Published online Jun 27, 2020. doi: 10.4240/wjgs.v12.i6.277
Peer-review started: February 29, 2020
First decision: April 7, 2020
Revised: April 13, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: June 27, 2020
Processing time: 110 Days and 18.5 Hours
There is an increased need for accurate staging for gastric cancer treatment. Consequently, it is necessary to carefully examine all dissected lymph nodes for precise staging. Recently, the fat-dissociation method has been developed as a quick and accurate method for harvesting dissected lymph nodes of colorectal cancer cases.
To investigate the usefulness of the fat-dissociation method for harvesting dissected lymph nodes of gastric cancer cases.
Fifty-six resected specimens from gastric cancer patients who underwent standard curative gastrectomy and lymph node dissection at our hospital were used. Group 2 lymph nodes were separated from each specimen, and the remaining adipose tissue containing the group 1 lymph nodes was used. Some resected specimens were subjected to the fat-dissociation method. One vial of Imofully® was dissolved in 50 mL of saline and injected into the tissue. The tissue was incubated for 1 h and the dissolved fat was removed. Subsequently, the nodes were identified, picked up with scissors, and mapped. The number of nodes in each lymphatic compartment and duration of lymph node harvest and mapping were compared.
The fat-dissociation method was used for 24 samples, while the conventional dissection method was used for 32 samples. The total number of harvested lymph nodes was 45.9 in the fat dissociation group and 44.3 in the control group, and there was no significant difference between the two groups. There were also no significant differences in the number of lymph nodes between the two groups based on a comparison of the lymphatic compartments. However, the total median duration of the fat-dissociation method was 38.2 min, reflecting a reduced duration of approximately 60 min compared to the control group.
Based on our results, the fat-dissociation method is effective in shortening the duration of lymph node harvest in gastric cancer surgery.
Core tip: Accurate examination of dissected lymph nodes is important for precise staging in gastric cancer treatment. We investigated the usefulness of the fat-dissociation method for lymph node harvest in gastric cancer. We used the fat-dissociation method on 24 resected specimens from patients with gastric cancer who underwent standard curative gastrectomy and lymph node dissection at our hospital, while the conventional method was used on 32 specimens. There were no differences in the numbers of harvested lymph nodes between these two groups; however, there was a 60 min reduction in the total median duration of the procedure when using the fat-dissociation method.
- Citation: Kinami S, Ohnishi T, Nakamura N, Jiang ZY, Miyata T, Fujita H, Takamura H, Ueda N, Kosaka T. Efficacy of the fat-dissociation method for nodal harvesting in gastric cancer. World J Gastrointest Surg 2020; 12(6): 277-286
- URL: https://www.wjgnet.com/1948-9366/full/v12/i6/277.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i6.277
In cancer surgery, the status of lymph node metastasis affects the prognosis after curative surgery and is an important factor in determining whether adjuvant therapy should be administered. Indeed, the number of lymph node metastases is also indispensable for staging in gastric cancer surgery[1]. In recent years, adjuvant chemotherapy after gastric cancer surgery has been proven to be effective in preventing recurrence[2,3]. Adjuvant chemotherapy in stage III cases has also improved[4]. There is an increased need for accurate staging of gastric cancer; therefore, it is necessary to accurately examine dissected lymph nodes. However, lymph node harvest is not emphasized in gastric cancer surgery[5].
More recently, the fat-dissociation method has been developed as a quick and accurate method for harvesting dissected lymph nodes[6]. This is a method of dissolving the fat of the mesentery with a specific reagent, which results in improved visibility of the lymph nodes and facilitates their harvest. The usefulness of the fat-dissociation method has been reported for colorectal cancer[6-8]; however, it has not yet been studied for gastric cancer.
In this study, we proceeded to harvest and detect lymph nodes in gastric cancer after fat dissociation and examined the usefulness of this method.
We started this trial after obtaining a commercial fat lysate reagent product in June 2016, at which time we began to prospectively recruit gastric cancer patients from our hospital who were undergoing standard radical gastrectomy with lymph node dissection for gastric cancer. The exclusion criteria included cases in which function preservation limited surgery or extended radical gastrectomy.
The treatment policy for these cases was in accordance with the Gastric Cancer Treatment Guidelines of the Japanese Gastric Cancer Association[9], and the descriptions of the findings comply with the Japanese Classification of Gastric Carcinoma[10]. All surgeries were performed by Kinami S or Kosaka T, who both had practiced gastric cancer surgery for over 25 years. Kinami S and Ohnishi T were responsible for performing the lymph node harvest, which was initiated within 3 h of gastric cancer surgery. Kinami S has performed lymph node harvests for over 25 years. Ohnishi T practices the same harvest technique as Kinami S and had performed more than 100 harvests before the start of the study.
