Published online Mar 25, 2016. doi: 10.4253/wjge.v8.i6.310
Peer-review started: November 25, 2015
First decision: December 22, 2015
Revised: January 2, 2016
Accepted: January 29, 2016
Article in press: January 31, 2016
Published online: March 25, 2016
Processing time: 118 Days and 21.4 Hours
AIM: To perform a systematic review comparing the outcomes of endoscopic, percutaneous and surgical pancreatic pseudocyst drainage.
METHODS: Comparative studies published between January 1980 and May 2014 were identified on PubMed, Embase and the Cochrane controlled trials register and assessed for suitability of inclusion. The primary outcome was the treatment success rate. Secondary outcomes included were the recurrence rates, re-interventions, length of hospital stay, adverse events and mortalities.
RESULTS: Ten comparative studies were identified and 3 were randomized controlled trials. Four studies reported on the outcomes of percutaneous and surgical drainage. Based on a large-scale national study, surgical drainage appeared to reduce mortality and adverse events rate as compared to the percutaneous approach. Three studies reported on the outcomes of endoscopic ultrasound (EUS) and surgical drainage. Clinical success and adverse events rates appeared to be comparable but the EUS approach reduced hospital stay, cost and improved quality of life. Three other studies compared EUS and esophagogastroduodenoscopy-guided drainage. Both approaches were feasible for pseudocyst drainage but the success rate of the EUS approach was better for non-bulging cyst and the approach conferred additional safety benefits.
CONCLUSION: EUS-guided drainage appeared to be advantageous in drainage of pancreatic pseudocysts located adjacent to the stomach or duodenum. In patients with unfavorable anatomy, surgical cystojejunostomy or percutaneous drainage could be considered. Large randomized studies with current definitions of pseudocysts and longer-term follow-up are needed to assess the efficacy of the various modalities.
Core tip: Pancreatic pseudocysts are traditionally managed by open surgical internal drainage. With continued improvements in medical technology, the uses of percutaneous, endoscopic and laparoscopic drainage were increasingly reported. Nevertheless, trials comparing these different approaches are lacking. In this systematic review, endoscopic ultrasound-guided drainage appeared to be advantageous in drainage of pancreatic pseudocysts located adjacent to the stomach or duodenum. In patients with unfavorable anatomy, surgical cystojejunostomy or percutaneous drainage could be considered. Large randomized studies with current definitions of pseudocysts and longer-term follow-up are needed to assess the efficacy of the various modalities.
- Citation: Teoh AYB, Dhir V, Jin ZD, Kida M, Seo DW, Ho KY. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J Gastrointest Endosc 2016; 8(6): 310-318
- URL: https://www.wjgnet.com/1948-5190/full/v8/i6/310.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i6.310
Pancreatic pseudocysts are amylase rich fluid collections in the peri-pancreatic tissues surrounded by a well-defined wall[1]. There should be absence of necrosis or solid component in the collections. The relative proportion of acute and chronic pseudocyst varies between reports and depends on how the pseudocysts are being defined[2]. The incidence is higher in patients suffering from chronic pancreatitis. Pancreatic pseudocysts are traditionally managed by open surgical internal drainage. With continued improvements in medical technology, less invasive options including percutaneous, endoscopic and laparoscopic drainage were increasingly reported. Nevertheless, trials comparing these different approaches are lacking and there is an absence in consensus on the best approach for management of this condition. Thus, the aim of the current systematic review was to evaluate the outcomes of comparatives studies on endoscopic, percutaneous and surgical pancreatic pseudocyst drainage and to summarize the findings of available data.
Eligible studies were comparative studies on endoscopic, percutaneous or surgical methods of pancreatic pseudocyst drainage. The definition of pseudocyst was according to the revised Atlanta’s classification[1] (Table 1). In brief, pseudocyst referred to a fluid collection in the peri-pancreatic tissues persisting for more than 4 wk on computed tomography, surrounded by a well-defined wall and contained no solid material. Studies describing the results of pancreatic necrosis or abscesses were excluded. The indications for treatment of pancreatic pseudocyst was if they persisted for more than 4 to 6 wk and are ≥ 6 cm in size, causing symptoms or complications[3,4].
