Published online Mar 10, 2016. doi: 10.4253/wjge.v8.i5.252
Peer-review started: May 7, 2015
First decision: July 17, 2015
Revised: October 24, 2015
Accepted: December 29, 2015
Article in press: January 1, 2016
Published online: March 10, 2016
Processing time: 309 Days and 1.4 Hours
Colorectal cancer (CRC) is the 2nd most common cancer in women and 3rd most common cancer in men worldwide. Most CRCs develop from adenomatous polyps arising from glandular epithelium. Tumor growth is initiated by mutation of the tumor suppressor gene APC and involves other genetic mutations in a stepwise process over years. Both hereditary and environmental factors contribute to the development of CRC. Screening has been proven to reduce the incidence of CRC. Screening has also contributed to the decrease in CRC mortality in the United States. However, CRC incidence and/or mortality remain on the rise in some parts of the world (Eastern Europe, Asia, and South America), likely due to factors including westernized diet, lifestyle, and lack of healthcare infrastructure. Multiple screening options are available, ranging from direct radiologic or endoscopic visualization tests that primarily detect premalignant or malignant lesions such as flexible sigmoidoscopy, optical colonoscopy, colon capsule endoscopy, computed tomographic colonography, and double contrast barium enema - to stool based tests which primarily detect cancers, including fecal DNA, fecal immunochemical test, and fecal occult blood test. The availability of some of these tests is limited to areas with high economic resources. This article will discuss CRC epidemiology, pathogenesis, risk factors, and screening modalities with a particular focus on new technologies.
Core tip: Multiple societies have issued screening guidelines for colorectal cancer (CRC). However, global CRC screening implementation can be challenging due to wide variability in healthcare infrastructure and resources in different countries. The practical implementation of CRC screening in a given area depends mainly upon availability of endoscopic resources. In areas with the greatest healthcare resources, colonoscopy remains the gold standard, although technological advances have provided alternative screening methods including computed tomographic colonography, fecal DNA testing, and colon capsule endoscopy. In areas with fewer healthcare resources, guaiac-based fecal occult blood testing is the predominant screening modality.
- Citation: El Zoghbi M, Cummings LC. New era of colorectal cancer screening. World J Gastrointest Endosc 2016; 8(5): 252-258
- URL: https://www.wjgnet.com/1948-5190/full/v8/i5/252.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i5.252
Colorectal cancer (CRC) is the second most common cancer in women and third most common cancer in men worldwide[1]. Globally, there is marked variation in CRC incidence and mortality[1,2]. Some countries in Eastern Europe and Asia have demonstrated increasing incidence rates (Slovakia, Czech Republic, Singapore, and Japan) which have been attributed to behavioral risk factors related to westernization of diet and lifestyle[3]. In addition, some countries (Brazil, Mexico, and Romania) have experienced increasing CRC mortality rates from CRC purportedly due to limited healthcare resources[4]. In the United States, CRC is the third leading cause of cancer death and accounts for approximately 7% and 9% of overall cancer deaths in females and males, respectively[5]. CRC incidence and mortality rates have been declining in the United States secondary to increased screening mainly via colonoscopy, which enables primary prevention and early detection[6,7]. In recent years, technological advances have led to the development of new, less invasive screening modalities including fecal immunochemical testing, computed tomographic colonography (CTC), stool DNA testing, and colon capsule endoscopy. This article will discuss CRC pathogenesis, risk factors, and screening with a particular focus on new screening methods.
Most colorectal carcinomas develop from adenomatous polyp arising from the glandular epithelium of the intestine[8]. Adenomas are initiated by somatic mutation of the tumor suppressor gene APC[9]. Additional genetic alterations of oncogenes and tumor suppressor genes are involved in a stepwise growth process that occurs over years[10-12]. The accumulation of genetic mutations in accordance with chromosomal instability, shifts the normal intestinal lining to an adenomatous polyp, then high-grade adenoma and finally to a carcinoma[13,14]. CRC can also arise from nonpolypoid and depressed lesions. Although these lesions are less common than that of the polypoid adenoma, they manifest more aggressive behavior and more rapid growth, and they are more difficult to diagnose[15,16].
