Published online Dec 27, 2019. doi: 10.4240/wjgs.v11.i12.443
Peer-review started: August 23, 2019
First decision: September 13, 2019
Revised: September 18, 2019
Accepted: November 7, 2019
Article in press: November 7, 2019
Published online: December 27, 2019
Processing time: 108 Days and 16.6 Hours
IgG4-related disease can manifest diversely, including autoimmune pancreatitis and IgG4-related cholangiopathy. We are reporting a very unusual cause of pancreatic cancer triggered in a previously unknown IgG4-related disease.
A 75-year-old man was diagnosed with a 43 mm × 33 mm pancreatic head tumor after consulting for abdominal pain and jaundice. A pancreaticoduodenectomy was carried out uneventfully, and the histopathology report showed an early stage of acinar-cell pancreatic cancer. The patient reconsulted on the 30th postoperative day with fever, jaundice and asthenia. Magnetic resonance cholangiopancreatography evidenced an extense bile duct stricture. A percutaneous biliary drainage proved to be ineffective, even after exchanging it with larger bore drainage. Reviewing the surgical specimen, features compatible with IgG4-related disease were observed. Consequently, empiric treatment with steroids was initiated achieving excellent results.
IgG4-related disease may cause chronic inflammation of the pancreas and can condition pancreatic malignancies.
Core tip: Until today, the relationship between autoimmune pancreatitis and pancreatic cancer was not clear, and there are no studies in this regard. We are reporting a very unusual cause of pancreatic cancer triggered in a previously unknown IgG4-related disease and conducted an up-to-date literature review.
- Citation: Glinka J, Calderón F, de Santibañes M, Hyon SH, Gadano A, Mullen E, Pol M, Spina J, de Santibañes E. Early pancreatic cancer in IgG4-related pancreatic mass: A case report. World J Gastrointest Surg 2019; 11(12): 443-448
- URL: https://www.wjgnet.com/1948-9366/full/v11/i12/443.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i12.443
IgG4-mediated disease is a systemic condition that can manifest in various clinical forms, including autoimmune pancreatitis (AIP type 1) and IgG4-related cholangiopathy[1-3]. It is well known that chronic pancreatitis is a physiopathological model for pancreatic adenocarcinoma (PAC), but there is no firm evidence that AIP can serve as a pathway for pancreatic neoplasm[4]. We are reporting a very unusual cause of pancreatic cancer (PC) triggered in a previously unknown IgG4-related disease.
Upper abdominal pain, pruritus and jaundice.
The patient had history of smoking, ischemic cardiomyopathy and type 2 diabetes mellitus.
A Caucasian 75-year-old man was diagnosed with pancreatic tumor in another center. Upon endoscopic retrograde cholangiopancreatography and plastic biliary stenting (7-Fr × 10 cm), the patient was referred to our institution for further treatment. Upon discussion in a multidisciplinary oncology meeting, surgical exploration was decided under the presumption of a potentially resectable PAC of the head and uncinate process. No preoperative biopsy was performed.
Physical examination was relevant for jaundice, minor skin lesions due to scratching and a mild upper abdominal tenderness without peritoneal signs.
Plasma laboratory was positive for total bilirubin: 7.6 mg/dL; direct bilirubin: 4.0 mg/dL; alkaline phosphatase: 900 IU; and plasma CA 19-9: 1380 IU.
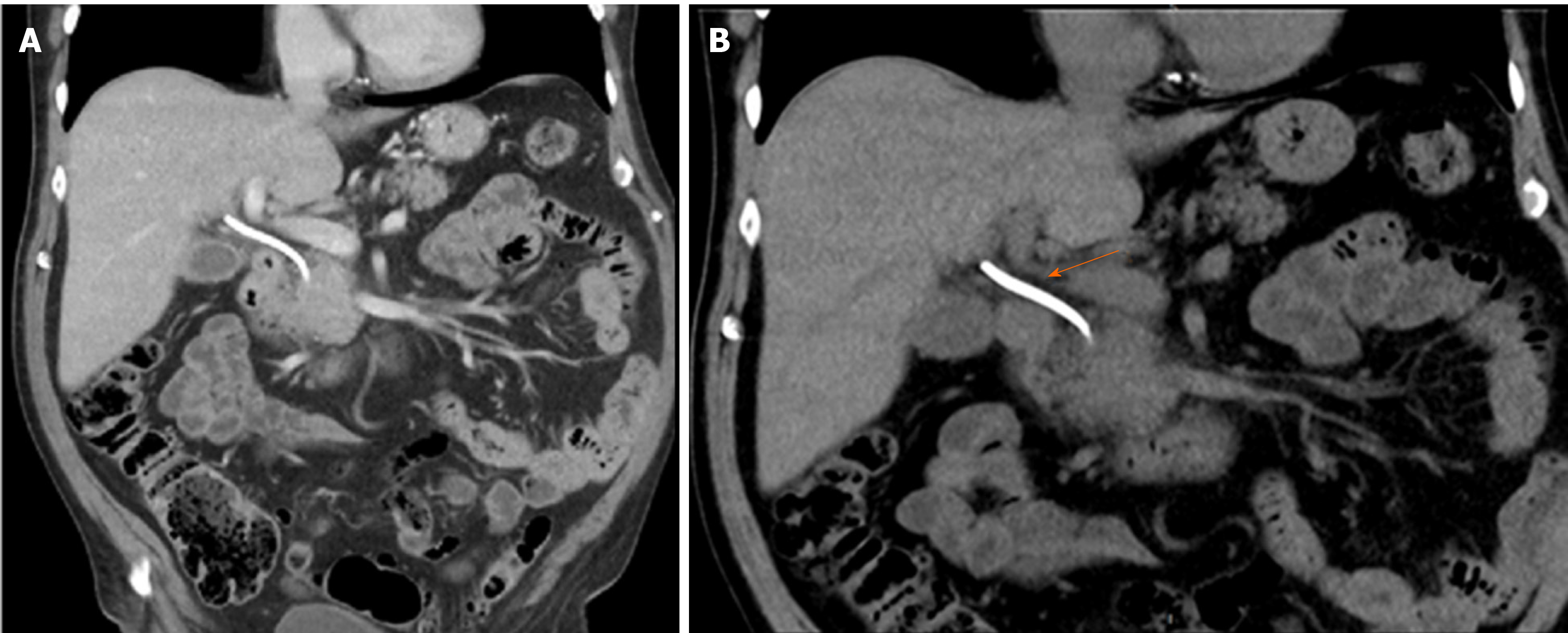
Abdominal computed tomography scan revealed a 43 mm × 33 mm mass embracing the pancreatic head and uncinate process with an uncertain superior mesenteric vein abutment. No metastatic disease was found in the routine work-up (Figure 1).
Early PC within a probable IgG4-related disease pancreatic mass.
A pancreaticoduodenectomy (Whipple procedure) was performed.
Surgery was carried out uneventfully, and the patient was discharged on the 7th day after surgery. Histopathology of the surgical specimen revealed a well differentiated acinar cell PC with negative margins. No perivascular or perineural invasion was observed, and none of the fourteen resected lymph nodes were positive for malignancy. Thirty days after surgery, the patient presented at the Emergency Department with fever, jaundice and a noteworthy asthenia. Laboratory exams revealed total bilirubin: 14.0 mg/dL; direct bilirubin: 8.6 mg/dL; alkaline phosphatase: 950 IU; aspartate aminotransferase: 147 IU; and alanine amino-transferase: 56 IU.
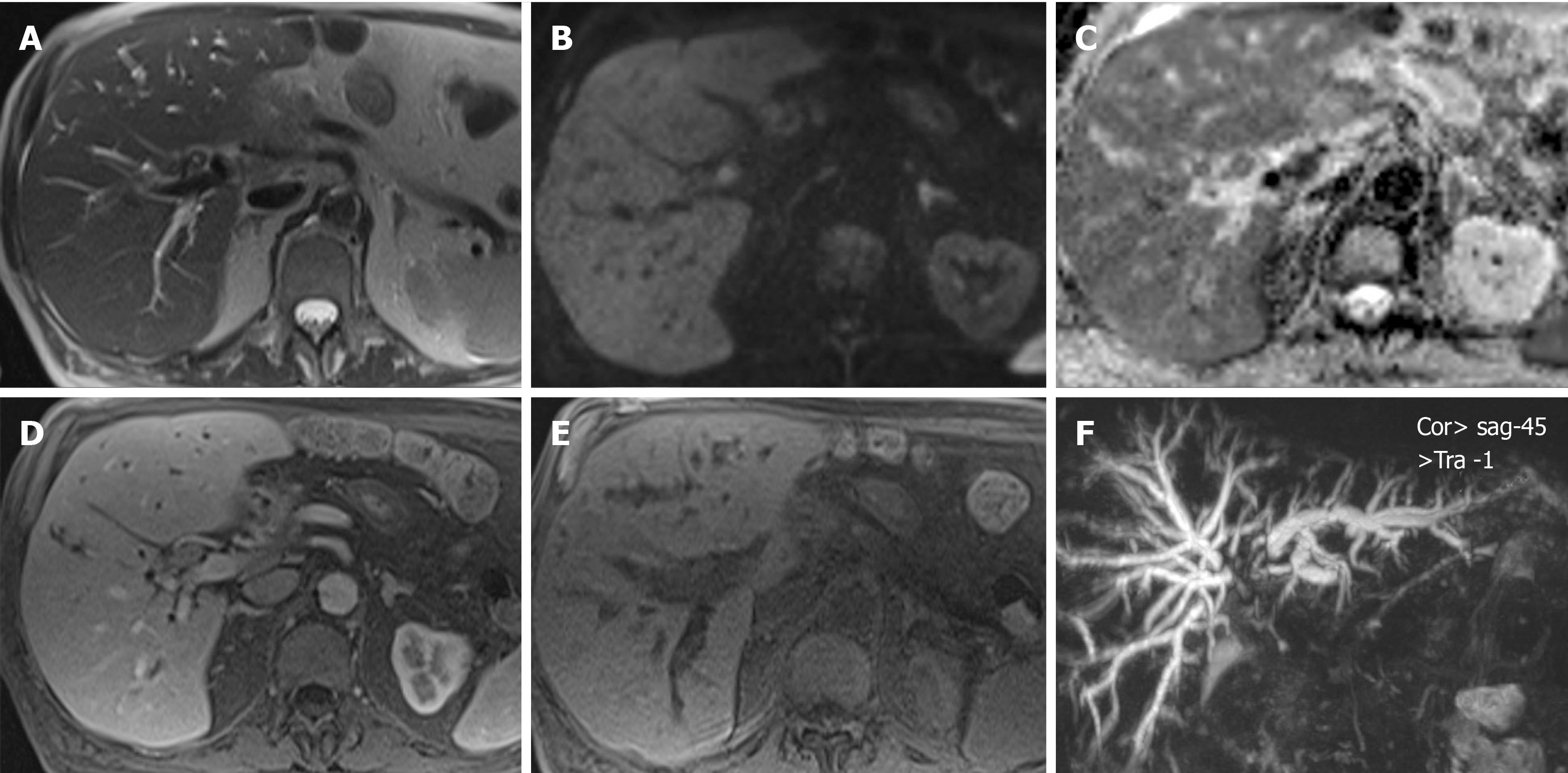
A magnetic resonance cholangiopancreatography showed intrahepatic bile duct dilation with a biliary stenosis extending from the hepatic carrefour to the hepatojejunostomy. Presuming an anastomotic stricture, a percutaneous transhepatic cholangiography was done, followed by insertion of an internal/external, 8.5-Fr biliary drainage with the pigtail locked into the jejunal loop. Plasma bilirubin worsened, reaching levels of total bilirubin: 20 mg/dL and direct bilirubin: 11 mg/dL at 7 d post procedure. The 8.5-Fr drain was replaced by a 10.2-Fr drain, but no improvement occurred.
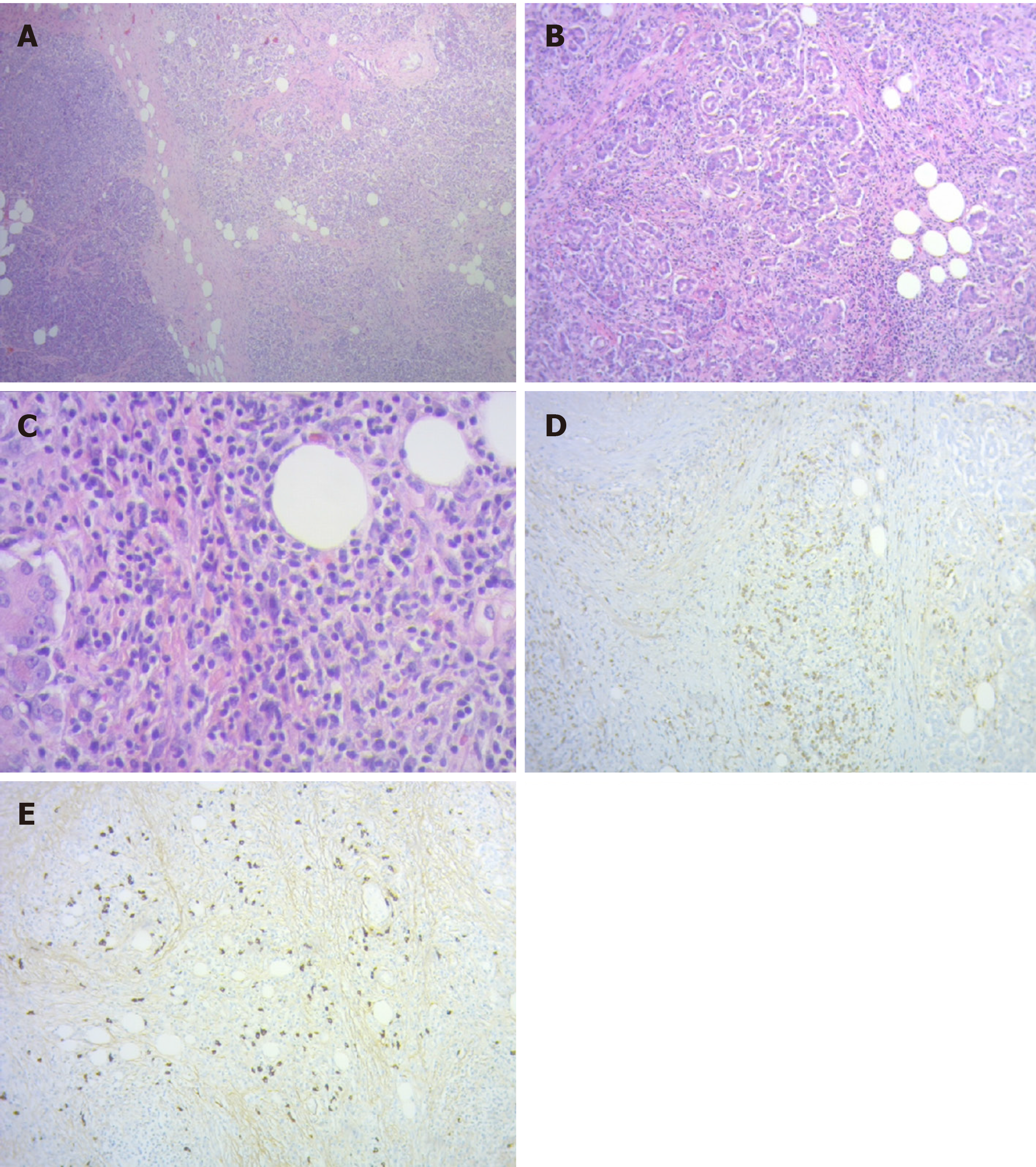
A new magnetic resonance cholangiopancreatography revealed persistent intrahepatic bile duct dilation and a common bile duct stricture from the biliary confluence to the anastomosis with an eccentric parietal growth from the common bile duct (Figure 2). On histopathological revision of the resected specimen and surrounding the neoplastic lesion, pathologic characteristics of IgG4-related disease could be recognized. There was an intense lymphoplasmacytic infiltrate predominantly on a periductal fashion, a trabecular fibrotic pattern and focal vascular structures with obliterative phlebitis.
After immunohistochemical evaluation, most of the plasma cells were IgG positive with more than 50 IgG4 positive plasma cells/high power field and an IgG4/IgG ratio greater than 40% (Figure 3). Because the lesion met most of the Honolulu Criteria a diagnosis of acinar cell pancreatic cancer in a context of a probable IgG4-related disease was made[5]. The clinical context in addition to the histopathology and immunohistochemistry allowed inferring a systemic IgG4-related disease. Autoimmune cholangiopathy was then presumed, which mandated starting steroid therapy with 0.6 mg/kg-body-weight of meprednisone daily (40 mg daily). Upon 7 d of treatment, liver function tests normalized, and all symptoms disappeared allowing gradual reduction in meprednisone dosage up to 8 mg daily.
The IgG4-related disease is considered a systemic condition, which has clinical manifestations in diverse organs. AIP and cholangiopathy are two of the many manifestations of this rare disorder[6]. Patients with AIP can often manifest a pancreatic mass simulating a PC. In this setting, the differential diagnosis is usually challenging and often confirmed after surgical resection[7,8].
In certain cases, determination of IgG4 serum level and the presence of a characteristic radiology may serve to differentiate between AIP and PC[9,10]. Even so, in the study reported by Pak et al[11], up to 9% of pancreatic masses due to PC have high serum IgG4. Therefore, overconfidence in this marker and the assumption that a pancreatic mass is an AIP may lead to dangerously misdiagnosing a PAC and consequently missing a curative chance. In the study by Macinga et al[12], their histopathology specimens of resected PAC were retrospectively analyzed. They found an impressive 40% of AIP coexisting with PAC. As the patient reported here presented with jaundice, weight loss, a pancreatic mass in the imaging studies and high CA 19-9 serum marker, a PC was assumed as the main diagnosis and neither specific autoimmunity studies nor a biopsy prior to surgery was performed.
After surgical resection of the mass and a confirmatory histopathology of acinar-cell PC, early jaundice occurred, which raised the suspicion of stricture of the biliodigestive anastomosis. However, the lack of response to effective biliary drainage as well as the development of new intrahepatic bile duct strictures led us to review the specific immunohistochemistry of the surgical specimen. Subsequent steroid therapy resulted in a spectacular improvement of cholestasis, confirming the diagnosis. Until today, the relationship between AIP and PC was not clear, and there are no studies in this regard[13].
Furthermore, in a recent review by Okamoto et al[4], not only the correlation between these entities is refused, but also AIP is considered as a paraneoplastic syndrome of PC and not its determinant. There is agreement that chronic inflammation is a major determinant of biliopancreatic malignancies due to chronic pancreatitis and autoimmune cholangiopathy being well recognized risk factors[14]. Therefore, it may not be surprising that a causal association in the natural history of AIP can be established with precision in the future.
An unsettled issue is how to differentiate patients who are in an intermediate stage; that is, determining who carries a pancreatic mass or a biliary stricture that will progress to malignancy. This is mandatory to provide each patient the best available treatment. Improving knowledge on this unusual disease may result in an earlier diagnosis and a timely therapy.
All efforts must be addressed to correctly diagnose patients with suspected IgG4-related disease so as to offer them the most appropriate and timely treatment. Misdiagnosing an AIP with a mass and a PC could be catastrophic. On the other hand, performing a pancreaticoduodenectomy in a patient with a pancreatitis is not harmless, even in specialized centers. Thus, further studies to determine a precise causal correlation between AIP and PC are needed.
| 1. | Okazaki K. Autoimmune Pancreatitis and IgG4-Related Disease: The Storiform Discovery to Treatment. Dig Dis Sci. 2019;64:2385-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Detlefsen S, Klöppel G. IgG4-related disease: with emphasis on the biopsy diagnosis of autoimmune pancreatitis and sclerosing cholangitis. Virchows Arch. 2018;472:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Hegade VS, Sheridan MB, Huggett MT. Diagnosis and management of IgG4-related disease. Frontline Gastroenterol. 2019;10:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Okamoto A, Watanabe T, Kamata K, Minaga K, Kudo M. Recent Updates on the Relationship between Cancer and Autoimmune Pancreatitis. Intern Med. 2019;58:1533-1539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the honolulu consensus document. Pancreatology. 2010;10:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Matsumori T, Shiokawa M, Kodama Y. Pancreatic Mass in a Patient With an Increased Serum Level of IgG4. Gastroenterology. 2018;155:269-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Vashi B, Khosroshahi A. IgG4-Related Disease with Emphasis on Its Gastrointestinal Manifestation. Gastroenterol Clin North Am. 2019;48:291-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Hsu WL, Chang SM, Wu PY, Chang CC. Localized autoimmune pancreatitis mimicking pancreatic cancer: Case report and literature review. J Int Med Res. 2018;46:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Sandrasegaran K, Menias CO. Imaging in Autoimmune Pancreatitis and Immunoglobulin G4-Related Disease of the Abdomen. Gastroenterol Clin North Am. 2018;47:603-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Courcet E, Beltjens F, Charon-Barra C, Guy F, Orry D, Ghiringhelli F, Arnould L. [An IgG4-related pancreatitis mimicking an adenocarcinoma: A case report]. Ann Pathol. 2015;35:511-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Pak LM, Schattner MA, Balachandran V, D'Angelica MI, DeMatteo RP, Kingham TP, Jarnagin WR, Allen PJ. The clinical utility of immunoglobulin G4 in the evaluation of autoimmune pancreatitis and pancreatic adenocarcinoma. HPB (Oxford). 2018;20:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Macinga P, Pulkertova A, Bajer L, Maluskova J, Oliverius M, Smejkal M, Heczkova M, Spicak J, Hucl T. Simultaneous occurrence of autoimmune pancreatitis and pancreatic cancer in patients resected for focal pancreatic mass. World J Gastroenterol. 2017;23:2185-2193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Dite P, Novotny I, Dvorackova J, Kianicka B, Blaho M, Svoboda P, Uvirova M, Rohan T, Maskova H, Kunovsky L. Pancreatic Solid Focal Lesions: Differential Diagnosis between Autoimmune Pancreatitis and Pancreatic Cancer. Dig Dis. 2019;37:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Hausmann S, Kong B, Michalski C, Erkan M, Friess H. The role of inflammation in pancreatic cancer. Adv Exp Med Biol. 2014;816:129-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vitali F, Yu XJ S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