| Name of the collection | Definition |
| Onset < 4 wk after initial attack | |
| Acute peripancreatic fluid collection | Fluid collections that develop in the early phase of pancreatitis. They do not have a well-defined wall, are homogeneous, are confined by normal fascial planes in the retroperitoneum |
| Acute necrotic collection | A collection containing variable amounts of fluid and necrotic tissue without a well-defined wall |
| Onset ≥ 4 wk after initial attack | |
| Pancreatic pseudocyst | A collection of fluid in the peripancreatic tissues surrounded by a well-defined wall and contains no solid material |
| Walled-of pancreatic necrosis | A mature, encapsulated collection of pancreatic and/or peripancreatic necrosis and has a well-defined inflammatory |
| Any time after initial attack | |
| Infected necrosis | Presence of superimposed infection of the necrotic pancreas. May be indicated by presence of gas in the collection |
A computerized systematic literature review from January 1980 to May 2014 on PubMed, Embase and the Cochrane controlled trials register was performed. Articles were selected using MeSH headings and text words related to pancreatic pseudocyst, pseudocyst drainage, cystogastrostomy, cystojejunostomy, transmural pseudocyst drainage, transpapillary pseudocyst drainage and percutaneous pseudocyst drainage. Only English comparative studies involving the concerned treatment approaches were included. Reference lists from eligible trials were checked to locate missing publications. The titles of the articles and abstracts located were evaluated (Anthony Yuen Bun, TEOH1 and Vinay DHIR2). Where the article fulfilled the selection criterion, a copy of the full manuscript was obtained. Full manuscripts were then reviewed and a final decision was made about the inclusion. Studies published only in abstract form, conference abstracts, symposium proceedings and case reports were not eligible for inclusion. Any disagreements were resolved by consensus.
Data were extracted using a standard extraction form. Parameters included were study methodology (including randomization and blinding), inclusion criteria, demographics, the indications of treatment and types of pancreatic fluid collection. Procedural data including the technical approaches, methods of anastomosis, catheters and stents used were also recorded. The primary outcome was the treatment success rate. Secondary outcomes included were the recurrence rates, re-interventions, lengths of hospital stay, adverse events and mortalities. Treatment success was defined as radiographic cyst resolution after the index intervention. Re-intervention was defined as the need for repeat interventions owing to persistent symptoms in association with a residual pseudocyst. Adverse events were defined according the individual study criteria.
Assessment of methodological quality and risk of bias of the included studies. Assessment of risk of bias were performed by AT and VD according to principles of the Cochrane Handbook for systemic reviews of interventions version 5.1[5]. For randomized trials, the assessment focused on sequence generation, allocation concealment, blinding, incomplete outcome data, follow-up losses, intention to treat method of analysis and selective reporting. For non-randomized comparative trials, quality assessments were according to the Newcastle-Ottawa scale and the studies were scored on 3 domains including: Case selection, comparability of cases and controls and outcome assessments[6]. The results of this study were reported according to the PRISMA guidelines[7].
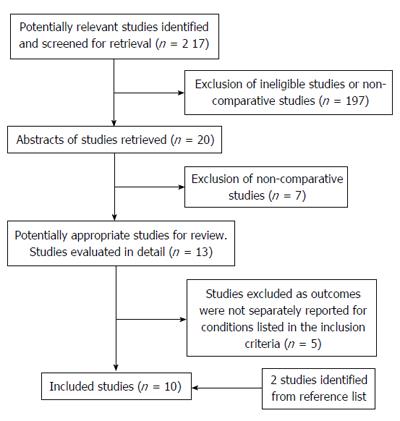
The search identified 217 potentially relevant publications and 20 articles were selected for reviewing of the abstracts. Seven studies were rejected as they were not comparative studies and the full manuscripts of the remaining 13 publications were reviewed. Two studies were further excluded as the outcomes for pseudocyst drainage were not separately reported and in 3 studies the outcomes of the different techniques were not reported individually. Two further articles were identified from the reference list of the included studies (Figure 1)[8-17]. Since there was significant heterogeneity amongst the study interventions, recruitment and outcome measurements, statistical pooling of the results was not performed.