Available tests for CRC screening are divided into 2 major types, stool-based tests or endoscopic and radiologic tests. The stool-based tests include the guaiac-based fecal occult blood test (gFOBT), fecal immunochemical test (FIT), and fecal DNA testing. These tests detect cell debris and blood shed by vascularized polyps, adenomas and cancers[17]. The endoscopic and radiologic examinations include optical colonoscopy, flexible sigmoidoscopy (FS or FSIG), double-contrast barium enema (DCBE), capsule endoscopy, and CTC and are based on direct or radiographic visualization of the polyp or cancer.
gFOBT detects the presence of blood in feces through a chemical reaction dependent upon the peroxidase activity of heme. It is an inexpensive test that can be mailed to patients. Annual or biennial gFOBT have shown to decrease CRC mortality rates by 15%-33%[18-20]. In the Minnesota Colon Cancer Control Study, a 30-year follow-up of patients randomly assigned to annual/or biennial gFOBT vs usual care showed a 32% decrease in CRC mortality. Furthermore, mortality reduction was more pronounced in men compared to women[21].
A disadvantage of gFOBT is the requirement for 3 different stool samples[22]. This makes collection more cumbersome to the patient, which results in lowered adherence and thus decreases its effectiveness as a screening test[23,24]. gFOBT endorses a risk of false-positive results if patients ingest animal products or vegetables prior to testing, or if the patient is on anticoagulants or antiplatelet agents[25]. On the other hand, a risk of false negative test arises if patient is on ascorbic acid or any other form of antioxidants[26].
FIT is an antibody-based test that detects and binds to the globin component of hemoglobin. The FIT sampling technique is simpler and easier to collect compared to that of gFOBT. Only one or two fecal samples are required and no dietary or medication restrictions are needed prior to the test. The overall accuracy of FIT for detection of CRC was 95% with 79% sensitivity and 94% specificity as been shown in systematic review and meta-analysis including 19 qualified studied performed by Lee et al[27]. FIT has been shown to have a greater sensitivity in detecting advanced adenomas and CRC than gFOBT[28-31].
A disadvantage of FIT is its more expensive cost compared to FOBT. Although FIT is easier to collect, its sensitivity decreases with any delay in mailing or processing of the sample. Furthermore, similar to other non-invasive tests, if the test is positive, a follow-up colonoscopy would be needed.
Fecal DNA testing, or Cologuard (Exact Sciences), is a non-invasive, easy to perform test based on a single stool sample, and does not require dietary or medication restriction. It is a composite test that includes an immunochemical assay similar to the one used in FIT, methylated markers and molecular mutations markers associated with CRC. In 2014, this test was approved by the United States Food and Drug Administration as a screening test for CRC.
One multicenter study on 9989 patients comparing fecal DNA test to FIT using colonoscopy as the gold standard showed that the fecal DNA test had a higher sensitivity than FIT for detecting CRC, (92% vs 74%), adenomas with high-grade dysplasia (69% vs 46%), and serrated sessile polyps (42% vs 5%). However, specificity was lower with fecal DNA test at 87%-90% compared to FIT at 95%-96%[32].
In a large multicenter case-control study, automated fecal DNA testing accurately detected CRC regardless of the site or the stage of the lesion with an overall sensitivity of more than 98%. Sensitivity for precancerous lesions increased in proportion to lesion size from 57% for lesions > 1 cm to 83% for those > 3 cm[33].
Disadvantages of fecal DNA testing include its expensive cost; the inconvenience stool sampling and shipment to the lab; and the need for colonoscopy if the test is positive.
