Published online Apr 27, 2019. doi: 10.4240/wjgs.v11.i4.237
Peer-review started: March 4, 2019
First decision: March 19, 2019
Revised: March 24, 2019
Accepted: April 9, 2019
Article in press: April 9, 2019
Published online: April 27, 2019
Processing time: 56 Days and 5.7 Hours
Pancreatitis with infected necrosis is a severe complication of acute pancreatitis and carries with it high rates of morbidity and mortality. The management of infected pancreatic necrosis alongside concomitant colorectal cancer has never been described in literature.
A 77 years old gentleman presented to the Emergency Department of our hospital complaining of ongoing abdominal pain for 8 h. The patient had clinical features of pancreatitis with a raised lipase of 3810 U/L, A computed tomography (CT) abdomen confirmed pancreatitis with extensive peri-pancreatic edema. During the course of his admission, the patient had persistent high fevers and delirium thought secondary to infected necrosis, prompting the commencement of broad-spectrum antibiotic therapy with Piperacillin/Tazobactam. Subsequent CT abdomen confirmed extensive pancreatic necrosis (over 70%). Patient was managed with supportive therapy, nutritional support and gut rest initially and improved over the course of his admission and was discharged 42 d post admission. He represented 24 d following his discharge with fever and chills and a repeat CT abdomen scan noted gas bubbles within the necrotic pancreatic tissue thereby confirming infected necrotic pancreatitis. This CT scan also revealed asymmetric thickening of the rectal wall suspicious for malignancy. A rectal cancer was confirmed on flexible sigmoidoscopy. The patient underwent two endoscopic necrosectomies and was treated with intravenous antibiotics and was discharged after 28 d. Within 1 wk post discharge, the patient commenced a course of neoadjuvant radiotherapy and subsequently underwent concomitant chemotherapy prior to undergoing a successful Hartmann’s procedure for treatment of his colorectal cancer.
This case highlights the efficacy of endoscopic necrosectomy, early enteral feeding and targeted antibiotic therapy for timely management of infected necrotic pancreatitis. The prompt resolution of pancreatitis permitted the patient to undergo neoadjuvant treatment and resection for his concomitant colorectal cancer.
Core tip: Early identification of infected pancreatic necrosis is critical to minimizing the associated high morbidity and mortality of this disease. A high index of clinical suspicion combined with radiological evidence will guide the discerning clinician towards instituting targeted antibiotic therapy and enteral feeding in a timely manner. As soon as it is clinically feasible, minimally invasive necrosectomy should be considered to achieve definitive source control. Efficient resolution of infection is especially important for patients who require urgent treatment for other life-threatening conditions, such as colorectal cancer.
- Citation: Choi K, Flynn DE, Karunairajah A, Hughes A, Bhasin A, Devereaux B, Chandrasegaram MD. Management of infected pancreatic necrosis in the setting of concomitant rectal cancer: A case report and review of literature. World J Gastrointest Surg 2019; 11(4): 237-246
- URL: https://www.wjgnet.com/1948-9366/full/v11/i4/237.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i4.237
Acute pancreatitis is a common and potentially deadly disease. Up to one in five patients diagnosed with acute pancreatitis will develop infected necrosis of the pancreatic parenchyma[1]. Infection of the necrotic area may lead to sepsis and multi-organ failure and is associated with a high mortality[2,3]. Early enteral feeding, targeted antibiotics and drainage of the necrotic area have been shown to improve outcomes. Colorectal cancer is similarly a common and potentially deadly disease that requires prompt intervention. There have been no published reports of these two conditions being managed concomitantly. Concurrent management of both conditions requires timely stabilization and treatment of the necrotizing pancreatitis prior to commencing treatment of colorectal cancer. We present herein, the first case of a patient with simultaneous infected necrotizing pancreatitis and colorectal cancer.
A 77 years old male presented to the emergency department complaining of lower abdominal pain with associated nausea and vomiting.
The patient described sudden onset severe, cramping lower abdominal pain that continued for 8 h prior to presentation. The patient also experienced several bouts of nausea and vomiting since onset of the pain.
The patient previously had a L3-5 laminectomy and a right total knee replacement. He was a non-smoker with a history of occasional social alcohol intake, but reported abstaining from alcohol for the preceding 6 mo. He also reported no previous episodes of pancreatitis and no contributory family history.
The patient was taking Oxycodone/Naloxone (5/2.5 mg) twice daily for lower back pain, prior to presentation.
On examination the patient had a heart rate of 65 beats per minute and a blood pressure of 175/78. His respiratory rate was 16 with saturations of 99% on room air. The patient was afebrile at 37.6 °C. The patient’s abdomen was mildly distended and diffusely tender across the lower abdomen.
Blood analysis showed an elevated lipase of 3810 U/L (< 60) and derangement of liver function tests. Bilirubin was 56 mmol/L (< 20 mmol/L), ALP 128 U/L (30-110 U/L), GGT 326 U/L (< 55 U/L), ALT 341 U/L (< 45 U/L) and AST 412 U/L (< 35 U/L). Hemoglobin was 139 g/L (120–180 g/L) and white blood count was raised at 16.7 × 109 /L (3.5-11.0 × 109/L). The patient’s serum C-reactive protein (CRP) was 6.2 (< 5).
The initial computed tomography (CT) abdomen scan showed extensive peri-pancreatic edema involving the neck and body of the pancreas. An abdominal ultrasound showed multiple mobile calculi within the gallbladder, measuring up to 7 mm. The common bile duct was dilated to a diameter of 9.6 mm however a calculus was unable to be visualized.
Throughout the first week of admission, the patient continued to have severe abdominal pain, fevers up to 39.4 oC and periods of delirium. Broad spectrum antibiotic therapy, i.e., IV Piperacillin/Tazobactam 4.5 g tds, was continued for the first 5 d. During this time the white cell count continued to rise and peaked at 20.4 × 109/L on day 4. Numerous blood cultures were also acquired during this period, but all samples demonstrated no growth.
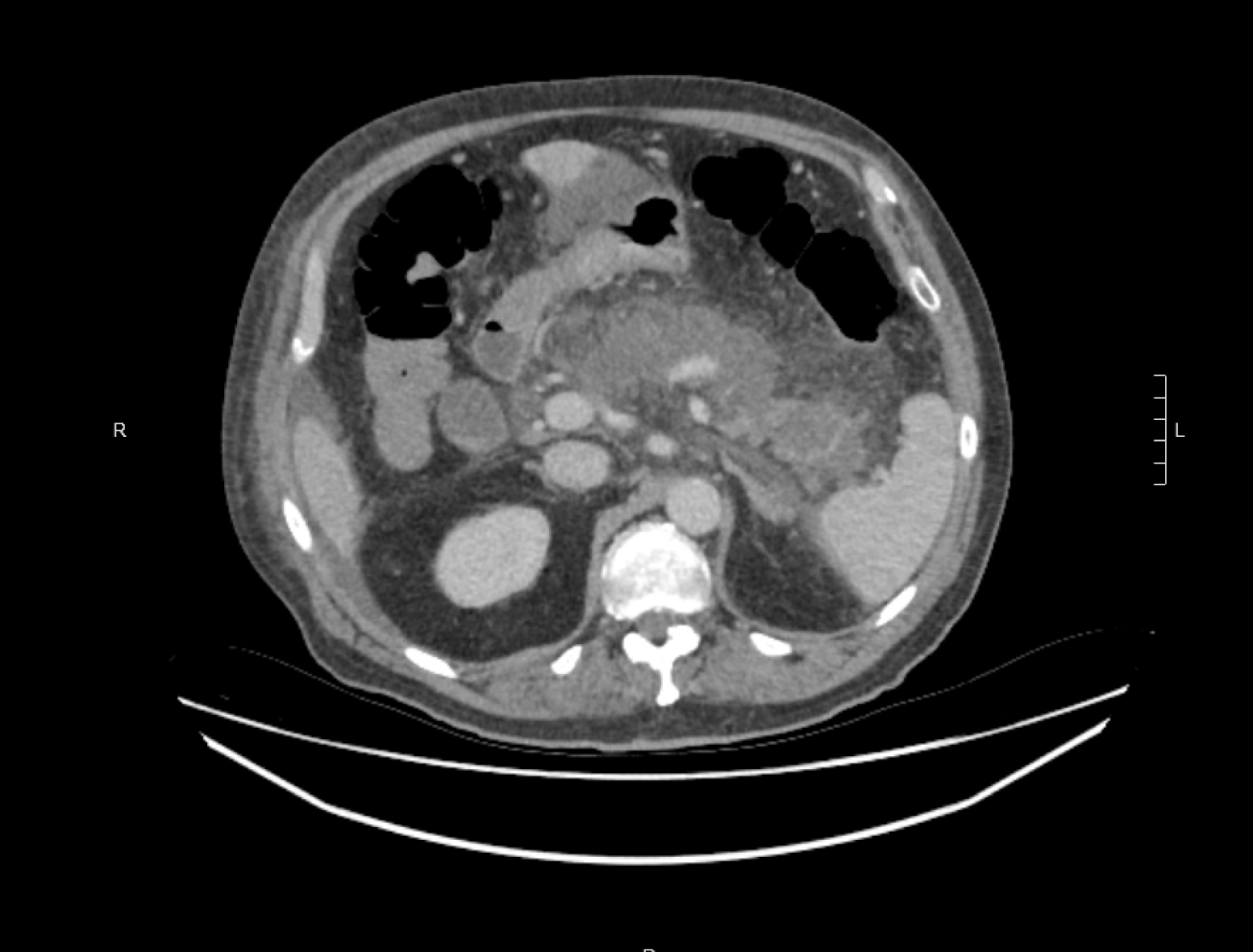
A CT abdomen scan on day 5 showed extensive necrosis involving over 70% of the pancreas (Figure 1). This significant degree of necrosis combined with worsening inflammatory markers and continuing fevers with delirium prompted suspicion for infected necrotic pancreatitis. Consequently, the antibiotic regimen was changed from IV Piperacillin/Tazobactam 4.5 g tds to IV Meropenem 1 g tds and IV Fluconazole 400 mg daily, on day 5 of admission.
Enteral feeding was also commenced via nasogastric tube on day 5, but had to be discontinued on day 6 due to development of ileus. Enteral feeds were reinstated on day 11 and continued until discharge without further complication.
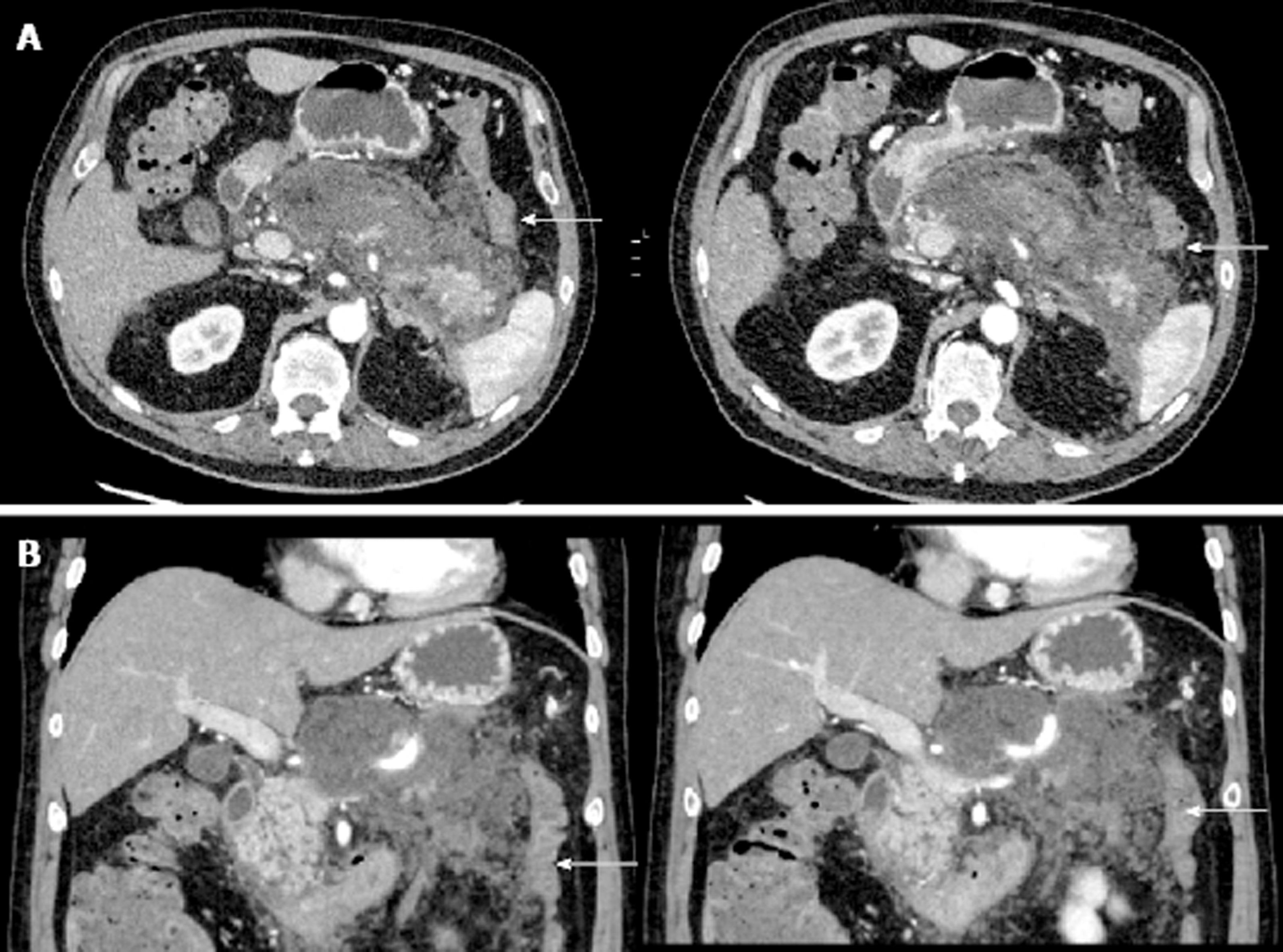
On day 10 of admission, the patient experienced one episode of small volume, painless bright red rectal bleeding. There was no palpable rectal mass on digital rectal examination. At this point in time, the rectal bleeding was presumed to be secondary to splenic flexure colitis or inflammatory changes in the mesocolon which were evident on CT abdomen scan (Figure 2).
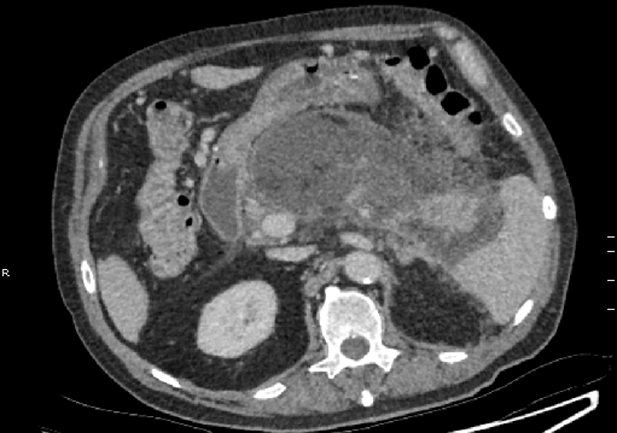
On day 19, antibiotic therapy was ceased due to clinical and biochemical improvement. However, 4 d later the patient developed worsening abdominal pain and fevers. The patient was re-started on IV Meropenem and IV Fluconazole. A progress CT abdomen scan was suggestive of infected necrosis around the pancreatic body as evidenced by an interval increase in size and peripheral enhancement, despite the absence of gas (Figure 3).
The antibiotic regime was continued for a further 17 d. During this time, the patient’s delirium resolved, he tolerated all enteral feeds, and he progressed to independent mobilisation. After maintaining a stable clinical status for 2 d post cessation of antibiotic therapy, the patient was discharged home on day 42. On discharge the patient’s serum CRP was 8.2. He was due to be followed up in clinic 4-6 wk following his discharge in the outpatient department. Our plan was to allow the pancreatic necrosis sufficient time to mature and wall off adequately, in order to facilitate drainage either endoscopically or surgically.
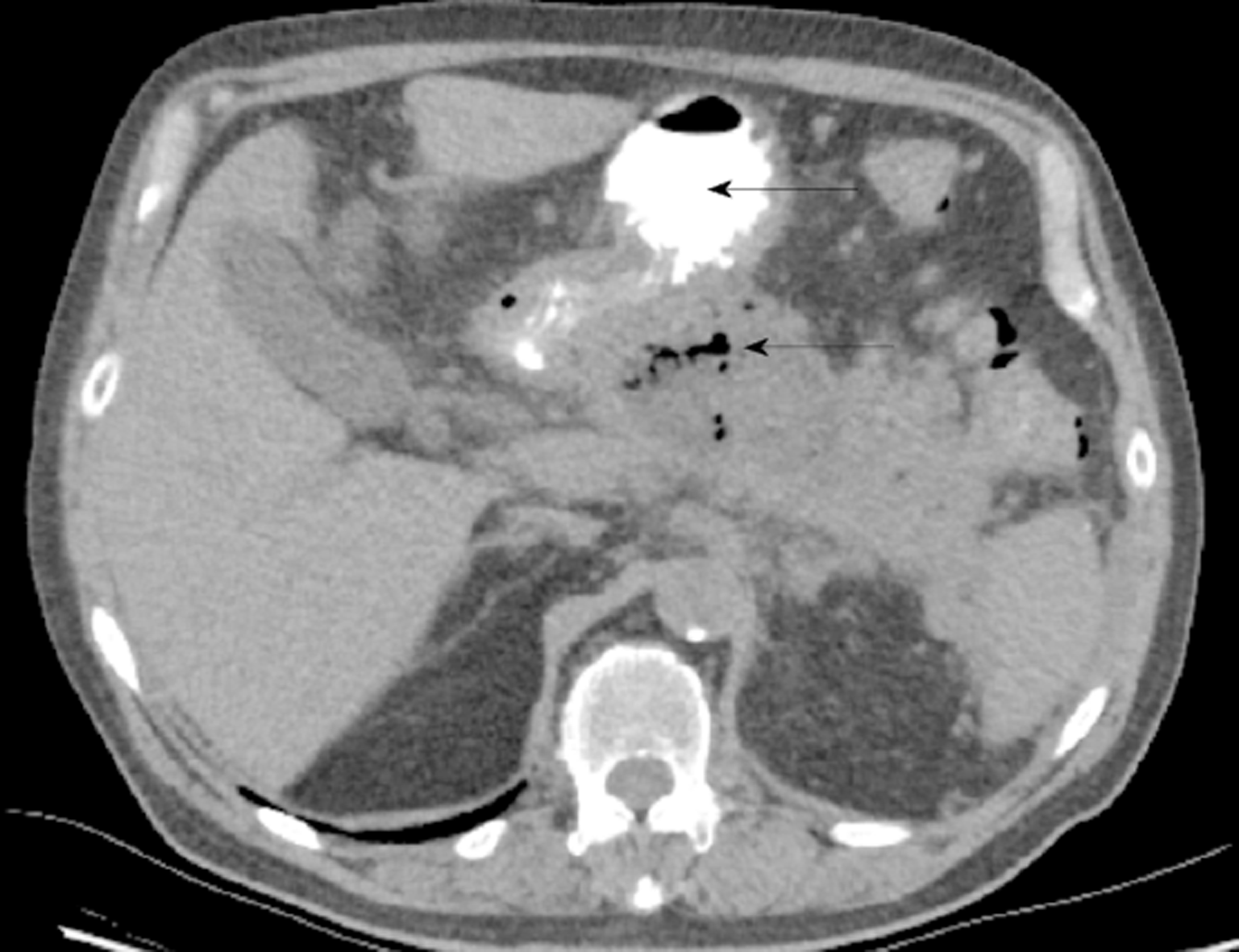
Unfortunately, 22 d following his discharge the patient represented to the Emergency Department with fever and rigors before he was seen in the outpatient department. He was hemodynamically stable, and his abdomen was non-tender, however the serum CRP had increased to 142 mg/L and white cell count was 14.6 × 109/L. Repeat CT abdomen revealed infected necrosis with the presence of gas locules within a large walled off cavity (Figure 4). Subsequently, the patient was admitted and commenced on IV Piperacillin/Tazobactam 4.5 g tds.
