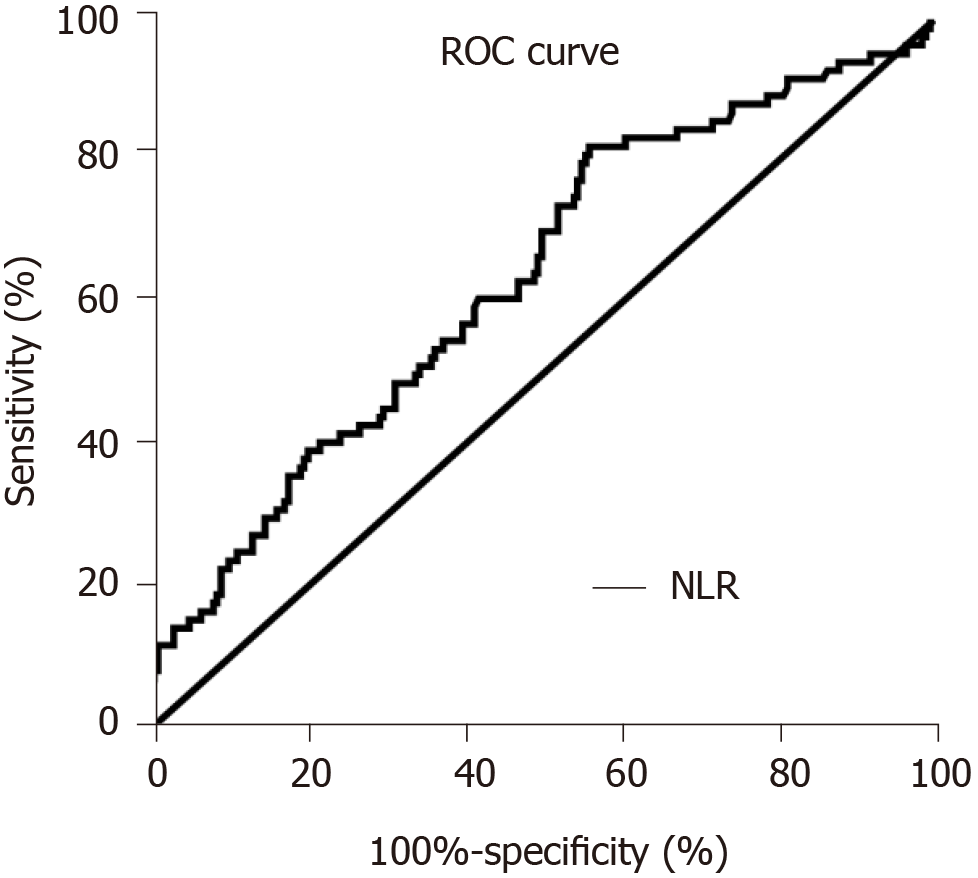
ROC curves analysis with occurrence of AKI was used to determine the optimal cut-off value of NLR. The value of NLR at the maximum value of the Youden index is taken as the optimal cut-off point. As shown in Figure 1, the optimal cut-off value of NLR was 8.380 [sensitivity of 82.14% and specificity of 43.43%, area under the curve = 0.634, 95%CI: 0.563-0.705, P = 0.0004]. The patients were divided into a high-NLR group (n = 181) and a low-NLR group (n = 101) by the optimal cut-off value of NLR (8.380).
As shown in Table 2, the average age of 282 patients in ICU was 60.48 ± 17.74 years. In the cohort, 24.11% of patients underwent hepatobiliary surgery. The age, sex, operative site and characteristics of surgery showed no significant differences between the high-NLR and low-NLR groups. Laboratory examination at ICU admission revealed that the high-NLR group exhibited higher white blood cell count (14.85 ± 10.25 vs 8.77 ± 5.65, P < 0.001) and neutrophil count (13.10 ± 7.91 vs 6.34 ± 4.32, P < 0.001), and lower lymphocyte count (0.78 ± 0.50 vs 1.49 ± 1.50, P < 0.001) and arterial lactate (2.91 ± 3.29 vs 4.40 ± 5.23, P = 0.006) than the low-NLR group. No significant differences were found in SOFA and APACHE II scores at ICU admission between the two groups. The most common coexisting conditions at ICU admission were smoking, hypertension and drinking. There were no differences in smoking, hypertension, drinking, diabetes mellitus, ischemic heart disease, stroke, and malignant diseases between the high and low-NLR patients. There was no significant difference in terms of ICU care, including ventilation, steroids, cardio-pulmonary resuscitation, vasopressor and transfusion, except that high-NLR patients used more dialysis.
