Published online Feb 27, 2018. doi: 10.4240/wjgs.v10.i2.21
Peer-review started: November 2, 2017
First decision: December 6, 2017
Revised: January 14, 2018
Accepted: February 6, 2018
Article in press: February 6, 2018
Published online: February 27, 2018
Processing time: 115 Days and 20.6 Hours
To investigate changes in hepatic and splenic stiffness in patients without chronic liver disease during liver resection for hepatic tumors.
Patients scheduled for liver resection for hepatic tumors were considered for enrollment. Tissue stiffness measurements on liver and spleen were conducted before and two days after liver resection using point shear-wave elastography. Histological analysis of the resected liver specimen was conducted in all patients and patients with marked liver fibrosis were excluded from further study analysis. Patients were divided into groups depending on size of resection and whether they had received preoperative chemotherapy or not. The relation between tissue stiffness and postoperative biochemistry was investigated.
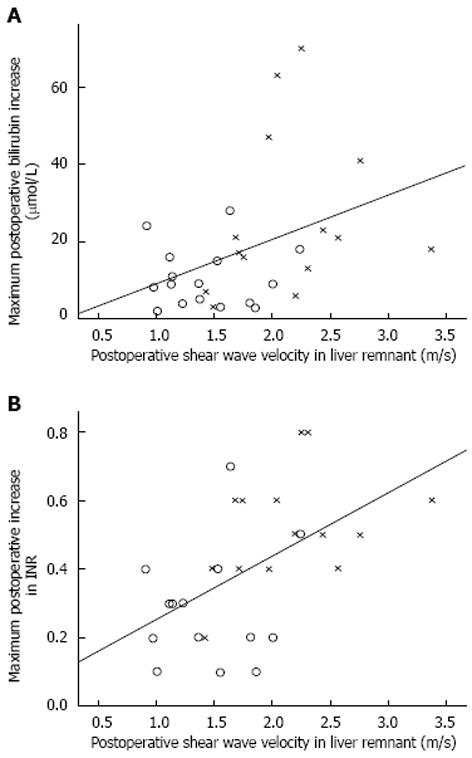
Results are presented as median (interquartile range). 35 patients were included. The liver stiffness increased in patients undergoing a major resection from 1.41 (1.24-1.63) m/s to 2.20 (1.72-2.44) m/s (P = 0.001). No change in liver stiffness in patients undergoing a minor resection was found [1.31 (1.15-1.52) m/s vs 1.37 (1.12-1.77) m/s, P = 0.438]. A major resection resulted in a 16% (7%-33%) increase in spleen stiffness, more (P = 0.047) than after a minor resection [2 (-1-13) %]. Patients who underwent preoperative chemotherapy (n = 20) did not differ from others in preoperative right liver lobe [1.31 (1.16-1.50) vs 1.38 (1.12-1.56) m/s, P = 0.569] or spleen [2.79 (2.33-3.11) vs 2.71 (2.37-2.86) m/s, P = 0.515] stiffness. Remnant liver stiffness on the second postoperative day did not show strong correlations with maximum postoperative increase in bilirubin (R2 = 0.154, Pearson’s r = 0.392, P = 0.032) and international normalized ratio (R2 = 0.285, Pearson’s r = 0.534, P = 0.003).
Liver and spleen stiffness increase after a major liver resection for hepatic tumors in patients without chronic liver disease.
Core tip: Point shear-wave elastography is an ultrasound-based technique which lets the user measure tissue stiffness. The technique has previously mostly been used to study patients with chronic liver disease and cirrhosis. In the current study we investigate changes in liver and spleen stiffness in patients without chronic liver disease undergoing chemotherapy and liver resection for liver tumors. A major liver resection resulted in a 42% increase in liver stiffness. Also, spleen stiffness increased more after a major than after a minor resection. However, there was no difference in tissue stiffness between patients who received preoperative chemotherapy or not.
- Citation: Eriksson S, Borsiin H, Öberg CF, Brange H, Mijovic Z, Sturesson C. Perioperative liver and spleen elastography in patients without chronic liver disease. World J Gastrointest Surg 2018; 10(2): 21-27
- URL: https://www.wjgnet.com/1948-9366/full/v10/i2/21.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v10.i2.21
Liver elastography implies reporting metrics related to the mechanical stiffness of the liver, using either ultrasound or magnetic resonance techniques[1]. Ultrasound-based techniques include virtual touch tissue quantification (VTTQ) (Siemens, Erlangen, Germany) which is a software based on point shear-wave elastography (SWE) technology, used to measure tissue elasticity[2-4]. Using standard ultrasound equipment, an acoustic pulse is applied to a region of interest within the tissue under investigation[5]. The pulse will cause small displacements of the tissue and generate shear waves perpendicular to the original pulse. The shear wave propagation velocity will differ depending on the elastic properties, i.e., stiffness, of the tissue[6]. High shear wave velocity denotes a stiffer tissue. In relation to surgical resection of liver tumors, a high preoperative liver stiffness has been shown to increase the risk of postoperative liver failure after resection of hepatocellular carcinoma in patients with chronic liver disease[7].
Changes in perioperative liver stiffness in patients without chronic liver disease undergoing liver resection for tumors have not been previously investigated. Surgical resection offers a potential cure for both primary liver tumors and liver metastasis[8,9]. The risk of postoperative liver failure is the most important factor for postoperative mortality limiting the size of the resection[10]. An otherwise healthy liver can withstand a larger resection than a liver with parenchymal damage, which requires a larger liver remnant to ensure a sufficient postoperative liver function. Parenchymal damage can be due to chronic liver disease because of hepatitis or alcohol abuse but also occurs in non-alcoholic fatty liver disease or because of chemotherapy[11-13]. Chemotherapy associated parenchymal damage include steatosis, steatohepatitis and sinusoidal obstruction syndrome which all have been suggested to increase postoperative morbidity or mortality[12,14,15].
The current study aimed to investigate changes in hepatic and splenic stiffness during liver resection for hepatic tumors in patients without chronic liver disease, effects of preoperative chemotherapy on tissue stiffness and its relation to early postoperative biochemistry with the aim to detect postoperative liver failure.
The study protocol was approved by the Regional Ethical Review Board. Patients scheduled for liver resection for hepatic tumors at a single center were considered for enrollment. Patients were given both written and oral information about the study and gave their written consent prior to enrollment. Patients’ clinical data were recorded from patient medical records. Patients with marked liver fibrosis were excluded from study analysis.
To study the effect of liver resection on liver stiffness patients were divided into two groups depending on whether they underwent a major resection (hemihepatectomy or extended hemihepatectomy) or a minor resection. Liver resection was performed as previously described[16]. If necessary, the blood flow of the portal vein and hepatic artery was temporarily occluded (Pringle’s maneuver). Preoperative chemotherapy was defined as receiving chemotherapy within 3 mo prior to surgery[17].
Measurements of liver and spleen stiffness were made using a Siemens ACUSON S2000 ultrasound system (Siemens Medical Solutions Inc., Mountain View, CA, United States) accompanied by the VTTQ software package. A 4C1 transducer (Siemens Medical Solutions Inc., Mountain View, CA, United States) was used. Patients were fasting 4 h before examination. To decrease movement artefacts patients were asked to hold their breath during the seconds of measurement. Measurements were conducted before and after liver resection.
Preoperative measurements were conducted in both the right and the left liver lobe as well as in the spleen. A region of interest within the respective parenchyma was chosen at a depth of 3-6 cm from the transducer[3]. The regions were chosen so that major blood vessels and bile ducts were avoided. For measurements in the right liver lobe intercostal transducer placement was used. Each region was measured 10 times and a median of the 10 measurements was calculated. Comparison between the pre- and postoperative measurements were made on the spleen and the remnant liver lobe, e.g., on the right liver lobe if the patient was undergoing a left hemihepatectomy. Tissue stiffness data was presented as the shear wave velocity (m/s).
Histological analysis of the resected liver specimen was conducted in all patients. The pathologist was blinded to stiffness results. Steatosis was graded (0-3), steatohepatitis (0-8) and fibrosis (0-4), according to the non-alcoholic fatty liver disease activity score, NAS[18]. A steatosis grade ≥ 2 was defined as steatosis. A NAS ≥ 5 was defined as steatohepatitis and fibrosis > 2 was defined as marked fibrosis. Sinusoidal obstruction syndrome was defined as a sinusoidal dilatation grade ≥ 2 according to Rubbia-Brandt et al[11].
Statistical analysis was performed using IBM SPSS Statistics version 23 (IBM, Armonk, NY, United States). The statistical methods were reviewed by a biomedical statistician. To compare continuous data the Mann-Whitney U-test or the Wilcoxon test for paired samples was used. Categorical data was compared with a χ2 test. Correlations were made using linear regression analysis and by calculating a Pearson’s correlation coefficient, r. A P-value < 0.05 was considered statistically significant. All results are presented as median (interquartile range) if not stated otherwise.
Forty-seven patients were enrolled in the study. Nine patients failed to complete the study protocol and were excluded from the study; 6 patients declined to participate after enrollment, mostly due to postoperative pain and 3 patients were transferred to a different hospital before the second measurement. In addition, 3 patients were excluded from study analysis because of marked fibrosis on histological analysis of the liver specimen, leaving 35 patients included for study analysis.
Postoperative liver measurements were made typical on postoperative day 2 (postoperative day 1-3). Patient characteristics are presented in Table 1. Median preoperative shear wave velocity in all patients in the right liver lobe was 1.33 (1.15-1.50) m/s and in the left liver lobe 1.41 (1.20-1.66) m/s. The shear wave velocity in the left lobe was higher than in the right, P = 0.026. Median preoperative shear wave velocity of the spleen was 2.76 (2.37-3.02) m/s.
| No resection | Minor resection | Major resection | |
| No. of patients | 4 | 16 | 15 |
| Gender (male:female) | 1:3 | 8:8 | 8:7 |
| Age (yr) | 69 (56-76) | 75 (66-79) | 66 (50-74) |
| BMI (kg/m²) | 23.5 (21.4-28.6) | 24.7 (21.8-26.8) | 26.8 (25.2-28.7) |
| Weight (kg) | 64 (53-86) | 72 (63-83) | 78 (70-90) |
| ASA physical status (1/2:3/4) | 3:1 | 10:6 | 10:5 |
| Preoperative bilirubin (µmol/L) | 5 (4-7) | 6 (5-10) | 7 (6-11) |
| Preoperative INR | 1.0 (0.9-1.0) | 1.0 (0.9-1.1) | 1.0 (1.0-1.1) |
| Diagnosis | |||
| Colorectal metastases | 3 | 13 | 11 |
| Other malignant tumors | 0 | 2 | 3 |
| Benign tumors | 1 | 1 | 1 |
| Number of hepatic tumors | 5 (1-7) | 1 (1-2) | 2 (2-6) |
| Largest hepatic tumor (mm) | 42 (17-57) | 30 (10-45) | 30 (23-51) |
| Preoperative chemotherapy | 2 | 6 | 12 |
| Oxaliplatin-based therapy | 2 | 4 | 9 |
| Liver lobe operated (right lobe:left lobe:both lobes) | - | 7:4:5 | 7:0:8 |
| Operating time (h) | 2.5 (2-3) | 3 (3-5.5) | 6 (4.5-7) |
| Operative bleeding (mL) | 125 (100-150) | 275 (150-500) | 650 (400-1100) |
| Length of hospital stay (d) | 3 (2-6) | 6 (3-9) | 6 (5-7) |
| Liver parenchyma damage | |||
| Steatosis | - | 0 | 1 |
| Steatohepatitis | - | 0 | 0 |
| SOS | - | 0 | 0 |
Four patients did not undergo resection because of intraoperatively discovered unexpected excessive liver tumor disease precluding resection (n = 2) and two patients without intraoperatively detectable tumor disease. No difference in pre- and postoperative liver or spleen stiffness was found for these patients (results not shown).
Liver and spleen stiffness measurements for the minor and major resection groups are presented in Table 2. There were no differences between groups regarding gender ratio, body mass index, American Society of Anesthesiologists (ASA) physical status classification or diagnosis. However, patients who underwent a minor resection were older than patients undergoing a major resection [75 (66-79) vs 66 (50-74) years, P = 0.033] and did not undergo preoperative chemotherapy as frequent as the patients who underwent a major resection (6 vs 12 patients, P = 0.017).
| Minor resection | Major resection | P value | |
| No. of patients | 16 | 15 | - |
| Future liver remnant (m/s) | 1.31 (1.15-1.52) | 1.41 (1.24-1.63) | 0.318 |
| Right liver lobe preoperative (m/s) | 1.29 (1.12-1.49) | 1.38 (1.14-1.57) | 0.423 |
| Left liver lobe preoperative (m/s) | 1.35 (1.06-1.71) | 1.41 (1.29-1.63) | 0.667 |
| Spleen preoperative (m/s) | 2.76 (2.36-2.91) | 2.69 (2.33-3.11) | 0.984 |
| Liver remnant postoperative (m/s) | 1.37 (1.12-1.77) | 2.20 (1.72-2.44) | < 0.001 |
| Spleen postoperative (m/s) | 2.83 (2.44-3.18) | 2.90 (2.63-3.50) | 0.216 |
| Relative difference in liver remnant (%) | 4 (-16-24) | 42 (33-71) | 0.001 |
| Relative difference in the spleen (%) | 2 (-1-13) | 16 (7-33) | 0.047 |
The stiffness of the liver remnant increased in patients undergoing a major resection (P = 0.001) as compared to preoperative measurements. There was no difference for patients undergoing a minor resection (P = 0.438).
Patients who underwent preoperative chemotherapy (n = 20) did not differ from others in preoperative right liver lobe [1.31 (1.16-1.50) vs 1.38 (1.12-1.56) m/s, P = 0.569] or spleen [2.79 (2.33-3.11) vs 2.71 (2.37-2.86) m/s, P = 0.515] stiffness.
There was no difference between patients preoperatively treated with oxaliplatin (n = 15) compared to others in preoperative right liver [1.31 (1.16-1.50) vs 1.38 (1.14-1.61) m/s P = 0.670] or spleen [2.76 (2.34-2.97) vs 2.76 (2.37-3.07) m/s, P = 0.892] stiffness.
The correlation between shear wave velocity in the liver remnant and maximum postoperative increase of bilirubin and international normalized ratio (INR) are presented in Figure 1 respectively.
The current study presents data on changes in liver and spleen stiffness after liver resection for hepatic tumors in patients without chronic liver disease. In patients who underwent a major resection, the stiffness of the liver remnant increased by 42% as measured with point SWE. No change in liver stiffness was found in patients who underwent a minor resection. The spleen stiffness increased by 16% after a major resection, more than after a minor resection (Table 2).
Liver elastography is most frequently used to non-invasively quantify the degree of liver fibrosis in patients with chronic liver disease[2]. As patients with liver fibrosis were excluded in the present study, the reasons for increase in liver stiffness found must be unrelated to histological fibrosis. The increase in tissue stiffness may be explained by a postoperative increase in portal pressure which causes a congestion in the smaller liver remnant[19]. In comparison, an elevated liver stiffness has been shown in patients with acute decompensated heart failure[20] and also in patients with extrahepatic biliary obstruction[21]. No comparative measurements of portal pressure were conducted in the current study. In animal models, increase in hepatic perfusion in small-for-size liver grafts has shown to be of importance in both liver regeneration and liver damage[22]. However, the significance of liver stiffness on liver regeneration is yet to be investigated. A postoperative increase in liver stiffness has previously been demonstrated after liver resection for living donor transplantation[19].
Mean shear wave velocity in healthy livers range about 0.8-1.7 m/s[5]. The present preoperative measurements are in alignment with these values. In addition, there was a significant difference between measurements in the right and left liver lobes. This has been observed previously[23], and may be due to the smaller volume of the left lobe or its close position to the heart, causing movement artefacts. The same authors have suggested that more reliable measurements are obtained at a greater depth than superficial measurements. For that reason measurements in the current study were conducted at a depth of 3-6 cm from the transducer[23].
Point SWE measurements allow fast and non-invasive measurements of tissue stiffness. Compared to transient elastography with Fibroscan®, another ultrasound-based tissue stiffness diagnostic technique, point SWE can be made using standard ultrasound equipment, without the need for an extra examination and a region of interest within the tissue can easily be defined by the operator using a real-time conventional B-mode image[5,24].
Measurements were done on the second postoperative day as earlier measurements were found difficult to make due to postoperative pain.
No differences in liver or spleen stiffness were found in patients undergoing preoperative chemotherapy. Chemotherapy-induced liver parenchyma damage could worsen outcome after a liver resection[12,14,15] and perioperative identification of parenchymal damage would be desirable. Oxaliplatin, often included in preoperative treatment of colorectal liver metastasis, has previously been shown to induce splenic enlargement[25], proposed as a result of induced sinusoidal obstruction syndrome[26]. In the present study, no differences were found in preoperative splenic or liver stiffness in patients who received oxaliplatin. However, only one patient showed histological signs of steatosis and none presented with sinusoidal obstruction syndrome or steatohepatitis, which is a considerably lower frequency than previously reported[11-13]. One limitation of the current study is the relative small number of patients included, which may explain the differences.
Postoperative liver failure has high morbidity and mortality rates and early detection is of great interest to rapidly initiate treatment measures[27]. There is currently no good method for its early diagnosis and signs of liver failure are first detected several days after surgery when patients develop high bilirubin and INR values[28]. The present measurements on the second postoperative day showed weak but significant correlations with maximum postoperative increase in bilirubin and INR, as shown in Figure 1. A study on living liver donors have presented similar results on maximum bilirubin[19]. In a small report on 3 patients with acute liver failure due to intoxication, liver stiffness was suggested to be higher than healthy controls but similar to patients with liver cirrhosis[29]. Point SWE measurements may play a role in the early detection of liver failure, however further study is needed on the dynamics of normal and pathological liver stiffness after liver resection.
In conclusion, liver and spleen stiffness changes after liver resection for hepatic tumors using point SWE measurements have been presented. The size of resection matters to the dynamics of liver stiffness. The potential of point SWE in the detection of chemotherapy induced liver damage and postoperative liver failure needs further investigation.
Surgical resection offers a potential cure for both primary liver tumors and liver metastases. The risk of postoperative liver failure is the most important factor for postoperative mortality and limits the size of the resection. An otherwise healthy liver can withstand a larger resection than a liver with parenchymal damage, which requires a larger liver remnant to ensure sufficient postoperative liver function. Liver elastography implies reporting metrics related to the mechanical stiffness of the liver. Liver elastography is most frequently used to non-invasively quantify the degree of liver fibrosis in patients with chronic liver disease. Changes in perioperative liver stiffness in patients without chronic liver disease undergoing liver resection for tumors have not been investigated.
Postoperative liver failure has high morbidity and mortality rates and early detection is of great interest to rapidly initiate treatment measures. There is currently no good method for its early diagnosis and signs of liver failure are first detected several days after surgery when patients develop high bilirubin and international normalized ratio values.
The current study aimed to investigate the changes in hepatic and splenic stiffness during liver resection for hepatic tumors in patients without chronic liver disease; and to investigate effects of preoperative chemotherapy on tissue stiffness and its relation to early postoperative biochemistry with the aim to detect postoperative liver failure.
Tissue stiffness measurements on liver and spleen were conducted before and two days after liver resection for hepatic tumors using point shear-wave elastography (SWE). Patients were divided into groups depending on size of resection and whether they had received preoperative chemotherapy or not.
The stiffness of the liver remnant increased by 42% as measured with point SWE in patients who underwent a major resection. In patients who underwent a minor resection, no change in liver stiffness was found. The spleen stiffness increased by 16% after a major resection, more than after a minor resection. In patients undergoing preoperative chemotherapy, no differences in liver or spleen stiffness were found. Remnant liver stiffness on the second postoperative day did not show strong correlations with maximum postoperative increase in bilirubin and international normalized ratio.
Liver and spleen stiffness increase after a major liver resection for hepatic tumors in patients without chronic liver disease. The potential of point SWE in the detection of chemotherapy induced liver damage and postoperative liver failure needs further investigation.
Point SWE measurements may play a role in the early detection of liver failure; however, further study is needed on the dynamics of normal and pathological liver stiffness after liver resection.
| 1. | Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: Part 1, Principles and Techniques. AJR Am J Roentgenol. 2015;205:22-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011;46:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (3)] |
| 4. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 5. | D’Onofrio M, Crosara S, De Robertis R, Canestrini S, Demozzi E, Gallotti A, Pozzi Mucelli R. Acoustic radiation force impulse of the liver. World J Gastroenterol. 2013;19:4841-4849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 652] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, Festi D, Pinna AD. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256:706-712; discussion 712-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN; Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 9. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 691] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 10. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1819] [Article Influence: 121.3] [Reference Citation Analysis (1)] |
| 11. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 773] [Article Influence: 35.1] [Reference Citation Analysis (1)] |
| 12. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 962] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 13. | Peppercorn PD, Reznek RH, Wilson P, Slevin ML, Gupta RK. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008-2011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Tamandl D, Klinger M, Eipeldauer S, Herberger B, Kaczirek K, Gruenberger B, Gruenberger T. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, Prasad KR. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Blind PJ, Andersson B, Tingstedt B, Bergenfeldt M, Andersson R, Lindell G, Sturesson C. Fast-track program for liver resection--factors prolonging length of stay. Hepatogastroenterology. 2014;61:2340-2344. [PubMed] |
| 17. | Sturesson C, Nilsson J, Eriksson S, Spelt L, Andersson R. Limiting factors for liver regeneration after a major hepatic resection for colorectal cancer metastases. HPB (Oxford). 2013;15:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8554] [Article Influence: 407.3] [Reference Citation Analysis (8)] |
| 19. | Ninomiya M, Shirabe K, Ijichi H, Toshima T, Harada N, Uchiyama H, Taketomi A, Yoshizumi T, Maehara Y. Temporal changes in the stiffness of the remnant liver and spleen after donor hepatectomy as assessed by acoustic radiation force impulse: A preliminary study. Hepatol Res. 2011;41:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Colli A, Pozzoni P, Berzuini A, Gerosa A, Canovi C, Molteni EE, Barbarini M, Bonino F, Prati D. Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology. 2010;257:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 471] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 22. | Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A, García-Valdecasas JC. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | D’Onofrio M, Gallotti A, Mucelli RP. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am J Roentgenol. 2010;195:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 24. | Crespo G, Fernández-Varo G, Mariño Z, Casals G, Miquel R, Martínez SM, Gilabert R, Forns X, Jiménez W, Navasa M. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Jung EJ, Ryu CG, Kim G, Kim SR, Park HS, Kim YJ, Hwang DY. Splenomegaly during oxaliplatin-based chemotherapy for colorectal carcinoma. Anticancer Res. 2012;32:3357-3362. [PubMed] |
| 26. | Park S, Kim HY, Kim H, Park JH, Kim JH, Kim KH, Kim W, Choi IS, Jung YJ, Kim JS. Changes in Noninvasive Liver Fibrosis Indices and Spleen Size During Chemotherapy: Potential Markers for Oxaliplatin-Induced Sinusoidal Obstruction Syndrome. Medicine (Baltimore). 2016;95:e2454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19:7983-7991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 172] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (3)] |
| 28. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The ”50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828; discussion 828-829. [PubMed] |
| 29. | Karlas TF, Pfrepper C, Rosendahl J, Benckert C, Wittekind C, Jonas S, Moessner J, Tröltzsch M, Tillmann HL, Berg T. Acoustic radiation force impulse (ARFI) elastography in acute liver failure: necrosis mimics cirrhosis. Z Gastroenterol. 2011;49:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Aoki H, Memeo R, Yu WB S- Editor: Wang JL L- Editor: A E- Editor: Yan JL