DCBE is a non-invasive radiological test, which provides a complete evaluation of the large intestine. The sensitivity and specificity of barium enema for polyps of any size is 38% and 86%, respectively[34]. One study comparing barium enema to CT colonography and colonoscopy showed that DCBE has the lowest sensitivity and specificity with sensitivity of 41% for lesions ≥ 6 mm and sensitivity and specificity of 48% and 90% respectively for lesions ≥ 10 mm[35]. These results are consistent with a meta-analysis comparing the performance of barium enema to that of CTC showing CTC is more sensitive and more specific than barium enema for large polyps (≥ 10 mm) and small polyps (6-9 mm) in average-risk and high-risk populations[36]. In the United States, CT colonography has largely replaced DCBE as a radiographic option for CRC screening. A disadvantage of DCBE is that the test must be followed by colonoscopy if abnormalities are found.
Optical colonoscopy entails direct visualization of the colonic mucosa from the cecum to the rectum with a flexible endoscope. Insufflation, irrigation, and suction facilitate careful inspection of the mucosa. Colonoscopy allows both detection and removal of polyps, which can be submitted for histopathological examination. Colonoscopy is routinely performed in some countries with sedation, whereas in others sedation is rarely used. Colonoscopy requires a bowel preparation with a laxative and clear liquid diet prior to the procedure. Split-dose protocols, in which patients ingest half the bowel preparation the day of the procedure, may encourage compliance and are now recommended for optimal bowel cleansing[37]. Procedural risks include cardiopulmonary complications due to sedation, the possibility of missed lesions, bleeding, and a 0.08% rate of perforation, which is typically related to polypectomy[38]. Although traditionally colonoscopy has been considered to be the gold standard for CRC screening, the miss rate for adenomas ≥ 1 cm was 6% in a tandem colonoscopy study[39]. Moreover, colonoscopy is less effective at reducing proximal compared with distal CRCs[40-43]. This finding may result from a combination of factors including inadequate bowel preparation, which is more likely to affect the right colon; incomplete colonoscopy; and a higher prevalence in the proximal colon of non-polypoid colorectal neoplasms, which are often more difficult to detect than traditional polypoid neoplasms[44]. Based on pooled data from several large North American studies, 0.6% of patients with adenomas developed CRC within an average of 4 years after clearing colonoscopy[45]. Fifty-two percent of these cancers were felt to be missed lesions, 19% were thought to be potentially incompletely resected lesions, and 24% were thought to be new lesions. These statistics reflect the fact that colonoscopy is operator dependent. Indeed, the development of interval cancers within 3 years after colonoscopy has been associated with performance of colonoscopy by non-gastroenterologists[46].
FS is used to visualize the left-sided or descending colon and the rectum where approximately 60% of all CRCs develop. Compared to colonoscopy, FS is safer, faster, and more easily tolerated procedure. Sedation is not required, and self-administered enemas are usually used in bowel preparation[47,48].
Screening with FS decreases the incidence and overall mortality of CRC[48,49]. A large randomized control trial involving 34272 participants between the ages of 55 and 64 years with a median follow-up of around 11 years showed a 31% decrease in the incidence of CRC and a 38% decrease in CRC mortality after one-time screening with FS, compared with no screening[49].
A disadvantage of FS is that follow-up colonoscopy is required given that about 3%-5% of patients with CRC in the distal colon will have lesions in the proximal colon[50]. In the United States, colonoscopy has largely replaced FS for CRC screening.
With capsule endoscopy (Pillcam COLON, Given Imaging Ltd, Yoqneam, Israel) the patient swallows a capsule which records digital images on 2 camera heads at a rate ranging from 4 to 35 frames per second for approximately 10 h. These images are then transmitted wirelessly to a recording device carried by the patient. The data are transferred from the device to a computer that uses a software (RAPID) to compile the video to be analyzed then by an experienced gastroenterologist[51]. Indications for colon capsule endoscopy have not been standardized; the use of CE is recommended in cases of colonoscopy contraindication, colonoscopy failure, or in patients unwilling to perform colonoscopy. In the United States, the Food and Drug Administration has approved Pillcam COLON 2 (second generation) for patients who have had an incomplete colonoscopy.
A recent prospective study conducted by Doug Rex on 884 patients comparing accuracy of PillCam COLON 2 to that of optical colonoscopy demonstrated 88% sensitivity and 82% specificity in detecting adenoma ≥ 6 mm in average risk screening population[52].
An advantage of capsule endoscopy compared to other non-invasive methods is the lack of radiation exposure. Disadvantages of capsule endoscopy include the need for a complex bowel preparation regimen and the risk, albeit low, of capsule retention, which may necessitate surgical removal.
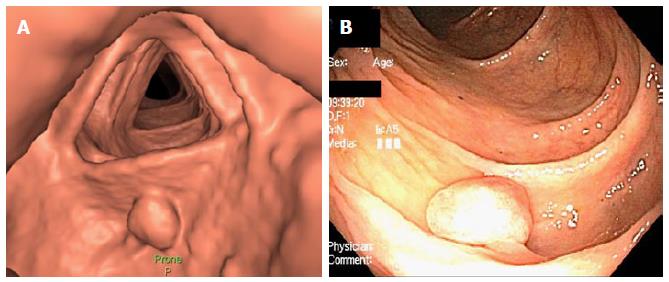
CTC, or virtual colonoscopy, is a radiographic imaging test in which two-dimensional or three-dimensional images of the colon and rectum are generated using specialized computer software and abdominal computed tomography scanning. It is offered to the patient if colonoscopy is incomplete or in the event of patients’ refusal or has additional risk factors. CT colonography every 5 years is a screening option according to some CRC screening guidelines (see below). Multiple steps are involved in completing CTC. The first step is the bowel preparation, which includes a fiber-free diet and ingestion of a laxative and contrast medium prior to the test. The second step is colonic insufflation, which is done by insufflation of CO2via a rectal catheter and bulb in a gradual manner with a controlled pressure to prevent perforation. The third step is acquisition of the radiographic images. An adjusted scout-view is obtained so that the entire colon is covered. Images are obtained in 2 positions, supine and prone; decubitus lateral positions are performed if patient is overweight.
The final step is interpretation of images; two dimensional interpretation identifies any lesion that is larger than a centimeter. The infracentimetric lesions are identified via three dimensional interpretation. After identification of a polyp-like lesion, its density should be determined. The lipoma or an inverted tumor is fatty, fecal residue is dense, and tumor tissue’s density is similar to that of the colonic wall. Each lesion is then classified by C-RAD, which specifies the site, the shape, type of density, and the largest diameter of the head of the polyp. A colonoscopy is indicated for lesions that are ≥ 10 mm or more than 3 lesions > 5 mm[53]. Figure 1 displays a polyp visualized on CT colonography and subsequent colonoscopy.
A multicenter trial enrolling 845 patients who underwent screening with CTC followed by colonoscopy showed 69% sensitivity and 91% specificity in detecting polyps > 6 mm[54]. CTC was found to accurately detect 90% of lesions > 10 mm in diameter[55]. The detection rate for advanced neoplasm was found to be similar for patients undergoing CTC compared to colonoscopy, while the rate of polypectomies and complications was considerably smaller in the CTC group compared to that of colonoscopy[56]. Radiation exposure is one of disadvantage of CTC[57]. In addition, perforation is still a risk, although it is less than that with colonoscopy[36].
In the United States, the two major guidelines for CRC screening are: (1) joint guidelines from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology; and (2) the US Preventive Services Task Force (USPSTF) guidelines. Other organizations have issued their own guidelines as well, such as the American College of Gastroenterology and the American College of Physicians. Table 1 summarizes the varying recommendations from these different sets of guidelines for average risk individuals. USPSTF guidelines were issued in 2008 and are in the process of being updated. On a global level, CRC screening can be challenging to implement due to wide variability in healthcare infrastructure and resources in different countries. The World Gastroenterology Organization practice guidelines on CRC screening provide differing recommendations for average risk screening depending upon the availability of endoscopic resources[58]. In areas with the lowest access to FS and colonoscopy, for example, biennial gFOBT or FIT is recommended, while colonoscopy every 10 years is recommended in areas with greater healthcare and endoscopic resources.
| Joint guidelines | USPSTF | ACG | ACP | |
| Flexible sigmoidoscopy | Every 5 yr | Every 5 yr, with high sensitivity FOBT every 3 yr | Every 5-10 yr | Every 5 yr |
| Colonoscopy | Every 10 yr | Every 10 yr | Every 10 yr | Every 10 yr |
| Barium enema | Every 5 yr | Not recommended | Not recommended | Every 5 yr |
| CT colonography | Every 5 yr | Insufficient evidence to recommend | Every 5 yr | Every 5 yr |
| gFOBT | Annual | Annual | Annual | Annual |
| FIT | Annual | Every year | Annual | Annual |
| sDNA | Uncertain | Insufficient evidence to recommend | Every 3 yr | Uncertain |
CRC screening is associated with decreased CRC incidence and mortality. CRC screening modalities include radiographic or endoscopic methods (colonoscopy, FS, CT colonography, double contrast barium enema, colon capsule endoscopy) and stool-based tests (fecal DNA test, gFOBT, and FIT). Options for screening also depend upon the healthcare infrastructure of the country including the availability of endoscopic resources. In offering CRC screening, the physician should discuss with the patient the advantages and disadvantages of each test and ascertain the patient’s preferences for better adherence.
We thank Luis Landeras, MD, for providing the CT colonographic image.
P- Reviewer: Tsuji Y S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21451] [Article Influence: 1950.1] [Reference Citation Analysis (6)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25598] [Article Influence: 1706.5] [Reference Citation Analysis (10)] |
| 3. | Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 873] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 4. | Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1907] [Article Influence: 119.2] [Reference Citation Analysis (1)] |
| 5. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9977] [Article Influence: 907.0] [Reference Citation Analysis (15)] |
| 6. | Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev. 2012;21:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1519] [Article Influence: 94.9] [Reference Citation Analysis (1)] |
| 8. | Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1013] [Article Influence: 53.3] [Reference Citation Analysis (3)] |
| 9. | Lamlum H, Papadopoulou A, Ilyas M, Rowan A, Gillet C, Hanby A, Talbot I, Bodmer W, Tomlinson I. APC mutations are sufficient for the growth of early colorectal adenomas. Proc Natl Acad Sci USA. 2000;97:2225-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (11)] |
| 10. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4489] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 11. | Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251-2270. [PubMed] |
| 12. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 13. | Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1389] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 14. | Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1417] [Article Influence: 83.4] [Reference Citation Analysis (12)] |
| 15. | Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S, Sanders DS, Lobo AJ. A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the United Kingdom. Am J Gastroenterol. 2003;98:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 17. | Carroll MR, Seaman HE, Halloran SP. Tests and investigations for colorectal cancer screening. Clin Biochem. 2014;47:921-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 1839] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 19. | Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1640] [Cited by in RCA: 1605] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 20. | Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2185] [Article Influence: 66.2] [Reference Citation Analysis (1)] |
| 21. | Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 661] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 22. | Deutekom M, van Rossum LG, van Rijn AF, Laheij RJ, Fockens P, Bossuyt PM, Dekker E, Jansen JB. Comparison of guaiac and immunological fecal occult blood tests in colorectal cancer screening: the patient perspective. Scand J Gastroenterol. 2010;45:1345-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Gellad ZF, Stechuchak KM, Fisher DA, Olsen MK, McDuffie JR, Ostbye T, Yancy WS. Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol. 2011;106:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Fenton JJ, Elmore JG, Buist DS, Reid RJ, Tancredi DJ, Baldwin LM. Longitudinal adherence with fecal occult blood test screening in community practice. Ann Fam Med. 2010;8:397-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Clarke P, Jack F, Carey FA, Steele RJ. Medications with anticoagulant properties increase the likelihood of a negative colonoscopy in faecal occult blood test population screening. Colorectal Dis. 2006;8:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Jaffe RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83:824-826. [PubMed] |
| 27. | Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 510] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 28. | Allison JE, Fraser CG, Halloran SP, Young GP. Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (FIT). Gut Liver. 2014;8:117-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Allison JE, Lawson M. Screening tests for colorectal cancer: a menu of options remains relevant. Curr Oncol Rep. 2006;8:492-498. [PubMed] |
| 31. | Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638-658. [PubMed] |
| 32. | Imperiale TF, Ransohoff DF, Itzkowitz SH. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;371:187-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Lidgard GP, Domanico MJ, Bruinsma JJ, Light J, Gagrat ZD, Oldham-Haltom RL, Fourrier KD, Allawi H, Yab TC, Taylor WR. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2013;11:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 34. | Winawer SJ, Stewart ET, Zauber AG, Bond JH, Ansel H, Waye JD, Hall D, Hamlin JA, Schapiro M, O’Brien MJ. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work Group. N Engl J Med. 2000;342:1766-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 327] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, Yee J, Henderson J, Hatten P, Burdick S. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 36. | Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA. Potentially Serious Adverse Events at CT Colonography in Symptomatic Patients: National Survey of the United Kingdom 1. Radiology. 2006;239:464-471. |
| 37. | Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, Robertson DJ, Boland CR, Giardello FM, Lieberman DA. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2014;147:903-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 317] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 38. | Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [PubMed] |
| 40. | Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6:1117-1121; quiz 1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Raginel T, Puvinel J, Ferrand O, Bouvier V, Levillain R, Ruiz A, Lantieri O, Launoy G, Guittet L. A population-based comparison of immunochemical fecal occult blood tests for colorectal cancer screening. Gastroenterology. 2013;144:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1184] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 43. | Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 44. | Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, Soetikno RM, Masclee AA, Sanduleanu S. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 46. | Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012;118:3044-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 47. | Atkin WS, Cook CF, Cuzick J, Edwards R, Northover JM, Wardle J. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 316] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 48. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 49. | Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103:1310-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 50. | Cappell MS. The pathophysiology, clinical presentation, and diagnosis of colon cancer and adenomatous polyps. Med Clin North Am. 2005;89:1-42, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Mamonov AV, Figueiredo IN, Figueiredo PN, Tsai YH. Automated polyp detection in colon capsule endoscopy. IEEE Trans Med Imaging. 2014;33:1488-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 52. | Rex DK, Adler SN, Aisenberg J, Burch WC, Carretero C, Chowers Y, Fein SA, Fern SE, Fernandez-Urien Sainz I, Fich A. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015;148:948-957.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 53. | Gandon Y. Screening for colorectal cancer: the role of CT colonography. Diagn Interv Imaging. 2014;95:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Heresbach D, Djabbari M, Riou F, Marcus C, Le Sidaner A, Pierredon-Foulogne MA, Ponchon T, Boudiaf M, Seyrig JA, Laumonier H. Accuracy of computed tomographic colonography in a nationwide multicentre trial, and its relation to radiologist expertise. Gut. 2011;60:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, Menias CO, Siewert B, Cheema JI, Obregon RG. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 712] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 56. | Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, Gopal DV, Reichelderfer M, Hsu RH, Pfau PR. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 467] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 57. | Lin OS. Computed tomographic colonography: hope or hype? World J Gastroenterol. 2010;16:915-920. [PubMed] |
| 58. | Winawer SJ, Krabshuis J, Lambert R, O’Brien M, Fried M. Cascade colorectal cancer screening guidelines: a global conceptual model. J Clin Gastroenterol. 2011;45:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |