Published online Apr 10, 2016. doi: 10.4253/wjge.v8.i7.319
Peer-review started: October 27, 2015
First decision: November 27, 2015
Revised: January 19, 2016
Accepted: February 14, 2016
Article in press: February 16, 2016
Published online: April 10, 2016
Processing time: 167 Days and 12 Hours
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related death in the United States. Due to the aggressive tumor biology and late manifestations of the disease, long-term survival is extremely uncommon and the current 5-year survival rate is 7%. Over the last two decades, endoscopic ultrasound (EUS) has evolved from a diagnostic modality to a minimally invasive therapeutic alternative to radiologic procedures and surgery for pancreatic diseases. EUS-guided celiac plexus intervention is a useful adjunct to conventional analgesia for patients with pancreatic cancer. EUS-guided biliary drainage has emerged as a viable option in patients who have failed endoscopic retrograde cholangiopancreatography. Recently, the use of lumen-apposing metal stent to create gastrojejunal anastomosis under EUS and fluoroscopic guidance in patients with malignant gastric outlet obstruction has been reported. On the other hand, anti-tumor therapies delivered by EUS, such as the injection of anti-tumor agents, brachytherapy and ablations are still in the experimental stage without clear survival benefit. In this article, we provide updates on well-established EUS-guided interventions as well as novel techniques relevant to pancreatic cancer.
- Citation: Oh SY, Irani S, Kozarek RA. What are the current and potential future roles for endoscopic ultrasound in the treatment of pancreatic cancer? World J Gastrointest Endosc 2016; 8(7): 319-329
- URL: https://www.wjgnet.com/1948-5190/full/v8/i7/319.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i7.319
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related death in the United States[1]. Only 20% of patients at diagnosis are amenable to surgical resection[2], which offers the best chance of long-term survival. As a result, the majority of patients are treated with palliative chemotherapy or best supportive care. From a histological standpoint, one of the defining features of pancreatic ductal adenocarcinoma is extensive desmoplastic stroma with fibrotic reaction around the tumor. The fibrotic stroma promotes tumor growth[3], induces resistance to chemotherapy and radiotherapy[4], and constitutes a barrier to the delivery of therapeutic agents[5]. Due to the aggressive tumor biology and late manifestations of the disease, long-term survival is extremely uncommon and the current 5-year survival rate is 7%[6].
Over the last two decades, endoscopic ultrasound (EUS) has evolved from a diagnostic modality to a minimally invasive therapeutic alternative to radiologic procedures and surgery for pancreatic diseases. EUS-guided celiac plexus neurolysis (CPN)/block are widely accepted techniques for pain management in patients with pancreatic cancer. Recently, EUS-guided biliary access in both malignant and non-malignant biliary obstruction has been increasingly utilized. As EUS offers dynamic images, unparalleled access to the pancreas and Doppler to avoid vascular structures, it has a theoretical advantage of targeting the tumor directly through the desmoplastic stroma while minimizing complications. This, coupled with the lack of effective systemic chemotherapies for pancreatic cancer, has prompted researchers to investigate local EUS-guided delivery of anti-tumor agents and ablative therapies over the last decade.
In this article, we provide updates on well-established EUS-guided interventions as well as novel techniques that are in the development for the treatment of pancreatic cancer.
CPN refers to permanent chemical ablation of the celiac plexus and is performed by injecting alcohol or phenol into or around the celiac plexus or ganglion. Celiac plexus block denotes inhibition of pain transmission via the celiac plexus by injecting a combination of a corticosteroid and a long acting local anesthetic. Injections can be delivered via a percutaneous, surgical or EUS-guided approach. EUS provides access to the celiac plexus which is located adjacent to the proximal gastric wall. The main advantage of this route over a percutaneous one is the ability to avoid vessels with Doppler, in addition to being able to undertake concomitantly at the time of another intervention such as an endoscopic retrograde cholangiopancreatography (ERCP) or fine needle aspiration of the primary mass.
Since the first report of EUS-CPN in 30 patients with intra-abdominal malignancy (25 with pancreatic cancer) showing significant improvement in pain scores[7], multiple randomized controlled and meta-analyses[8-12] have demonstrated that EUS-CPN provided effective pain relief in patients with pancreatic cancer compared with conventional analgesia. There is also evidence that CPN reduces analgesia use. Two meta-analyses showed that CPN (either EUS or percutaneous approach) was associated with a significant reduction in narcotic use[8,11]. Additionally, a randomized controlled trial involving 96 patients with advanced pancreatic cancer reported that morphine consumption was lower at 3 mo in the EUS-CPN group compared to placebo[11]. Nonetheless, approximately 15% of patients may see no reduction in their use of narcotics, and in this group, a repeat EUS-CPN has not been shown to be effective. A study of 24 patients with pancreatic cancer undergoing repeat EUS-CPN showed that repeat CPN was not as effective as index procedure in pain control (67% after the initial CPN vs 29% at 1 mo follow-up)[13].