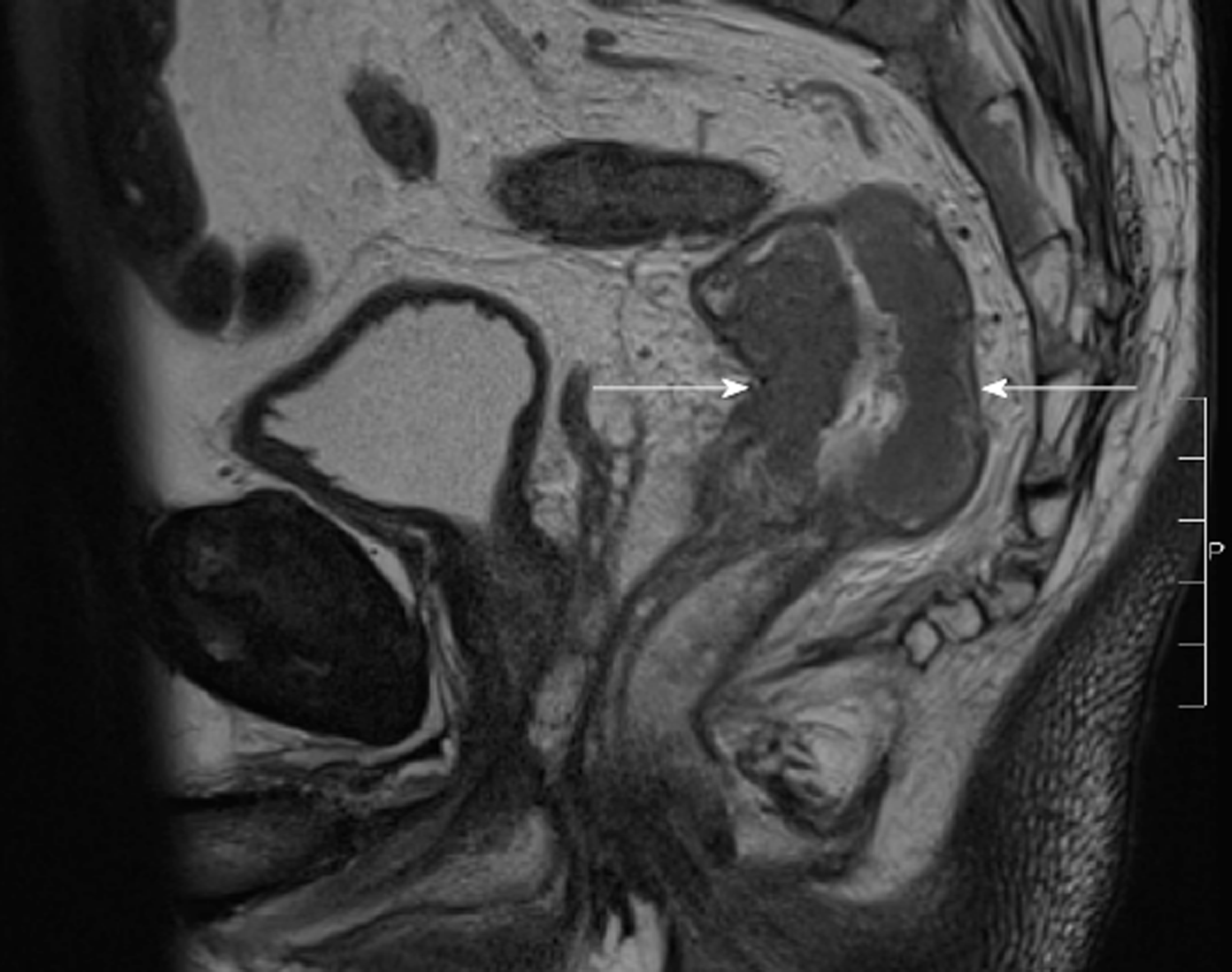
The CT scan of his abdomen on readmission raised a new concern of a possible rectal malignancy with thickening of the rectal wall, which had not been appreciated on previous imaging. The patient underwent a flexible sigmoidoscopy on day 4 of his second admission, which revealed a fungating but non-obstructing 8 cm lesion in the rectum. Biopsy of the mass, magnetic resonance imaging of the rectum and staging CT confirmed a T3bN1aM0 adenocarcinoma of rectum (Figure 5). This finding led to an increased level of urgency to definitively manage the infected pancreatic necrosis to facilitate treatment of the rectal cancer.
The decision to drain the infected necrosis at this stage was driven primarily because he had presented with clinical features of infection and had developed new radiological features of infected necrosis with gas locules within the area of walled off necrosis. It was also an appropriate time for intervention given the collection was sufficiently mature and walled off making it a safe time to do so. The rectal cancer made it crucial for us to adequately drain his collection but the decision to intervene at this stage was driven primarily by his clinical features of infection and radiological features which supported this being an appropriate time to intervene.
By this stage (approximately 10 wk post-presentation), the patient had developed a sufficiently mature and walled off collection on imaging. This could have been approached endoscopically or surgically via a transgastric route. The authors preferred the endoscopic approach over surgery as this was less invasive and would have avoided the added insult of surgery and inherent morbidity associated with this. Surgery was reserved at this stage only if the endoscopic approach failed consistent with a step-up approach.