| Variables | Overall (n = 282) | High-NLR group (n = 181) | Low-NLR group (n = 101) | P value |
| Age (yr) | 60.48 ± 17.74 | 62.62 ± 17.44 | 58.42 ± 18.17 | 0.146 |
| Sex (male/female) | 180/102 | 114/67 | 66/35 | 0.695 |
| Operative site | 0.330 | |||
| Gastrointestinal surgery, n (%) | 214 (75.89) | 134 (74.03) | 80 (79.21) | |
| Hepatobiliary surgery, n (%) | 68 (24.11) | 47 (25.97) | 21 (20.79) | |
| Characteritics of surgery | 0.398 | |||
| Emergency surgery | 55 (19.5) | 38 (21.0) | 17 (16.8) | |
| Non-emergency surgery | 227 (80.5) | 143 (79.0) | 84 (83.2) | |
| Lab values at ICU admission | ||||
| PLT (109/L) | 144.17 ± 112.61 | 146.84 ± 114.94 | 139.40 ± 108.70 | 0.596 |
| WBC (109/L) | 12.67 ± 9.34 | 14.85 ± 10.25 | 8.77 ± 5.65 | < 0.001 |
| ALT (U/L) | 169.28 ± 356.37 | 194.10 ± 413.23 | 126.53 ± 259.71 | 0.140 |
| AST (U/L) | 258.83 ± 683.02 | 310.81 ± 822.44 | 169.28 ± 308.64 | 0.098 |
| TB (μmol/L) | 87.42 ± 132.18 | 90.95 ± 126.59 | 81.14 ± 142.01 | 0.554 |
| Albumin (g/L) | 28.63 ± 6.42 | 28.14 ±5.90 | 29.49 ± 7.20 | 0.091 |
| Creatinine (μmol/L) | 131.44 ± 135.27 | 138.84 ± 122.81 | 118.20 ± 154.87 | 0.225 |
| K (mmol/L) | 3.95 ± 0.76 | 4.03 ± 0.77 | 3.82 ± 0.73 | 0.034 |
| Na (mmol/L) | 137.55 ± 7.72 | 137.68 ± 7.54 | 137.32 ± 8.05 | 0.708 |
| Neutrophil (109/L) | 10.68 ± 7.57 | 13.10 ± 7.91 | 6.34 ± 4.32 | < 0.001 |
| Lymphocyte (109/L) | 1.04 ± 1.04 | 0.78 ± 0.50 | 1.49 ± 1.50 | < 0.001 |
| PaO2/FiO2 | 3.24 ± 2.08 | 3.30 ± 1.75 | 3.14 ± 2.63 | 0.576 |
| Arterial lactate (mmol/L)1 | 3.43 ± 4.13 | 2.91 ± 3.29 | 4.40 ± 5.23 | 0.006 |
| Procalcitonin (ng/mL)2 | 15.85 ± 35.73 | 17.11 ± 39.68 | 13.37 ± 26.29 | 0.453 |
| SOFA score at ICU admission | 7.74 ± 5.01 | 7.63 ± 4.11 | 7.93 ± 6.34 | 0.806 |
| APACHE II at ICU admission | 16.04 ± 7.78 | 15.95 ± 7.49 | 16.19 ± 8.31 | 0.630 |
| Coexisting conditions | ||||
| Smoking (yes/no) | 91/191 | 60/121 | 31/70 | 0.672 |
| Drinking (yes/no) | 65/217 | 43/138 | 22/79 | 0.706 |
| Hypertension (yes/no) | 71/211 | 44/137 | 27/74 | 0.653 |
| Diabetes mellitus (yes/no) | 36/246 | 27/154 | 9/92 | 0.147 |
| Ischemic heart disease (yes/no) | 24/258 | 16/165 | 8/93 | 0.791 |
| Stroke (yes/no) | 26/256 | 15/166 | 11/90 | 0.469 |
| Malignant diseases (yes/no) | 62/220 | 43/138 | 19/82 | 0.336 |
| ICU care | ||||
| Ventilation (yes/no) | 169/113 | 103/78 | 66/35 | 0.166 |
| Dialysis (yes/no) | 72/210 | 54/127 | 18/83 | 0.027 |
| Steroids (yes/no) | 81/201 | 52/129 | 29/72 | 0.998 |
| CPR (yes/no) | 58/224 | 35/146 | 23/78 | 0.494 |
| Vasopressor (yes/no) | 131/151 | 84/97 | 47/54 | 0.984 |
| Transfusion | 173/109 | 112/69 | 61/40 | 0.806 |
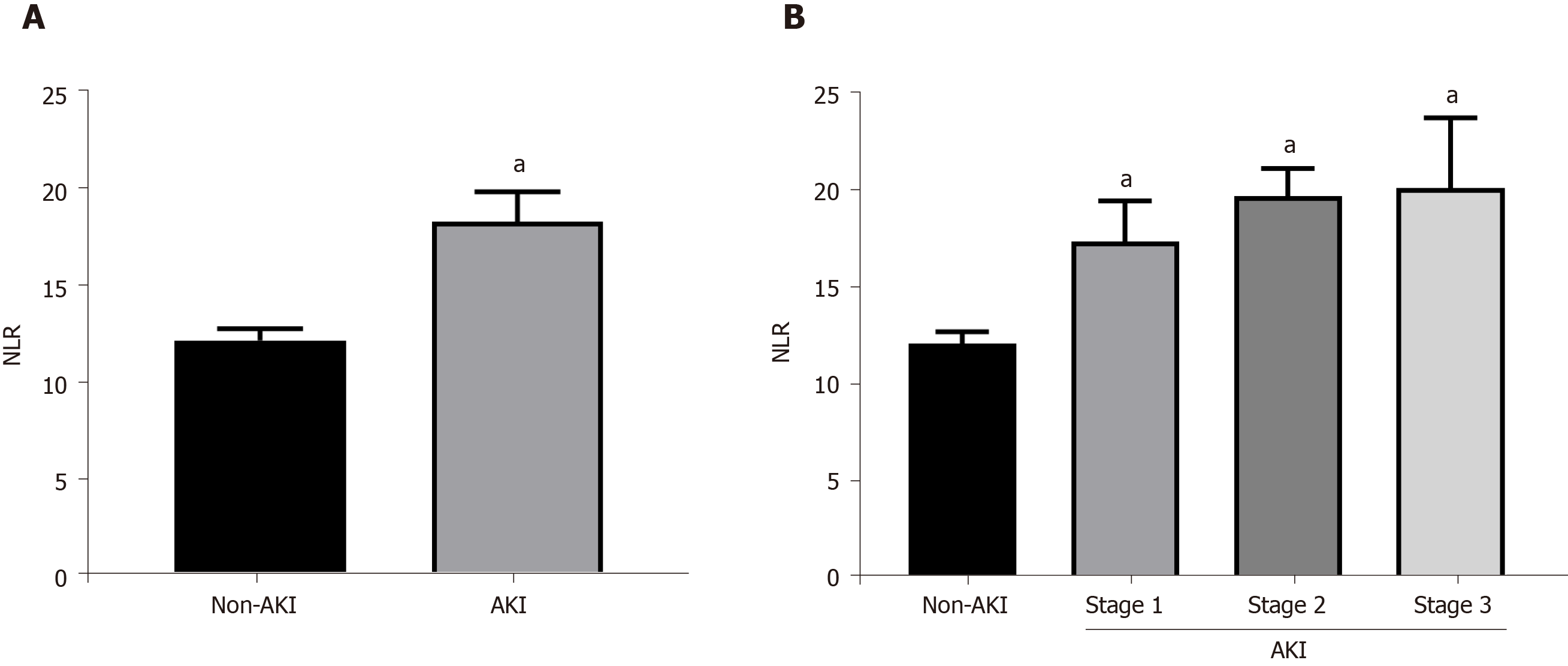
To further clarify the relationship between postoperative AKI occurrence and NLR, we analyzed the NLR levels in the AKI patients and non-AKI patients. The results showed that AKI patients had higher NLR value compared with the non-AKI patients (18.210 ± 14.179 vs 12.121 ± 8.499, P < 0.001) (Figure 2A). Additionally, NLR increased with the severity of AKI (stage 1: 17.356 ± 15.686, stage 2: 19.697 ± 5.080, stage 3: 20.113±13.937) and was significantly higher in all three stages than that in the non-AKI group (Figure 2B).