The resected specimens were allocated to one of two groups. The fat-dissociation group was designated as group F, in which lymph nodes were detected using the fat-dissociation method. Although no case selection criteria were initially established, the specimens of group F were particularly high in visceral fat. The other group was designated group C for conventional harvest, in which specimens were harvested using the conventional method. Sample collection was completed in December 2018.
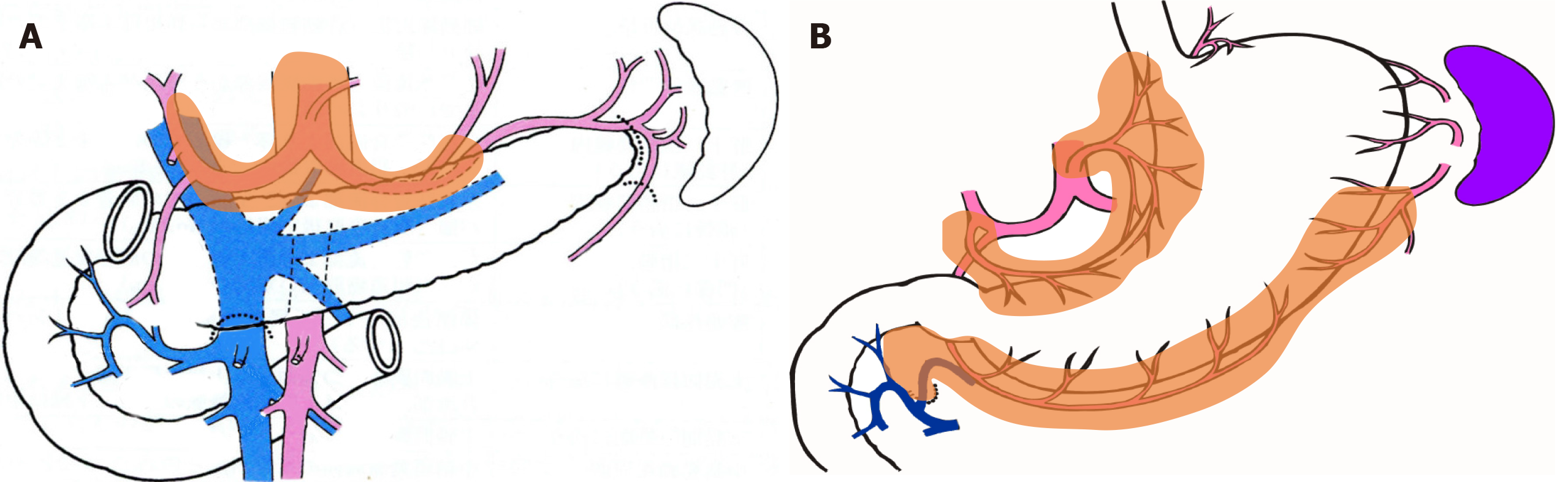
In both groups, the group 2 lymph nodes (i.e., lymph nodes Nos. 8a, 9, 10, 11p, 11d, and 12a) and easily identifiable group 1 lymph nodes (i.e., nodes Nos. 2 and 4sa) were separated with scissors, numbered, and submitted to pathology as per the usual procedure. The remaining adipose tissue containing group 1 lymph nodes was used in this study (Figure 1).
Typically, lymph node harvest is performed as follows: Initially, adipose tissue including the lymph nodes is spread over a cork plate with pins, while remaining attached to the stomach wall. The capsule surrounding the tissue is then peeled off using scissors. Next, the fat tissue is scraped off with scissors and blood vessels, nerves, and lymph nodes remain. Lymph nodes can be distinguished from adipose tissue using color and morphology. If it is difficult to identify lymph nodes with the naked eye, they can be identified by palpating the tissue. In this study, each lymph node was picked up and mapped one by one.
