©The Author(s) 2025.
World J Gastrointest Surg. Jul 27, 2025; 17(7): 105136
Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.105136
Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.105136
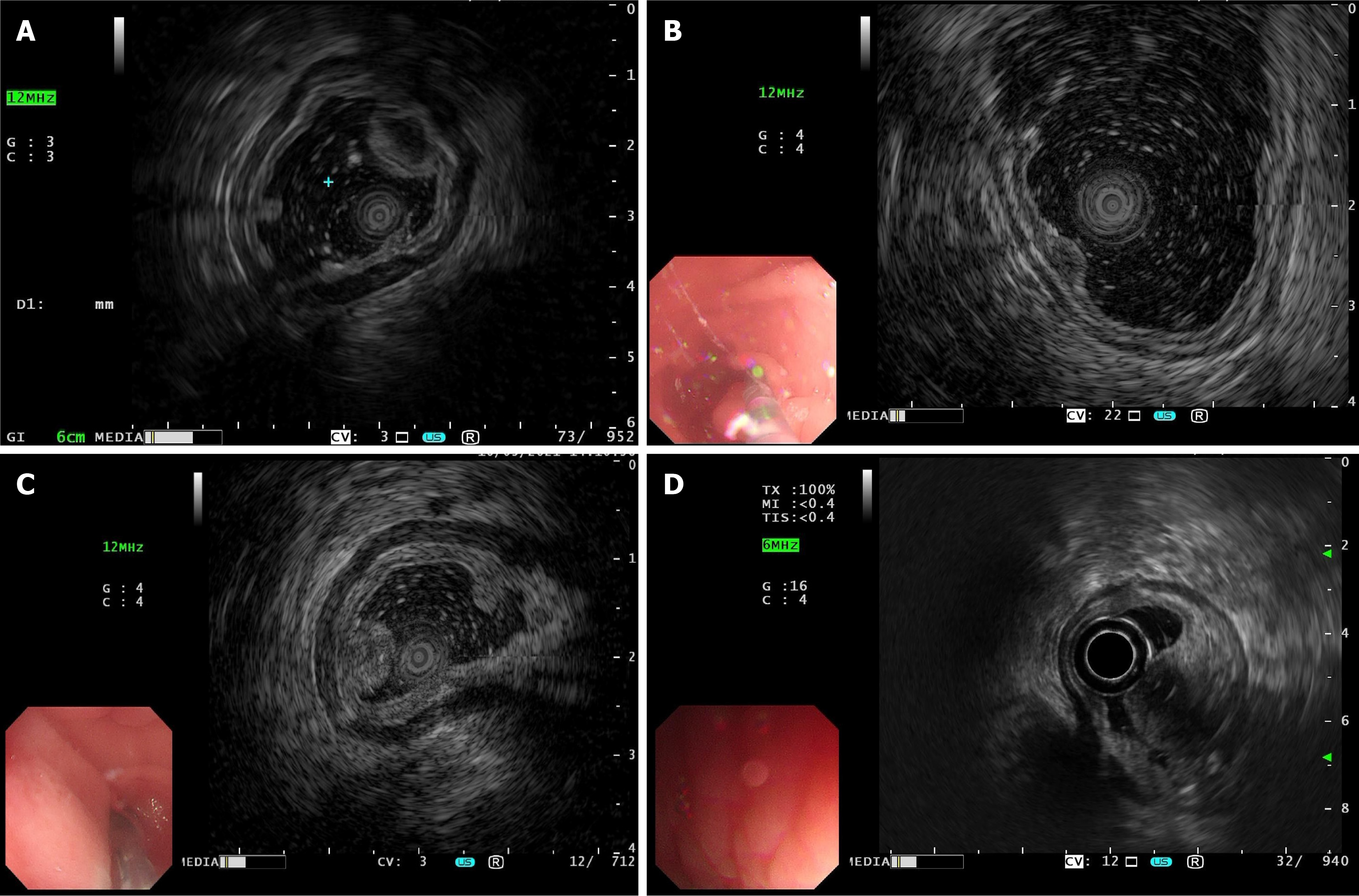
Figure 1 Different manifestations of gastric inflammatory fibroid polyps under endoscopic ultrasonography.
A: The lesion originated from the third layer, with low and homogeneous echo and indistinct margin, and diagnosed as a gastric neuroendocrine tumor by preoperative endoscopic ultrasonography (EUS), while the pathological results suggested a gastric inflammatory fibroid polyps (IFP) in the fibrovascular stage; B: The lesion originated from the second layer, with medium-low and homogeneous echo and distinct margin, and diagnosed as gastric polyp preoperatively by EUS while the pathological results suggested a gastric IFP in the fibrovascular stage; C: The lesion originated from the second layer, with medium-high and homogeneous echo and indistinct margin, and diagnosed as a gastric adenoma by preoperative EUS, while the pathological results suggested a gastric IFP in the fibrovascular stage; D: The lesion involved both the second and third layers, with high and heterogeneous echo and indistinct margin, and was diagnosed as a ectopic pancreas of the stomach by EUS preoperatively, while the pathological results suggested a gastric IFP in the fibrovascular stage.
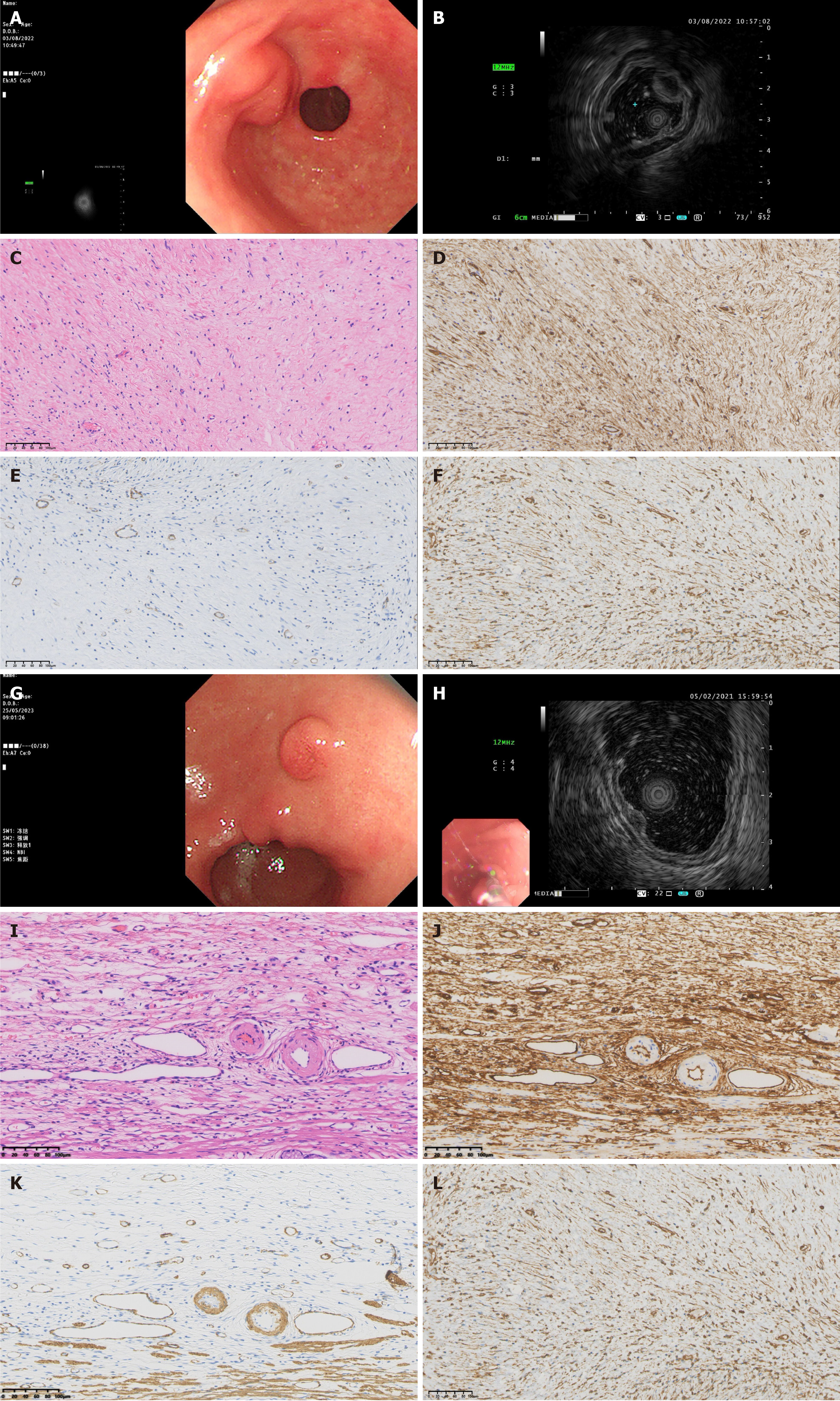
Figure 2 Endoscopic, ultrasonic endoscopic and pathological characteristics of gastric inflammatory fibroid polyps.
A-F: Nodular stage; A: General endoscopic features: A submucosal protrusion on the anterior wall of the gastric antrum near the pylorus, with smooth and intact surface mucosa; B: Endoscopic ultrasonography (EUS) features: The lesion originated from the third layer, with low echo, homogeneous echo and indistinct margin; C: Pathological characteristics: Immature fibroblastic-like spindle cell proliferation, few inflammatory cells such as eosinophils, and a small amount of thin-walled vascular proliferation, hematoxylin-eosin (HE): 200 ×; D: Immunohistochemical cluster of differentiation (CD) 34 +: 200 ×; E: Immunohistochemical smooth muscle actin (SMA) +: 200 ×; F: Immunohistochemical vimentin +: 200 ×; G-L: Fibrovascular stage; G: General endoscopic features: A submucosal protrusion on the lesser curvature of the gastric antrum, with hyperemia of the surface mucosa; H: EUS features: Lesion originated from the second layer, with medium-low echo, homogeneous echo and indistinct margin; I: Pathological characteristics: Mature fibroblastic-like spindle cell proliferation, thin- and thick-walled blood vessels are visible, and there is a significant eosinophil proliferation around the blood vessels. The proliferating spindle cells exhibit a classic “onion skin” pattern around small blood vessels, HE: 200 ×; J: Immunohistochemical CD34 +: 200 ×; K: Immunohistochemical SMA +: 200 ×; L: Immunohistochemical vimentin + 200 ×.
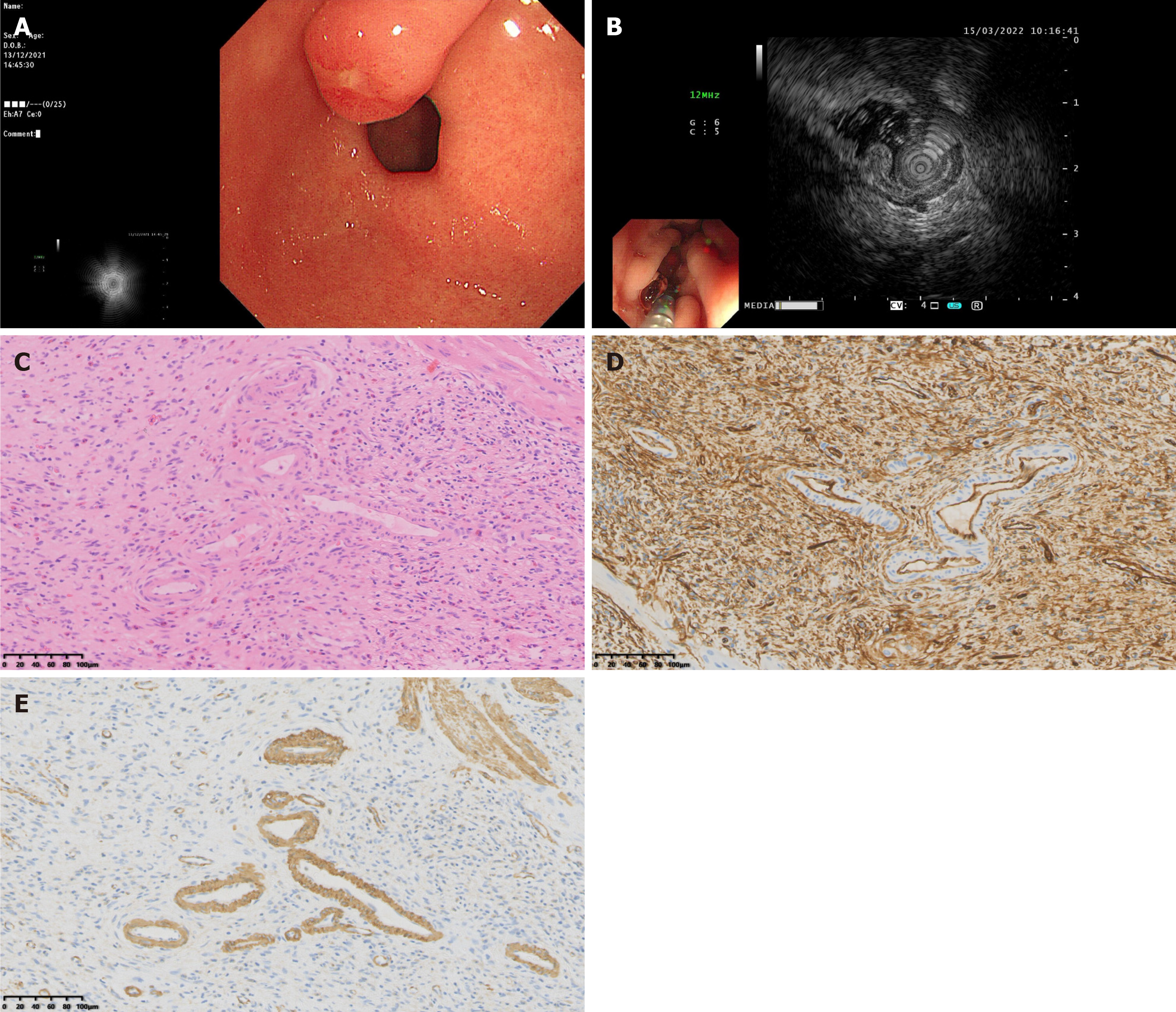
Figure 3 Endoscopic, ultrasonic endoscopic and pathological characteristics of sclerotic stage gastric inflammatory fibroid polyps.
A: General endoscopic features: A submucosal protrusion on the anterior wall of the gastric antrum, the surface mucosa was congested with ulcer formation on the top; B: Endoscopic ultrasonography features: The lesion involved both the second and third layer, with medium-high echo, heterogeneous echo and indistinct margin; C: Pathological features: Collagen fiber proliferation with concomitant hyalinization, thickened blood vessels, and extensive eosinophil infiltration, hematoxylin-eosin: 200 ×; D: Immunohistochemical cluster of differentiation 34 +: 200 ×; E: Immunohistochemical smooth muscle actin + 200 ×.
- Citation: Zhang FM, Ning LG, Wang XX, Du HJ, Zhu HT, Chen HT. Comparison of endoscopic ultrasonography features and pathological staging of gastric inflammatory fibroid polyps. World J Gastrointest Surg 2025; 17(7): 105136
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/105136.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.105136