©The Author(s) 2025.
World J Gastrointest Surg. Mar 27, 2025; 17(3): 100951
Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.100951
Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.100951
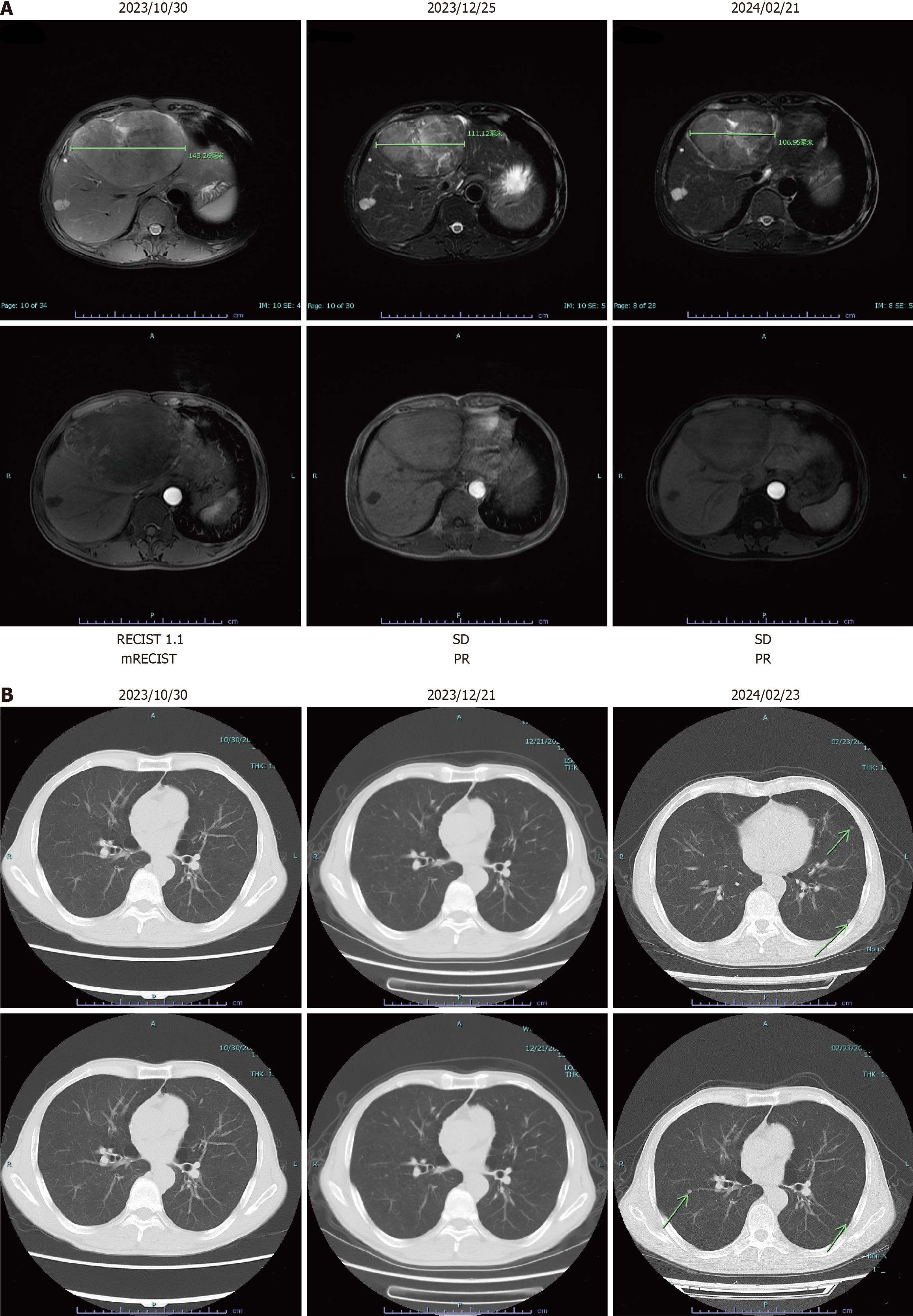
Figure 1 Imaging of liver and lung during different periods.
A: Radiologic assessments showed a partial response based on the modified Response Evaluation Criteria in Solid Tumors criteria; B: Computed tomography imaging examination indicated multiple metastatic sites in the lung on February 23, 2024. mRECIST: Modified Response Evaluation Criteria in Solid Tumors; PR: Partial response; SD: Stable disease.
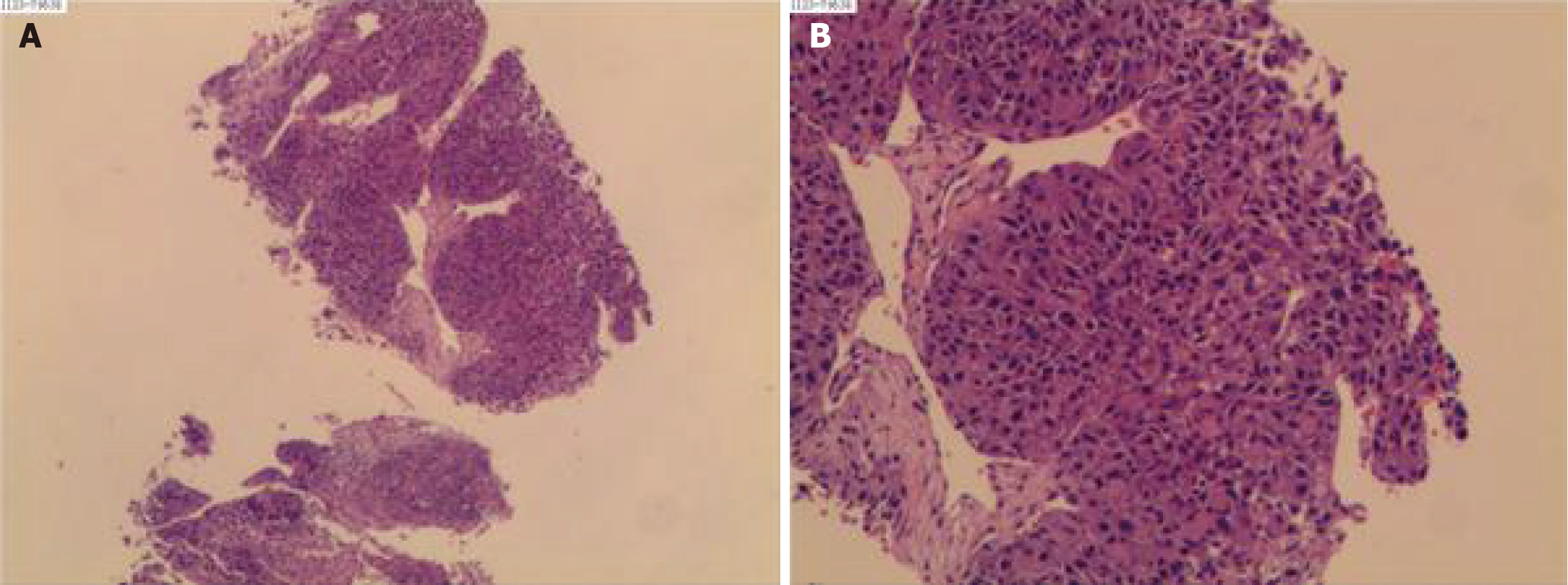
Figure 2 Liver Biopsy.
The outcome confirmed the diagnosis of hepatocellular carcinoma (poor differentiation) on December 06, 2023. A and B: The microscopic view of the liver biopsy.
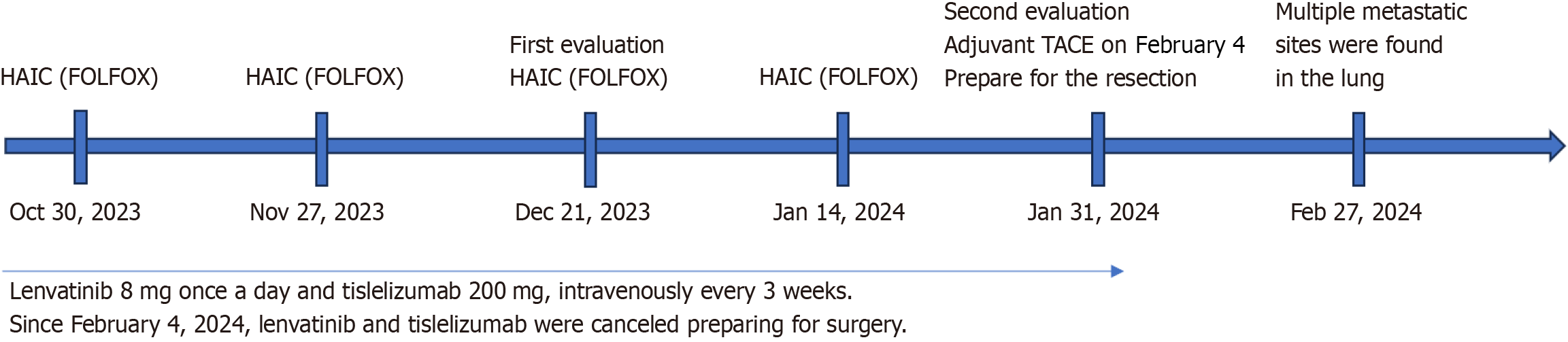
Figure 3 Timeline of treatment.
After four courses of the hepatic arterial infusion chemotherapy regimen, lenvatinib and tislelizumab were canceled, preparing for surgery. Multiple metastatic sites were found in the lung on February 27, 2024. HAIC: Hepatic arterial infusion chemotherapy; TACE: Transcatheter arterial chemoembolization.
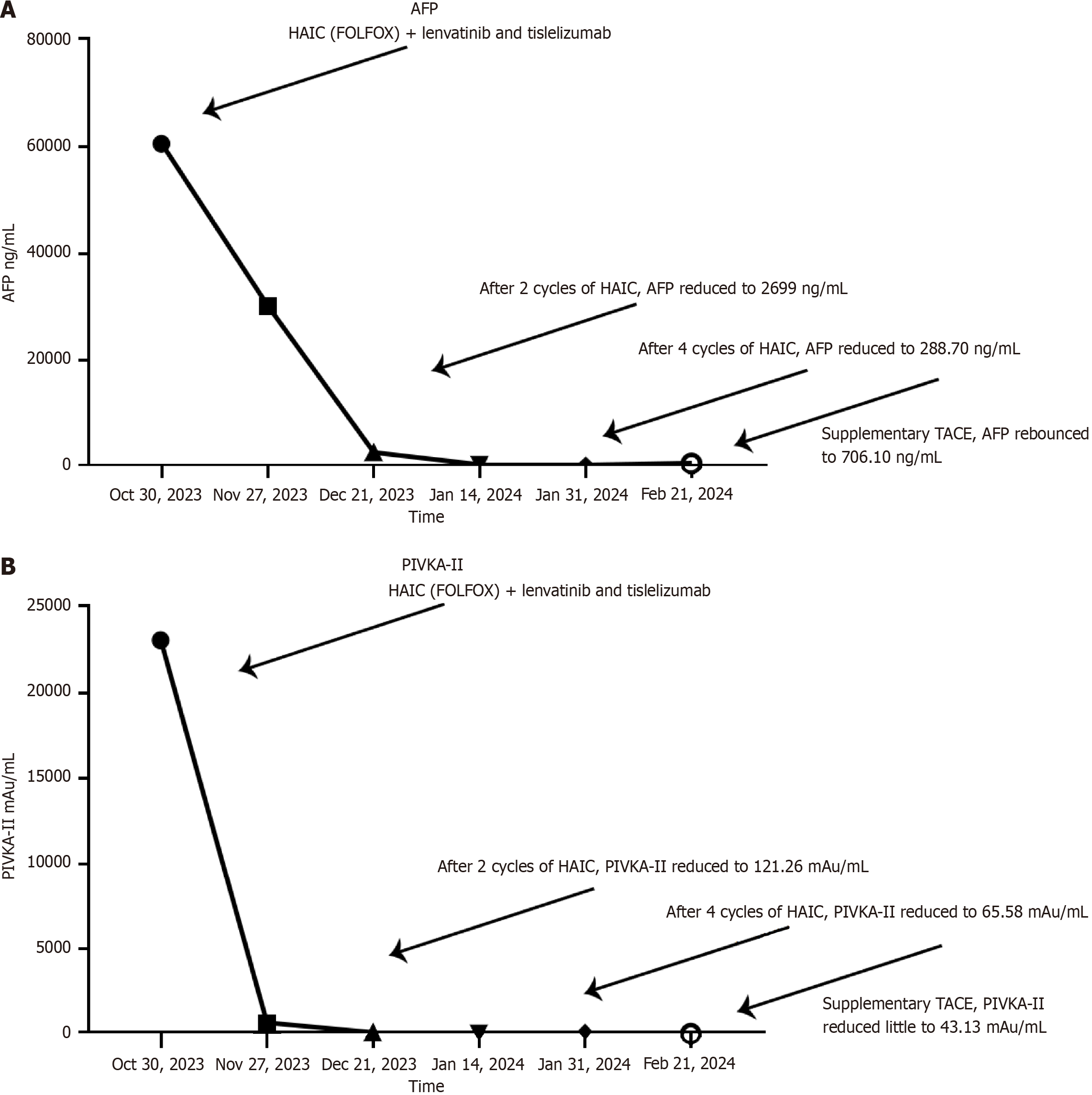
Figure 4 Trends of tumor markers during the treatment.
A: Alpha-fetoprotein levels of different periods, drastic decrease could be observed during the combined therapy, while a decrease was observed after supplementary transcatheter arterial chemoembolization on February 4, 2024, replacing hepatic arterial infusion chemotherapy with lenvatinib and tislelizumab; B: Prothrombin Induced by Vitamin K Absence or Antagonism Type II levels of different periods, continuous decline was observed. HAIC: Hepatic arterial infusion chemotherapy; TACE: Transcatheter arterial chemoembolization; AFP: Alpha-fetoprotein; PIVKA-II: Prothrombin Induced by Vitamin K Absence or Antagonism Type II.
- Citation: Wang CD, Liu RD, Liu MJ, Song J. Lung metastasis following temporary discontinuation of lenvatinib and tislelizumab in hepatocellular carcinoma: A case report. World J Gastrointest Surg 2025; 17(3): 100951
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/100951.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.100951