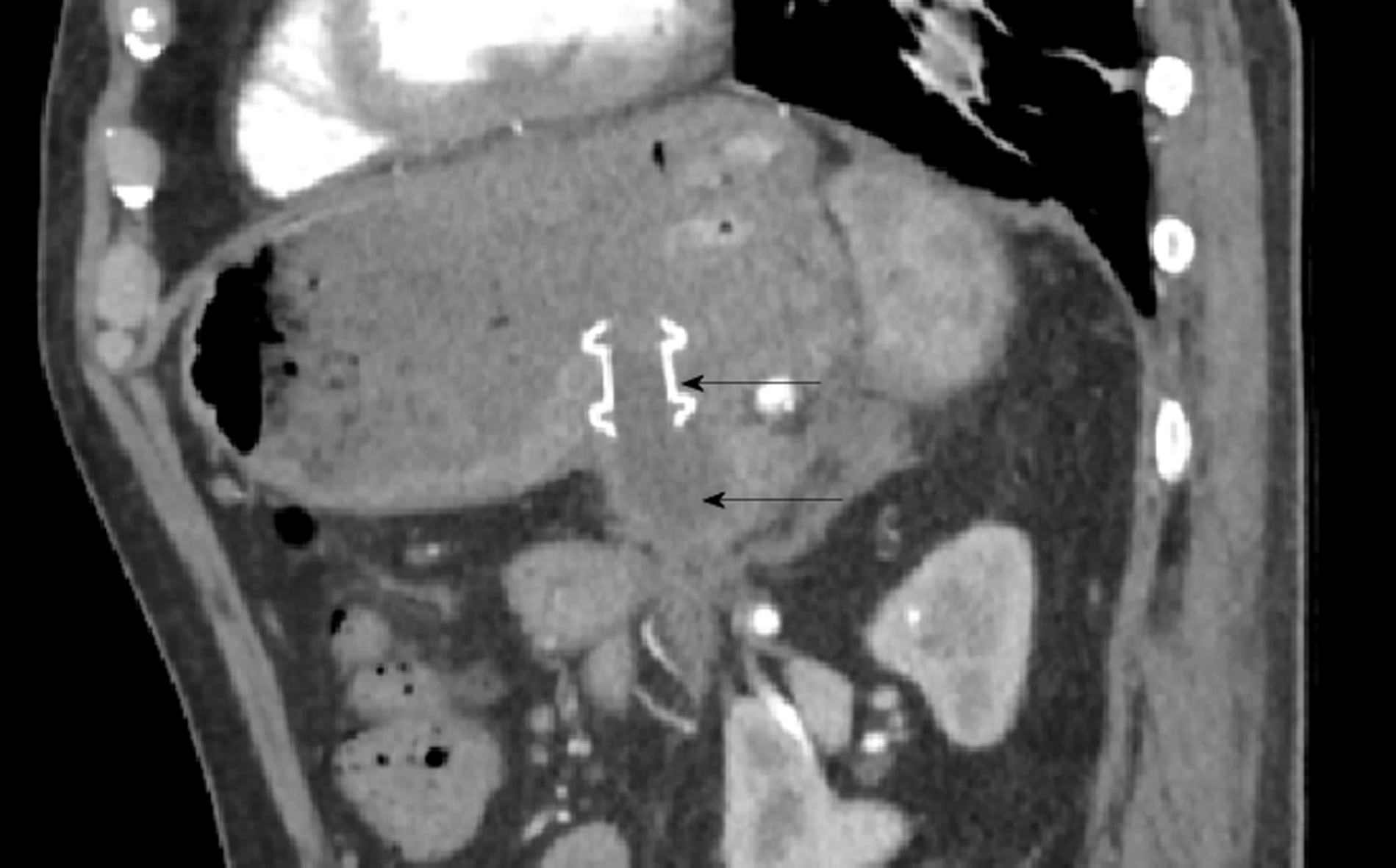
The patient was referred for endoscopic drainage and debridement of his walled off necrosis by the therapeutic gastroenterology team. On day 11 of his second admission he underwent endoscopic ultrasound guided Hot-AXIOS stent placement (Boston Scientific, Marlborough, MA, United States) and insertion (Figure 6). Further endoscopic debridement and removal of the nasocystic catheter was performed on day 18. At this procedure, pulsations of an arterial vessel were visible in the center of the necrotic cavity raising concern of possible arterial erosion and major hemorrhage. The AXIOS stent was therefore removed and two plastic, double-pigtail stents were positioned across the cyst-gastrostomy to ensure patency and ongoing drainage.
The patient’s recovery post-endoscopic necrosectomy was uncomplicated and he was discharged on day 28. Notably, he had received IV antibiotic therapy for the entire admission. The case was discussed at the oncology multidisciplinary meeting where it was initially decided to commence neoadjuvant radiotherapy without chemotherapy due to the presence of infected pancreatic necrosis.
The patient started neoadjuvant radiotherapy (50 Gy in 25 fractions) for his rectal cancer one week following his discharge. Given that he tolerated this without adverse effects and with effective ongoing drainage of his walled off pancreatic necrosis, he was then trialed on chemotherapy (Capecitabine) 10 d following his radiotherapy. He also tolerated the chemotherapy well.
Ten weeks post completion of chemoradiotherapy, the patient underwent a laparoscopic ultra-low Hartmann’s procedure and cholecystectomy. At the time of surgery, both the splenic flexure and mesocolon were fixed secondary to the inflammatory change from pancreatitis. These operative findings reinforced the decision to perform a non-restorative procedure, which had been discussed with the patient prior to surgery. Post-operative recovery was uneventful.