Clinical outcomes of patients in the high and low NLR groups are shown in Table 3. A total of 84 patients (29.79%) and 28 patients (9.92%) were complicated with AKI and severe AKI, respectively. The incidence of AKI and severe AKI in the high-NLR group was significantly higher than those in the low-NLR group (AKI: 38.12% vs 14.85%, P < 0.001; severe AKI: 14.36% vs 1.98%, P = 0.001). The difference in the occurrence of sepsis between two groups showed a strong tendency of statistical significance (17.68% vs 10.89%, P = 0.083). Additionally, length of ICU stays, ICU re-admission, ICU mortality and 28-d overall mortality exhibited no significant differences between the high-NLR and low-NLR groups.
| Variables | Overall (n = 282) | High-NLR group (n = 181) | Low-NLR group (n = 101) | P value |
| Renal | ||||
| AKI, n (%) | 84 (29.79) | 69 (38.12) | 15 (14.85) | < 0.001 |
| Severe AKI | 28 (9.92) | 26 (14.36) | 2 (1.98) | 0.001 |
| Sepsis, n (%) | 45 (15.96) | 32 (17.68) | 11 (10.89) | 0.083 |
| Length of ICU stay (d) | 11.36 ± 13.37 | 11.36 ± 12.58 | 11.38 ± 14.74 | 0.990 |
| ICU re-admission, n (%) | 40 (14.18) | 24 (13.25) | 16 (15.84) | 0.551 |
| ICU mortality, n (%) | 41 (14.54) | 24 (13.25) | 17 (16.83) | 0.415 |
| 28-d overall mortality (%)1 | 80 (28.4) | 55 (30.4) | 25 (24.8) | 0.314 |
In this study, the multivariate analysis showed NLR at admission was an independent risk factor for occurrence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in ICU. Patients with NLR ≥ 8.380 exhibited significantly higher incidences of postoperative AKI and severe AKI. NLR at admission could be a predictor of AKI occurrence in patients with gastrointestinal and hepatobiliary surgery and should be included in the routine assessment of AKI occurrence.
AKI is one of the most common critical illnesses with high morbidity and poor prognosis. The causes of AKI are extremely complicated, including renal hypoperfusion such as hypovolemia and reduced cardiac output, nephrotoxicity drugs and urinary obstruction[16]. Different causes eventually lead to hypoxia, inflammation, oxidative stress and innate immune system activation and cell death[17]. AKI is a common complication after abdominal surgery. The mortality of patients with postoperative AKI after abdominal surgery increased by 3.5 times[18]. A large number of previous studies have shown that preoperative renal insufficiency is the most important risk factor for AKI after abdominal surgery, and other risk factors include preoperative dehydration, intra-abdominal hypertension, blood transfusion, and use of nephrotoxic drug[19]. Our study found a 29.79% incidence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in ICU. Univariate analysis exhibited that sex, coexisting condition of ischemic heart disease, SOFA score at ICU admission, APACHE II at ICU admission, serum creatinine, serum K concentration and NLR were significantly associated with occurrence of postoperative AKI. However, multivariate analysis revealed that only NLR was the independent risk factor for occurrence of postoperative AKI in surgical ICU.
Intrarenal and systemic response plays a key role in postoperative AKI. A large number of inflammatory factors and inflammatory cells promote oxidative stress and apoptosis, eventually leading to renal insufficiency[20]. Many anti-inflammatory drugs have significant effects on AKI and have entered clinical trials[20]. Early detection of AKI has great influence on the prognosis of postoperative patients. Examinination of indicators of renal insufficiency, such as creatinine and urea nitrogen, is the most accurate test, but changes often occur at a later stage[21]. Some new tests are either too expensive or too difficult to implement, making them difficult for clinical use[9]. NLR is a marker of inflammation reflecting the progress of inflammation-related disease. Extensive studies have shown that NLR can predict the outcome of cardiac surgery, sepsis, and cancer[10-12]. Our study showed that NLR at admission was an independent risk factor for occurrence of postoperative AKI and patients with NLR ≥ 8.380 exhibited significantly higher incidences of postoperative AKI and severe AKI. NLR, characterized by easy accessibility, objectivity, and noninvasiveness, could be a better predictor of AKI occurrence in patients with gastrointestinal and hepatobiliary surgery.