Surgical drainage procedures: Cystogastrostomy, cystoduodenostomy and cystojejunostomy: Surgical drainage of pseudocysts is traditionally performed by the open approach[18,19]. However in recent years, laparoscopic pseudocyst drainage is increasingly reported[9,20]. For the open approach, midline or bilateral subcostal incisions were employed. The type of surgical drainage depended on the location of the cysts and whether it was adherent to the stomach or duodenum. When adhered to the posterior wall of the stomach, a cystogastrostomy were performed. If the cyst were not adhered to the stomach or duodenum, then a Roux-en Y cystojejunostomy would be fashioned. It is acknowledged that resectional procedures are sometimes required for patients with concomitant pancreatic ductal pathologies or complicated pseudocyst. However, resectional procedures do not have comparable endoscopic counterparts and these are not considered in this review.
In laparoscopic drainage procedures, various techniques have been described to replicate their open equivalents[9,20]. These include intragastric, transgastric or exogastric approaches and they differ in the method of accessing the posterior wall of the stomach to create a cystogastrostomy. The anastomosis is usually created with a laparoscopic stapler and the enterostomy closed by laparoscopic suturing. Laparsocopic cystojejunostomy is also possible for pseudocysts that protrude into the infracolic compartment and this is usually drained by a Roux-en Y jejunal loop.
Percutaneous drainage can be performed by ultrasound or computed tomography (CT) guidance and this can be achieved by the retroperitoneal route or transperitoneally[15-17]. The appropriate drainage site is first identified, followed by progressive track dilation and insertion of a 7 to 12 Fr drainage catheter into the pseudocyst. In patients that received transperitoneal drainage, a transgastric needle puncture can be performed and the passage through the stomach could allow subsequent exchange of a double pigtail stent and internalization into the stomach. In patients with retroperitoneal drainage, the pigtail stents would be connected to an external bag for free drainage.
Endoscopic drainage can be performed transpapillary or transmurally[21]. Transpapillary drainage can be performed if the pseudocyst communicates with the pancreatic duct on endoscopic retrograde cholangiopancreatography (ERCP) and a transpapillary stent is passed through the pancreatic duct into the pseudocyst. In patients with pancreatic ductal leak or ductal stricture, the stent may also serve to bridge the leak or stricture site[22].
Endoscopic transmural drainage can be performed with or without endoscopic ultrasound (EUS) guidance[11-13]. A prerequisite is that the pseudocyst is in direct apposition with the gastric or duodenal wall. When performed under esophagogastroduodenoscopy (EGD) guidance, the location of the pseudocyst is usually identified by the presence of bulging on the stomach wall. This is then confirmed by needle puncture, aspiration of the fluid and injection of contrast. A catheter and guide-wire is then passed into the pseudocyst. The fistula track is dilated with a balloon catheter and 1 or 2 plastic stents would be inserted. When performed under EUS guidance, the puncture site of the pseudocyst is chosen away from intervening vessels or structures. The pseudocyst is then punctured with a 19-gauge needle and a guide-wire passed to form 2 or more loops. The needle tract is dilated and plastic stents would be inserted. Recently, the use of metallic stents for draining pseudocyst has also been described but results from comparative studies are lacking[23,24]. All the studies included in the current review used plastic stents.
The identified studies covered a heterogeneous group of patients and mostly included small numbers from a single center (Table 2). In only one study, the outcomes of percutaneous drainage were compared to surgical drainage on a national level. Amongst the 10 included studies, 3 were randomized controlled trials[8,10,12]. One compared EUS drainage with open cystogastrostomy and 2 compared EGD vs EUS guided-drainage. The remaining seven studies were non-randomized trials, 1 compared laparoscopic, endoscopic and open cystogastrotomies[9], 1 study compared EUS drainage with open cystogastrostomy[10], 1 study compared EGD and EUS-guided drainage and 4 studies compared percutaneous and open surgical drainage[13-17]. The definition of pseudocyst was clearly stated in all the randomized studies and in 6 out of 7 non-randomized studies. The indications for intervention were defined in all the randomized studies and 2 non-randomized studies.
| Ref. | Design | Study duration | Follow-up duration1 | Interventions | Sample size | Pseudocyst defined | Inclusion criteria or indications for intervention |
| Varadarajulu et al[8] (United States) | Single center RCT | Jan 2009-Dec 2009 | 24 | EUS vs open cystogastrostomy | 20:20 | Yes | Pseudocyst > 6 cm and adjacent to stomach |
| History of acute or chronic pancreatitis | |||||||
| Persistent pain | |||||||
| Complications of pseudocyst | |||||||
| Melman et al[9] (United States) | Single center retrospective | Mar 1999-Aug 2007 | 9.5 | EUS vs laparoscopic vs open cystogastrostomy | 45:16:22 | Yes | Symptomatic pseudocyst |
| Varadarajulu et al[10] (United States) | Single center retrospective | Jul 2005-Jun 2007 | 24 | EUS vs Open cystogastrostomy | 20:10 | Yes | NA |
| Park et al[11] (South Korea) | Single center RCT | Jan 2004-Dec 2007 | 25 - 27 | EGD ± R-EUS vs EUS | 29:31 | Yes | Symptomatic pseudocyst > 4 wk |
| Varadarajulu et al[12] (United States) | Single center RCT | May 2007-Oct 2007 | NA | EGD vs EUS | 15:15 | Yes | Symptomatic pseudocyst > 4 wk |
| Kahaleh et al[13] (United States) | Single center retrospective | 2000-2005 | 11 | EGD vs EUS | 53:46 | Yes | NA |
| Morton et al[14] (United States) | National multicenter retrospective | Jan 1997-Dec 2001 | NA | Percutaneous vs Surgical drainage | 8121:6409 | Yes | NA |
| Heider et al[15] (United States) | Single center retrospective | 1984-1995 | NA | Percutaneous vs Surgical drainage | 66:66 | Yes | NA |
| Adams et al[16] (United States) | Single center retrospective | 1965-1991 | NA | Percutaneous vs Surgical drainage | 52:42 | No | Percutaneous drainage: Symptomatic pseudocyst > 5 cm without PD dilation |
| Lang et al[17] (United States) | Single center retrospective | Jan 1978-Jun 1988 | NA | Percutaneous vs Surgical drainage | 12:14 | Yes | Wall thickness < 3 mm |
The risks of bias in the randomized trials were assessed according to the principles of the Cochrane Handbook for systemic reviews of interventions (Table 3). None of the studies blinded the assessor of the outcomes. In one study comparing EGD vs EUS drainage[11], the patients randomized to the EGD arm also received EUS when the pseudocyst could not be located. This resulted in a hybrid technique and may contaminate the data in the EGD arm resulting in contamination bias. The risks of bias in non-randomized trials were assessed using the Newcastle-Ottawa scale (Table 4). Most studies were of moderate quality and scored between 4 to 7 stars out of 10.
Percutaneous vs surgical drainage: Four retrospective studies were included (Table 5). The largest United States study included more than 14000 patients (Percutaneous: 8121 and surgical: 6409) that were identified using a US national database[14]. Significant differences in background demographics between the groups were noted, including the cause of pseudocyst, the percentage of patients that received CT or ERCP and the proportion of patients that were treated in a teaching hospital. After adjusting for these confounding variables, a reduction in mortality was still observed in the surgical drainage arm (OR = 1.37, 95%CI: 1.12-1.68). Both emergency admission and acute pancreatitis increased the odds of in-patient mortality (OR = 2.45, 95%CI: 1.87-2.30 and OR = 2.36, 95%CI: 1.89-2.96, respectively) and the use of ERCP yielded a protective effect (OR = 0.68, 95%CI: 0.51-0.9). This study was the largest and most statistically robust amongst all the included studies. Yet, there is also a risk of selection biases, as the patients who were poor candidates for surgery tended to receive percutaneous drainage.
| Ref. | Sample size | Size (cm)1 | Clinical success | Hospital stay (d)1 | Reintervention | Mortalities | Adverse events | Bleeding | Intra-abdominal infection |
| Morton et al[14] | Perc: 8121 | - | - | 21 (22)2 | 5.9%2 | - | 9.64%2 | 6.8%2 | |
| Surg: 6409 | - | - | 15 (15) | 2.8% | - | 8.96% | 4.54% | ||
| Heider et al[15] | Perc: 66 | 8.2 (1.1) | 42% | 45 (5) | 50% | 9.1% | 64%2 | 9.1% | 45.5% |
| Surg: 66 | 7.4 (1.3) | 88% | 18 (2) | 12% | 0 | 27% | 4.5% | 15.2% | |
| Adams et al[16] | Perc: 52 | - | - | 36.7 | 9.5% | 2 | 7.7% | 1.9% | 1.9% |
| Surg: 42 | - | - | 39.8 | 19.2% | 7.1% | 16.7% | 4.8% | 4.8% | |
| Lang et al[17] | Perc: 26 | - | 76.9% | - | 11.5% | 3.8% | 3.8% | 3.8% | 0 |
| Surg: 26 | - | 73.1% | - | 23.1% | 3.8% | 0 | 0 | 0 |
Heider et al[15] compared the results of expectant treatment with percutaneous and open surgical drainage. No statistical analysis of the results was performed (no P-values given). The patients that were treated by percutaneous drainage had a re-intervention rate of 50%, adverse events rate of 67% and mortality rate of 9.1% and the results were worse than surgery. On the contrary, two smaller studies favored the percutaneous approach. Adams noted higher risk of mortalities, morbidities and re-interventions in patients that were treated with surgical drainage[16]. Whilst in another study, similar risks of mortalities and adverse events were observed in both groups but the patients that underwent surgery required more subsequent re-interventions[17].
It is worthwhile to note that the definition of pseudocyst in some of the older studies may not be according to the Atlanta’s classification and thus, the study population could include some patients with pancreatic necrosis and the results of these may need to be interpreted with caution. Based on the results of the national study, surgical drainage appeared to reduce mortality and adverse events risk as compared to the percutaneous approach. The lack of an external catheter also reduced risk developing pancreatic fistula and wound site infection. However, the validity of these results in the current era needs to be confirmed by a modernized randomized trial with updated definitions.
EUS vs surgical drainage: One randomized trial and two retrospective studies were included (Table 6). Varadarajulu et al[10] first published a retrospective case-matched study comparing EUS and open cystogastrostomy. No differences in treatment success, adverse events or re-interventions were noted between the groups. The same author then followed-up with the first randomized study, comparing 20 patients that received EUS drainage with an equal number receiving open cystogastrostomy[8]. The time to pseudocyst recurrence was used as the main outcome measurement. However, none of the patients in the EUS group developed recurrence, thus raising the issue of an underpowered study. Nevertheless, similar rates of clinical success, mortalities and morbidities were observed between the two groups. In addition, the EUS group was associated with significantly lower hospital costs (mean difference of -$8040 USD) and better quality of life scores (physical component scores and mental component scores). Hence, favoring the EUS approach over open cystogastrostomy.
| Ref. | Sample size | Size (cm) | Clinical success | Hospital stay (d) | Reintervention | Mortalities | Adverse events | Bleeding | Intra-abdominal infection |
| Varadarajulu et al[8] | EUS: 20 | 10.5 (9-14.9)1 | 95% | 2 (1-4)13 | 5% | 0 | 0 | 0 | 0 |
| Open: 20 | 11 (8.4-14.5)1 | 100% | 6 (5-9)1 | 5% | 0 | 2% | 1 | 0 | |
| Melman et al[9] | EUS: 45 | 9.1 (0.4) | 51.1%2 | 3.9 (0-25)2 | - | 0 | 15.6% | 2.2% | 0 |
| Lap: 16 | 10.4 (0.5) | 87.5% | 6.9 (3-23)2 | - | 0 | 25% | 12.5% | 0 | |
| Open: 22 | 9.5 (0.8) | 81.2% | 10.8 (4-82)2 | - | 0 | 22.7% | 0 | 0 | |
| Varadarajulu et al[10] | EUS: 20 | 9.8 | 95% | 2.6 (1-11)23 | 0 | 0 | 0 | 0 | 0 |
| Open: 10 | 8.9 | 100% | 6.5 (4-20)2 | 10% | 0 | 0 | 0 | 0 |
In another study comparing EUS, laparoscopic and open cystogastrostomy, a significantly higher rate of clinical success was observed in the surgery arm. However, the rate of clinical success in the EUS group was unusually low at 51.1% and grade 2 or above complications occurred in up to 15.6% of the patients. Three patients required urgent laparotomy and 2 experienced a gastric perforation. These results reflect that the endoscopist performing the procedures may still be overcoming their learning curves and the difference in outcomes may not be truly representative of the techniques. Nevertheless, this study was the only comparative study that incorporated the results of laparoscopic cystogastrostomy.
EUS vs EGD drainage: Two randomized trials and 1 retrospective comparison were included (Table 7)[11-13]. Kahaleh performed a retrospective comparison of patients that underwent EUS or EGD drainage[13]. Those with bulging pseudocyst underwent EGD drainage whilst patients with non-bulging cyst or those at risk of bleeding underwent EUS drainage. No difference in clinical success and adverse event rates were observed between the two groups. In a Korean randomized study, EUS was compared to a modified EGD approach[11]. In patients with bulging cyst, a blind EGD puncture was performed. Whilst in patients with the absence of bulging, radial EUS was employed to mark the site of puncture. This resulted in hybrid EUS-EGD approach in some of the patients. The trial found a significant difference in technical success rates in favor of the EUS approach (94% vs 72%, P = 0.039). The patients with failed EGD approach then crossed over to EUS drainage and this was successful in all patients. No differences in adverse events were observed in both arms. The third study was also a randomized study comparing EUS with pure EGD drainage of pseudocyst[12]. The EUS approach was shown to have significantly higher success rate as compared to the pure EGD technique (100% vs 33.3%, P < 0.001) and all patients with failed EGD drainage were successfully drained with the EUS technique. However, of more concern was that 2 patients in the EGD arm suffered from severe bleeding after drainage. One patient died within 4 h after the procedure due to massive bleeding into the cyst and another required endoscopic hemostasis and blood transfusion.
| Ref. | Sample size | Size (cm)1 | Clinical success | Hospital stay (d) | Reintervention | Mortalities | Adverse events | Bleeding | Intra-abdominal infection |
| Park et al[11] | EUS: 31 | 8.2 (3.8) | 89% | - | 6.5% | 0 | 7% | 3.2% | - |
| EGD: 29 | 7.4 (4) | 86% | - | 6.5% | 0 | 10% | 6.9% | - | |
| Varadarajulu et al[12] | EUS: 15 | 6.5 (5-12)2 | 100%5 | 2 (1-9)2 | - | 0 | 0 | 0 | - |
| EGD: 15 | 7 (4.2-13)2 | 33%4 | 1 (1-8)2 | - | 6.7% | 13.3% | 13.3% | - | |
| Kahaleh et al[13] | EUS: 46 | 8.6 (4-20)3 | 84% | - | 10.9% | 0 | 19.6% | 4.3% | 8.7% |
| EGD: 53 | 9.5 (3-20)3 | 91% | - | 9.4% | 0 | 18.9% | 1.9% | 7.5% |
Hence, the results of these studies suggest that although a blind EGD pseudocyst drainage is technically feasible, it may result in life-threatening adverse events. The success rate of the EUS approach was better for non-bulging cyst and the approach conferred additional safety benefits by allowing visualization of extraluminal structures.
Although the current review has established a strict criterion for inclusion, the included studies incorporated a heterogeneous group of patients that were treated with a number of different approaches. Thus, the results were not directly comparable and statistical analysis in a form of meta-analysis was inappropriate. Nevertheless, a number of conclusions could still be made. EUS-guided drainage has similar efficacy to surgery but the EUS approach may reduce hospital stay, costs of the procedure and improve quality of life. EGD and EUS-guided drainages are both feasible but the success rate of the EUS approach is better for non-bulging cyst and it may offer additional safety benefit. Whether surgical internal drainage of pancreatic pseudocyst is preferred over percutaneous drainage needs to be validated, as no results from a modern study are available. However, surgical cystogastrostomy may still be preferred it avoids the need of an external catheter and reduces the risk developing an external pancreatic fistula. Consequently, the EUS approach is preferred when anatomy of the pseudocyst allows for direct drainage into the stomach or duodenum. However, if the pseudocyst is located away from the stomach or duodenum, surgical cystojejunostomy or percutaneous drainage could be considered. In addition, it is acknowledged that laparoscopic drainage is the modern minimally invasive approach for surgical drainage. However, results from comparative studies were lacking and the long-term outcomes of the treatment approaches could not be made.
The current study is the only systematic review comparing percutaneous, endoscopic and surgical drainage of pseudocyst. A prior systematic review compared endoscopic and laparoscopic internal drainage by summarizing the results from cohort studies without direct statistical comparison[20]. No randomized or comparative studies were available. The review concluded that both approaches were safe and the laparoscopic approach appeared to have a higher success rate, lower morbidity and recurrence. In a meta-analysis comparing EGD and EUS-guided drainage, 2 randomized studies and 2 prospective studies were included[25]. Technical success was higher for EUS drainage (RR = 12.38, 95%CI: 1.39-110.22) and adverse events were similar between the two techniques. The review concluded that for bulging pseudocysts, both approaches could be selected whereas for non-bulging pseudocyst, portal hypertension or coagulopathy, EUS drainage is the preferred modality.
There were some limitations to the current study. Firstly, the numbers of high quality comparative studies assessing the 3 approaches were lacking. Hence, the robustness of the results generated in this review is limited by the quality of the original studies. Furthermore, with regards to the available randomized trials, all were single center studies with small sample sizes and they were not designed to detect differences in recurrence rates or adverse event rates between the modalities. Thus, the results were prone to type II error. In addition, the literature search failed to identify any comparative studies involving endoscopic transpapillary drainage and laparoscopic internal drainage. Therefore, conclusions regarding these approaches could not be made. Furthermore, it was observed that many of the studies did not report on the follow-up time or only reported a very short follow-up period. This may not be adequate to detect longer-term recurrence. Lastly, the definitions of pseudocyst has changed over time and may be different for each study, thus of the patients included in the current review may not be suffering from the modern definition of pseudocyst and the outcomes of treatment may be affected by the definition.
Currently, there is a lack of consensus in the best practice for pseudocyst drainage. A number of professional bodies have attempted to establish guidelines regarding the management of complications of acute pancreatitis including infected pseudocyst and pancreatic necrosis[26]. However, none of these guidelines have received widespread acceptance. In a systemic review of 16 guidelines published by profession bodies, it was observed that the guidelines lacked consensus and few were graded according to the strength of evidence. In addition, there were wide variations in the recommendations regarding the role of percutaneous and endoscopic drainage of pancreatic fluid collections. For infected pseudocyst, percutaneous drainage was recommended by 6 guidelines, 1 did not recommend its use and for endoscopic drainage, the approach was recommended by 7 guidelines. A recent guideline published by the International Association of Pancreatology and the American Association of Pancreateology, represented the best evidenced-based recommendations concerning key aspects the management of acute pancreatitis[27]. However, the optimal management of pseudocysts were not discussed and there is still a pressing need for more randomized studies to establish the best approach for management of this condition.
In conclusion, significant heterogeneity was present in the included studies and a clear conclusion could not be made. However, EUS-guided drainage appeared to be advantageous in drainage of pancreatic pseudocysts located adjacent to the stomach or duodenum. In patients with unfavorable anatomy, surgical cystogastrostomy or percutaneous drainage could be considered. Large randomized studies with current definitions of pseudocysts and longer-term follow-up are needed to assess the efficacy of the various modalities.
The authors would like to extend the deepest gratitude to all Asian EUS group members for their support to the group. We would also like to thank Mr. Steven Chan and his team in their excellent support during all AEG related activities.
Pancreatic pseudocysts are traditionally managed by open surgical internal drainage. With continued improvements in medical technology, the uses of percutaneous, endoscopic and laparoscopic drainage were increasingly reported. Nevertheless, trials comparing these different approaches are lacking. Thus, the aim of this study is to perform a systematic review comparing the outcomes of endoscopic, percutaneous and surgical pancreatic pseudocyst drainage.
Currently, there is a lack of consensus in the best practice for pseudocyst drainage. A number of professional bodies have attempted to establish guidelines regarding the management of complications of acute pancreatitis including infected pseudocyst and pancreatic necrosis. However, the guidelines lacked consensus and few were graded according to the strength of evidence.
Endoscopic ultrasound (EUS)-guided pseudocyst drainage is an endoscopic approach for establishing internal transmural drainage of a pseudocyst. The approach allows visualization of extra-mural structures to allow precise placement of internal stents.
In the current study, the authors conclude that EUS-guided drainage appeared to be advantageous in drainage of pancreatic pseudocysts located adjacent to the stomach or duodenum. In patients with unfavorable anatomy, surgical cystojejunostomy or percutaneous drainage could be considered. Large randomized studies with current definitions of pseudocysts and longer-term follow-up are needed to assess the efficacy of the various modalities.
Pseudocyst are fluid collections in the peri-pancreatic tissues persisting for more than 4 wk on computed tomography, surrounded by a well-defined wall and contained no solid material after an attack of pancreatitis.
The manuscript gives an overview of publications on outcome of endoscopic drainage of pancreatic pseudocysts, compared with percutaneous and/or surgical drainage.
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4724] [Article Influence: 363.4] [Reference Citation Analysis (48)] |
| 2. | Aghdassi AA, Mayerle J, Kraft M, Sielenkämper AW, Heidecke CD, Lerch MM. Pancreatic pseudocysts--when and how to treat? HPB (Oxford). 2006;8:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Yeo CJ, Bastidas JA, Lynch-Nyhan A, Fishman EK, Zinner MJ, Cameron JL. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet. 1990;170:411-417. [PubMed] |
| 4. | Bradley EL, Clements JL, Gonzalez AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg. 1979;137:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 256] [Article Influence: 5.4] [Reference Citation Analysis (3)] |
| 5. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. 2011;. |
| 6. | Wells GA, Shea B, O’Connell D, Peterson J, Welch WV, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed 2014 May]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. |
| 7. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13822] [Article Influence: 813.1] [Reference Citation Analysis (3)] |
| 8. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (2)] |
| 9. | Melman L, Azar R, Beddow K, Brunt LM, Halpin VJ, Eagon JC, Frisella MM, Edmundowicz S, Jonnalagadda S, Matthews BD. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc. 2009;23:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, Christein JD. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 284] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Morton JM, Brown A, Galanko JA, Norton JA, Grimm IS, Behrns KE. A national comparison of surgical versus percutaneous drainage of pancreatic pseudocysts: 1997-2001. J Gastrointest Surg. 2005;9:15-20; discussion 20-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Heider R, Meyer AA, Galanko JA, Behrns KE. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999;229:781-787; discussion 787-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg. 1992;215:571-576; discussion 576-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Lang EK, Paolini RM, Pottmeyer A. The efficacy of palliative and definitive percutaneous versus surgical drainage of pancreatic abscesses and pseudocysts: a prospective study of 85 patients. South Med J. 1991;84:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Frey CF. Pancreatic pseudocyst--operative strategy. Ann Surg. 1978;188:652-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Aranha GV, Prinz RA, Freeark RJ, Kruss DM, Greenlee HB. Evaluation of therapeutic options for pancreatic pseudocysts. Arch Surg. 1982;117:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Aljarabah M, Ammori BJ. Laparoscopic and endoscopic approaches for drainage of pancreatic pseudocysts: a systematic review of published series. Surg Endosc. 2007;21:1936-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Binmoeller KF, Seifert H, Walter A, Soehendra N. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 207] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Varadarajulu S, Noone TC, Tutuian R, Hawes RH, Cotton PB. Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placement. Gastrointest Endosc. 2005;61:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Weilert F, Binmoeller KF, Shah JN, Bhat YM, Kane S. Endoscopic ultrasound-guided drainage of pancreatic fluid collections with indeterminate adherence using temporary covered metal stents. Endoscopy. 2012;44:780-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012;75:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 25. | Panamonta N, Ngamruengphong S, Kijsirichareanchai K, Nugent K, Rakvit A. Endoscopic ultrasound-guided versus conventional transmural techniques have comparable treatment outcomes in draining pancreatic pseudocysts. Eur J Gastroenterol Hepatol. 2012;24:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Loveday BP, Mittal A, Phillips A, Windsor JA. Minimally invasive management of pancreatic abscess, pseudocyst, and necrosis: a systematic review of current guidelines. World J Surg. 2008;32:2383-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1095] [Article Influence: 84.2] [Reference Citation Analysis (10)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Boulay B, Buanes TA, De Palma GD, Osawa S, Thomopoulos KC, Wilcox CM S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