EUS-guided injection can be given centrally into the space between the aorta and the origin of the coeliac trunk, or bilaterally on either side of the coeliac axis. To date, one randomized trial comparing the two techniques demonstrated no difference in the duration of pain relief (11 wk vs 14 wk), complete pain relief (2/29 patients vs 2/21 patients) or reduction in pain medication (9/29 patients vs 7/21 patients)[14]. The decision to inject centrally or bilaterally often depends on the personal preference and experience of an endosonographer and further prospective studies are needed to determine which approach is superior. On the other hand, a Japanese group investigated the efficacy of broad plexus neurolysis (BPN) extending over the superior mesenteric artery with the aim of delivering a larger amount of neurolytic agents[15]. The study found that EUS-BPN patients had significantly greater reductions at days 7 and 30 on the visual analog pain scale scores compared with EUS-CPN group. This technique, however, is yet to be validated in a large, prospective trial.
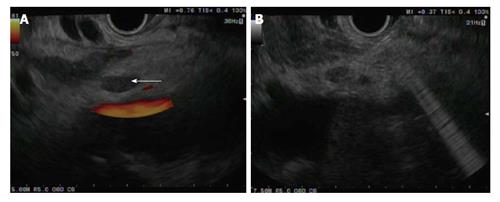
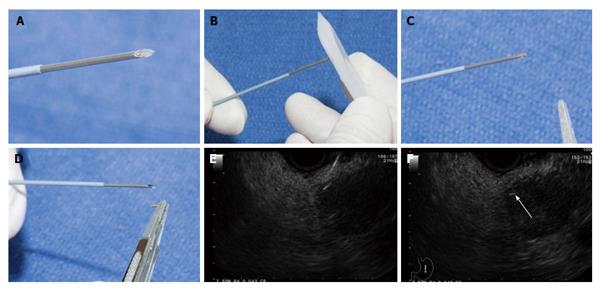
There has been an interest in direct celiac ganglia injection to improve the efficacy of CPN (Figure 1). Celiac ganglia appear as an oval, hypo to isoechoic structures around the celiac axis and are visible in upwards of 80% of the general population[16,17]. A recent study randomized 34 patients to EUS-celiac ganglia neurolysis vs EUS-CPN showed that celiac ganglion neurolysis was associated with more effective pain relief compared with CPN (73.5% vs 45.5%, respectively; P = 0.026) with a smaller volume of alcohol needed for the ablation[18].
Contraindications to celiac plexus interventions include coagulopathy (international normalized ratio > 1.5), thrombocytopenia (platelets < 50000/L), and hemodynamic or respiratory instability prohibiting adequate sedation. Otherwise, EUS-guided celiac plexus intervention is generally safe. Diarrhea, abdominal pain and hypotension due to the disruption of the autonomic nervous system are usually self-limiting. A paradoxical increase in pain has been shown to occur in 9% of cases but generally resolves spontaneously[19]. Serious adverse events including paralysis from anterior spinal cord infection[20,21], necrotic gastric perforation[22], and celiac artery thrombosis causing infarction[23,24] are rare.
ERCP for biliary access and drainage is successful in 90% to 95% of cases and is the preferred method of stenting the bile duct in obstructive jaundice from pancreatic cancer. In cases of unsuccessful ERCP due to difficult cannulation or altered anatomy, the alternatives have been precut papillotomy, percutaneous transhepatic biliary drainage (PTBD) and surgical bypass. Recently, EUS-guided biliary drainage has emerged as an alternative to these options. EUS-guided approach spares patients the discomfort of an external drain, and can be performed at the time of an unsuccessful ERCP, reducing the need for additional percutaneous interventions.
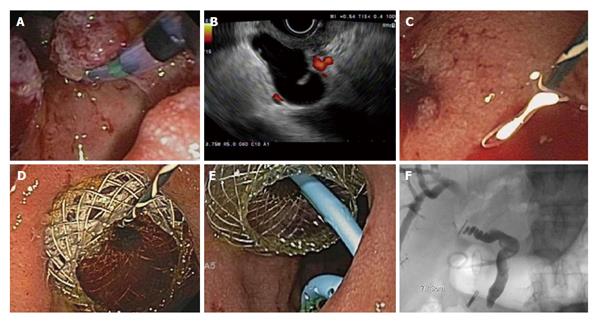
Three main approaches for EUS-guided biliary drainage have been described. Rendezvous technique is where a guidewire is placed into the intra or extrahepatic bile duct and passed through the papilla for retrieval by duodenoscopy for retrograde biliary intervention. Direct transgastric (hepaticogastrostomy) or transduodenal (choledochoduodenostomy) route involves the dilation of the tract followed by stenting for transmural biliary drainage (Figure 2). This obviates biliary access via the papilla. A third, less frequently performed intervention, involves the antegrade placement of a stent across the papilla via a transduodenal approach[25,26]. The transduodenal approach requires at least an intact duodenal bulb[27] and can sometimes be performed after placement of a duodenal stent for gastric outlet obstruction. In patients with obstruction at the level of the pylorus, the transgastric approach almost always requires a dilated intrahepatic biliary system[28].
Available evidence suggests excellent technical and clinical success with EUS-guided biliary drainage in 87% of cases, however, adverse events up to 10%-20% have been reported[29-40]. One of the major shortcomings of the rendezvous technique is a failure rate of 25%, and this can be associated with prolonged procedure times and higher risk of bile leak[31,36,37,40,41]. In contrast, transluminal stenting can be complicated by stent migration or occlusion, bile leak and biliary peritonitis, cholangitis, hemobilia and pneumoperitoneum[27,33-35].
Alternatively, EUS-guided gallbladder drainage may be an option when the previously mentioned approaches are not feasible. As the gallbladder presents a large target in close proximity to the gastric antrum and duodenal bulb, this technique can be performed more easily. However, it would not be beneficial in a non-dilated gallbladder suggesting cystic duct invasion by tumor[42]. Excellent technical success, clinical success and safety profiles with EUS-guided gallbladder drainage in patients with acute cholecystitis have been demonstrated in a randomized controlled trial[43] and its use in the setting of malignancy has been described in case reports and small series[44,45].
At present, experts recommend that EUS-guided biliary drainage should be performed by an advanced endoscopist with expertise in both ERCP and EUS in a tertiary center, where surgery and radiology unit can provide support to manage adverse events if they arise[46,47].
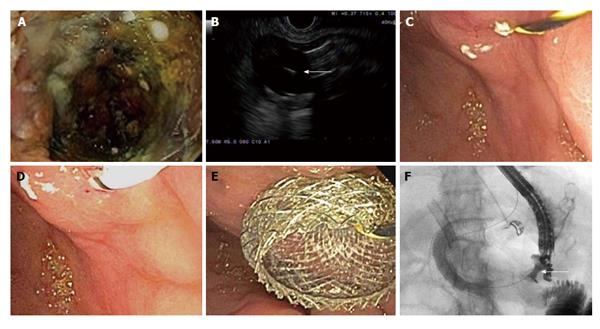
Gastric outlet obstruction is a common late manifestation of cancer in the head of the pancreas. When endoscopic gastroduodenal stent placement is unsuccessful in relieving obstruction, bypass surgery can be performed to accomplish the anastomosis between the stomach and jejunum. However, in poor surgical candidates, the EUS-guided approach may offer a minimally invasive means of establishing an anastomosis. In this technique, a gastrojejunal fistula is created by obtaining an access to the jejunum via EUS-guided needle, placing a guidewire through the needle and dilating the tract over the wire using a dilator catheter, balloon and/or electrical cautery needle. Subsequently, a lumen-apposing stent is placed across the fistula (Figure 3). This has been described in 2 recent case reports[48,49]. EUS-guided gastrojejunostomy using a double-balloon enteric tube to distend the jejunum between the two balloons at the EUS-guided needle puncture has also been reported[50,51].
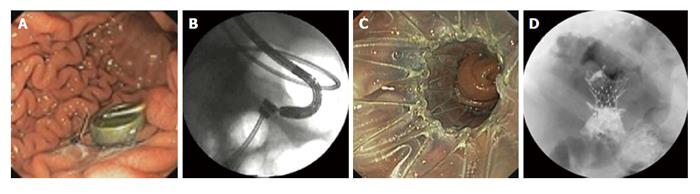
The use of magnetic compression devices through oral, percutaneous, and surgical introduction of magnets to create gastroenterostomy and cholecystoenteric anastomosis in animal models has been reported[52,53] (Figure 4). Encouraged by the favorable outcomes of the experimental studies, two human trials of endoscopic gastroenteric anastomosis have been performed. The first study evaluated 15 patients with malignant obstruction undergoing gastroenteric anastomosis using magnetic compression devices and a yoyo stent and found that the procedure was successful in 13 (87%) patients[54]. One perforation occurred and was attributed to manipulation of the recently formed fistula. Three stents migrated (2 distal and 1 proximal) and no mortality was reported. Subsequently, a prospective multicenter study evaluated 18 patients who had gastroenteric anastomosis using magnetic compression device and self-expandable stent[55]. The procedure was successful in 12 (67%) patients but the study was terminated after inclusion of 18 patients due to a fatal perforation in 1 patient. Three (25%) patients experienced stent migration. This technique is usually performed by forward-viewing endoscope but can also be performed under the guidance of EUS combined with fluoroscopy. Creation of magnetic biliary anastomoses using endoscopic and radiologic techniques has also been described in case reports[56,57] but there are no large trials to date.
Through-the-scope device for EUS-guided suturing and tissue approximation between two organs has been tested in porcine models[58,59]. A suturing device was developed for suturing under EUS guidance to the desired depth. The device allowed multiple sutures to be placed without withdrawing the echoendoscope. Stitching, knot tying, and thread cutting were achieved through an accessory channel in the echoendoscope. Traction for the insertion of stents and other devices was provided through the lumen of both organs. Within 4 to 7 d, anastomoses had formed between the small intestine and the stomach, and between the gallbladder and the stomach. The initial diameter of the anastomoses ranged from 3 to 9 mm, and no adverse events were reported.
Cytoimplant: An allogenic mixed lymphocyte culture (Cytoimplant) induces cytokine production and activates the host immune effector mechanism. EUS-fine needle injection (EUS-FNI) of Cytoimplant was examined in a phase I trial of 8 patients with advanced pancreatic cancer[60]. The median survival was 13.2 mo, with 2 partial responses (> 50% reduction in tumor size measured on imaging) and 1 minor response (tumor size reduction of < 50%). The technique was feasible and no major complications were seen.
Immunotherapy/dendritic cells: Immature dendritics cells can stimulate primary T-cell response against tumor antigens. To date, 2 pilot trials have been conducted on EUS-FNI of dendritics for the treatment of unresectable pancreatic cancer. The use of EUS-FNI of immature dendritic cells was reported in a study of 7 patients with advanced pancreatic cancer who previously failed gemcitabine. Injections of 10 billion or more dendritic cells at two to three sites were performed. There was 1 complete response, 3 partial responses and 2 patients had stable disease with a median survival of 9.9 mo. No adverse events were seen[61]. Later, the use of combined systemic gemcitabine and EUS-FNI of OK432-pulsed dendritic cells, followed by intravenous lymphokine-activated killer cells was reported in 5 patients with unresectable pancreatic cancer. One patient showed a partial response and 2 patients had stable disease over 6 mo[62].
Tumor necrosis factor erade: Tumor necrosis factor (TNF)erade is a replication-deficient adenovirus vector that expresses human TNF-alpha gene regulated by promoter Egr-1, which is inducible by chemotherapy and radiation. Preliminary results from a phase I/II trial of intratumoral TNFerade injection (either EUS or percutaneous approach) in combination with systemic 5-fluorouracil and radiotherapy in 50 patients with locally advanced pancreatic cancer demonstrated encouraging results[63]. One complete response, 3 partial responses and 12 stable disease with a median survival of 297 d was noted. Interestingly, seven patients had surgical resection, 6 with negative margins, 1 with complete pathologic response and 3 surviving more than 2 years. However, a subsequent large randomized multicenter trial involving 304 patients with locally advanced pancreatic cancer showed no survival benefit of combining intratumoral TNFerade injection with 5-fluorouracil and radiotherapy compared with chemoradiation alone[64]. In addition, the study used either EUS-guided or a percutaneous approach for the injection of TNFerade and found that EUS-FNI was associated with inferior progression-free survival. This was thought to be the operator-dependent nature of EUS-FNI.
ONYX-015: ONYX-015 is a modified adenovirus (deletion in the E1B gene) which preferentially replicates in tumor cells leading to cell death. In a phase I/II trial using EUS-FNI of ONYX-015 in 21 patients with locally advanced pancreatic cancer, patients received 8 injections and the last injection was administered with systemic gemcitabine[65]. The mean survival was 7.5 mo. Serious adverse events included duodenal perforations and sepsis in 2 patients each, raising concerns over its safety.
BC-819: BC-819 is a DNA plasmid that targets the expression of diphtheria-toxin gene under the control of H19 regulatory sequences and has the potential to treat pancreatic cancer that overexpresses the H19 gene. In a phase I/IIa trial, EUS or computed tomography (CT)-guided FNI of BC-819 was performed in 9 patients with advanced pancreatic cancer treated with concurrent chemoradiation[66]. Three patients achieved partial response and 2 were successfully downstaged for surgery. No serious adverse events were reported.
EUS-guided brachytherapy: Brachytherapy involves the insertion of a radioactive seed directly into the tumor for local destruction. Iodine-125 (125I) is the most common radioactive seed used and has a half-life of 59.7 d and tissue penetration of 1.7 cm[67]. EUS-guided implantation of 125I into pancreatic tumor was first reported in a pilot study of 15 patients with unresectable pancreatic cancer[68]. The study showed partial response in 27%, minimal response in 20% and stable disease in 33%. Reduction in pain was noted in 30% but the effect was short-lived. Two further studies examined the efficacy of combined EUS-brachytherapy and systemic gemcitabine-based chemotherapy in patients with advanced pancreatic cancer, both demonstrating no significant survival benefit but improvement in pain was again noted[69,70].
Stereotactic body radiotherapy and fiducial placement: The main benefit of stereotactic body radiotherapy (SBRT) is that it limits the field of radiation to the organ of interest thereby minimizing irradiation of adjacent normal tissue[71]. One prospective[71] and two retrospective studies[72,73] showed that local tumor control and overall survival following SBRT were comparable with the outcomes of external beam radiotherapy.
Placement of fiducial markers prior to SBRT acts as a landmark and enables precise tumor targeting. Fiducial markers are available in different forms, including radiopaque spheres, coils or seeds and were traditionally placed in or near the tumor using surgical or radiological techniques (Figure 5[74]). However, two recent prospective studies have demonstrated that EUS-guided placement of fiducial markers in pancreatic tumors had excellent technical success rates (88% to 90%) and safety[74,75]. EUS-guided placement is performed by passing fiducials through a 19G or 22G needle and deploying them by using stylet or injecting sterile water into the needle after the needle is punctured to the desired depth[76]. Different types of fiducial markers have also been studied. Khashab et al[77] evaluated the EUS-placement of traditional vs coiled fiducials in a study of 39 patients with locally advanced pancreatic cancer. Visibility score was significantly better for traditional compared with coil fiducials but no difference in migration rate, number of fiducials placed, technical success or complication rate were seen. The authors recommended the placement of traditional fiducials whenever possible.
Radiofrequency ablation: Radiofrequency ablation (RFA) works by passing electrical current in the range of radio waves between a needle electrode positioned in the tumor, and grounding pads placed on the patient’s skin. Radiofrequency current produces a high level of heat within the tumor leading to protein desaturation and loss of fluids (coagulative necrosis)[78]. Several studies have demonstrated the feasibility of RFA via open, percutaneous and laparoscopic approaches in patients with locally advanced pancreatic cancer[79,80].
The application of EUS-guided RFA in porcine models was shown to be effective in destroying pancreatic tissue[78,81,82]. Complications included pancreatitis[81], intestinal wall adhesion[82], and retroperitoneal fibrosis in an adjacent organ[83]. To date, there is only one study that reported the use of EUS-RFA in humans. The study used a cryothermal probe which is a large bore flexible bipolar device that combines radiofrequency with cryogenic cooling in the same session. The probe was successfully applied under EUS guidance in 73% (16/22) of patients with locally advanced pancreatic cancer and the procedure was well tolerated in all patients. In 6/16 patients, reduction in tumor size was noted on follow-up CT[83].
Photodynamic therapy: Photodynamic therapy (PDT) is a technique where a specific wavelength of light is delivered via optical fibers threaded through a needle placed in the target tissue[84]. Wavelength light is then activated by a photosensitizing agent which is usually administered intravenously. Photosensitizer is also present in pancreatic cancer at a sevenfold greater concentration compared with normal tissue[85]. The combination of a photosensitizing agent and wavelength light in the presence of oxygen leads to the generation of reactive oxygen species that can damage cellular constituents leading to cell death[86]. Unlike RFA, PDT is collagen sparing and preserves normal tissue architecture[87].
Promising results of PDT on cholangiocarcinoma have been reported including survival benefit[88-94] however its use in pancreatic cancer is still at an experimental stage. Three pilot trials of PDT in patients with locally advanced pancreatic cancer have demonstrated its feasibility and safety[86,95,96].
EUS-guided celiac plexus intervention is a useful adjunct to conventional analgesia for pain management in patients with pancreatic cancer. Direct injection into the celiac ganglia may result in a better response.
EUS-guided biliary drainage has emerged as a viable alternative to PTBD in patients who have failed ERCP. However, it should be performed by an interventional endoscopist with expertise in both ERCP and EUS at a tertiary center where surgery and radiology can provide support in case of adverse events.
EUS-guided anastomosis is in the preliminary stage of development and the majority of studies are limited to animal models. Major advancements in technique and prospective human trials are needed before it becomes a feasible alternative to surgery in patients at high risk of operative complications.
Results of trials with EUS-guided anti-tumor injection therapy have been disappointing. The lack of effective anti-tumor agents is a significant barrier to the development in this field.
EUS-guided brachytherapy and fiducial placement can be performed safely and easily. However, there is no available data to suggest clear survival benefit, although clinical benefit from pain relief has been noted in some studies.
The use of EUS-guided ablative therapies is still at an experimental stage. Further human trials are need to determine its clinical benefit.
To summarize, EUS is an indispensable tool in pancreatic cancer not only for tissue diagnosis and disease staging but also for therapeutic purposes. Although some EUS-guided therapies have become widely accepted interventions for patients with pancreatic cancer, others have yet to evolve. Given the lack of effective systemic treatment for pancreatic cancer at present, further research in this field is warranted.
P- Reviewer: Buanes TA, Kitano M, Sadik R S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10465] [Article Influence: 654.1] [Reference Citation Analysis (2)] |
| 2. | Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI. Pancreatic cancer. Curr Probl Cancer. 2002;26:176-275. [PubMed] |
| 3. | Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 394] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 4. | Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 947] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 5. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2601] [Article Influence: 153.0] [Reference Citation Analysis (22)] |
| 6. | Sun H, Ma H, Hong G, Sun H, Wang J. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981-2010. Sci Rep. 2014;4:6747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656-662. [PubMed] |
| 8. | Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430-438. [PubMed] |
| 9. | Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011;CD007519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | McGreevy K, Hurley RW, Erdek MA, Aner MM, Li S, Cohen SP. The effectiveness of repeat celiac plexus neurolysis for pancreatic cancer: a pilot study. Pain Pract. 2013;13:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, Johnson C, Howard TJ, Lillemoe KD, DeWitt J. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Sakamoto H, Kitano M, Kamata K, Komaki T, Imai H, Chikugo T, Takeyama Y, Kudo M. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol. 2010;105:2599-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Ascunce G, Ribeiro A, Reis I, Rocha-Lima C, Sleeman D, Merchan J, Levi J. EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pancreatic malignancy (with video). Gastrointest Endosc. 2011;73:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Gleeson FC, Levy MJ, Papachristou GI, Pelaez-Luna M, Rajan E, Clain JE, Topazian MD. Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy. 2007;39:620-624. [PubMed] |
| 18. | Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316-324. [PubMed] |
| 20. | Fujii L, Clain JE, Morris JM, Levy MJ. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E265-E266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Mittal MK, Rabinstein AA, Wijdicks EF. Pearls & amp; oy-sters: Acute spinal cord infarction following endoscopic ultrasound-guided celiac plexus neurolysis. Neurology. 2012;78:e57-e59. [PubMed] |
| 22. | Loeve US, Mortensen MB. Lethal necrosis and perforation of the stomach and the aorta after multiple EUS-guided celiac plexus neurolysis procedures in a patient with chronic pancreatitis. Gastrointest Endosc. 2013;77:151-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Jang HY, Cha SW, Lee BH, Jung HE, Choo JW, Cho YJ, Ju HY, Cho YD. Hepatic and splenic infarction and bowel ischemia following endoscopic ultrasound-guided celiac plexus neurolysis. Clin Endosc. 2013;46:306-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 26. | Artifon EL, Safatle-Ribeiro AV, Ferreira FC, Poli-de-Figueiredo L, Rasslan S, Carnevale F, Otoch JP, Sakai P, Kahaleh M. EUS-guided antegrade transhepatic placement of a self-expandable metal stent in hepatico-jejunal anastomosis. JOP. 2011;12:610-613. [PubMed] |
| 27. | Itoi T, Yamao K. EUS 2008 Working Group document: evaluation of EUS-guided choledochoduodenostomy (with video). Gastrointest Endosc. 2009;69:S8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Savides TJ, Varadarajulu S, Palazzo L. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59. [PubMed] |
| 30. | Maranki J, Hernandez AJ, Arslan B, Jaffan AA, Angle JF, Shami VM, Kahaleh M. Interventional endoscopic ultrasound-guided cholangiography: long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy. 2009;41:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010;42:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Fabbri C, Luigiano C, Fuccio L, Polifemo AM, Ferrara F, Ghersi S, Bassi M, Billi P, Maimone A, Cennamo V. EUS-guided biliary drainage with placement of a new partially covered biliary stent for palliation of malignant biliary obstruction: a case series. Endoscopy. 2011;43:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Komaki T, Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasonography-guided biliary drainage: evaluation of a choledochoduodenostomy technique. Pancreatology. 2011;11 Suppl 2:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, Binmoeller KF. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest Endosc. 2012;75:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Park do H, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest Endosc. 2013;78:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 40. | Khashab MA, Dewitt J. EUS-guided biliary drainage: is it ready for prime time? Yes! Gastrointest Endosc. 2013;78:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Ogura T, Higuchi K. Does endoscopic ultrasound-guided biliary drainage really have clinical impact? World J Gastroenterol. 2015;21:1049-1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Jang JW, Lee SS, Song TJ, Hyun YS, Park do H, Seo DW, Lee SK, Kim MH, Yun SC. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012;142:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 44. | Itoi T, Binmoeller K, Itokawa F, Umeda J, Tanaka R. Endoscopic ultrasonography-guided cholecystogastrostomy using a lumen-apposing metal stent as an alternative to extrahepatic bile duct drainage in pancreatic cancer with duodenal invasion. Dig Endosc. 2013;25 Suppl 2:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Widmer J, Alvarez P, Gaidhane M, Paddu N, Umrania H, Sharaiha R, Kahaleh M. Endoscopic ultrasonography-guided cholecystogastrostomy in patients with unresectable pancreatic cancer using anti-migratory metal stents: a new approach. Dig Endosc. 2014;26:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Luz LP, Al-Haddad MA, Sey MS, DeWitt JM. Applications of endoscopic ultrasound in pancreatic cancer. World J Gastroenterol. 2014;20:7808-7818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Gavini H, Lee JH. Endoscopic ultrasound-guided endotherapy. J Clin Gastroenterol. 2015;49:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Tyberg A, Kumta N, Karia K, Zerbo S, Sharaiha RZ, Kahaleh M. EUS-guided gastrojejunostomy after failed enteral stenting. Gastrointest Endosc. 2015;81:1011-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Ikeuchi N, Itoi T, Tsuchiya T, Nagakawa Y, Tsuchida A. One-step EUS-guided gastrojejunostomy with use of lumen-apposing metal stent for afferent loop syndrome treatment. Gastrointest Endosc. 2015;82:166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Itoi T, Itokawa F, Uraoka T, Gotoda T, Horii J, Goto O, Moriyasu F, Moon JH, Kitagawa Y, Yahagi N. Novel EUS-guided gastrojejunostomy technique using a new double-balloon enteric tube and lumen-apposing metal stent (with videos). Gastrointest Endosc. 2013;78:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Itoi T, Ishii K, Tanaka R, Umeda J, Tonozuka R. Current status and perspective of endoscopic ultrasonography-guided gastrojejunostomy: endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy (with videos). J Hepatobiliary Pancreat Sci. 2015;22:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (35)] |
| 52. | Cope C. Evaluation of compression cholecystogastric and cholecystojejunal anastomoses in swine after peroral and surgical introduction of magnets. J Vasc Interv Radiol. 1995;6:546-552. [PubMed] |
| 53. | Cope C. Creation of compression gastroenterostomy by means of the oral, percutaneous, or surgical introduction of magnets: feasibility study in swine. J Vasc Interv Radiol. 1995;6:539-545. [PubMed] |
| 54. | Chopita N, Vaillaverde A, Cope C, Bernedo A, Martinez H, Landoni N, Jmelnitzky A, Burgos H. Endoscopic gastroenteric anastomosis using magnets. Endoscopy. 2005;37:313-317. [PubMed] |
| 55. | van Hooft JE, Vleggaar FP, Le Moine O, Bizzotto A, Voermans RP, Costamagna G, Devière J, Siersema PD, Fockens P. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010;72:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Mimuro A, Tsuchida A, Yamanouchi E, Itoi T, Ozawa T, Ikeda T, Nakamura R, Koyanagi Y, Nakamura K. A novel technique of magnetic compression anastomosis for severe biliary stenosis. Gastrointest Endosc. 2003;58:283-287. [PubMed] |
| 57. | Itoi T, Yamanouchi E, Ikeda T, Sofuni A, Kurihara T, Tsuchiya T, Tsuchida A, Kasuya K, Moriyasu F. Magnetic compression anastomosis: a novel technique for canalization of severe hilar bile duct strictures. Endoscopy. 2005;37:1248-1251. [PubMed] |
| 58. | Fritscher-Ravens A, Mosse CA, Mills TN, Mukherjee D, Park PO, Swain P. A through-the-scope device for suturing and tissue approximation under EUS control. Gastrointest Endosc. 2002;56:737-742. [PubMed] |
| 59. | Fritscher-Ravens A, Mosse CA, Mukherjee D, Mills T, Park PO, Swain CP. Transluminal endosurgery: single lumen access anastomotic device for flexible endoscopy. Gastrointest Endosc. 2003;58:585-591. [PubMed] |
| 60. | Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325-1335. [PubMed] |
| 61. | Irisawa A, Takagi T, Kanazawa M, Ogata T, Sato Y, Takenoshita S, Ohto H, Ohira H. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: a pilot study. Pancreas. 2007;35:189-190. [PubMed] |
| 62. | Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Hecht JR, Farrell JJ, Senzer N, Nemunaitis J, Rosemurgy A, Chung T, Hanna N, Chang KJ, Javle M, Posner M. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc. 2012;75:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 64. | Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. 2013;31:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 65. | Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561. [PubMed] |
| 66. | Hanna N, Ohana P, Konikoff FM, Leichtmann G, Hubert A, Appelbaum L, Kopelman Y, Czerniak A, Hochberg A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012;19:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 67. | Jin Z, Chang KJ. Endoscopic ultrasound-guided fiducial markers and brachytherapy. Gastrointest Endosc Clin N Am. 2012;22:325-331, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399-403. [PubMed] |
| 69. | Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 70. | Du Y, Jin Z, Jin H, Meng H, Zou D, Chen J, Liu Y, Zhan X, Wang D, Liao Z. Long-term effect of gemcitabine-combined endoscopic ultrasonography-guided brachytherapy in pancreatic cancer. J Interv Gastroenterol. 2013;3:18-24. |
| 71. | Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320-323. [PubMed] |
| 72. | Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, Yu A, Neerchal N, Rabinowitz S. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, Bahary N, Quinn A, Burton SA. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 76. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [PubMed] |
| 77. | Khashab MA, Kim KJ, Tryggestad EJ, Wild AT, Roland T, Singh VK, Lennon AM, Shin EJ, Ziegler MA, Sharaiha RZ. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Campagnol M, Ambrosi A, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy. 2008;40:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392-395. [PubMed] |
| 80. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2007;96:89-90. [PubMed] |
| 81. | Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392-401. [PubMed] |
| 82. | Kim HJ, Seo DW, Hassanuddin A, Kim SH, Chae HJ, Jang JW, Park do H, Lee SS, Lee SK, Kim MH. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Vesper BJ, Colvard MD. Photodynamic therapy (PDT): an evolving therapeutic technique in head and neck cancer treatment. Head & Neck Cancer: Current Perspectives, Advances, and Challenges. Springer Netherlands 2013; 649-676. |
| 85. | Chatlani PT, Nuutinen PJ, Toda N, Barr H, MacRobert AJ, Bedwell J, Bown SG. Selective necrosis in hamster pancreatic tumours using photodynamic therapy with phthalocyanine photosensitization. Br J Surg. 1992;79:786-790. [PubMed] |
| 86. | Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AW. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549-557. [PubMed] |
| 87. | Fan BG, Andrén-Sandberg A. Photodynamic therapy for pancreatic cancer. Pancreas. 2007;34:385-389. [PubMed] |
| 88. | McCaughan JS, Mertens BF, Cho C, Barabash RD, Payton HW. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch Surg. 1991;126:111-113. [PubMed] |
| 89. | Ortner MA, Liebetruth J, Schreiber S, Hanft M, Wruck U, Fusco V, Müller JM, Hörtnagl H, Lochs H. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology. 1998;114:536-542. [PubMed] |
| 90. | Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355-1363. [PubMed] |
| 91. | Witzigmann H, Berr F, Ringel U, Caca K, Uhlmann D, Schoppmeyer K, Tannapfel A, Wittekind C, Mossner J, Hauss J. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230-239. [PubMed] |
| 92. | Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100:2426-2430. [PubMed] |
| 93. | Pereira SP, Ayaru L, Rogowska A, Mosse A, Hatfield AR, Bown SG. Photodynamic therapy of malignant biliary strictures using meso-tetrahydroxyphenylchlorin. Eur J Gastroenterol Hepatol. 2007;19:479-485. [PubMed] |
| 94. | Wolfsen HC. Uses of photodynamic therapy in premalignant and malignant lesions of the gastrointestinal tract beyond the esophagus. J Clin Gastroenterol. 2005;39:653-664. [PubMed] |
| 95. | Chan HH, Nishioka NS, Mino M, Lauwers GY, Puricelli WP, Collier KN, Brugge WR. EUS-guided photodynamic therapy of the pancreas: a pilot study. Gastrointest Endosc. 2004;59:95-99. [PubMed] |
| 96. | Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, Kent E, Bown SG, Hasan T, Pogue BW. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110:1698-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 312] [Article Influence: 26.0] [Reference Citation Analysis (0)] |