Sepsis is life threatening organ dysfunction caused by the host's harmful response to infection. Patients with AKI significantly increased sepsis mortality[22]. Studies have shown that sepsis is associated with 50% of AKI, and up to 60% of sepsis patients develop organ dysfunction including AKI[23]. Mechanism of sepsis-induced AKI is that deleterious inflammatory cascade of sepsis causes kidney damage[10,24]. Several studies have shown that NLR is a predictor of AKI occurrence in patients with sepsis. They showed that NLR ≥ 9.11 in sepsis had a high risk of AKI occurrence[24]. In this study, the difference in the occurrence of sepsis between high-NLR group and low-NLR group showed a strong tendency of statistical significance (17.68% vs 10.89%, P = 0.083). The high tendency of sepsis in the NLR ≥ 8.380 group may be one of the important reasons for NLR as an independent risk factor for AKI in patients with gastrointestinal and hepatobiliary surgery in ICU.
High levels of arterial lactate reflect tissue microcirculatory insufficiency. Numerous studies have considered lactic acid levels as a risk factor in critically ill patients[25,26]. However, in this study, the multivariate analysis showed that arterial lactate at admission was not an independent risk factor for occurrence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in ICU. Intraoperative tissue ischemia and postoperative coagulation and sepsis may lead to changes in lactate levels[27]. In addition, severe inflammatory response in the tissue can lead to elevated lactic acid levels[28]. Our study showed that patients with NLR ≥ 8.380 had high arterial lactate levels (2.91 ± 3.29 mmol/L vs 4.40 ± 5.23 mmol/L, P = 0.006). Increased lactic acid levels may be due to postoperative inflammation under such conditions.
This study has several limitations. First, this is a single-center retrospective cohort study. The results might be influenced by selection bias, recall bias and some residual confounding. A further multiple-center data was needed to clarify the relationship between NLR and the occurrence of AKI in patients with gastrointestinal and hepatobiliary surgery in ICU. Second, this study retrospectively analyzed the electronic medical records of 282 patients after gastrointestinal and hepatobiliary surgery in the surgical ICU. The conclusion is only based on a small number of patients. A further large sample sized study is needed in the future. Additionally, this research mainly clarified the phenomenon that NLR at admission is a predictor of AKI occurrence, and the specific mechanism needs further study.
In conclusion, NLR at admission was an independent risk factor and could be a predictor for occurrence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in ICU. NLR should be included in the routine assessment of AKI occurrence.
Postoperative acute kidney injury (AKI) is one of the most common complications after gastrointestinal and hepatobiliary surgery. Neutrophil-to-lymphocyte ratio (NLR) is a marker of inflammation that can be calculated directly from a patient's complete blood count. Extensive studies have shown that NLR can predict the outcome of cardiac surgery, sepsis, and cancer.
The risk factors and early diagnosis of postoperative AKI have always been urgent problems in clinic.
To clarify the relationship between NLR and the occurrence of AKI in patients with gastrointestinal and hepatobiliary surgery in the intensive care unit (ICU).
This study retrospectively analyzed the electronic medical records of 282 patients after gastrointestinal and hepatobiliary surgery in ICU to clarify the relationship between NLR at admission and the postoperative AKI occurrence.
Postoperative AKI occurred in 29.79% of patients receiving ICU care. NLR value at admission was higher in AKI patients compared with the non-AKI patients and increased with the severity of AKI. Patients with NLR ≥ 8.380 exhibited significantly higher incidences of postoperative AKI and severe AKI than patients with NLR < 8.380. The multivariate analysis showed that NLR at admission was an independent risk factor for occurrence of postoperative AKI in patients with gastrointestinal and hepatobiliary surgery in ICU.
NLR at admission is a predictor of AKI occurrence in patients with gastrointestinal and hepatobiliary surgery in ICU.
NLR should be included in the routine assessment of AKI occurrence.
We are indebted to all individuals who participated in or helped with this research project.
| 1. | Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37:2552-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 312] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Srisawat N, Sileanu FE, Murugan R, Bellomod R, Calzavacca P, Cartin-Ceba R, Cruz D, Finn J, Hoste EE, Kashani K, Ronco C, Webb S, Kellum JA; Acute Kidney Injury-6 Study Group. Variation in risk and mortality of acute kidney injury in critically ill patients: a multicenter study. Am J Nephrol. 2015;41:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1211] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Li Q, Ma T, Liu X, Wang B, Wu Z, Dang S, Lv Y, Wu R. Transfusion of Older Red Blood Cells Increases the Risk of Acute Kidney Injury After Orthotopic Liver Transplantation: A Propensity Score Analysis. Anesth Analg. 2018;127:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Trongtrakul K, Sawawiboon C, Wang AY, Chitsomkasem A, Limphunudom P, Kurathong S, Prommool S, Trakarnvanich T, Srisawat N. Acute kidney injury in critically ill surgical patients: Epidemiology, risk factors and outcomes. Nephrology (Carlton). 2019;24:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1731] [Cited by in RCA: 1664] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 7. | Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 611] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 8. | Jiang L, Zhu Y, Luo X, Wen Y, Du B, Wang M, Zhao Z, Yin Y, Zhu B, Xi X; Beijing Acute Kidney Injury Trial (BAKIT) workgroup. Epidemiology of acute kidney injury in intensive care units in Beijing: the multi-center BAKIT study. BMC Nephrol. 2019;20:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, Hunn BH. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J Am Soc Nephrol. 2017;28:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Bu X, Zhang L, Chen P, Wu X. Relation of neutrophil-to-lymphocyte ratio to acute kidney injury in patients with sepsis and septic shock: A retrospective study. Int Immunopharmacol. 2019;70:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, Postow MA, Coit DG, Ariyan CE. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer. 2020;126:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Green J, Bin Mahmood SU, Mori M, Yousef S, Mangi AA, Geirsson A. Stability across time of the neutrophil-lymphocyte and lymphocyte-neutrophil ratios and associations with outcomes in cardiac surgery patients. J Cardiothorac Surg. 2019;14:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Du Z, Dong J, Bi J, Bai R, Zhang J, Wu Z, Lv Y, Zhang X, Wu R. Predictive value of the preoperative neutrophil-to-lymphocyte ratio for the development of hepatocellular carcinoma in HBV-associated cirrhotic patients after splenectomy. PLoS One. 2018;13:e0195336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 14. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 20116] [Article Influence: 1547.4] [Reference Citation Analysis (9)] |
| 15. | Okusa MD, Davenport A. Reading between the (guide)lines--the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int. 2014;85:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 1308] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 17. | Hultström M, Becirovic-Agic M, Jönsson S. Comparison of acute kidney injury of different etiology reveals in-common mechanisms of tissue damage. Physiol Genomics. 2018;50:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Kim M, Brady JE, Li G. Variations in the risk of acute kidney injury across intraabdominal surgery procedures. Anesth Analg. 2014;119:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | An Y, Shen K, Ye Y. Risk factors for and the prevention of acute kidney injury after abdominal surgery. Surg Today. 2018;48:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative Consensus XIII Work Group. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J Am Soc Nephrol. 2016;27:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 458] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 21. | Gameiro J, Fonseca JA, Neves M, Jorge S, Lopes JA. Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care. 2018;8:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, Liang X, Fu P, Liu ZH, Mehta RL. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin J Am Soc Nephrol. 2015;10:1324-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 536] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 24. | Ni J, Wang H, Li Y, Shu Y, Liu Y. Neutrophil to lymphocyte ratio (NLR) as a prognostic marker for in-hospital mortality of patients with sepsis: A secondary analysis based on a single-center, retrospective, cohort study. Medicine (Baltimore). 2019;98:e18029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Lokhandwala S, Andersen LW, Nair S, Patel P, Cocchi MN, Donnino MW. Absolute lactate value vs relative reduction as a predictor of mortality in severe sepsis and septic shock. J Crit Care. 2017;37:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Bou Chebl R, El Khuri C, Shami A, Rajha E, Faris N, Bachir R, Abou Dagher G. Serum lactate is an independent predictor of hospital mortality in critically ill patients in the emergency department: a retrospective study. Scand J Trauma Resusc Emerg Med. 2017;25:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | O'Connor E, Fraser JF. The interpretation of perioperative lactate abnormalities in patients undergoing cardiac surgery. Anaesth Intensive Care. 2012;40:598-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Pucino V, Bombardieri M, Pitzalis C, Mauro C. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur J Immunol. 2017;47:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tajiri K, Tchilikidi KY S-Editor: Wang J L-Editor: MedE-Ma JY E-Editor: Wang LL