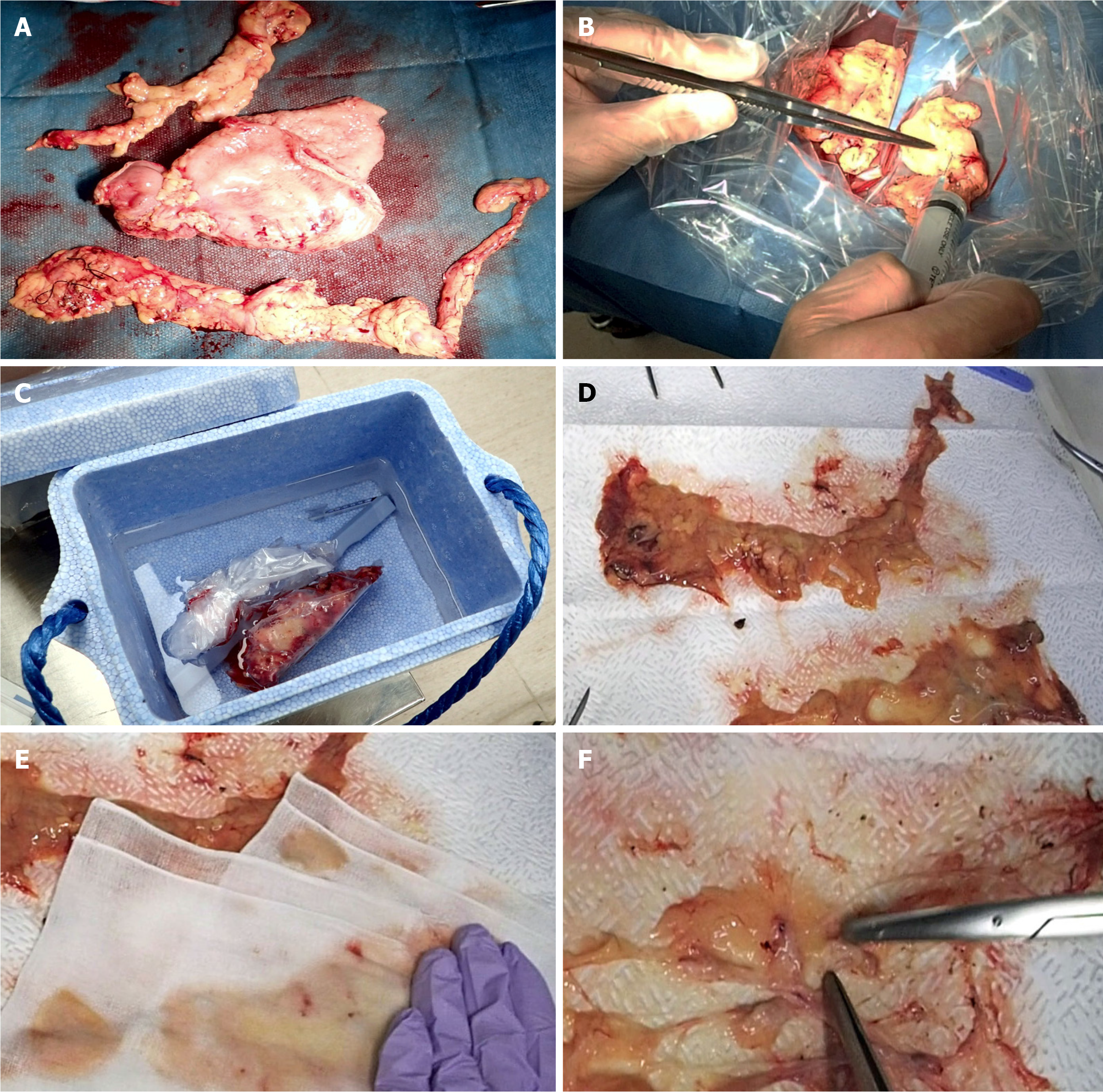
The fat-dissociation method was performed as follows (Figure 2): First, adipose tissue with lymph nodes was detached from the stomach wall with scissors. The adipose tissue was detached en bloc, taking care not to leave any fat on the stomach wall and to avoid cutting into the tissue. A commercially available reagent (Imofully®; Sysmex Corporation, Kobe, Japan) was used as the fat-dissolving solution. One vial of Imofully® was dissolved in 50 mL of saline, and the lysate was injected into the tissue using a syringe equipped with a 22 G injection needle. The tissue was placed into a nylon bag and rubbed manually from the outside. Then, the nylon bag was soaked in hot water at 42 °C and incubated for 1 h. After incubation, the tissue was carefully spread on a water-absorbing paper (Kim Towel®; Nippon Paper Crecia, Tokyo, Japan). Using a surgical scalpel, several incisions were made in the capsule surrounding the adipose tissue. At this time, a surgeon carefully cut the capsule, avoiding the blood vessels and lymph nodes. A water-absorbing paper or gauze was applied, and pressure was exerted from above to remove dissolved fat from the water-absorbing paper. After the fat droplets had been sufficiently absorbed, the paper was carefully peeled off, leaving the blood vessels, lymph nodes, and a small amount of fat. The lymph nodes were identified, picked up with scissors, and mapped.
All harvested lymph nodes were submitted for pathology analysis. These nodes were fixed in 10% buffered formalin, embedded in paraffin, sectioned at the maximum plane, and subjected to H&E staining. Lymph node identification was confirmed using a microscope.
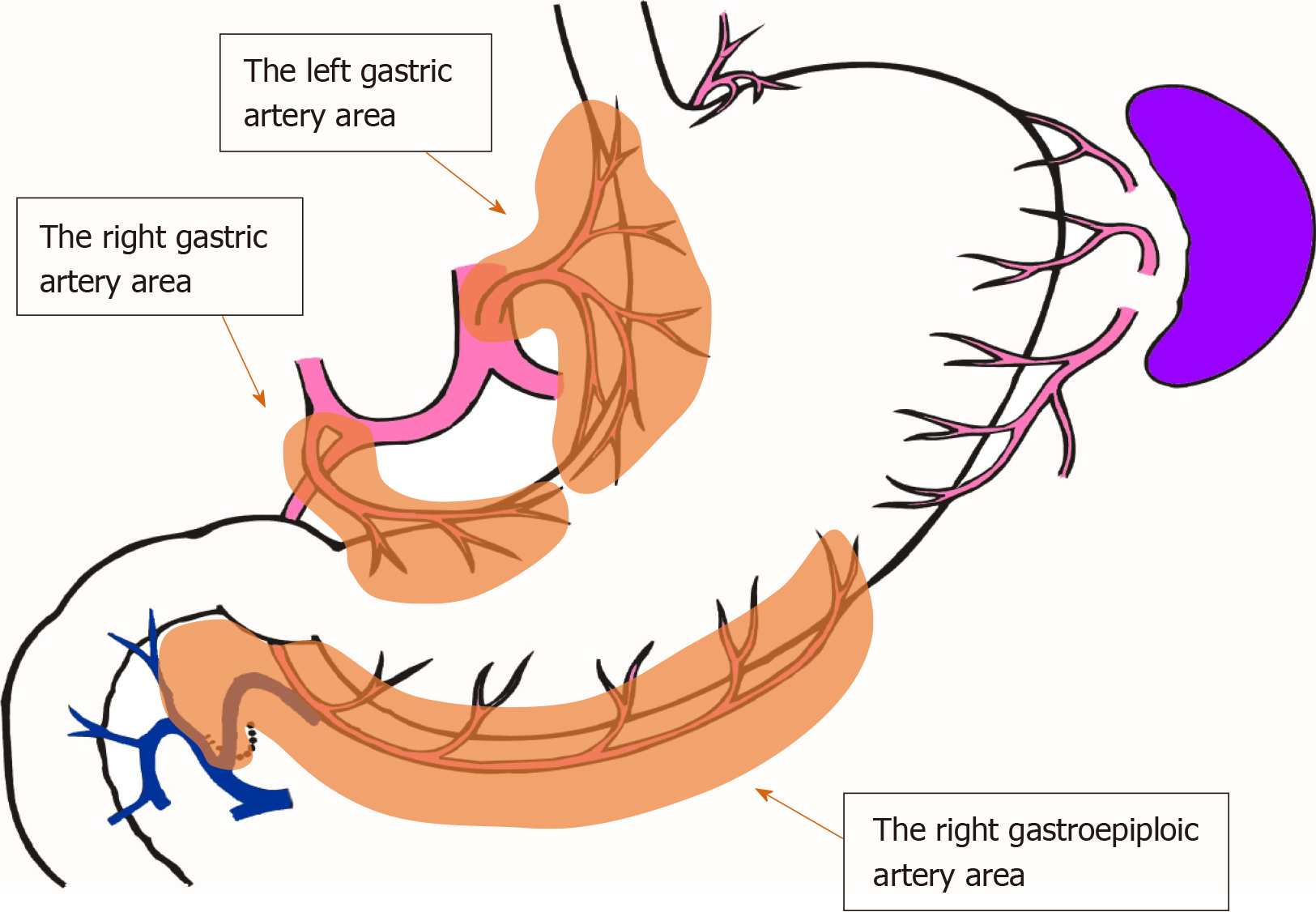
The number of lymph nodes and the time required for mapping were compared between groups F and C. Since the number of lymph nodes differs depending on the site of dissection, lymph nodes were grouped along the lymphatic compartment[11] (shown in Figure 3), and the numbers of lymph nodes belonging to each compartment of the left gastric artery area, right gastric artery area, and right gastroepiploic artery area were compared. The time required for mapping was determined based on the time needed for harvesting and mapping all lymph nodes, including group 2 nodes. For group F, the time required for incubation and fat removal was subtracted.
This study was approved by the ethics committee of Kanazawa Medical University and was conducted in accordance with the Good Clinical Practice guidelines and Declaration of Helsinki. All patients provided written informed consent for surgery and use of their data. Regarding data use for lymph node mapping, patients were given the opportunity to opt out of the study at any time.
The chi-square test was used to compare background factors. The Student t-test or Welch's test was used to compare the number of harvested lymph nodes and the time required for lymph node mapping. P values < 0.05 were considered significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface of R (The R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of R Commander designed to add statistical functions frequently used in biostatistics[12].
The number of resected specimens was 24 for group F and 32 for group C. Table 1 shows the background factors between the two groups. There were no statistically significant differences between the two groups.
| Group F | Group C | P value | |
| n | 24 | 32 | |
| Age, median (range) | 69 (51-82) | 66 (39-90) | NS |
| Sex, male/female | 18 /6 | 25/7 | NS |
| BMI, median (range) | 23.2 (15.9-28.7) | 21.9 (14.6-27.7) | NS |
| Location, U/M/L | 2/12/10 | 5/12/15 | NS |
| Tumor size, mm (mean ± SD) | 50.9 ± 31.3 | 54.6 ± 26.1 | NS |
| McT, 0/1/2/3/4/5 | 12/0/3/7/1/1 | 14/1/7/4/3/3 | NS |
| sT factor, 1/2/3/4 | 8/1/3/12 | 12/2/8/10 | NS |
| sN factor, 0/1/2/3 | 9/4/6/5 | 20/7/3/2 | NS |
| Approach, open/laparoscopic | 17/7 | 27/5 | NS |
| Surgery, DG/TG | 22/2 | 26/6 | NS |
| D number, 1+/2 | 14/10 | 19/13 | NS |
| pN factor. 0/1/2/3 | 8/5/2/9 | 16/5/7/4 | NS |
Table 2 shows the number of harvested lymph nodes. There was no significant difference in the total number of harvested lymph nodes. However, the fat-dissociation method was applied only to group 1 nodes. Table 2 shows the number of nodes in each lymphatic compartment in order to correctly compare the lymph node harvest performances. The left gastric artery area contained the nodes of station numbers 1, 3a, and 7; the right gastric artery area contained numbers 3b and 5; and the right gastroepiploic artery area contained numbers 4d and 6. There were no statistically significant differences in the numbers of lymph nodes between the two groups based on a comparison of the lymphatic compartments.
| Group F | Group C | P value | |
| Total number of harvested nodes | 45.9 (21-82) | 44.3 (20-89) | NS |
| Number of the left gastric artery, area nodes (No. 1, 3a, 7) | 16.9 (8-29) | 14.1 (7-26) | NS |
| Number of the right gastric artery, area nodes (No. 3b, 5) | 2.2 (0-9) | 2.8 (0-10) | NS |
| Number of the right gastroepiploic, artery area nodes (No. 4d, 6) | 16.9 (7-27) | 16.8 (8-39) | NS |
| Time for harvesting and mapping (min) | 38.2 (25-55) | 101.1 (50-160) | < 0.0001 |
In addition, the duration of lymph node harvesting and mapping was compared between the two groups. Since the duration of lymph node harvest cannot be measured only in the case of group 1 nodes, the total duration of the harvest was compared between the two groups. In group F, the mean duration was 38.2 min, which was about 60 min shorter than group C of 101.1 min, and there was a significant difference (P = 4.8158 × 10-8). The duration of lymph node harvest in group 2 was generally short, and the procedure was the same between the two groups. The difference between the two groups may have been due to the fat-dissociation method performed when harvesting the group 1 nodes.
We concluded from this study that the usefulness of the fat-dissociation method in gastric cancer surgery was to shorten the duration of lymph node harvest. This is the first report to examine the usefulness of the fat-dissociation method in gastric cancer surgery.
In the traditional Japanese classification of gastric carcinoma of the 20th century, the N-stage of gastric cancer was determined by the location of the lymph node metastasis[13]. In this era, staging is possible if the presence or absence of metastasis of the most distant lymph nodes can be determined; therefore, it is not necessary to harvest all dissected lymph nodes. However, by this method, accurate N-number cannot be determined without knowing the lymph node grouping and performing an adequate range of lymph node dissection. Alternatively, it has been reported that the prognosis of gastric cancer is well correlated with the number of lymph node metastases[14-16], and the N-number was altered to reflect the number of lymph node metastases[10,17]. While this was useful in generalizing staging, it was changed so that all dissected lymph nodes had to be sent to pathology for staging. With evidence of the efficacy of adjuvant chemotherapy after gastric cancer surgery to prevent recurrence[2-4], the need to submit all dissected lymph nodes to pathology without leaking increases.
How many regional lymph nodes should be investigated in gastric cancer surgery? The number of lymph nodes increases when examined at a specialized cancer center[18]. In the cancer staging manual of the American Joint Committee on Cancer, recommendations have been made to investigate 15 or more lymph nodes[17]. As a result, in general clinical practice, lymph nodes are often harvested using an investigation of approximately 15 nodes as a guide[18,19]. However, the actual number of lymph nodes is indeed much higher. In recent reports, it appears that overall, more than 35 lymph nodes have been identified in distal gastrectomy D2[20,21]. In gastric cancer, the regional lymph nodes are often small, and lymph node metastasis of gastric cancer may also occur in small lymph nodes[22]. Half of lymph node metastases in gastric cancer are microscopic peripheral-type metastases[23,24]. There are many reports that an increased number of harvested lymph nodes is associated with a better prognosis[19,25-27]. This is thought to reflect increased accuracy due to the increased number of nodes being examined. As previously stated, to accurately determine the N-number and properly apply adjuvant chemotherapy to improve the prognosis of gastric cancer, any small lymph node must be harvested and submitted for pathological investigation.
Few studies have detailed the methods of lymph node harvest in gastric cancer. The palpation method, in which palpation is used to distinguish lymph nodes, is probably the most practiced method worldwide. However, 2-3 mm lymph nodes were not identified on palpation. Worldwide, lymph node harvest is likely performed by a pathologist. The palpitation method can be performed by a pathologist, but it is believed that a surgeon will harvest a greater number of nodes[28,29]. A packet submission method has been proposed as an improved method to secure the number of harvested lymph nodes, even if the harvest is performed by a pathologist[5,29,30]. In addition, a fat cleaning method has been proposed[31], but it is somewhat complicated to perform. The method used in this study is a traditional method used by surgeons at Kanazawa University and is called “the fat removal method”. Although this method is technically difficult, it can detect lymph nodes smaller than approximately 1 mm. In Japan, lymph node harvest is often performed by young surgeons. The reason for this is that, like martial arts, this technique was assigned as scissors training. However, this procedure is technically demanding and time consuming, and therefore can be burdensome for non-surgeons and unacceptable for young surgeons due to the procedure time duration.
Lymph node harvest is important in colorectal cancer as well as in gastric cancer. Lymph node harvest for colorectal cancer is also often performed using the palpitation method, but the variation in accuracy seems to be larger than that for gastric cancer. To overcome this problem, the fat-dissociation method was developed by Fujino et al[6]. The fat-dissociation method requires incubation, but it increases the accuracy of lymph node harvest and does not require fixing or staining the sample. Fujieda et al[8] reported that by this method, small lymph nodes could be detected and there was an increased number of harvested nodes compared to the conventional method.
However, lymph node harvest in gastric cancer presents some differences compared to that in colorectal cancer. For example, the distribution of lymph nodes in colon cancer is flat and fan shaped. Lymph node harvest in rectal cancer is also easier if the regional lymph nodes are flattened by removing the mesentery from the rectal wall. Alternatively, the lymphatic system involved in gastric cancer is somewhat complex, since it is three-dimensional and multi-directional compared to colorectal cancer. In particular, the group 2 nodes are located near the arteries and pancreas, and it is difficult to determine the orientation of the nodes after surgery. On the other hand, since the thickness of adipose tissue around the stomach is generally thinner than that of the colorectal mesentery, identification of nodes is easier than in colorectal cancer.
We applied the fat-dissociation method only to the group 1 nodes. The location of the group 2 nodes is difficult to determine, but as the fat is small and easy to sort, there is no need to use the fat-dissociation method for this group of nodes. In addition, the harvest is quicker and more accurate when performed by the surgeon in the operating room immediately after surgery than when performed by a pathologist or novice surgeon.
In our study, the usefulness of the fat-dissociation method in gastric cancer was to reduce the duration of lymph node harvest. The fat-dissociation method is also considered useful in reducing the difficulty of harvest as well as improving accuracy and eliminating variations in the technique, although it is difficult to quantify objectively. In this study, there was no difference in the number of harvested nodes between the two groups, presumably because of the surgical skill of Kinami S and Ohnishi T in the lymph node harvest procedure. The fat-dissociation method does not affect the pathological diagnosis[6-8]. In addition, the identification and grouping of lymph nodes are easier and more accurate than the conventional method because of the ease of identifying the relationship between the lymph nodes and blood vessels. We believe that if a novice is responsible for lymph node harvest, the fat-dissociation method will increase the lymph node count and accuracy of the procedure.
The disadvantages of the fat-dissociation method include the drug cost and the incubation time. The process of removing the dissolved fat also requires time, but this is only about 5 min. Conversely, a 60 min incubation period is also required. However, we do not view this as a drawback. The physician’s workload is only nodal harvesting; incubation does not contribute to the working hours. We performed postoperative patient briefing, bedside management, and specimen fixation during the incubation period.
There were some limitations to this study. The number of cases used in this study was insufficient. The prognosis was not yet known. The skilled surgeons performed the lymph node harvest. Additionally, this study was a retrospective study of prospectively recruited patients, rather than a randomized controlled trial. Given the cost of the reagents, the fat-dissociation method was only applied to difficult cases that presented increased visceral fat. However, it is still unclear whether a randomized trial is still needed, since the usefulness of this method is apparent from the results of this study. Another problem is that the commercial lipolysis reagent “Imofully®” is currently difficult to obtain. However, we refer readers to the first report[6] for details on creating this reagent.
In conclusion, in gastric cancer surgery, the fat-dissociation method is effective in reducing the duration of the lymph node harvest. Although difficult to quantify, the fat-dissociation method may reduce the labor and difficulty of the harvest procedure. However, if the surgeon is sufficiently skilled, the fat-dissociation method may not result in improved accuracy. Therefore, the application of the fat-dissociation method is not considered an absolute necessity in the context of lymph node harvest. It is thought that the adoption of this method should occur based on the skills and workload of the surgeons performing the harvest procedure, as well as the cost of the reagents required to perform the fat-dissociation method.
In gastric cancer surgery, the status of lymph node metastasis affects the prognosis after curative surgery and is an important factor in determining whether adjuvant therapy should be administered. Therefore, it is necessary to accurately examine dissected lymph nodes. However, lymph node harvest is not emphasized in gastric cancer surgery.
Recently, the fat-dissociation method has been developed as a quick and accurate method for harvesting dissected lymph nodes. This is a method of dissolving the fat of the mesentery with a specific reagent, which results in improved visibility of the lymph nodes and facilitates their harvest.
This study aimed to investigate the usefulness of the fat-dissociation method for harvesting dissected lymph nodes of gastric cancer cases.
Fifty-six resected specimens from gastric cancer patients who underwent standard curative gastrectomy and lymph node dissection were used. Group 2 lymph nodes were separated from each specimen, and the remaining adipose tissue containing the group 1 lymph nodes was used. Some resected specimens were subjected to the fat-dissociation method. One vial of Imofully® was dissolved in 50 mL saline and injected into the tissue. The tissue was incubated, and the dissolved fat was removed. The number of nodes in each lymphatic compartment and duration of lymph node harvest were compared.
The fat-dissociation method was used for 24 samples, while the conventional dissection method was used for 32 samples. The total number of harvested lymph nodes was 45.9 in the fat dissociation group and 44.3 in the control group, and there was no significant difference between the two groups. There were also no significant differences in the numbers of lymph nodes between the two groups based on a comparison of the lymphatic compartments. However, the total median duration of the fat-dissociation method was 38.2 min, reflecting a reduced duration of approximately 60 min compared to the control group.
The fat-dissociation method is effective in shortening the duration of lymph node harvest in gastric cancer surgery. Although difficult to quantify, the fat-dissociation method may reduce the labor and difficulty of the harvest procedure. However, if the surgeon is sufficiently skilled, the fat-dissociation method may not result in improved accuracy.
The adoption of the fat-dissociation method should occur based on the skills and workload of the surgeons performing the harvest procedure, as well as the cost of the reagents required to perform the fat-dissociation method.
| 1. | Deng JY, Liang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20:3967-3975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 142] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 2. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 3. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 798] [Article Influence: 66.5] [Reference Citation Analysis (1)] |
| 4. | Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, Kaji M, Okitsu H, Nomura T, Matsui T, Yoshikawa T, Matsuyama J, Yamada M, Ito S, Takeuchi M, Fujii M. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol. 2019;37:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 5. | Ikoma N, Estrella JS, Hofstetter WL, Ajani JA, Fournier KF, Mansfield PF, Skibber JM, Badgwell BD. Surgeon Assessment of Gastric Cancer Lymph Node Specimens with a Video of Technique. J Gastrointest Surg. 2018;22:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Fujino S, Miyoshi N, Ohue M, Noura S, Tomita Y, Yano M, Sakon M. New enhanced and effective method for staging cancer to detect lymph nodes after fat-dissociation. Oncol Rep. 2014;32:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Maeda H, Okamoto K, Oba K, Shiga M, Fujieda Y, Namikawa T, Hiroi M, Murakami I, Hanazaki K, Kobayashi M. Lymph node retrieval after dissolution of surrounding adipose tissue for pathological examination of colorectal cancer. Oncol Lett. 2018;15:2495-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Fujieda Y, Maeda H, Oba K, Okamoto K, Fukudome I, Shiga M, Kawanishi Y, Akimori T, Kuroiwa H, Nishimoto H, Namikawa T, Murakami I, Kobayashi M, Hanazaki K. Lymph node retrieval after colorectal cancer surgery: a comparative study of the efficacy between the conventional manual method and a new fat dissolution method. Surg Today. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018. 5th ed. Tokyo, Kanehara-shuppan, 2018. |
| 10. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma 2017. 15th ed. Tokyo, Kanehara-shuppan, 2018. |
| 11. | Kinami S, Fujimura T, Ojima E, Fushida S, Ojima T, Funaki H, Fujita H, Takamura H, Ninomiya I, Nishimura G, Kayahara M, Ohta T, Yoh Z. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol. 2008;13:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 14448] [Article Influence: 1111.4] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 964] [Reference Citation Analysis (0)] |
| 14. | Adachi Y, Kamakura T, Mori M, Baba H, Maehara Y, Sugimachi K. Prognostic significance of the number of positive lymph nodes in gastric carcinoma. Br J Surg. 1994;81:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. The number of metastatic lymph nodes: a promising prognostic determinant for gastric carcinoma in the latest edition of the TNM classification. J Am Coll Surg. 1998;187:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Hayashi H, Ochiai T, Suzuki T, Shimada H, Hori S, Takeda A, Miyazawa Y. Superiority of a new UICC-TNM staging system for gastric carcinoma. Surgery. 2000;127:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual 8th edition. New York, Springer: Springer International Publishing, 2017. |
| 18. | Morgan JW, Ji L, Friedman G, Senthil M, Dyke C, Lum SS. The role of the cancer center when using lymph node count as a quality measure for gastric cancer surgery. JAMA Surg. 2015;150:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 486] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 20. | Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg. 2015;39:2734-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 253] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 21. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 548] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 22. | Noda N, Sasako M, Yamaguchi N, Nakanishi Y. Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg. 1998;85:831-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Fujimura T, Yonemura Y, Taniguchi K, Bando E, Miyashita T, Fujita H Michiwa Y, Fushida S, Nishimura G, Miwa K, Miyazaki I. Preoperative diagnosis by computed tomography for lymph node metastasis in gastric cancer (Japanese). Hokuriku Geka Gakkaishi. 1997;16:23-26. |
| 24. | Yanagita S, Natsugoe S, Uenosono Y, Arima H, Kozono T, Ehi K, Arigami T, Higashi H, Aikou T. Morphological distribution of metastatic foci in sentinel lymph nodes with gastric cancer. Ann Surg Oncol. 2008;15:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Macalindong SS, Kim KH, Nam BH, Ryu KW, Kubo N, Kim JY, Eom BW, Yoon HM, Kook MC, Choi IJ, Kim YW. Effect of total number of harvested lymph nodes on survival outcomes after curative resection for gastric adenocarcinoma: findings from an eastern high-volume gastric cancer center. BMC Cancer. 2018;18:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Deng J, Yamashita H, Seto Y, Liang H. Increasing the Number of Examined Lymph Nodes is a Prerequisite for Improvement in the Accurate Evaluation of Overall Survival of Node-Negative Gastric Cancer Patients. Ann Surg Oncol. 2017;24:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Huang CM, Zhou ZW. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 2017;24:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Bunt AM, Hermans J, van de Velde CJ, Sasako M, Hoefsloot FA, Fleuren G, Bruijn JA. Lymph node retrieval in a randomized trial on western-type versus Japanese-type surgery in gastric cancer. J Clin Oncol. 1996;14:2289-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Cao Y, Xiong L, Deng S, Shen L, Li J, Wu K, Wang J, Tao K, Wang G, Cai K. The effect of perigastric lipolymphatic tissue grouping by surgeon on the number of pathologic sampled lymph nodes after radical gastrectomy. Medicine (Baltimore). 2018;97:e11411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Wang P, Zhang K, Xi H, Liang W, Xie T, Gao Y, Wei B, Chen L. Lymph Node Yield Following Packet Submission After Isolation By Surgeon During Gastrectomy. Cancer Manag Res. 2019;11:9871-9881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Candela FC, Urmacher C, Brennan MF. Comparison of the conventional method of lymph node staging with a comprehensive fat-clearing method for gastric adenocarcinoma. Cancer. 1990;66:1828-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen JQ, Matowicka-Karna J S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wu YXJ