Histopathology revealed a T3 N0 low grade rectal adenocarcinoma with Grade 1 (moderate response) to neoadjuvant treatment. There was no lymphatic, vascular or perineural invasion and the margins of excision were clear. The patient attended regular general surgical outpatient appointments for review of his pancreatic necrosis as well as his rectal cancer. At 1 year follow up, there were no complications from the pancreatitis or evidence of rectal cancer recurrence.
Infected pancreatic necrosis and concomitant T3bN1aM0 adenocarcinoma of rectum adenocarcinoma of rectum.
The patient had endoscopic drainage of their infected pancreatic necrosis and stabilization of this in order to enable receipt of neoadjuvant chemoradiotherapy prior to resection of their rectal cancer.
The patient has recovered well and remains disease free at one year follow-up.
Pancreatic necrosis occurs in up to 30% of patients with acute pancreatitis and carries a high morbidity and mortality[3-8]. Our patient developed extensive necrosis of over 70% his pancreas. Necrosis of greater than 30% of the pancreas is associated with a morbidity of greater than 70% and a mortality rate of 11%–25%[9].
Infection of the necrotic area may lead to sepsis and multi-organ failure thereby compounding morbidity and mortality. The management of necrotizing pancreatitis has changed drastically over the past several decades, with an associated im-provement in morbidity and mortality rates[10-12].
Interestingly, management of necrotizing pancreatitis with concomitant colorectal cancer has never been described in literature and presents several unique challenges in order to facilitate stabilization of pancreatitis, timely therapy and surgical resection of the colorectal cancer. In this case, several factors contributed to shortening the clinical course of the infected necrotizing pancreatitis, namely: Use of endoscopic step up procedure for necrosectomy management, early enteral feeding, early antibiotic treatment and staged chemotherapy and resection. Swift resolution of pancreatitis permitted the patient to undergo prompt neoadjuvant chemotherapy and surgical resection of the colorectal cancer.
The use of a minimally invasive endoscopic “step up approach” for management of infected necrosis consists of endoscopic transluminal drainage followed by endoscopic necrosectomy if the initial drainage doesn’t result in clinical improvement. This technique has been performed since the early 2000’s[13] and has been shown in several trials[14,15] be superior to outcomes of surgical necrosectomy with regards to major complications and mortality. Our patient’s case was amenable to endoscopic debridement given the stable, walled off necrosis surrounding the pancreas and was undertaken to facilitate a quicker recovery than if the patient had undergone a surgical necrosectomy. The endoscopic approach also helped to avoid further complications from general anesthesia and surgical intervention which would prolong progression onto chemoradiotherapy and colorectal resection.
Nutritional support is an essential component in the treatment of patients with severe pancreatitis due to the hyper-catabolic state and negative nitrogen balance associated with the disease[16-19]. Furthermore acute malnutrition secondary to acute pancreatitis has been shown to result in immunological disturbances, septic complications, and delayed healing of wounds[20]. Multiple randomized control trials (RCTs) and meta analyses have demonstrated the benefit of early enteral nutrition in necrotizing pancreatitis[18,21-24]. Early enteral feeding is associated with beneficial outcomes in necrotizing pancreatitis, lower rates of mortality, systemic infections and multi-organ failure[25]. This is thought to be due to enteral nutrition maintaining the integrity of the gut barrier as a means of preventing bacterial translocation, which is the most likely cause of the systemic inflammatory response seen in necrotizing pancreatitis[26-30]. Our patient developed an ileus shortly after institution of enteral nutrition. Following resolution of the ileus, the patient was restarted on enteral feeds and tolerated them well throughout the remainder of his admission. The early enteral feeding helped to maintain gut wall integrity, possibly delaying superinfection in our patient.
Prophylactic antibiotic use in severe pancreatitis has been a contentious issue since the 1970’s. Death from infected necrosis ensues in anywhere from 20%-80% of patients and occurs in approximately 40%-70% of patients with acute necrotizing pancreatitis[28,31]. Although the mechanism is unclear, clinical data suggests that organism transmission from GI tract to the pancreas is the most likely source of infection[32]. Within the past decade, several RCTs and meta-analyses have examined the use of prophylactic antibiotics in the context of acute necrotising pancreatitis and have shown no statistically significant reduction in rates of infected necrosis or mortality[33-35]. With our case, antibiotics and anti-fungal agents were commenced early in the course as there was significant clinical suspicion of active infective necrosis given the patients consistently high temperatures, persistent periods of delirium and areas of enlarging necrotic tissue on imaging.
The implementation of the endoscopic step up procedure, early enteral support and antibiotics facilitated our patient’s recovery to a point where he was able to undergo therapy for his colorectal cancer. Definitive source control achieved by debridement of the infected necrotic tissue facilitated his neoadjuvant radiotherapy treatment. The success of this treatment then facilitated progression onto neoadjuvant chemotherapy (which was initially withheld) and finally on to surgical resection. Due to scarring from the inflammatory process around the splenic flexure and the difficulty in mobilizing the bowel, a Hartmann’s procedure was performed. This was deemed the most appropriate long term solution for the patient in view of the significant intraabdominal scarring.
The management of infected pancreatic necrosis with concomitant rectal cancer has not been described and presents unique challenges. Management of infected pancreatic necrosis with adequate nutritional support, antimicrobial therapy and ultimately a minimally invasive endoscopic necrosectomy, facilitated resolution and adequate drainage of the infected pancreatic necrosis. This enabled the patient to go on to receive neoadjuvant treatment for his rectal cancer prior to surgical resection.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Granero-Castro B S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | van Brunschot S, Fockens P, Bakker OJ, Besselink MG, Voermans RP, Poley JW, Gooszen HG, Bruno M, van Santvoort HC. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg Endosc. 2014;28:1425-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 481] [Article Influence: 32.1] [Reference Citation Analysis (2)] |
| 3. | Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, Rosman C, Ploeg RJ, Brink MA, Schaapherder AF, Dejong CH, Wahab PJ, van Laarhoven CJ, van der Harst E, van Eijck CH, Cuesta MA, Akkermans LM, Gooszen HG; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 879] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 4. | Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 299] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 324] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Kokosis G, Perez A, Pappas TN. Surgical management of necrotizing pancreatitis: an overview. World J Gastroenterol. 2014;20:16106-16112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | El Boukili I, Boschetti G, Belkhodja H, Kepenekian V, Rousset P, Passot G. Update: Role of surgery in acute necrotizing pancreatitis. J Visc Surg. 2017;154:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1175] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 9. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 979] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 10. | Boumitri C, Brown E, Kahaleh M. Necrotizing Pancreatitis: Current Management and Therapies. Clin Endosc. 2017;50:357-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20:13879-13892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 238] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (5)] |
| 12. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 503] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 13. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1070] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 14. | Haghshenasskashani A, Laurence JM, Kwan V, Johnston E, Hollands MJ, Richardson AJ, Pleass HC, Lam VW. Endoscopic necrosectomy of pancreatic necrosis: a systematic review. Surg Endosc. 2011;25:3724-3730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Kumar N, Conwell DL, Thompson CC. Direct endoscopic necrosectomy versus step-up approach for walled-off pancreatic necrosis: comparison of clinical outcome and health care utilization. Pancreas. 2014;43:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Zhao G, Wang CY, Wang F, Xiong JX. Clinical study on nutrition support in patients with severe acute pancreatitis. World J Gastroenterol. 2003;9:2105-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Shaw JH, Wolfe RR. Glucose, fatty acid, and urea kinetics in patients with severe pancreatitis. The response to substrate infusion and total parenteral nutrition. Ann Surg. 1986;204:665-672. [PubMed] |
| 18. | Gupta R, Patel K, Calder PC, Yaqoob P, Primrose JN, Johnson CD. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II > or =6). Pancreatology. 2003;3:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Chandrasegaram MD, Plank LD, Windsor JA. The impact of parenteral nutrition on the body composition of patients with acute pancreatitis. JPEN J Parenter Enteral Nutr. 2005;29:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Wereszczynska-Siemiatkowska U, Swidnicka-Siergiejko A, Siemiatkowski A, Dabrowski A. Early enteral nutrition is superior to delayed enteral nutrition for the prevention of infected necrosis and mortality in acute pancreatitis. Pancreas. 2013;42:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Petrov MS, van Santvoort HC, Besselink MG, van der Heijden GJ, Windsor JA, Gooszen HG. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of randomized trials. Arch Surg. 2008;143:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Petrov MS, Whelan K. Comparison of complications attributable to enteral and parenteral nutrition in predicted severe acute pancreatitis: a systematic review and meta-analysis. Br J Nutr. 2010;103:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 23. | Quan H, Wang X, Guo C. A meta-analysis of enteral nutrition and total parenteral nutrition in patients with acute pancreatitis. Gastroenterol Res Pract. 2011;2011:698248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336-344; discussion 344-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;CD002837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, Imrie CW. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol. 2005;100:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Wang G, Wen J, Xu L, Zhou S, Gong M, Wen P, Xiao X. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J Surg Res. 2013;183:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Mazaki T, Ishii Y, Takayama T. Meta-analysis of prophylactic antibiotic use in acute necrotizing pancreatitis. Br J Surg. 2006;93:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Piciucchi M, Merola E, Marignani M, Signoretti M, Valente R, Cocomello L, Baccini F, Panzuto F, Capurso G, Delle Fave G. Nasogastric or nasointestinal feeding in severe acute pancreatitis. World J Gastroenterol. 2010;16:3692-3696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Singh N, Sharma B, Sharma M, Sachdev V, Bhardwaj P, Mani K, Joshi YK, Saraya A. Evaluation of early enteral feeding through nasogastric and nasojejunal tube in severe acute pancreatitis: a noninferiority randomized controlled trial. Pancreas. 2012;41:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291:2865-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Runkel NS, Moody FG, Smith GS, Rodriguez LF, LaRocco MT, Miller TA. The role of the gut in the development of sepsis in acute pancreatitis. J Surg Res. 1991;51:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 34. | Sainio V, Kemppainen E, Puolakkainen P, Taavitsainen M, Kivisaari L, Valtonen V, Haapiainen R, Schröder T, Kivilaakso E. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995;346:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 260] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Nordback I, Sand J, Saaristo R, Paajanen H. Early treatment with antibiotics reduces the need for surgery in acute necrotizing pancreatitis--a single-center randomized study. J Gastrointest Surg. 2001;5:113-8; discussion 118-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |