Published online Jun 16, 2017. doi: 10.4253/wjge.v9.i6.282
Peer-review started: January 19, 2017
First decision: March 8, 2017
Revised: March 30, 2017
Accepted: April 23, 2017
Article in press: April 24, 2017
Published online: June 16, 2017
Processing time: 149 Days and 5.3 Hours
An 87-year-old-man with prostate-cancer-stage-T1c-Gleason-6 treated with radiotherapy in 1996, recurrent prostate cancer treated with leuprolide hormonal therapy in 2009, and bladder-urothelial-carcinoma in situ treated with Bacillus-Calmette-Guerin and adriamycin in 2010, presented in 2015 with painless, bright red blood per rectum coating stools daily for 5 mo. Rectal examination revealed bright red blood per rectum; and a hard, fixed, 2.5 cm × 2.5 cm mass at the normal prostate location. The hemoglobin was 7.6 g/dL (iron saturation = 8.4%, indicating iron-deficiency-anemia). Abdominopelvic-CT-angiography revealed focal wall thickening at the bladder neck; a mass containing an air cavity replacing the normal prostate; and adjacent rectal invasion. Colonoscopy demonstrated an ulcerated, oozing, multinodular, friable, 2.5 cm × 2.5 cm mass in anterior rectal wall, at the usual prostate location. Histologic and immunohistochemical analysis of colonoscopic biopsies of the mass revealed poorly-differentiated-carcinoma of urothelial origin. At visceral angiography, the right-superior-rectal-artery was embolized to achieve hemostasis. The patient subsequently developed multiple new metastases and expired 13 mo post-embolization. Comprehensive literature review revealed 16 previously reported cases of rectal involvement from bladder urothelial carcinoma, including 11 cases from direct extension and 5 cases from metastases. Patient age averaged 63.7 ± 9.6 years (all patients male). Rectal involvement was diagnosed on average 13.5 ± 11.8 mo after initial diagnosis of bladder urothelial carcinoma. Symptoms included constipation/gastrointestinal obstruction-6, weight loss-5, diarrhea-3, anorexia-3, pencil thin stools-3, tenesmus-2, anorectal pain-2, and other-5. Rectal examination in 9 patients revealed annular rectal constriction-6, and rectal mass-3. The current patient had the novel presentation of daily bright red blood per rectum coating the stools simulating hemorrhoidal bleeding; the novel mechanism of direct bladder urothelial carcinoma extension into rectal mucosa via the prostate; and the novel aforementioned colonoscopic findings underlying the clinical presentation.
Core tip: Comprehensive literature review revealed 16 reported cases of bladder-urothelial-carcinoma involving rectum. None of these cases presented with daily rectal bleeding. Among 11 cases with direct extension, none had pathologically-proven rectal mucosal involvement. A case is reported of recurrent bladder-urothelial-carcinoma presenting with daily bright red blood per rectum coating stools from bladder-urothelial-carcinoma involving rectal mucosa. A hemorrhagic, multinodular, rectal mass, identified by colonoscopy, from direct extension of Bladder-Urothelial-Carcinoma via prostate to rectal mucosa underlies the presentation with daily bright red blood per rectum. This report shows that bladder-urothelial-carcinoma can cause rectal bleeding by directly extending to rectal mucosa.
- Citation: Aneese AM, Manuballa V, Amin M, Cappell MS. Bladder urothelial carcinoma extending to rectal mucosa and presenting with rectal bleeding. World J Gastrointest Endosc 2017; 9(6): 282-295
- URL: https://www.wjgnet.com/1948-5190/full/v9/i6/282.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i6.282
Bladder urothelial carcinoma rarely spreads to the gastrointestinal (GI) tract, with comprehensive literature review revealing only 33 previously reported cases; even more rarely surrounds or extends into the rectum with only 16 previously reported cases; and has not been previously reported to appear at colonoscopy as a rectal mucosal nodular mass from direct cancer extension from the bladder via the prostate (3 prior patients had rectal mucosal metastases without direct extension) (Tables 1-3)[1-21]. A novel case is reported in which bladder urothelial carcinoma directly extended to rectal mucosa, via the prostate; mimicked hemorrhoidal bleeding in presenting with chronic, daily, painless, bright red blood per rectum coating the stools; and demonstrated novel, colonoscopic findings underlying the clinical presentation.
| Patient No., age and sex | Prior oncologic history | Clinical presentation with GI involvement | Radiologic imaging subsequent endoscopy/surgery | Metastatic location:Pathologic diagnosis | Treatment | Outcome | Ref. |
| 1. 87-year-old man | Nineteen years PTA underwent external beam radiotherapy and leuprolide hormonal therapy for prostate cancer stage T1c Gleason 6. Five years PTA underwent Bacillus Calmette-Guérin immunotherapy and adriamycin chemotherapy for bladder urothelial carcinoma in situ stage Ta G1-2 | Painless, bright red blood coating stools for 5 mo. Rectal exam: Bright red blood per rectum and large, hard, fixed, multinodular, “prostate” mass. Hemoglobin = 7.6 g/dL | CT angiography: Mass containing air-fluid cavity replacing prostate, with rectal invasion. Colonoscopy: Ulcerated, friable, oozing, multinodular, hemorrhagic, 2.5 cm × 2.5 cm mass on anterior rectal wall, just proximal to dentate line | Rectum: Poorly differentiated carcinoma of urothelial origin | Abdominopelvic angiography: Successful right-superior-rectal-artery embolization using embolospheres | Stopped bleeding for 3 mo. Subsequently rebled. Underwent palliative colostomy for the rebleeding. Died 13 mo after diagnosis of rectal lesion | Current report |
| 2. 64-year-old man | Sixteen month PTA, underwent radical cystectomy, left nephroureterectomy, and right ureterocutaneostomy for Grade 3 urothelial carcinoma Stage pT3aN0. 11 mo PTA, received 3 courses of MVAC chemotherapy for lymph node metastases | Anorexia, tenesmus | Abdominopelvic CT: Focal, annular thickening of rectal wall | Rectum: Urothelial carcinoma | Fecal diversion | Died 2 mo later | Katayama et al[1] |
| 3. 60-year-old man | Prior high grade bladder urothelial carcinoma | Anal pain, fatigue, weight loss, and anorexia. Rectal exam: Hard, fixed, annular, constrictive mass, 6 cm from anal verge. Hemoglobin = 11.6 g/dL | Pelvic CT: Mass posterior to bladder. Perirectal wall thickening | Rectum: Grade 4 urothelial carcinoma | Chemotherapy with VP-16 and cisplatin in 3 mo cycles and external beam RT | Died 9 mo after initiating RT | Stillwell et al[2] |
| 4. 58-year-old man | Two year PTA underwent partial cystectomy for grade 3 N0 bladder urothelial carcinoma | Anorexia, weight loss, fatigue, straining with bowel movements, narrow-caliber stools, rectal pain, and tenesmus for several months. Rectal exam: hard, annular, constrict-ing lesion with a narrowed lumen, at 8 cm from anal verge | Pelvic CT: Large mass encircling rectum, lytic lesion in third lumbar vertebra, and bilateral hydronephrosis. Proctoscopy: Constricting lesion with normal overlying mucosa, suggestive of extrinsic compression. Exploratory laparotomy: Hard mass extending from posterior bladder wall, obliterating rectovesical pouch, and encompassing rectum | Rectum: Biopsy during proctos-copy showed normal mucosal tissue. Transrectal (deep) and transperineal biopsy: Poorly differentiated grade 3 urothelial cancer | Sigmoid loop colostomy, RT to pelvis and lumbar spine, followed by single dose of cisplatin | Died 3 mo later from liver metastasis | Stillwell et al[2] |
| 5. 73-year-old man | Three years PTA underwent radical cystoprostatectomy, with clear margins, and ileal loop urinary diversion for Stage pT3a N0 bladder urothelial carcinoma. At that time, biopsy also demonstrated areas of adenocarcinoma and signet ring cell carcinoma | Diarrhea, rectal pain, fatigue, weight loss, and fecal incontinence for 1 mo. Physical exam: Thin elderly male, bilateral lower extremity edema. Rectal exam: rectal stenosis 1 cm from anal verge. Guaiac negative stool | Abdominopelvic CT: annular rectal mass. Exploratory laparoscopy: Solid pelvic tumor adherent to sacrum | Rectum: Urothelial cancer invading muscularis propria of rectal wall | Diverting loop colostomy | Chemotherapy planned, but patient developed lower extremi- ty ischemia, requiring leg amputation. Died shortly thereafter | Langenstroer et al[3] |
| 6. 76-year-old man | Underwent left nephroureter-ectomy. 1 mo PTA underwent right ureteral diverting cutaneostomy for grade 3 bladder urothelial carcinoma. Bladder mass firmly attached to pelvic wall and to thickened lateral pedicles | Symptoms of rectal obstruction. Rectal exam: Stenosis with intact rectal mucosa | Pelvic CT: Annular thickening of rectal wall and thickened lateral pedicles, bilaterally | Rectum: Urothelial carcinoma | Diverting colostomy and unspecified immunotherapy | Died 5 mo later | Kobayashi et al[4] |
| 7. 66-year-old man | No prior oncologic history | Rectal exam: Severe rectal stenosis with intact rectal mucosa | Abdominopelvic CT: Thickened bladder and rectal walls, bilateral hydronephrosis. Colonoscopy: Narrow rectal lumen with edematous mucosa, suggesting extrinsic compression | Rectum: Grade 3 urothelial carcinoma | Ileal-conduit and colostomy | Died 3 mo after surgery | Kobayashi et al[4] |
| 8. 51-year-old man | 1 mo PTA underwent ureterocutaneostomy for unresectable grade 3 bladder urothelial carcinoma attached to pelvic wall, causing bilateral hydronephrosis | Thin stools. Rectal exam: Narrow rectal lumen | Pelvic CT: Annular constriction of rectum | Rectum: Grade 3 urothelial carcinoma | Diverting colostomy and one course of M-VAC chemotherapy | Died 10 mo after surgery | Kobayashi et al[4] |
| 9. 74-year-old man | Eleven months PTA underwent radical cystectomy for grade 3 urothelial carcinoma of bladder | Continuous watery rectal discharge and thin stools | Barium enema: Stenosis of lower rectum Pelvic MRI: Thickened rectal mucosa and muscle layer without evident tumor | Rectum: Grade 3 pT3a urothelial carcinoma | Colostomy, MVAC chemotherapy, and radiation | Died 7 mo after presentation | Ito et al[5] |
| 10. 54-year-old man | Underwent radical cystoprostatectomy with neobladder for grade 3 bladder urothelial carcinoma | Presumed refractory ulcerative proctitis | Pelvic MRI: Circumferential thickening of rectum. Endoscopy: Circumferential rectal wall thickening 11 cm from anal verge. EUS: Circumferential hypoechoic infiltrate extending through all rectal wall layers | Rectum: Urothelial carcinoma | Chemotherapy | NR | Gleeson et al[6] |
| 11. 55-year-old man | Underwent radical cystoprostatectomy with neobladder for grade 3 bladder urothelial carcinoma | Constipation | Abdominopelvic CT: No evident metastasis Endoscopy: Circumferential rectal wall thickening with stricture 16 cm from anal sphincter EUS: Diffuse circumferential thickening of rectal wall | Rectum: urothelial carcinoma | Chemotherapy | NR | Gleeson et al[6] |
| 12. 60-year-old man | Underwent radical cystoprostatectomy with neobladder, for grade 3 urothelial cancer of bladder | Constipation | Abdominopelvic CT: Abnormal perirectal lymph nodes. Endoscopy: Circumferential rectal wall thickening. EUS: Diffuse circumferential thickening of all layers of rectal wall with several hypoechoic lymph nodes in extraluminal space | Rectum: Urothelial carcinoma | Chemotherapy | NR | Gleeson et al[6] |
| Patient age, sex | Prior oncologic history | Clinical presentation with GI involvement | Radiologic imaging, endoscopy, surgery | Metastasis location: Pathologic diagnosis | Treatment | Outcome | Ref. |
| 1. 63-year-old man | Ten months PTA underwent radical cystectomy and MVAC chemotherapy for bladder urothelial carcinoma | Painless jaundice, 5-kg weight loss, and constipation for 2 wk. Physical exam: mild right upper quadrant tenderness. Laboratory: Elevated liver function tests | Abdominopelvic CT: Concentric thickening of rectal wall; bile duct hilar stricture with diffuse intrahepatic ductal dilation. MRI: Diffusely thickened common hepatic duct with extension into secondary branch ducts suspicious for cholangiocarcinoma. Colonoscopy: Concentric rectal constriction blocking colonoscopic intubation. ERCP: Strictures of common hepatic and right intrahepatic ducts; obstructed left intrahepatic duct | Rectum and hepatic duct: Micropapillary variant of transitional cell (urothelial) carcinoma | Rectal tumor: RT with external beam and brachy-therapy. Hepatic tumor: Polyethylene stent placed in intrahepatic bile duct. RT is planned | Alive at 4 mo | Hong et al[7] |
| 2. 55-year-old man | Fifteen months PTA underwent TURBT and 6 wk of mitomycin C, followed by 4 rounds of gemcitabine and cisplatin chemotherapy for high grade urothelial carcinoma of bladder with iliac lymph node chain involvement. Six months PTA underwent radical cystoprostatectomy with neobladder creation and pelvic lymphadenectomy | Worsening constipation, abdominal distention, anorexia, and dyschezia. Rectal exam: palpable mass 3 cm from anal verge | Abdominopelvic CT: Pelvic and omental nodules. PET: Increased uptake at these nodules. Flexible sigmoidoscopy: 3 cm wide rectal lesion near anal verge | Rectum, omentum, other pelvic structures: Urothelial carcinoma | Diverting loop colostomy | Brain and lung metastases | Asfour et al[8] |
| 3. 77-year-old man | Eleven years PTA underwent resection of papillary bladder urothelial carcinoma. Eight years PTA underwent TURBT and RT for recurrence. Underwent periodic cystoscopies and bladder biopsies thereafter | Progressive constipation, weight loss, and malaise | Barium enema: barium could not pass beyond sigmoid colon. Laparotomy: Sigmoid colon obstructed due to adherent tumor of terminal ileum and cecum | Sigmoid, right-transverse colon, cecum, ileum, appendix, omentum: Urothelial carcinoma | Ileotransverse colostomy and loop colostomy of descending colon | NR | Aigen et al[9] |
| 4. 60-year-old man | Five months PTA underwent radical cystectomy with ileal conduit for invasive bladder urothelial carcinoma | Painless hematochezia. Rectal exam: Red blood in rectal vault. No externally visible or palpable hemorrhoids. Hemoglobin declined from 11.6 g/dL to 8.7 g/dL | Necrotic pelvic lesions suspicious for metastases vs abscess. Colonoscopy: Irregular, friable, partially obstructing mass at splenic flexure | Splenic flexure: Urothelial carcinoma | None | Refused treatment. Transferred to hospice | Kumar et al[10] |
| 5. 57-year-old man | Five years PTA underwent total cystectomy for bladder urothelial carcinoma. One year PTA underwent lymph node resection, RFA, bone cement injection, and chemotherapy for left obturator lymph node and several pulmonary and left pelvic bone metastasis. Five months PTA underwent RT for regrowth of left obturator lymph node metastasis | Massive melena, HR = 120 beats/min, BP = 76/39 mmHg, Hemoglobin = 9.2 g/dL | Abdominopelvic CT: Malignant lymph node invading sigmoid colon, with pseudoaneurysm of mesenteric artery supplying sigmoid Colonoscopy: Large, oozing, ulcerated colonic tumor | Sigmoid colon: NA | Pelvic angiogram: 10 mm × 8 mm pseudoaneurysm of left inferior gluteal artery successfully embolized using microcoils and vasopressin | Alive at 5 mo | Kakizawa et al[11] |
| 6. 83-year-old man | No prior oncologic history | Diarrhea and weight loss during prior 6 mo. Rectal exam: Mass 3 cm from anal verge | Abdominopelvic CT: Thickened right posterior wall of bladder, circumferential rectal wall thickening, and infiltrative lesions in multiple skeletal muscles Proctoscopy: Mass 3 cm from rectal verge | Rectum and skeletal muscles: Poorly differentiated urothelial carcinoma | Chemotherapy (regimen not specified) | NR | Ying-Yue et al[12] |
| 7. 54-year-old man | Two years PTA underwent radical cystectomy and ileal neobladder reconstruction for grade 3 bladder urothelial carcinoma | Change in bowel habits | Abdominopelvic MRI: Circumferential thickening and high-grade stenosis of rectal wall. Sigmoidoscopy: Luminal narrowing with erythematous and edematous folds. EUS: Hypoechoic, circumferential, rectal wall thickening, mimicking primary rectal cancer. No evident direct cancer extension from bladder | Rectum: Urothelial carcinoma | Chemotherapy (regimen not specified) | NR | Yusuf et al[13] |
| 8. 73-year old man | Two years PTA underwent resection of grade 2 bladder urothelial carcinoma | Severe constipation | Sigmoidoscopy: Friable, erythematous, and thickened distal rectal wall, with nearly obstructed lumen. EUS: Hypoechoic, symmetric, circumferential wall thickening, with loss of deep wall layers, and pseudopodia-like extensions into perirectal tissues. No evident direct tumor extension from bladder | Rectum: Poorly differentiated urothelial carcinoma | Total pelvic exenteration and chemotherapy (regimen not specified) | NR | Yusuf et al[13] |
| 9. 67-year-old man | Eighteen months PTA underwent transurethral excisional biopsy of bladder cancer. Ten months PTA underwent partial cystectomy, chemotherapy with gemcitabine, and RT. 1 mo PTA, nephrostomy tubes inserted for bilateral hydronephrosis | Massive rectal bleeding and altered mental status for one day. HR = 106 beats/min, BP = 65 mmHg/palpable. Rectal exam: Rectal mass and large amount of bright red blood and clots. Hemoglobin = 8.0 g/dL | Selective angiography of celiac trunk, superior mesenteric artery and inferior mesenteric artery: No bleeding source identified. Colonoscopy: Large amount of bright red blood in colon. Emergency laparotomy: Indurated, fixed, mass involving cecum, right lower retroperitoneum, and right pelvic side wall. Dilated colon. Active bleeding from fistula between iliac artery and cecum | Cecum: Urothelial carcinoma | Resection of cecum and terminal ileum, ligation of right external iliac artery, end-ileostomy | Alive at 6 mo | Chin et al[14] |
| Patient age and sex | Prior oncologic history | Clinical presentation with GI involvement | Radiologic imaging, endoscopy, surgery | Metastasis location: Pathologic diagnosis | Treatment | Outcome | Ref. |
| 1. 55-year-old man | 1 mo PTA underwent total cystoprostatectomy, bilateral ilio-obturator lymphadenectomy, and bladder reconstruction for bladder urothelial carcinoma pT3-GIII, N0 | Hematemesis 8 d after surgery | Chest and abdominopelvic CT: Esophageal mass. EGD: 2-cm-wide mass in proximal esophagus. EUS: No lymphadenopathy | Esophagus: Urothelial carcinoma infiltrating submucosa | Chemotherapy with M-VAC, and RT of metastasis | Developed radiation pericarditis but recovered. Alive at 2 yr | Jung et al[15] |
| 2. 66-year-old man | No prior oncologic history | Dysphagia, anorexia, weight loss, headaches, and lightheadedness for 6 wk. Palpable, tender, 2 cm mass on left neck | Neck and thoracic CT: 3 cm × 2 cm soft tissue mass with dilation and thickening of proximal esophagus. EGD: Focal stricture at 25 cm from incisors with a 2 cm × 1 cm ulcer with irregular margins | Esophagus: Poorly differentiated urothelial carcinoma | None | Died 10 d after discharge from hospital | Dy et al[16] |
| 3. 80-year-old man | Four years PTA underwent RT and chemotherapy (after declining radical cystectomy) for bladder urothelial carcinoma. Three years PTA underwent lung lobe wedge resection for solitary lung metastasis. 1 mo PTA had a normal EGD and colonoscopy in evaluation of anemia | Malaise, dizziness, dyspnea, melena. Rectal exam: Positive occult blood in stool. Hemoglobin = 5.4 g/dL | Small bowel enteroscopy: 3 cm, ulcerated, infiltrating tumor in distal duodenum. Tumor has an adherent, friable, clot | Duodenum: High-grade urothelial carcinoma | Duodenectomy and duodenomy jejunostomy | PET scan 2 mo later: Metastases to liver and lungs. Patient expired soon thereafter from cardiac arrhythmia | Girotra et al[17] |
| 4. 62-year-old man | Two years PTA underwent partial cystectomy with lymph node dissection and adjuvant chemotherapy for stage IIIb bladder urothelial carcinoma | Hematemesis, hemoglobin = 7.0 g/dL | EGD: Large bleeding mass in descending duodenum. Treated with proton pump inhibitor therapy. Repeat EGD 4 d later: large partly obstructing, 7-cm-long mass in descending duodenum | Duodenum: Poorly differentiated urothelial carcinoma | Palliative radiation | Died 6 wk later | Chan et al[18] |
| 5. 74-year-old man | Four years PTA underwent exploratory laparotomy which demonstrated nodal metastasis. Completed preoperative chemotherapy, but declined surgical resection | Abdominal pain, bloating, distention, nausea, and vomiting | Serial pelvic CT (to monitor cancer progression): Stable bladder wall thickening Small bowel barium contrast radiography: Narrowing of third portion of duodenum Gastroscopy: Fluid-filled, dilated, stomach without obstruction. EGD: Luminal narrowing with overlying normal mucosa in third portion of duodenum. EUS: Circumferential wall thickening | Duodenum: urothelial carcinoma | Enteral stent and palliative chemotherapy | Died 9 mo later | Yusuf et al[13] |
| 6. 42-year-old woman | No prior oncologic history | Nausea, vomiting, abdominal discomfort, and 6-kg weight loss for 2 mo | Barium meal: Abrupt stricture at junction between second and third portion of duodenum. Abdominopelvic CT: Infiltrative soft tissue mass around duodenum, calcified bladder wall. No pelvic lymphadenopathy. EGD: Gastric outlet obstruction with distorted and erythematous duodenum without ulceration, or mucosal tumor | Duodenum: Micropapillary variant of poorly differentiated urothelial carcinoma | Duodenal stent and RT to periduode-nal lesion. Administered palliative gemcitabine and carboplatin | Died 15 mo after diagnosis | Hawtin et al[19] |
| 7. 87-year-old man | Sixteen months PTA underwent TURBT for grade 3, pT2bN0M0, bladder urothelial carcinoma | Ileus | Abdominopelvic CT: Pneumoperitoneum due to GI perforation Laparotomy: Elastic hard tumor at site of ileal perforation | Ileum: Metastatic urothelial carcinoma | Partial resection of ileum | NA | Hoshi et al[20] (in Ja-panese) |
| 8. 53-year-old man | No prior oncologic history | Gross hematuria | Abdominopelvic CT: Bladder tumor invading prostate. Cystoscopy: Non-papillary, broad based, tumor in right wall of bladder | Ileum and prostate: Urothelial carcinoma pT4aN1M0 | Total cystec--tomy and creation of ileal conduit. Neoadjuvant chemotherapy | NA | Hoshi et al[20] (article in Japanese) |
| 9. 56-year-old man | Fifty-nine months PTA underwent TURBT for bladder urothelial carcinoma | Abdominal pain and GI perforation | NA | Small intestine, lymph nodes, lung, and liver: Urothelial carcinoma | NA | NA | Hoshi et al[20] (Case from table 2) |
| 10. 63-year-old woman | Seven months PTA underwent total cystectomy for pT3b bladder urothelial carcinoma | Abdominal pain | NR | Small intestine: Urothelial carcinoma | NR | NR | Hoshi et al[20] (Case from table 2) |
| 11. 46-year-old man | Thirty-eight months PTA underwent RT and chemotherapy for pT3b bladder urothelial carcinoma | Ileus | NR | Small intestine: Urothelial carcinoma | NR | NR | Hoshi et al[20] (Case from table 2) |
| 12. 71-year-old man | Thirty-six months PTA underwent total cystectomy for bladder urothelial carcinoma | Melena and anemia | NR | Small intestine: Urothelial carcinoma | NR | NR | Hoshi et al[20] (Case from table 2) |
| 13. 55-year-old man | Seven years PTA underwent total cystectomy, pelvic lymphadenectomy, and neobladder reconstruction. Underwent two cycles of adjuvant chemotherapy for pT3apN0 G2 bladder urothelial carcinoma | Massive melena, HR = 120 beats/min, BP = 72/36 mmHg. Hemoglobin = 7.9 g/dL | Abdominopelvic CT: Right hydronephrosis from external iliac lymph node metastasis invading ileum. Angiography: Right external iliac artery successfully embolized using microcoils and n-butyl cyanoacrylate. Then developed ischemic colitis, treated with iliac artery bypass grafting, and right common and internal iliac artery embolization | Ileum: NR | Three cycles of unspecified chemotherapy | Died 4 mo after embolization | Honda et al[21] |
The literature was systematically reviewed using PubMed with the following medical subject heading (MeSH) or keywords: {“rectum” or “rectal” or “sigmoid” or “descending colon” or “transverse colon” or “ascending colon” or “cecum” or “large intestine” or “large bowel” or “colon” or “duodenum” or “jejunum” or “ileum” or “jejunoileum” or “small intestine” or “small bowel” or “stomach” or “gastric” or “esophageal” or “esophagus”} and {“bladder cancer” or “bladder carcinoma” or “urothelial carcinoma” or “transitional cell carcinoma”}. Two authors independently reviewed the literature, and decided by consensus which articles to incorporate in this study. Patients with bladder adenocarcinoma; renal cell carcinoma, even with bladder involvement; or urothelial (transitional cell) carcinoma solely extrinsic to the bladder were excluded. One article written in French was professionally translated[15]. Data about 2 cases in one article, written in Japanese, were obtained from the abstract written in English[20]. Data regarding 4 cases were derived from a table present in one reference[20]. This case report received exemption/approval by the IRB at William Beaumont Hospital, Royal Oak, on August 27, 2015. Informed consent for publication was unobtainable from the patient because he had expired prior to writing this case report.
An 87-year-old, severely debilitated man with a 20-year-long oncologic history presented in 2015 with daily, painless, bright red blood per rectum coating the stool for five months. He had been treated in 1996 for prostate adenocarcinoma stage-T1c-Gleason-6 with external beam radiotherapy; retreated in 2009 for recurrent prostate adenocarcinoma with leuprolide hormonal therapy every 3 mo; and treated in 2010 for bladder urothelial carcinoma in situ stage-TCC-Ta-G1-2 with bacillus-Calmette-Guerin and adriamycin. In 2014 the patient underwent suprapubic catheter placement for severe urinary frequency, urinary incontinence, recurrent urethral stricture, and hematuria. Four months prior to admission the patient underwent cystoscopy for refractory, severe hematuria, which demonstrated a small, contracted bladder, chronic urethral stricture, and friable, hemorrhagic tissue at the bladder neck felt most likely secondary to recurrent (invasive) urothelial carcinoma, but radiation cystitis could not be excluded. The hemorrhagic area was treated with electrocautery during cystoscopy, and by continuous bladder irrigation administered for 24 h. No biopsies were obtained during cystoscopy because the patient refused aggressive therapy of chemotherapy or surgery based on his old age, severe chronic debilitation, and multiple prior cancers.
Physical examination revealed normal vital signs; a soft, nontender, abdomen; and no palpable abdominal mass. Rectal examination revealed a hard, fixed, multinodular, 2.5 cm × 2.5 cm mass, at the normal prostate location; gross bright red blood on the examining finger; and no visible or palpable hemorrhoids. The hemoglobin = 7.6 gm/dL, with iron deficiency anemia (iron = 26 mcg/dL, total iron binding capacity = 301 mcg/dL, iron-saturation = 8.4%). Hemoglobin increased to 9.7 gm/dL after transfusing 2 units of packed erythrocytes. The prostate specific antigen (PSA) = 43.3 ng/mL (normal < 2.5 ng/mL). Urinalysis revealed significant hematuria, trace proteinuria, nitrite positivity, and bacteriuria with 26-50 leukocytes/high power field (hpf). There were 10000-50000 colony forming units/mL of Enterobacter cloacae isolated from a urine culture. The patient was administered ceftriaxone 1 g/24 h for the bacteriuria.
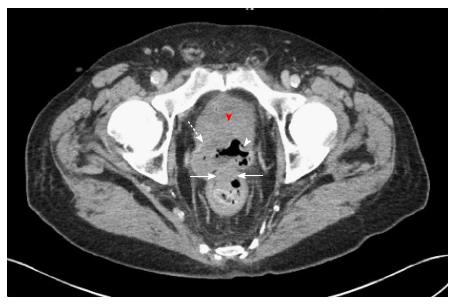
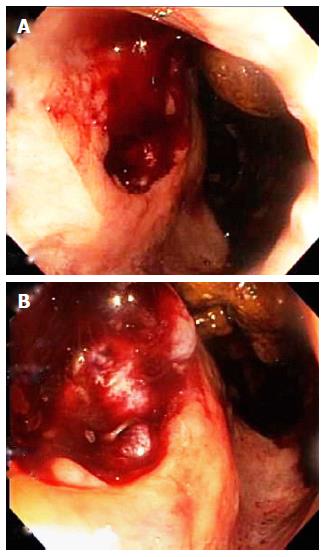
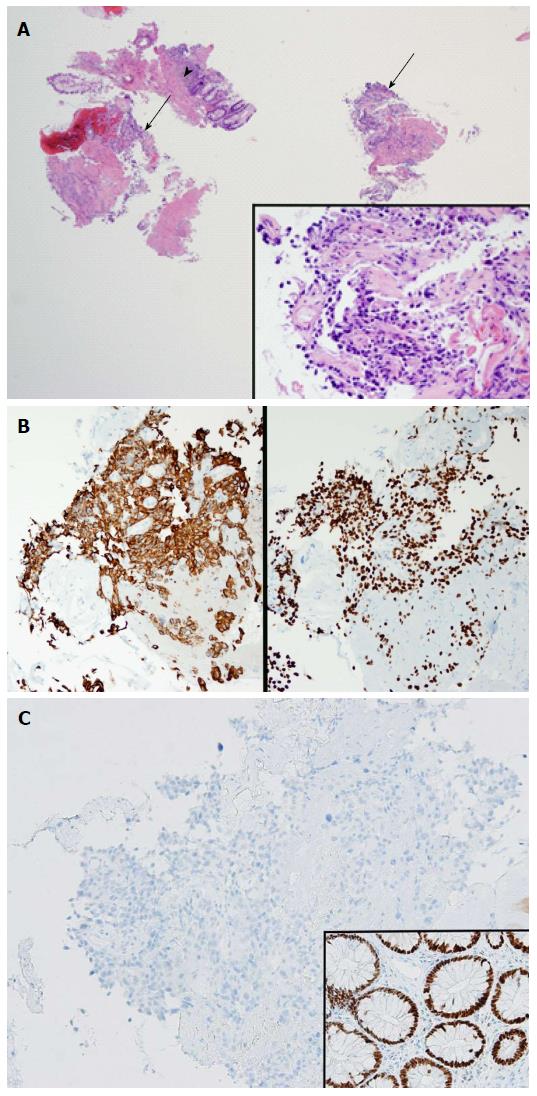
Abdominopelvic CT angiography revealed focal thickening of the bladder wall at its neck; a mass containing an air cavity replacing most of the prostate; and adjacent rectal invasion (Figure 1). Colonoscopy, with rectal retroflexion, demonstrated an ulcerated, friable, multinodular, 2.5 cm × 2.5 cm mass in the anterior rectal wall, just proximal to the dentate line, at the usual prostate location (Figure 2), no internal hemorrhoids, and no mucosal telangiectasia or other signs of radiation proctitis despite prior prostate radiotherapy. Histologic examination of rectal mucosal biopsies revealed poorly differentiated carcinoma (Figure 3A). Immunohistochemical analysis demonstrated the tumor cells stained positively with cytokeratin 20, indicating either a colonic or bladder (urothelial) primary (Figure 3B). Additional diffuse positivity for cytokeratin 7, 34bE12, and GATA-3 (Figure 3B); and focal positivity for CK5/6 strongly supported urothelial origin. Negative immunohistochemical staining for CDX2 (Caudal Type Homeobox 2) confirmed that this tumor did not arise from colonic adenocarcinoma (Figure 3C). The diffuse positivity for cytokeratin 20 and only focal positivity for CK5/6 (< 20% of cells positive) excluded anorectal squamous carcinoma. Immunohistochemical markers for prostate carcinoma, including PSA, PAP and P501S, were all negative. The history of non-invasive urothelial carcinoma in bladder biopsies in 2010, further supported the link between the cancers located in the bladder and rectum. The pathologic diagnosis was therefore poorly-differentiated carcinoma of urothelial origin.
At visceral angiography, the right-superior-rectal-artery was successfully embolized using 900-1200-micron embolospheres to achieve rectal hemostasis. However, the patient experienced recurrent rectal bleeding three months later requiring periodic packed erythrocyte transfusions and eventually requiring palliative colostomy. CT scans of chest, abdomen, and pelvis ten months post-embolization identified new hepatic and pulmonary metastases. At this time the patient also had progression of the bladder urothelial carcinoma with bilateral ureteral obstruction, prominent rectovesical fistula, and bilateral hydronephrosis that required bilateral nephrostomy tube placement. The patient expired 3 mo thereafter.
Bladder urothelial carcinoma, the most common urinary tract cancer, constitutes the fourth leading cause of cancer mortality in males, and the ninth leading cause of cancer mortality in females[10]. Urothelial carcinoma is the predominant histologic type in the United States and Europe, accounting for 90% of bladder cancers, but non-urothelial carcinomas are relatively more common in other countries[17].
This cancer most commonly metastasizes to lungs, liver, and bone, via lymphogenous or hematogenous routes. It rarely extends or metastasizes to the GI tract[10]. Comprehensive literature review revealed only 33 previously reported cases (or 34 cases including the present case) of GI involvement including: Direct extension to rectum-12 (Table 1); metastases to rectum-5, cecum-1, splenic flexure-1, sigmoid colon-1, and multiple colonic segments-1 (Table 2); and metastases to duodenum-4, ileum-3, esophagus-2, appendix-1, and unspecified-3 (Table 3). No metastases occurred in the stomach. The mean patient age was 64.0 ± 11.3 years (SD). Thirty-two patients were male, and only 2 patients were female. Patients developed GI extension/metastases on average, 28.5 ± 30.3 mo after the initial diagnosis of bladder urothelial carcinoma. Therapies for the primary bladder urothelial carcinoma prior to GI extension/metastases included radical or partial cystectomy-19, adjuvant chemotherapy-11, and radiotherapy-6 (including patients receiving multiple therapies). Presenting symptoms with GI involvement included: (1) systemic/extraintestinal symptoms of weight-loss-8, malaise/fatigue-5, anorexia-5, dizziness-2, dyspnea-2, and encephalopathy-1; (2) generalized abdominal symptoms of abdominal pain-4, abdominal distention-2, ileus-2, and GI obstruction-1; (3) GI bleeding including hematemesis-4, melena-4, and hematochezia-3; (4) upper GI symptoms of nausea/vomiting-2, and dysphagia-1; and (5) lower GI symptoms of constipation-7, diarrhea-4, anal pain-3, tenesmus-2, and fecal incontinence-1. Rectal examination, performed in 12 patients, revealed rectal stenosis-6, rectal mass-4, bright red blood per rectum-2, and fecal occult blood-1. The average hemoglobin on presentation was 8.8 ± 2.2 g/dL. Three patients presented with profound hypovolemia, manifesting as severe tachycardia or hypotension.
Colonoscopy, performed in 9 patients, revealed extrinsic colorectal constriction-5, ulcerated/friable mass-3, and profuse bright red blood per rectum-1 (Tables 1 and 2). Flexible sigmoidoscopy, performed in 5 patients, revealed extrinsic constriction-4, and rectal mass-1 (Table 2). Esophagogastroduodenoscopy, performed in 6 patients, revealed stricture of esophagus or duodenum-2; a moderately large, non-obstructive, mass in esophagus or duodenum-2; gastric outlet obstruction-1, and normal findings-1 (Table 3). Enteroscopy revealed a large, ulcerated, duodenal mass in one patient (Table 3). Endoscopic ultrasound performed in 7 patients, revealed hypoechoic, circumferential, rectal wall thickening in 6 patients (Tables 1 and 2).
On presentation with GI metastases, three patients had pathologically-proven extra-intestinal metastases, including one patient with common hepatic duct and intrahepatic duct strictures, identified by endoscopic retrograde cholangiopancreatography. Three other patients had suspected extraintestinal metastases identified by radiologic imaging.
Thirteen patients underwent surgery, including, diverting surgery (such as colostomy and ileostomy)-8, and local bowel resection-5. Seven patients underwent radiotherapy with external beam or brachytherapy. Sixteen patients underwent chemotherapy with carboplatin, cisplatin, etoposide, and gemcitabine; or with methotrexate, vinblastine, adriamycin and cisplatin. The prognosis remains poor for metastatic bladder urothelial carcinoma. Six patients expired from the cancer at a mean of 6.0 ± 4.5 mo. Four patients were reported alive at a mean of 9.8 ± 9.5 mo of follow-up. Most patients, however, had limited follow-up.
Bladder urothelial carcinoma has been previously reported to involve the rectum in 16 cases, including 11 cases by direct extension, as demonstrated by abdominopelvic CT-8, rectal endoscopic ultrasound (EUS)-3, and abdominopelvic magnetic resonance imaging (MRI)-2; and including 5 cases by metastases as demonstrated by abdominopelvic CT-2, EUS-2, MRI-1, and positron emission tomography (PET) scan-1 (1 patient had 2 diagnostic tests) (Tables 1 and 2). Rectal involvement was pathologically proven at surgery-6, by fine needle aspiration during EUS-5, colonoscopic biopsies-3, and transrectal needle biopsy-2. None of the 11 patients with direct bladder extension to rectum had pathologically-proven involvement of rectal mucosa; three of the 5 patients with rectal metastases had pathologically-proven mucosal involvement. Three patients had proven extraintestinal metastases at the time of diagnosis of rectal involvement, including hepatic duct-1, omentum and pelvic organs-1, and skeletal muscles-1. The average patient age at diagnosis of rectal involvement was 63.7 ± 9.6 years. All 16 patients were male. Rectal involvement was diagnosed on average 13.5 ± 11.8 mo after the bladder urothelial carcinoma was first diagnosed (includes 2 patients with simultaneous diagnosis of primary bladder urothelial carcinoma and rectal involvement, excludes 4 cases in which time interval between the two diagnoses was not reported). Patient symptoms included constipation or GI obstruction-6, weight loss-5, diarrhea-3, anorexia-3, pencil thin stools-3, tenesmus-2, anorectal pain-2, fatigue-1, straining with bowel movements-1, fecal incontinence-1, abdominal distention-1, and change in bowel habits-1. Rectal examination, performed in 9 patients, revealed annular rectal stenosis in 6, and rectal mass in 3. One patient presented with mild anemia.
This case report describes the novel colonoscopic appearance of bladder urothelial carcinoma extension, via the prostate, into the rectum, forming a multinodular, oval, ulcerated, friable mass. Bladder carcinoma extension via the prostate underlies the colonoscopic detection of rectal invasion only over the prostate location, just as the colonoscopic finding of a friable, multinodular, hemorrhagic rectal mass underlies the clinical presentation with daily bright red blood covering the stools. Invasion via the prostate may have been facilitated by prior radiotherapy and hormonal therapy for prostate cancer, or perhaps by prostate tissue being receptive to urothelial metastases. Bladder (and prostate) cancer have been reported to cause annular rectal constriction, presenting as constipation or GI obstruction, via contiguous spread to the rectal submucosal or muscular layers, but the current report of extension to rectal mucosa is novel. Bladder urothelial carcinoma is believed to break through the bladder wall, and follow along, the fascia of Denonvillier[2], or spread locally along the lateral pedicles of the bladder to reach the posterior rectal wall, to cause rectal constriction[4]. The current case, like all the prior 16 cases of rectal involvement, occurred in men. Female internal reproductive organs and their corresponding ligaments and fascia might serve as barriers that protect the rectum from local invasion by bladder urothelial carcinoma, whereas the prostate might be a weak barrier to protect the rectum from local invasion because of susceptibility to urothelial invasion. Metastasis, to other GI regions, have, likewise, been reported much more frequently in men than women, partly explained by bladder urothelial carcinoma being four times as common in men than women[22], but other potential contributing factors require further investigation.
The currently reported patient had two reported risk factors for bladder urothelial carcinoma: Prior prostate cancer[23], and prior radiotherapy for prostate cancer[24]. The current case demonstrates that the clinical symptoms of rectal mucosal invasion can mimic that of hemorrhoids: chronic, daily, bright red blood per rectum coating the stools. However, the currently reported patient also had an enlarged, hard, fixed, “prostate” on rectal examination, and had prior prostate and bladder cancers, findings suggesting recurrent bladder or prostate cancer.
This study is limited by reporting a single case and by reporting it retrospectively. This study is also somewhat limited by not entirely excluding prostate cancer as contributing to the pelvic mass. However, the cystoscopic findings of friable, hemorrhagic tissue at the bladder neck identified 4 mo prior to admission; the colonoscopic findings of a friable, multinodular rectal mass; the histologic and immunohistochemical findings of multiple rectal biopsies revealing bladder urothelial carcinoma; the CT demonstration of a mass extending from the bladder neck through the prostate into the rectum; and the absence of immunohistochemical markers for prostate cancer in the rectal biopsies all favor the diagnosis of bladder urothelial carcinoma over prostate cancer. In conclusion, a case of bladder urothelial carcinoma penetrating into the rectum via the prostate is reported, with apparently previously unreported, but likely characteristic colonoscopic findings. In the previously reported cases, rectal lesions were metastatic, presumably via lymphogenous or hematogenous routes, or caused rectal constriction from direct rectal wall extension without rectal mucosal involvement.
An 87-year-old man was treated in 1996 for prostate adenocarcinoma stage-T1c-Gleason-6 with external beam radiotherapy recurrent prostate cancer treated with leuprolide hormonal therapy in 2009, and bladder-urothelial-carcinoma in-situ treated with Bacillus-Calmette-Guerin and adriamycin in 2010, presented in 2015 with painless, bright red blood per rectum coating stools daily for 5 mo. Rectal examination revealed bright red blood per rectum; and a hard, fixed, 2.5 cm × 2.5 cm mass at the normal prostate location.
The symptom of daily, painless, bright red blood per rectum for 5 mo without other symptoms suggests hemorrhoidal bleeding. First, hemorrhoidal bleeding is very common and is typically unassociated with other symptoms. Second, hemorrhoids generally cause bright red blood because hemorrhoidal blood, despite being venous, is relatively well oxygenated. Third, hemorrhoidal bleeding is generally painless in the absence of hemorrhoidal thrombosis. Rectal examination did not, however, support this diagnosis. No external hemorrhoids were identified by anal inspection, and no internal hemorrhoids were palpated on digital rectal examination. Moreover, rectal examination revealed a hard, fixed, multinodular, mass at the normal location of the prostate, and gross red blood on the examining finger, findings suspicious for rectal bleeding from prostate cancer. This diagnosis is further suggested by the prior history of prostate cancer in 1996, prostate cancer recurrence in 2009 six years before the current clinical presentation, and palliative hormonal therapy for the cancer recurrence in 2009. The diagnosis of recurrent bladder urothelial cancer is also possible given the prior diagnosis of bladder urothelial carcinoma in situ in 2010; recurrent bladder urothelial carcinoma could cause rectal bleeding from metastases or direct extension to the rectum. Radiation proctitis must be included in the differential diagnosis because the patient had undergone external beam radiotherapy for prostate cancer in 1966, 19 years before the clinical presentation. Chronic radiation proctitis can cause rectal bleeding from telangiectasias caused by radiation-induced endothelial injury. Anal fissure must also be included in the differential diagnosis of bright red blood per rectum, but is unlikely in this case because anal fissure is typically very painful and this patient had no anorectal pain. Also the patient did not have the classic symptoms of anorectal bleeding commencing after passing a large, hard stool, the patient had daily rectal bleeding for 5 mo which is atypical for rectal fissure, and anal fissure is relatively uncommon.
The key finding in the blood tests is a hemoglobin level of 7.6 gm/dL, with iron saturation of 9%, indicating iron deficiency anemia. Hemorrhoidal bleeding tends to produce minimal-to-mild anemia because of minimal daily bleeding, whereas prostate or bladder cancer invading rectal mucosa can cause much more significant blood loss and more severe anemia.
Abdominopelvic computed tomography (CT) angiography revealed focal thickening of the bladder wall at its neck; a mass containing an air cavity replacing most of the prostate; and adjacent rectal invasion. These imaging findings strongly support the diagnosis of recurrent bladder urothelial carcinoma penetrating rectal mucosa via the prostate, or less likely support the diagnosis of recurrent prostate cancer penetrating rectal mucosa. These CT findings do not support the diagnosis of hemorrhoids. Either of these malignancies would be more likely to produce iron deficiency anemia from chronic blood loss than hemorrhoidal bleeding. Colonoscopy demonstrated an ulcerated, friable, multinodular, oval, hemorrhagic, 2.5 cm × 2.5 cm mass in the anterior rectal wall, just proximal to the dentate line, at the usual anatomic location of the prostate, no hemorrhoids, and no signs of radiation proctitis, such as mucosal telangiectasia despite the prior prostate radiotherapy. These colonoscopic findings are highly consistent with cancer invading rectal mucosa. These CT findings are most compatible with bladder urothelial carcinoma invading rectal mucosa by direct extension.
Histologic examination of colonoscopic biopsies of rectal tissue biopsies revealed poorly differentiated carcinoma. Immunohistochemical analysis demonstrated the tumor cells stained positively with cytokeratin 20, indicating either a colonic or bladder (urothelial) primary. Additional diffuse positivity for cytokeratin 7, 34bE12, and GATA-3; and focal positivity for CK5/6 strongly support urothelial origin. Negative immunohistochemical staining for CDX2 (Caudal Type Homeobox 2) confirms that this tumor does not arise from colonic adenocarcinoma. The diffuse positivity for cytokeratin 20 and only focal positivity for CK5/6 (< 20% of cells positive) excludes anorectal squamous carcinoma. Immunohistochemical markers for prostate carcinoma, including PSA, PAP and P501S, were all negative. The pathologic diagnosis was therefore poorly-differentiated carcinoma of urothelial origin. This pathology explains all the findings: Clinical presentation of painless, daily bright red blood per rectum from friable rectal mucosa from malignant invasion; iron deficiency anemia from chronic GI bleeding from rectal metastases; CT findings of direct cancer extension to rectal mucosa; and colonoscopic findings of an ulcerated, friable, multinodular, mass in the anterior rectal wall.
The patient received palliative therapy for the daily rectal bleeding. The right-superior-rectal-artery was successfully embolized during visceral angiography using embolospheres to achieve hemostasis. The patient did not undergo curative therapy in accordance with the patient’s wishes, because of the minimal likelihood of cure given that the patient presented with recurrent urothelial carcinoma spreading beyond the bladder, previously had recurrent prostate cancer, and was very elderly. The patient experienced recurrent rectal bleeding requiring periodic packed erythrocyte transfusions three months after embolectomy that required palliative colostomy. The patient expired 13 mo after embolization from widespread metastases from the advanced cancer with rectal penetration treated with palliative therapy.
Comprehensive literature review revealed 16 previously reported cases of rectal involvement of bladder urothelial carcinoma, including 11 cases of direct cancer extension and 5 cases of metastases. The current case is novel in that the bladder urothelial carcinoma directly penetrated into rectal mucosa; in that rectal involvement caused daily bright red blood per rectum and iron-deficiency anemia; and in the colonoscopic findings that were in accord with the clinical presentation of daily bright red blood per rectum and the CT findings.
This work demonstrates the novel findings that bladder urothelial carcinoma can directly extend to rectal mucosa via the prostate, can cause daily, painless, bright red blood per rectum mimicking hemorrhoidal bleeding; and produce colonoscopic findings of a multinodular rectal mucosal mass from cancer extension.
The authors reported on a single case of urothelial bladder cancer extending to rectal mucosa via the prostate and mimicking hemorrhoidal bleeding, and reviewed the literature on this subject. The case is well described and of clinical interest. The background literature review is comprehensive and well done.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sacco E, Zhang X, Vynios D S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Katayama H, Mituzuka K, Kawasaki Y, Kato S. Annular rectal constriction caused by infiltrating bladder carcinoma: a case report. Hinyokika Kiyo. 2010;56:229-231. [PubMed] |
| 2. | Stillwell TJ, Rife CC, Lieber MM. Bladder carcinoma presenting with rectal obstruction. Urology. 1989;34:238-240. [PubMed] |
| 3. | Langenstroer P, Zacharias A, Almagro U, Dewire D. Annular constriction of the rectum secondary to transitional cell carcinoma of the bladder. Urology. 1996;47:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Kobayashi S, Kato H, Iijima K, Kinebuchi Y, Igawa Y, Nishizawa O. Annular rectal constriction due to infiltration by bladder cancer. Hinyokika Kiyo. 2006;52:569-572. [PubMed] |
| 5. | Ito Y, Nishimoto K, Ogata K, Fujioka T. Annular constriction of the rectum secondary to urothelial carcinoma of the bladder. Hinyokika Kiyo. 2008;54:553-555. [PubMed] |
| 6. | Gleeson FC, Clain JE, Rajan E, Topazian MD, Wang KK, Wiersema MJ, Zhang L, Levy MJ. Secondary linitis plastica of the rectum: EUS features and tissue diagnosis (with video). Gastrointest Endosc. 2008;68:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Hong SP, Park SW, Lee SJ, Chung JP, Song SY, Chung JB, Kang JK, Cho NH. Bile duct wall metastasis from micropapillary variant transitional cell carcinoma of the urinary bladder mimicking primary hilar cholangiocarcinoma. Gastrointest Endosc. 2002;56:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Asfour R, Stettler G, Pinzon MM, Hayden D, Eberhardt J, Saclarides T, Slogoff M. Recurrent urothelial cell carcinoma presenting with gastrointestinal symptoms. Am Surg. 2014;80:E240-E242. [PubMed] |
| 9. | Aigen AB, Schapira HE. Metastatic carcinoma of prostate and bladder causing intestinal obstruction. Urology. 1983;21:464-466. [PubMed] |
| 10. | Kumar N, Raju M, Fass R. Bladder cancer presenting as lower-GI bleeding. Dig Dis Sci. 2009;54:2047-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Kakizawa H, Toyota N, Mita K, Fujimura Y, Hieda M, Hirai N, Tachikake T, Ito K. Pseudoaneurysm embolization and vasopressin infusion for lower gastrointestinal bleeding due to recurrence of urinary bladder carcinoma. Radiat Med. 2006;24:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ying-Yue J, Shen SH, Wang JH. Unusual presentation of urothelial carcinoma of the bladder with noncontiguous rectal and diffuse muscular skeletal metastases. J Urol. 2010;184:1163-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Yusuf TE, Levy MJ, Wiersema MJ. EUS features of recurrent transitional cell bladder cancer metastatic to the GI tract. Gastrointest Endosc. 2005;61:314-316. [PubMed] |
| 14. | Chin CC, Yeh CY, Kuo YH, Wang JY. Massive lower gastrointestinal bleeding from an external iliac artery fistula in a patient with bladder cancer. Chang Gung Med J. 2008;31:612-615. [PubMed] |
| 15. | Jung JL, Abouelfadel Z, Prevot-Maupoix M, Villeval C. Esophageal metastasis of cancer of the bladder. Ann Urol (Paris). 1997;31:205-206. [PubMed] |
| 16. | Dy RM, Slocum TL, Fidler ME, Taylor RJ, Quigley EM. Metastatic spread of transitional cell carcinoma of the bladder to the esophagus. J Clin Gastroenterol. 1998;26:81-82. [PubMed] |
| 17. | Girotra M, Jani N. Bladder cancer metastasis to duodenum: an unusual presentation of obscure GI bleed. Dig Dis Sci. 2010;55:1801-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Chan CH, Al-Busafi SA, Waschke KA. Massive upper gastrointestinal bleeding secondary to duodenal metastasis of transitional cell carcinoma of the urinary bladder. Case Rep Gastroenterol. 2011;5:246-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Hawtin K, Kent A, Collins C, Blunt D. Metastatic bladder cancer presenting as duodenal obstruction. Ann Acad Med Singapore. 2009;38:914-915. [PubMed] |
| 20. | Hoshi A, Tokunaga M, Usui Y, Yamashita H, Sasaki H, Kobayashi Y, Shima M, Miyakita H, Terachi T. Metastatic small intestinal tumor associated with transitional cell carcinoma: a report of 2 cases and review of cases in Japan. Hinyokika Kiyo. 2005;51:41-44. [PubMed] |
| 21. | Honda M, Watanabe T, Miyagawa I. Combined treatment involving unilateral common iliac embolization and femoro-femoral arterial bypass for lower gastrointestinal bleeding due to recurrent bladder cancer. Int J Urol. 2010;17:894-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Centers for Disease Control: Bladder Cancer. [accessed 2016 Aug 3]. Available from: http//www.cdc.gov/cancer/bladder. |
| 23. | Joung JY, Lim J, Oh CM, Jung KW, Cho H, Kim SH, Seo HK, Park WS, Chung J, Lee KH. Risk of Second Primary Cancer among Prostate Cancer Patients in Korea: A Population-Based Cohort Study. PLoS One. 2015;10:e0140693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Huang J, Kestin LL, Ye H, Wallace M, Martinez AA, Vicini FA. Analysis of second malignancies after modern radiotherapy versus prostatectomy for localized prostate cancer. Radiother Oncol. 2011;98:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |